Abstract
Recurrence of idiopathic focal segmental glomerulosclerosis (FSGS) after renal transplantation is believed to be caused by a circulating factor(s). We detected cardiotrophin-like cytokine 1 (CLCF1), a member of the IL-6 family, in the plasma from patients with recurrent FSGS. We hypothesized that CLCF1 contributes to the effect of FSGS serum on the glomerular filtration barrier in vitro. Presently, we studied the effect of CLCF1 on isolated rat glomeruli using an in vitro assay of albumin permeability (Palb). CLCF1 (0.05–100 ng/mL) increased Palb and caused maximal effect at 5–10 ng/mL (P<0.001). The increase in Palb was analogous to the effect of FSGS serum. Anti-CLCF1 monoclonal antibody blocked the CLCF1-induced increase in Palb and significantly attenuated the effect of FSGS serum (P<0.001). The heterodimer composed of CLCF1 and co-secreted molecule cytokine receptor-like factor-1 (CRLF1) attenuated the increase in Palb caused by CLCF1 or FSGS serum. Western blot analysis showed that CLCF1 upregulated phosphorylation of STAT3 (Tyr705) in glomeruli. This effect was diminished by the heterodimer CLCF1-CRLF1. JAK2 inhibitor BMS-1119543 or STAT3 inhibitor Stattic significantly blocked the effect of CLCF1 or FSGS serum on Palb (P<0.001). These novel findings suggest that while monomeric CLCF1 increases Palb, the heterodimer CLCF1-CRLF1 may protect the glomerular filtration barrier. We speculate that albuminuria in FSGS is related to qualitative or quantitative changes in the CLCF1-CRLF1 complex and, that JAK2 or STAT3 inhibitors may be novel therapeutic agents to treat FSGS.
Keywords: Focal segmental glomerulosclerosis, Glomerular filtration barrier, Cardiotrophin-like cytokine 1 Factor, Cytokine Receptor-Like Factor-1, JAK/STAT signaling pathway
INTRODUCTION
Focal segmental glomerulosclerosis (FSGS) describes a characteristic histological pattern of focal and segmental scarring of the glomerulus in renal biopsy. FSGS is associated with nephrotic syndrome in patients with a variety of etiologies including familial, viral agents, drug abuse or as part of systemic disease. Current treatment strategies include immunosuppression, modulation of renal hemodynamics and suppression of fibrosis. However, current empirical pharmacological interventions are only partially successful. Idiopathic FSGS remains a difficult medical problem with unknown etiology and only empirical treatment strategies and, recurs in at least a third of transplant recipients1,2.
Recurrence of proteinuria after renal transplantation and remission after repeated plasma exchange strongly suggest a role of plasma factor(s) but the identity of the causative plasma factor(s) remains to be confirmed3. Several candidate permeability factors have been proposed during the last forty years. These include a vascular permeability factor- a T-cell derived protein4, hemopexin- a plasma protein with protease activity5,6, angiopoietin-like4, an adipokine7 and soluble urokinase-type plasminogen activator receptor (suPAR)- an inflammation related molecule8,9. Urokinase (uPA) binds to uPAR and activates plasminogen to plasmin. Increased levels of suPAR have been interpreted as an indication of its role as the causative circulating factor in FSGS. Ongoing efforts in this direction are summarized and evaluated in recent reviews10–12.
We have pursued the hypothesis that one or more plasma components cause the initial damage to podocytes resulting in glomerular dysfunction. We have developed and extensively used an in vitro assay to study the effect of FSGS serum, plasma or plasma fractions on isolated rat glomeruli. Using this assay, we have established that recurrent FSGS plasma, serum or specific plasma fractions increase albumin permeability (Palb) of isolated glomeruli and, that increased Palb precedes proteinuria after injecting FSGS plasma fraction in rats13–14. Proteomic analysis of the active fraction using LC-MS/MS led us to identify cardiotrophin-like cytokine factor 1 (CLCF1)15.
Cardiotrophin-like cytokine factor 1 (CLCF1), a member of the IL-6 family of cytokines, is also known as novel neurotrophin1 (NNT1) and B cell stimulating factor (BSF3)16–17. CLCF1 is believed to be secreted and present in circulation as a heterodimeric composite cytokine with either of two proteins, namely cytokine receptor-like factor-1 (CRLF1) or soluble receptor alpha for ciliary neurotrophic factor (sCNTF Rα). Co-expression of CLCF1 with CRLF1 or sCNTF-Rα is considered a requisite for the efficient secretion of CLCF1 and formation of composite cytokines CLCF1-CRLF1 (CLC-CLF) and CLCF1-sCNTFRα, respectively18–19.
The role of CLCF1 in the regulation of podocyte structure and function is not known. Studies using cultured neurons show that CLCF1-CRLF1 heterodimer interacts with cells that express the tripartite receptor complex composed of CNTFRα, gp130 and leukemia inhibitory factor-β (LIFRβ) and primarily activates the Janus Tyrosine Kinases/ signaling transducers and activators (JAK/STAT) signaling pathway18. The heterodimer supports the survival of embryonic motor and sympathetic neurons and induces differentiation of fetal neuroepithelial cells to astrocytes18,20. Studies using B cells demonstrated the role of CLCF1 as an effector of JAK/STAT signaling16,18 and its regulatory function in the immune system through stimulation of B cell proliferation and immunoglobulin production21. Also, CLCF1-CRLF1 complex is required for fetal kidney development22,23. Thus, CLCF1 may affect the glomerular filtration barrier through direct interaction with glomerular cells or through indirect mechanisms. However, the effects of CLCF1-CRLF1 heterodimer complex or CLCF1 monomer on glomerular barrier function are not known.
Since CLCF1 is believed to circulate as a heterodimer, its monomeric and heterodimeric forms may cause similar or distinct effects on key elements of the JAK/STAT pathway and modulate glomerular filtration barrier function. Presently, we planned to compare the glomerular effect of monomeric recombinant CLCF1 with that of the recombinant heterodimer CLCF1-CRLF1. Increasing evidence highlights the role of JAK/STAT signaling pathway in glomerular disease24 which makes JAK and/or STAT as potential targets for treating glomerular disease. In some experiments we compared the effect of CLCF1 with that of sera from FSGS patients on glomerular albumin permeability in vitro using anti-CLCF1 antibody or inhibitors of JAK2 and STAT3. Results show that while monomeric CLCF1 or FSGS serum increased Palb, the heterodimer CLCF1-CRLF1attenuated this effect. We also found that commercially available JAK2 or STAT3 inhibitors blocked the effect of CLCF1 or FSGS serum on Palb. Opposite effects of heterodimer CLCF1-CRLF1 and CLCF1 are in contrast to the reported similarities in their effects on neuronal cells and suggest cell-type specificity. These results provide an exciting opportunity to study the role of CLCF1 and related molecules in the etiology of recurrent FSGS and to explore the potential application of JAK2 and STAT3 inhibitors for treating FSGS and other glomerular diseases.
METHODS AND MATERIALS
Animals
Adult male Sprague-Dawley rats (7–8 weeks old) were obtained from Harlan (Madison, WI) and maintained at the Animal Resource Facility (ARF), KC VA Medical Center, Kansas City, MO, under 12/12 hour light/dark cycle with unrestricted access to food and water. The ARF is approved by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Institutional Animal Care and Use Committee (IACUC), Safety Subcommittee and the Research and Development (R&D) Committee at the KC VA Medical Center, Kansas City, MO approved the protocol prior to start of these studies. The work presented in this manuscript conforms to the relevant ethical guidelines for human and animal research.
Human serum
Protocol was approved by the Institutional Review Board (IRB). Serum samples were from de-identified recurrent FSGS patients whose serum specimens caused an increase in Palb value (≥0.6). Twenty microliter aliquots of each serum sample were used.
Reagents and solutions
Recombinant human CLCF1 (rhCLCF1) and CLCF1-CRLF1 (rhCLCF1-CRLF1) and monoclonal anti-CLCF1 antibody were obtained from R&D Systems, Minneapolis, MN. Buffers and media were prepared using chemicals obtained from Sigma-Aldrich (St Louis, MO). Working solutions were prepared in a medium containing 5% BSA. JAK2 inhibitor BMS-911543 was obtained from Chemietek, Indianapolis, IN. STAT3 inhibitor Stattic was obtained from Selleck Chemicals, Boston, MA. Stock solutions were prepared and stored following supplier’s/manufacturer’s guidelines.
Glomerular albumin permeability (Palb) assay
Glomeruli from Sprague Dawley rats were used to study changes in glomerular filtration barrier characteristics using an in vitro assay established in our laboratory13,25. Briefly, rat glomeruli were isolated as previously described and suspended in a physiological buffer solution (pH 7.4) containing bovine serum albumin (BSA) 5 gm/dL (isolation/incubation buffer). Aliquots of isolated glomeruli were treated with control or test agents in the medium containing 5% BSA for 15 minutes at 37°C in 1 mL total volume. Glomeruli were observed using video microscopy. A second image of each glomerulus was obtained after changing BSA concentration in the bath medium to 1%. Change of the medium produces an oncotic gradient across the glomerular capillary wall (5% BSA in the lumen vs. 1% BSA in the bathing medium) that causes a net fluid influx and an increase in glomerular volume.
Glomerular diameter was measured at four coordinates 45° apart. The average of 4 diameters was used to calculate the volume of each glomerulus in 5% BSA and 1% BSA, respectively. The change in volume (ΔV) of each glomerulus in response to the oncotic gradient was calculated as: ΔV = (Vfinal-Vinitial)/ Vinitial X 100 % .The increase in glomerular volume (ΔV) is directly related to the oncotic gradient (DP) applied across the capillary wall. We apply this principle to calculate reflection coefficient (σalb) using the ratio of ΔV of experimental to ΔV of control glomeruli in response to identical oncotic gradient: σalb= ΔVexperimental/ΔVcontrol Convectional albumin permeability (Palb) is defined as (1-σalb) and describes the movement of albumin consequent to water flow. When σalb is zero, albumin moves at the same rate as water and Palb is 1.0. When σalb is 1.0, albumin cannot cross the membrane with water and Palb is zero.
Western Blotting
Glomeruli were incubated with CLCF1 (0.05–100 ng/mL) with or without CLCF1-CRLF1 as indicated. Glomeruli were homogenized in a lysis buffer containing Sigma Fast Protease Inhibitor (S8820, 119K8203 Sigma-Aldrich) and phosphatase inhibitors (P5726 and P0044, Sigma-Aldrich) using a sonicator and the lysate was centrifuged at 12,000g for 5 minutes. Total protein was determined using a kit based on Lowry’s assay (BioRad, Hercules, CA). The supernatant was frozen at −70°C. Phospho-STAT3 (pSTAT3, Tyr 705) was determined by Western blotting followed by imaging and density analysis. Beta-actin (β-actin) was used as the loading control.
Total glomerular protein lysate was electrophoresed by SDS-PAGE using TGX gels (BioRad) followed by electro-transfer to PVDF membrane and detection using specific primary antibodies. Rabbit anti-pSTAT3 (Tyr705 D3A7, Cell Signaling catalog# 9131, 1:1000 dilution) was used in 5% BSA TBST. Mouse anti-β-Actin (Sigma catalog# A5441, 1:10000) was used in 5% dry milk in TBST. HRP-conjugated secondary antibodies for pSTAT3 (Tyr 705) and β-actin were goat anti-rabbit HRP conjugate (BioRad, catalog# 1705046, 1:10,000 dilution) and goat anti-mouse HRP conjugate (BioRad, catalog# 170–5047, 1:10,000), respectively. ECL Prime Western Blotting Detection reagent (GE Healthsciences, Piscataway, NJ) was used for chemiluminescence reaction and images were obtained using Kodak Gel Logic 2200 imaging system (Carestream Health Inc., New Haven CT). Image intensity data were normalized by loading control β-actin. Normalized intensity ratios were used to present data as bar graphs shown. The effect of CLCF1 on STAT3 (Tyr 705) phosphorylation and its attenuation by CLCF1-CRLF1 at various concentrations were determined.
Statistical analyses
Descriptive statistics such as means and proportions were used. Mean values were compared using Student’s t-test. P<0.05 was accepted as significant.
RESULTS
CLCF1 increases Palb in a dose-dependent manner and anti-CLCF1 antibody blocks the increase in Palb
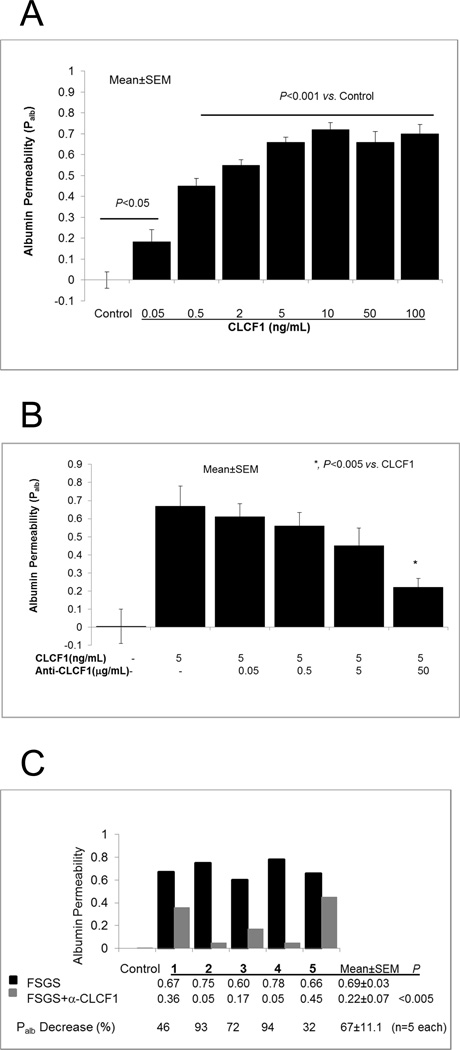
Recombinant human CLCF1 (rhCLCF1, CLCF1, 0.05–100 ng/mL) increased glomerular Palb in a dose-dependent manner within 15 minutes (Figure 1A). Significant increase in Palb was evident at CLCF1 concentrations as low as 0.05 ng/mL. A maximal increase was observed at 5–10 ng/mL (P<0.001) and CLCF1 was used at these concentrations in other experiments to study its effect on glomerular filtration barrier. These results show that, CLCF1 alters glomerular filtration barrier characteristics at sub-nanomolar concentrations.
Figure 1.
1A. CLCF1 caused an increase in glomerular albumin permeability (Palb). Isolated rat glomeruli were incubated with recombinant CLCF1 (0.05–100ng) for 15 minutes at 37°C. CLCF1 at 0.05ng/mL concentration caused significant increase in Palb (P<0.05 vs. control). Maximal increase was observed at 5–10 ng/mL (P<0.001). N= 15 glomeruli from 3 rats (5 glomeruli from each rat) in each group.
1B. Anti-CLCF1 antibody blocked the effect of CLCF1 on Palb. Isolated rat glomeruli were incubated with recombinant CLCF1 (5ng/mL) or with a mixture of CLCF1 and anti-CLCF1 monoclonal antibody (0.05–50µg /mL) for 15 minutes at 37°C. Control group included 5 µg human IgG. CLCF1 caused significant increase in Palb (P<0.001 vs. Control) that was maximally blocked by antibody at 50 µg /mL concentration (*, P<0.005 vs. CLCF1 alone). N= 15 glomeruli from 3 rats (5 glomeruli from each rat) in each group.
1C. Anti-CLCF1 antibody blocked the effect of sera from patients with recurrent FSGS. Isolated glomeruli were incubated with FSGS serum samples from five individuals (20 µL/mL each) or with a mixture of each serum sample and anti-CLCF1 antibody (50µg/mL) for 15 minutes at 37°C. Control group included 20µL/mL of pooled normal serum. Each pair of black and grey bars represents one sample. Black bars show that each FSGS serum sample caused significant increase in Palb (P<0.001 vs. Control). Each grey bar shows the effect of anti-CLCF antibody on the corresponding FSGS sample. Numeric values for Palb and percent change are shown under each pair of black and grey bars for each sample. Average of all five samples with SEM (mean±SEM) shows that anti-CLCF1 antibody significantly blocked the effect of FSGS serum (P<0.005, n=5 specimens). Five glomeruli from 1 rat were observed to calculate Palb for each sample.
Anti-CLCF1 monoclonal antibody blocked the increase in Palb caused by CLCF1 (Figure 1B). Glomeruli were incubated with CLCF1 (5ng/mL) or with a mixture of CLCF1 and anti-CLCF1 antibody (0.05–50mg/mL) for 15 min. Control group included 5 µg/mL human IgG. CLCF1 caused a significant increase in Palb (P<0.001 vs. Control) that was progressively blunted by increasing amounts of the antibody with a maximum effect at 50 µg antibody (P<0.005 vs. CLCF1 alone).
Anti-CLCF1 monoclonal antibody also blocked the increase in Palb caused by sera from five patients with recurrent FSGS (Figure 1C). Incubation with each FSGS serum caused increase in Palb with an average of 0.69 (P<0.001 vs. control). Pre-mixing the serum with anti-CLCF1 antibody blocked the effect of FSGS serum and the final average Palb was 0.22 (P<0.005 vs. FSGS serum alone, n=5). Thus, the effect of anti-CLCF1 antibody on different FSGS serum specimens varied between samples and resulted in 32–94% attenuation of Palb.
CLCF1-CRLF1 complex does not affect Palb and blocks the effect of CLCF1
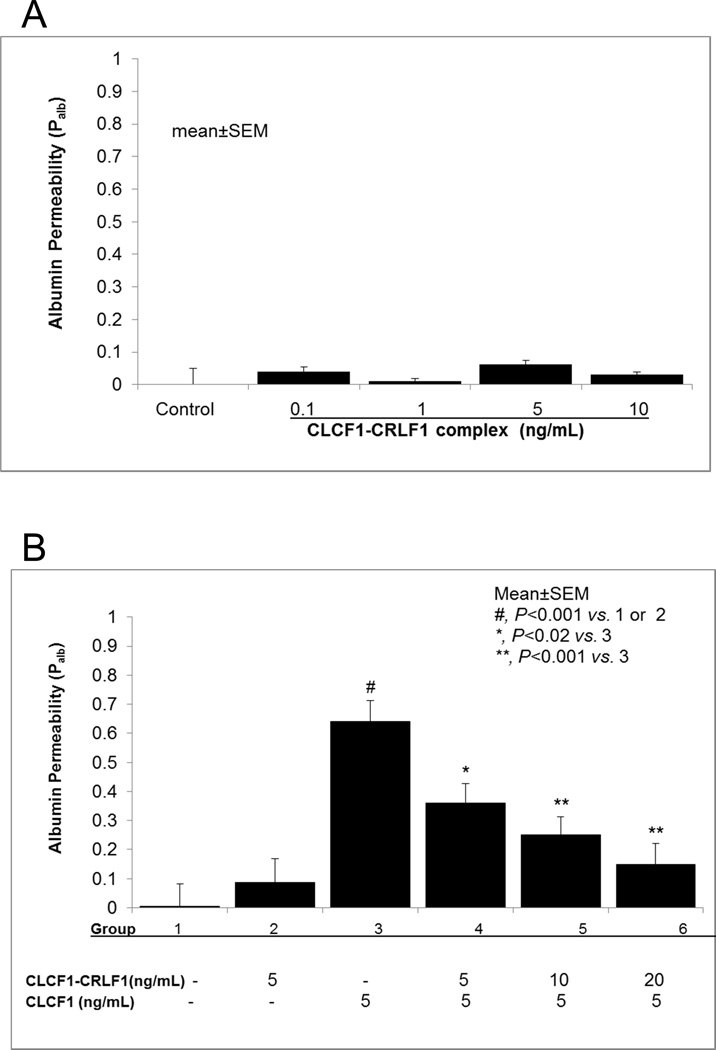
Incubation with the heterodimer CLCF1-CRLF1 (0.1–10ng/mL) did not increase glomerular Palb (Fig 2A). This finding contrasts the effect of CLCF1 monomer that caused a significant increase in Palb (Figure 1A). These findings led to the following experiments to determine the effect of CLCF1 on Palb in presence of the heterodimer CLCF1-CRLF1.
Figure 2.
2A. Heterodimer CLCF1-CRLF1 complex did not increase Palb. Isolated rat glomeruli were incubated with recombinant CLCF1-CRLF1 (0.1–10ng/mL) for 15 minutes at 37°C. CLCF1-CRLF1 did not affect Palb at any of the concentrations used. N=15 glomeruli from 3 rats (5 glomeruli from each rat) in each group.
2B. Heterodimer CLCF1-CRLF1 heterodimer blocked the effect of CLCF1 on Palb. Isolated rat glomeruli were pre-incubated with CLCF1-CRLF1 (5–20ng/mL) for 15 minutes followed by addition of CLCF1 (5ng/mL) for 15 minutes at 37°C. CLCF1 alone caused a significant increase in Palb (#, P<0.001 vs. control or CLCF1-CRLF1 alone) and pre-treatment with CLCF1-CRLF1 blocked the effect of CLCF1 in a dose-dependent manner (*, P<0.02 vs. CLCF1 alone; **, P<0.005 vs. CLCF1 alone). N=15 glomeruli from 3 rats (5 glomeruli from each rat) in each group.
Figure 2B shows the blocking effect of CLCF1-CRLF1 on the CLCF1-induced increase in Palb. Glomeruli were incubated with CLF1 or CLCF1-CRLF1 for 15 minutes as before. Additionally, glomeruli were incubated with CLCF1-CRLF1 heterodimer (5–20 ng/mL) for 15 min followed by addition of CLCF1 (5 ng/mL) and further incubation for 15 minutes. While CLCF1 alone caused a significant increase in Palb, the CLCF1-CRLF1 heterodimer did not affect Palb confirming results of earlier experiments (Figure 2A). Pre-incubation with 5 ng/mL CLCF1-CRLF1 attenuated the effect of CLCF1 on Palb (P<0.02 vs. CLCF1 alone). Higher concentrations of CLCF1-CRLF1 complex (10 or 20ng/mL) caused greater attenuation of Palb (P<0.005 vs. control).
These results show that pre-treatment with the heterodimer CLCF1-CRLF1 prevents the effect of CLCF1 and protects the glomerular filtration barrier from CLCF1 induced increase in Palb. These findings are in contrast to previously reported effect of CLCF1 and the heterodimer CLCF1-CRLF1 on neuronal cells. CLCF1-CRLF1 complex was found to have greater neuropoietic/neurotrophic effect. We conclude that the receptor complex and/or signaling events initiated in glomerular podocytes are unique and differ from those in neurons or B cells.
Pre-incubation of glomeruli with CLCF1-CRLF1 blocks the effect of FSGS serum on Palb
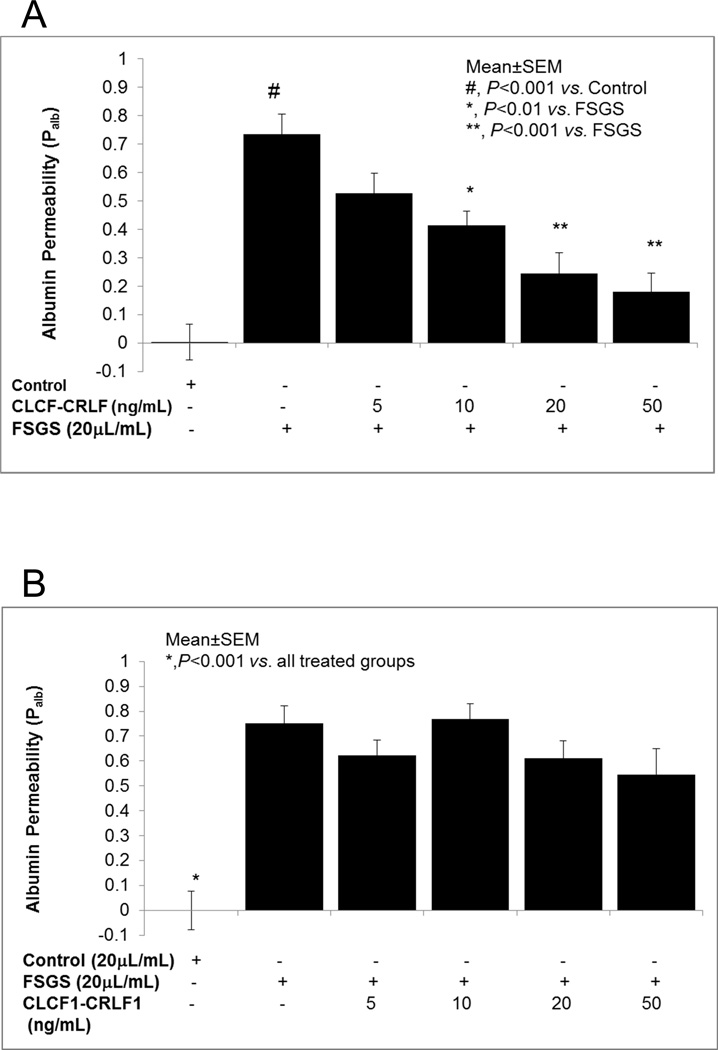
We sought to corroborate the blocking effect of CLCF1-CRLF1 on CLCF1-induced increase in Palb using FSGS serum. Figure 3A shows that CLCF1-CRLF1 blocked the increase in Palb caused by FSGS serum. Isolated glomeruli were pre-incubated with CLCF1-CRLF1 heterodimer (5–50 ng/mL) for 15 minutes followed by addition of FSGS serum (20 mL/mL) and further incubation for 15 minutes. FSGS serum caused a significant increase in Palb (#, P<0.001 vs. control) and CLCF1-CRLF1 attenuated the effect of FSGS serum. CLCF1-CRLF1 complex at 10 (P<0.02 vs. FSGS alone), 20 ng/mL (P<0.005 vs. FSGS alone) and 50 ng/mL (P<0.005 vs. FSGS alone) blocked the effect of FSGS serum.
Figure 3.
3A. Pre-treatment of glomeruli with CLCF1-CRLF1 heterodimer blocked the effect of FSGS serum on Palb. Isolated glomeruli were pre-incubated with CLCF1-CRLF1 complex (5–50ng/mL) for 15 minutes followed by addition of FSGS serum (20µL/mL) and incubation for 15 minutes at 37°C. FSGS serum alone caused a significant increase in Palb (#, P<0.001 vs. Control) that was blocked by 10ng/mL (*, P<0.02 vs. FSGS alone), 20ng or 50ng/mL (**, P<0.005 vs. FSGS alone). N= 15 glomeruli from 3 rats (5 glomeruli from each rat) in each group.
3B. Pre-treatment with FSGS serum prevented the protective effect of CLCF1-CRLF1 heterodimer on Palb. Isolated glomeruli were pre-incubated with FSGS serum (20µ/mL) for 15 minutes at 37°C followed by addition of CLCF1-CRLF1 (5–50 ng/mL) for 15 minutes at 37°C. FSGS serum with or without CLCF1-CRLF1 increased Palb significantly (*, P<0.001 vs. control). N= 15 glomeruli from 3 rats (5 glomeruli from each rat) in each group.
We planned to further confirm that the active component in FSGS serum and the heterodimer CLCF1-CRLF1 interact with the same site on the glomerulus. Figure 3B shows results of experiments using an alternate strategy to determine the effect of CLCF1-CRLF1 complex on FSGS serum-induced increase in Palb. For this purpose, we changed the order of adding FSGS serum and the heterodimer to glomerular suspension. Isolated glomeruli were pre-incubated with FSGS serum for 15 minutes followed by addition of CLCF1-CRLF1 (5–50 ng) and further incubation for 15 minutes. FSGS serum with or without CLCF1-CRLF1 increased Palb significantly (*, P<0.001 vs. control). These data show that pre-incubation with FSGS serum prevented the interaction between the heterodimer and the glomerular binding site. Thus, CLCF1-CRLF1 failed to prevent the effect of FSGS serum factor shown in Figure 3A. Results summarized in Figures 3A and 3B suggest that FSGS serum factor and the heterodimer CLCF1-CRLF1 appear to bind to the same glomerular site(s) and each prevents the binding of the other. These results also imply that CLCF1 and FSGS serum component(s) interact with the same receptor proteins that are blocked by the heterodimer.
Effect of the heterodimer CLCF1-CRLF1 is receptor specific
i. CLCF1-CRLF1 heterodimer does not block the effect of TNFa or IL6
In the next set of experiments, we selected two cytokines, namely TNFα and IL6 that individually can increase glomerular Palb. TNFα and IL6 are ligands for specific receptors that activate distinct signaling pathways and each can increase Palb. TNFα acts through its unique receptors, and IL6 binds to IL6Rα-gp130 complex. CLCF1 is a member of the IL6 family of cytokines and shares gp130 as a receptor component with other members of the IL6 family26.
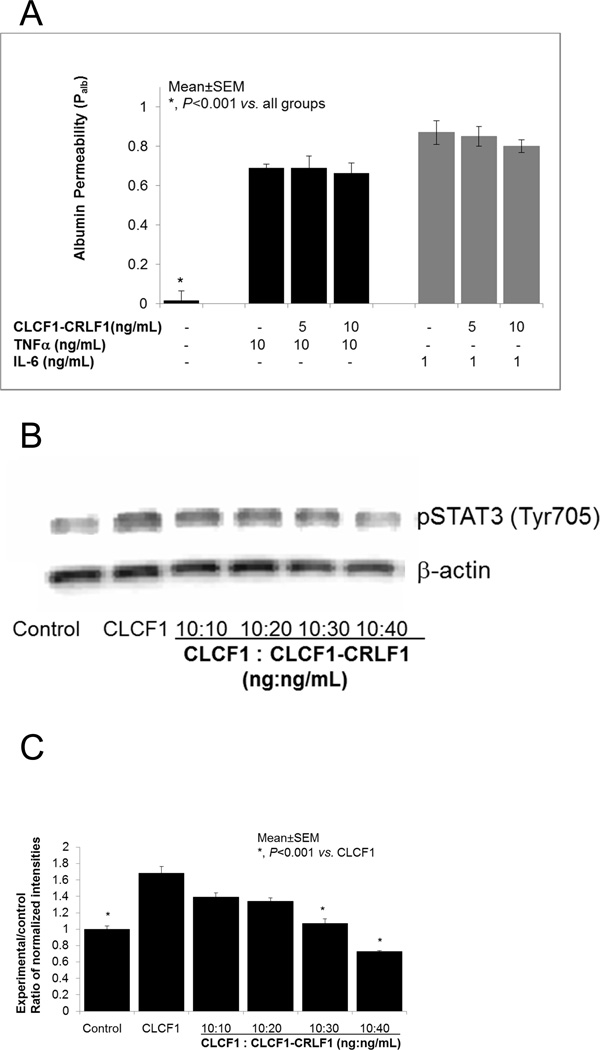
Figure 4A shows that pre-incubation of glomeruli with CLCF1-CRLF1 (5–20 ng/mL) for 15 minutes followed by addition of TNFα (10 ng/mL) or IL6 (1 ng/mL) did not block the increase in Palb caused by either of these cytokines. These results are in contrast to the effect of CLCF1-CRLF1 on CLCF1-induced increase in Palb where the heterodimer blocked the effect of CLCF1.
Figure 4.
4A. Pre-incubation of glomeruli with CLCF1-CRLF1 heterodimer did not block the effect of TNFα or IL6. Isolated rat glomeruli were pre-incubated with CLCF1-CRLF1 (5–20 ng/mL) for 15 minutes followed by addition of TNFa (10ng/mL) or IL6 (1 ng/mL) and further incubation for 15 minutes at 37°C. TNFα and IL6 each caused a significant increase in Palb compared to control (*, P<0.001 vs. control). CLCF1-CRLF1 did not block the increase in Palb caused by TNFα or IL6. N=15 glomeruli from 3 rats (5 glomeruli from each rat) in each group.
4B and 4C. CLCF1-CRLF1 heterodimer blocked the CLCF1- induced phosphorylation of STAT3. Figure 4B shows a representative Western blot image to demonstrate the upregulation of STAT3 (Tyr705) phosphorylation by CLCF1 and the blocking effect of CLCF1-CRLF1 heterodimer. Control group represents untreated glomeruli. Glomeruli were incubated with CLCF1 (10ng/mL) for 15 minutes in one group. In additional groups, glomeruli were pre-incubated with heterodimer CLCF1-CRLF1 (10–40ng/mL) for 15 minutes followed by addition of CLCF1 (10ng/mL) and incubation for 15 minutes at 37°C. Thus, the ratio of CLCF1 to the heterodimer CLCF1-CRLF1 ranged from 1:1 to 1:4 (ng:ng) or a molar ratio of approximately 1:0.3 to 1:1.25. Total protein lysates were resolved by SDS-PAGE followed by Western blotting using anti-pSTAT3 (Tyr705) as the primary antibody.
Figure 4C. The bar graph shows results of quantitative analysis of protein band intensities. Changes in STAT3 phosphorylation (Tyr705) were determined by semi-quantitative image analysis. Background subtracted intensities were normalized by the loading control β-actin. Ratios of intensities (Experimental/control) are presented in the bar graph. CLCF1 caused significant increase in pSTAT3 (Tyr705) (*, P<0.001 control vs. CLCF1 alone). CLCF1-induced increase in pSTAT3 (Tyr705) was attenuated by pretreatment with 30 or 40 ng/mL CLCF1-CRLF1 (*, P<0.001 vs. CLCF1 alone). Mean±SEM of three separate experiments are shown.
These results suggest that the monomeric CLCF1 and the heterodimer CLCF1-CRLF1 compete for the receptor complex CNTFRα-gp130-LIFRβ, and that TNFα and IL6 do not mediate their effects on Palb through this receptor complex. The antagonistic effect of CLCF1-CRLF1 heterodimer against CLCF1 is therefore receptor specific. Thus, we confirmed the specificity of the blocking effect of CRLF1-CLCF1 complex using two other cytokines that do not interact with the tripartite receptor complex for CLCF1.
ii. CLCF1-CRLF1 heterodimer blocks the CLCF1-induced upregulation of STAT3 phosphorylation
FigureS 4B and 4C summarize the results that demonstrate a specific effect of the heterodimer CLCF1-CRLF1 on CLCF1-induced activation of the JAK/STAT signaling pathway. Isolated rat glomeruli were pre-incubated with CLCF1 (10 ng/mL, MW ~ 25 kDa) for 15 minutes or with CLCF1-CRLF1 (10–40 ng/mL, MW. ~72 kDa) for 15 minutes followed by addition of CLCF1 (10 ng/mL) and incubation for 15 minutes at 37°C. Thus, the ratio of CLCF1 to the heterodimer CLCF1-CRLF1 ranged from 1:1 to 1:4 (ng:ng). These amounts provided an approximate molar ratio of 1:0.3–1:1.25 (CLCF1 : CLCF1-CRLF1).
Results of SDS-PAGE followed by Western blotting for pSTAT3 (Tyr705) are presented in Figure 4B. Blots were analyzed by semi-quantitative image analysis and experimental/control ratios of normalized intensities for pSTAT3 (Tyr705) are presented in Figure 4C. These results show that pre-treating glomeruli with CLCF1-CRLF1 complex attenuated the CLCF1-induced increase in STAT3 phosphorylation (*, P<0.001 vs. CLCF1 alone).
Earlier in this series of experiments, data presented in Figures 2 and 3 showed that the heterodimer CLCF1-CRLF1 blocked the effect of CLCF1 on Palb in a manner that suggested their action through a shared binding site on the tripartitereceptor complex. Since CLCF1 activates the JAK/STAT signaling pathway, results in Figures 4B and 4C lead us to surmise that the heterodimer CLCF1-CRLF1 attenuates the CLCF1-induced increase in Palb through down regulation of STAT3 phosphorylation.
JAK2 inhibitor blocks the effect of CLCF1 and FSGS serum on Palb
CLCF1 binding to the tripartite receptor activates JAK/STAT signaling17,18. The binding of CLCF1 to the receptor complex results in phosphorylation of JAK2 at specific sites18. To further determine the role of JAK/STAT pathway in CLCF1-induced increase in Palb we used BMS-911543 (BMS), a commercially available JAK2 specific small molecular weight inhibitor (IC50 ≤ 2 nM)27. Isolated rat glomeruli were pre-incubated with JAK2 inhibitor BMS-911543 (BMS, 1–10 nM) for 15 minutes followed by addition of CLCF1 (10 ng/mL) or FSGS serum (20 µL/mL) for 15 minutes.
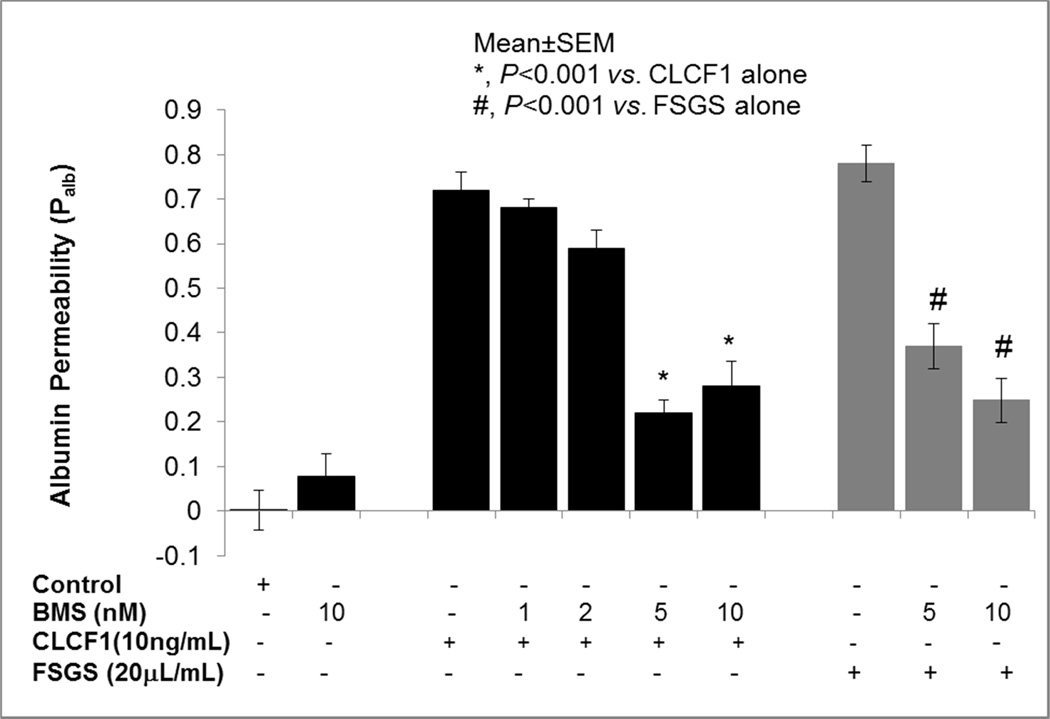
Figure 5 shows that BMS (1–10 nM) alone did not affect Palb and CLCF1 or FSGS serum caused increase in Palb. Pre-treatment of glomeruli with BMS significantly blocked the effect of CLCF1 (P<0.001 vs. CLCF1 alone) or FSGS (P<0.001 vs. FSGS serum alone). These results show that CLCF1 activates JAK2 and the resulting increase in Palb is significantly blocked by a JAK2-specific inhibitor. Thus, CLCF1-induced phosphorylation of JAK2 confirms receptor-ligand interaction that can be blocked by a specific inhibitor at very low concentrations.
Figure 5.
JAK2 inhibitor blocked the effect of CLCF1 or FSGS serum on Palb. Isolated rat glomeruli were pre-incubated with JAK2 inhibitor BMS-911543 (BMS, 1–10nM) for 15 minutes followed by addition of CLCF1 (10ng) or FSGS serum (20µL) for 15 minutes at 37°C. BMS blocked the effect of CLCF1 or FSGS serum at 5 and 10 nM concentrations and increased Palb. (*, P<0.001 vs. CLCF1 alone; #, P<0.001 vs. FSGS alone; Mean±SEM, N= 15 glomeruli from 3 rats, 5 glomeruli from one rat in each group).
Table 1 summarizes the effect of JAK2 inhibitor BMS911543 on sera from five patients with recurrent FSGS. Earlier experiments showed that CLCF1-induced increase in Palb is comparable to that caused by FSGS sera (Figures 1A, 1C). Pre-incubation with JAK2 inhibitor significantly but partially blocked the effect of FSGS sera on Palb. Average increase in Palb dropped from 0.78 (range 0.69 to 0.82) to 0.44 (range 0.42 to 0.50) following pre-treatment of glomeruli with JAK2 inhibitor. Thus, pretreatment with JAK2 inhibitor resulted in a 43% (28–49%) attenuation of the effect of FSGS serum on Palb.
Table 1.
| 1 | 2 | 3 | |
|---|---|---|---|
| Palb FSGS seruma Untreated |
Palb FSGS Serum + JAK2 Inhibitora,b (percent decrease, 1–2) |
Palb FSGS Serum + STAT3 Inhibitora,c (percent decrease, 1–3) |
|
| Recurr (M) | 0.75 | 0.44 (41) | 0.32 (57) |
| Recurr (M) | 0.82 | 0.42 (49) | 0.35 (57) |
| Recurr (M) | 0.77 | 0.42 (46) | 0.36 (53) |
| 2nd Recurr (M) | 0.69 | 0.50 (28) | 0.41 (43) |
| Recurr (M) | 0.88 | 0.42 (52) | 0.47 (47) |
| Mean | 0.78 | 0.44 (43) | 0.38 (51) |
| SD | 0.06 | 0.031 | 0.0526 |
| SEM | 0.028 | 0.0138 | 0.0235 |
| P value 1 vs. 2 or 3 | <0.001 | <0.001 |
Each value represents the mean of 5 glomeruli observed.
Glomeruli were incubated with JAK2 inhibitor BMS 911543 (5µM) for 15 minutes followed by addition of FSGS serum and incubation for 15 minutes at 37°C.
Glomeruli were incubated with STAT3 inhibitor Stattic (1µM) for 15 minutes followed by addition of FSGS serum and incubation for 15 minutes at 37°C.
Abbreviations: M- Male; Recurr- Recurrence
Control Palb 0.003±0.041
STAT3 inhibitor blocks the effect of CLCF1 and FSGS serum on Palb
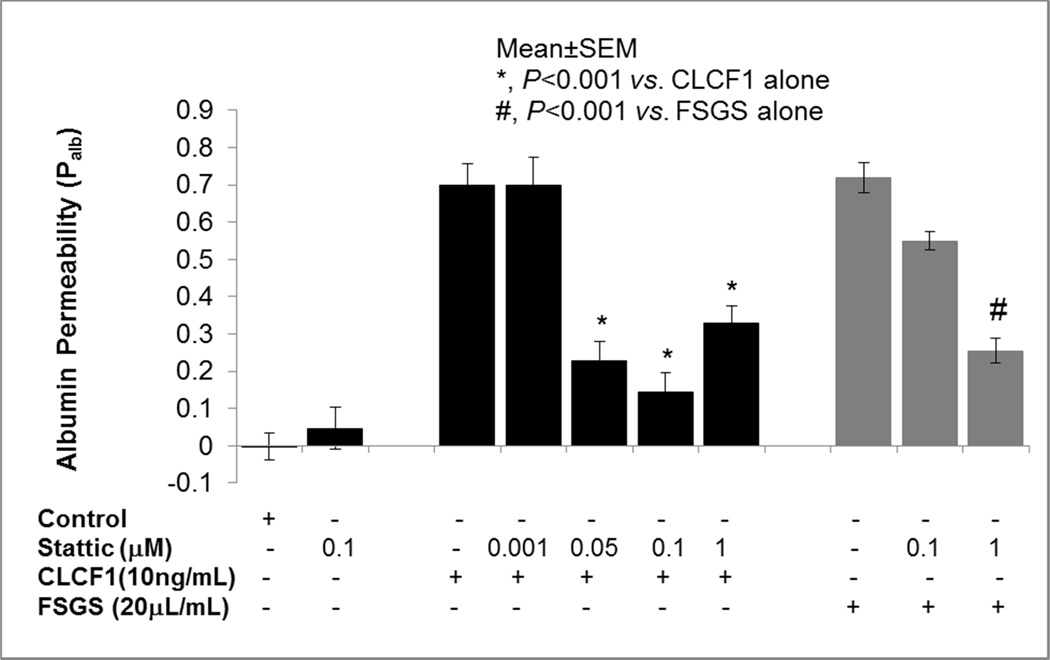
Low molecular weight compound Stattic is a specific inhibitor of STAT3 activation28. We tested its effect on CLCF1 and FSGS serum-induced increase in Palb. Isolated rat glomeruli were pre-incubated with Stattic (0.05–1 µM) for 15 minutes followed by addition of CLCF1 (10 ng/mL). In separate experiments, glomeruli were pre-incubated with Stattic (0.1 or 1 µM) for 15 minutes followed by FSGS serum (20 µL/mL) for 15 minutes. Pre-incubation with Stattic (1 µM) blocked the effect of FSGS serum on Palb (P<0.001).
Table 1 also summarizes the effect of STAT3 inhibitor Stattic on FSGS serum samples from five patients with recurrent FSGS. Pre-incubation with Stattic inhibitor significantly (P<0.001) blocked the effect of FSGS sera on Palb. Average increase in Palb dropped from 0.78 (range 0.69 to 0.82) to 0.38 (range 0.32 to 0.47) after pre-treatment of glomeruli with Stattic. Thus, pretreatment with STAT3 inhibitor resulted in a 51% (43–57%) attenuation of the effect of FSGS serum on Palb.
DISCUSSION
Three main findings emerged from these studies. First, we discovered that monomeric recombinant CLCF1 increased Palb comparable to FSGS serum. A monoclonal anti-CLCF1 antibody attenuated the effect of CLCF1 or FSGS serum on Palb. Second, the heterodimer CLCF1-CRLF1 blocked the effect of CLCF1 or FSGS serum on Palb but showed no effect on Palb by itself and did not attenuate the effect of TNFα or IL6. Finally, commercially available inhibitors of JAK2 or STAT3 blocked the effect of CLCF1 or FSGS serum on Palb. We believe these novel data provide valuable information on the role of circulating CLCF1 in idiopathic FSGS and an opportunity to investigate the potential repurposing of available JAK inhibitors to treat the effect of the circulating plasma factor on glomerular function.
A circulating factor was postulated about forty years ago and putative candidates have included cytokines released from activated T cells4,29–32. Ongoing efforts in different laboratories are focused on circulating molecules with diverse ontologic roles that appear to converge on glomerular pathophysiology. Current candidate molecules include vascular permeability factor4, hemopexin5,6, angiopoietin-like47 and suPAR8,9 Several studies have addressed the clinical and molecular aspects of suPAR as a circulating factor in FSGS. Several issues including its significance as a causative factor or specific indicator of FSGS and, its role as a mediator of glomerular/renal changes are subjects of ongoing research and discussion33,34. Soluble uPAR is associated with the uPA/uPAR plasminogen activation mechanism and its levels positively correlate with several inflammatory conditions including, cancer, infection and systemic disease35.
Since the role of CLCF1 in glomerular pathophysiology is not known, we planned to first determine its effect on glomerular barrier function using an in vitro assay developed in our laboratory. The glomerular permeability (Palb) assay is a functional assay based on glomerular response an experimental oncotic gradient in the presence/absence of potential mediator(s) of glomerular injury or protection. The assay detects diminished reflection coefficient that corresponds to increased convectional permeability by measuring the effect of FSGS plasma (or other reagent) on glomerular filtration in response to an experimental oncotic gradient. This assay serves to demonstrate the earliest changes in the glomerular filtration barrier that lead to increased filtration of albumin and eventually to proteinuria. Currently, it is the only assay that detects the changes in glomerular function that result from a circulating substance in FSGS serum/plasma. This assay has been used for testing of >2200 serum samples of pre- and post-transplant patients from across the world. Specifically, the assay detects robust Palb increasing effect of recurrent FSGS and the pre-transplant sera. Results have consistently shown that a Palb value of ≥0.5 is strongly associated with recurrence of FSGS36. Plasmapheresis decreases permeability activity in a manner consistent with the removal of a molecule that is present primarily in the plasma space. Activity gradually returns to prior levels several weeks after multiple plasma exchange treatments. We have shown that this assay discriminates between the effect of sera from recurrent FSGS and other glomerular disease including post-transplant membranous glomerular nephropathy and minimal change disease36. In other studies, we have shown that glomerular albumin permeability increases before proteinuria occurs. These include experiments performed after injecting FSGS plasma preparation14, rat models of hypertension37 and radiation nephropathy38. In each of these models, glomerular permeability increased prior to increase in Up/Uc ratio. We have used this assay extensively to determine the effect of cytokines, antibodies, metabolites and free radicals. We have also used this technique to detect the permeability factor in FSGS plasma and to follow the permeability activity in enriched plasma fractions. Recent reviews summarize the applications of this assay in our studies on recurrent FSGS, identification of agents that are protective or injurious to the glomerular protein permeability barrier3,39.
A comparable increase in Palb by CLCF1 and sera from patients with recurrent FSGS (Figure-1A, 1C), and a significant blockade of increase in Palb by anti-CLCF1 antibody (Figure 1B, 1C) suggested a potential role for CLCF1 in the early events leading to loss of glomerular barrier function observed in recurrent FSGS. A distinct role of monomeric CLCF1 in glomerular pathophysiology is not clear since previous studies using neurons have highlighted its role as a heterodimer with CRLF140. First, CLCF1 shows optimum neuropoietic and neurotrophic effects as a heterodimer with CRLF118,41. Second, CLCF1 is essential for lumbar and facial motoneuron differentiation42,43 and, mutations in CLCF1 result in cold-induced sweating syndrome (CISS) 45,46. Mutations in CRLF1 gene are also associated with CISS and Crisponi syndrome46. In one recent case study CISS presented with complications in the urinary system including a smaller than normal right kidney and persistent mild hypernatremia that were interpreted to indicate a role of CLCF1 in renal development47. Additionally, CRLF1 is required for normal kidney development during embryogenesis22,23. CRLF1 and CLCF1 appear to function as a heterodimer in neuronal differentiation and renal development but the role of monomeric CLCF1 in glomerular function is not known.
In contrast to its effect on neurons, the CLCF1-CRLF1 composite cytokine did not augment or parallel the effect of CLCF1 on Palb. Instead, it antagonized the effect of both monomeric CLCF1 (Figure 2B) and FSGS serum (Figure 3A). In each case, inhibition was dose-dependent and apparently competitive. CLCF1 and CLCF1-CRLF1 appear to interact with the same molecular component of the receptor complex since pre-treatment of glomeruli with FSGS serum prevented the blocking effect of the heterodimer (Figure 3B). We addressed the specificity of the effect of CLCF1-CRLF1 on CLCF1-mediated increase in Palb by demonstrating that the heterodimer does not block the increase in Palb caused by unrelated cytokines TNFα or IL6 (Figure 4A). The antagonistic effect of the CLCF1-CRLF1 complex on the CLCF1-induced increase in Palb is a novel and significant finding that will serve to further explore the diversity of gp130-associated receptor complexes and signaling elements. These observations have led us to conclude that the effect of CLCF1-CRLF1 complex on the glomerular filtration barrier differs from its effect on neurons where it causes similar and stronger response compared to monomeric CLCF1. These observations prompted the use of CLCF1-CRLF1 complex as a natural antagonist of CLCF1 in studies involving JAK/STAT signaling in glomeruli. Further, CLCF1-CRLF1 will be a valuable tool for future studies on the JAK/STAT pathway in podocytes.
Receptor activation by CLCF1 causes conformational changes in the intracellular regions of receptor proteins and phosphorylation of JAK218. JAK isoforms serve as sentinels of the JAK/STAT signaling pathway in mediating the cellular effects of several ligands through phosphorylation of STATs48. Our preliminary work showed that JAK2 and STAT3 are dominant isoforms in glomeruli and podocytes (data not presented here). JAK2 may be a potential target for regulating the CLCF1-induced over-activation of JAK/STAT signaling pathway. BMS-911543, used in the present experiments is a potent and selective small molecule inhibitor of the JAK2 (IC50 ≤ 2nM)27. We found that BMS911543 blocks the effect of both CLCF1 and FSGS serum. To our knowledge, this is the first report demonstrating the effect of BMS-911543 on glomerular barrier function but other JAK2 inhibitors have been tested in animal models. For example, in the mouse model of adriamycin-induced nephrotic syndrome, post-injury administration of JAK2 inhibitor AG 490 for 6 weeks decreased proteinuria, serum creatinine, glomerulosclerosis, tubulointerstitial lesions and renal alpha-smooth muscle actin (α-SMA) expression. AG490 also inhibited the expression of monocyte chemoattractant protein (MCP-1) mRNA and reduced interstitial infiltration of macrophages and T cells49. BMS-911543 and several small molecules are at advanced stages of clinical trials (Tofacitinib, Pfizer; INCB28050, Lilly/Incyte). Tofacitinib (Xeljanz, Pfizer) and Ruxolinitib/Jakafi, (Novartis/Incyte) have been approved by the US FDA. Potential off-label applications of these compounds are already under consideration50.
JAK2-mediated phosphorylation of STAT3 leads to transcriptional regulation of a number of genes. The diverse effects of STAT3 appear to be determined by post-translational modifications, dimerization and by whether or not it translocates to the nucleus. Briefly, activation of STAT3 involves phosphorylation at tyrosine 705 (Tyr705/Y705), translocation to the nucleus and binding to interferon-gamma-activated sequences for transcription initiation51–54. In addition, phosphorylation of serine (S) residues in STATs (S727 in STAT3) may affect STAT translocation positively or negatively depending upon the cell type and activation status of STAT, nature of the gene promoter and extracellular factors55. Phosphorylation of serine without tyrosine phosphorylation51 and phosphorylation of mitochondrial STAT356–58 are other modes of STAT3 activation. Additional post-translational modifications of STAT3 may influence its function. For example, acetylation of STAT3 in response to certain stimuli51 is responsible for its role in oxidative changes in diabetic nephropathy59 and in the down regulation of autophagy60,61. Recent data show that native unphosphorylated STAT3 can also move between the nucleus and cytoplasm suggesting that phosphorylation may not be a critical requirement, at least in certain cells62,63. Thus, STAT3 can influence both nuclear and cytoplasmic processes with or without phosphorylation or acetylation64,65. We limited the present studies to CLCF1-induced phosphorylation of STAT3 at tyrosine 705 but changes at other sites of STAT3 molecule and their role in the cellular effects of CLCF1 need to be studied.
Over-activation of STAT3 in podocytes is associated with glomerular disease and its down-regulation attenuates the glomerulonephritis induced by nephrotoxic serum66, HIVAN67 and diabetic nephropathy59. The diverse nature and wide range of its effects through multiple mechanisms has made STAT3 a highly sought after target for potential inhibitors derived from peptides, non-peptide small molecules, oligonucleotides, natural and synthetic molecules68,69. Stattic, a synthetic small molecule, specifically inhibits (IC50= 5.1µM) activation, dimerization, and nuclear translocation of STAT3, and increases the apoptotic rate of STAT3-dependent breast cancer cell18. We found that Stattic concentrations below its IC50 significantly protected against increase in Palb (Figure 6). Thus, concentrations of STAT3 inhibitor(s) required may depend on the cell type and reflect constitutive overexpression or induced over-activation of STAT3. In addition, our results also show that CLCF1-induced upregulation of STAT3 phosphorylation was attenuated by the heterodimer CLCF1-CRLF1 (Figure 4B–C). Thus, loss of equilibrium between the levels of circulating CLCF1 and the heterodimer CLCF1-CRLF1 may result in over-activation of STAT3 phosphorylation.
Figure 6.
STAT3 inhibitor blocked the effect of CLCF1 and FSGS serum on Palb. Isolated rat glomeruli were pre-incubated with STAT3 inhibitor Stattic (0.001–1µM) for 15 minutes followed by addition of CLCF1 (10ng/mL). In separate experiments, glomeruli were pre-incubated with Stattic (0.1 or 1µM) for 15 minutes followed by FSGS serum (20µL/mL) for 15 minutes at 37°C. Pre-treatment of glomeruli with Stattic (0.05–1µM) significantly blocked the effect of CLCF1 (P<0.001 vs.CLCF1 alone). Pre-incubation with Stattic (1µM) blocked the effect of FSGS serum on Palb. (#, P<0.001 vs. FSGS alone). N=15 glomeruli from 3 rats (5 glomeruli from each rat) in each group.
In summary, CLCF1, a member of the IL6 family of cytokines, or FSGS plasma increases Palb and activates the JAK2/STAT3 pathway in glomeruli. In contrast, the heterodimer CLCF1-CRLF1 blocks these effects of CLCF as well as the comparable effect of FSGS serum on Palb. Opposing effects of CLCF1 and CLCF1-CRLF1 on glomerular filtration barrier are in contrast to their parallel neurotrophic/neuropoietic and immune-modulatory effects. We interpret the increase in Palb by CLCF1 or FSGS sera and an upregulation of JAK/STAT activation as evidence in support of a central role of the JAK/STAT pathway in glomerular response to FSGS serum/plasma. The finding that inhibitors of JAK2 or STAT3 activation prevent FSGS-induced increase in Palb suggests a potential value of these molecules in treating chronic glomerular disease.
Brief Commentary.
Background: A circulating factor is believed to cause recurrence of idiopathic focal segmental glomerulosclerosis (FSGS) after renal transplantation. Recently we identified cardiotrophin-like cytokine 1 (CLCF1) in the plasma from recurrent FSGS patients. Present studies show that recombinant CLCF1 and FSGS serum induce comparable increase in glomerular albumin permeability in vitro that can be blocked by JAK2 or STAT3 inhibitors.
Translational significance: These novel findings suggest a significant role of CLCF1 in the early glomerular pathophysiology of recurrent FSGS. FDA approved JAK inhibitors may be useful in regulating the JAK2/STAT3 signaling to treat recurrent FSGS and other glomerular disease.
ACKNOWLEDGMENT
We thank Ms. Maohui Chen for laboratory assistance.
The study was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, VA BX001037 (Savin), NIH grants R01 DK 43752 and DK R21 00292588 (Savin), DK 1RO1 DK064969 (McCarthy) and funds from the Midwest Biomedical Research Foundation (Savin, Sharma).
We thank Dr. Peter Mundel and Dr. Jochen Reiser for providing immortalized mouse podocytes. The work presented in this manuscript conforms to the relevant ethical guidelines for human and animal research.
JAK2/STAT3 inhibitors protect glomerular function (45/45 characters)
- CLCF1
Cardiotrophin-like cytokine Factor-1
- CNTF
Ciliary Neurotrophic Factor
- CNTFRα
Ciliary Neurotrophic Factor Receptor alpha
- sCNTFRα
Soluble Cilliary Neurotrophic Factor Receptor alpha
- CRLF1
Cytokine Receptor-Like Factor-1
- FSGS
Focal Segmental Glomerulosclerosis
- gp130
Glycoprotein130
- IL6
Interleukin −6
- JAK
Janus Kinase
- LIFRβ
Leukemia Inhibitory Factor Receptor-β
- STAT
Signal Transducer and Activator of Transcription
- TNFβ
Tumor Necrosis Factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Parts of the results were presented at the annual meeting of the American Society of Nephrology, 2014.
All authors have read the journal’s Authorship Agreement.
CONFLICT OF INTEREST STATEMENT
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
All named authors have reviewed and approved the manuscript. All authors have read the journal’s policy on conflicts of interest. None of the authors have any conflict of interest to declare regarding the contents of this paper.
REFERENCES
- 1.Deegens JK, Steenbergen EJ, Wetzels JF. Review on diagnosis and treatment of focal segmental glomerulosclerosis. Neth J Med. 2008;66:3–12. [PubMed] [Google Scholar]
- 2.D’Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N Engl J Med. 2011;365:2398–411. doi: 10.1056/NEJMra1106556. [DOI] [PubMed] [Google Scholar]
- 3.Savin VJ, McCarthy ET, Sharma M. Permeability factors in nephrotic syndrome and focal segmental glomerulosclerosis. Kidney Res Clin Prac. 2012;31:205–213. doi: 10.1016/j.krcp.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lagrue G, Xheneumont S, Branellec A, Hirbec G, et al. A vascular permeability factor elaborated from lymphocytes. I. Demonstration in patients with nephrotic syndrome. Biomedicine. 1975;23:37–40. [PubMed] [Google Scholar]
- 5.Bakker WW, Borghuis T, Harmsen MC, et al. Protease activity of plasma hemopexin. Kidney Int. 2005;68:603–610. doi: 10.1111/j.1523-1755.2005.00438.x. [DOI] [PubMed] [Google Scholar]
- 6.Lennon R, Singh A, Welsh GI, et al. Hemopexin induces nephrin-dependent reorganization of the actin cytoskeleton in podocytes. J Am Soc Nephrol. 2008;19:2140–2149. doi: 10.1681/ASN.2007080940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clement LC, Macé C, Avila-Casado C, et al. Circulating angiopoietin-like 4 links proteinuria with hypertriglyceridemia in nephrotic syndrome. Nat Med. 2014;20:37–46. doi: 10.1038/nm.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei C, Moller CC, Altintas MM, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14:55–63. doi: 10.1038/nm1696. [DOI] [PubMed] [Google Scholar]
- 9.Wei C, El Hindi S, Li J, et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952–960. doi: 10.1038/nm.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiser J, Nast CC, Alachkar N. Permeability factors in focal and segmental glomerulosclerosis. Adv Chronic Kidney Dis. 2014;21:417–421. doi: 10.1053/j.ackd.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maas RJ, Deegens JK, Wetzels JF. Serum suPAR in patients with FSGS: trash or treasure? Pediatr Nephrol. 2013;28:1041–1048. doi: 10.1007/s00467-013-2452-5. [DOI] [PubMed] [Google Scholar]
- 12.Reiser J, Wei C, Tumlin J. Soluble urokinase receptor and focal segmental glomerulosclerosis. Curr Opin Nephrol Hypertens. 2012;21:428–432. doi: 10.1097/MNH.0b013e328354a681. [DOI] [PubMed] [Google Scholar]
- 13.Sharma M, Sharma R, McCarthy ET, Savin VJ. “The FSGS factor:” enrichment and in vivo effect of activity from focal segmental glomerulosclerosis plasma. J Am Soc Nephrol. 1999;10:552–561. doi: 10.1681/ASN.V103552. [DOI] [PubMed] [Google Scholar]
- 14.Sharma M, Sharma R, McCarthy ET, Savin VJ. The focal segmental glomerulosclerosis permeability factor: biochemical characteristics and biological effects. Exp Biol Med. 2004;229:85–98. doi: 10.1177/153537020422900111. [DOI] [PubMed] [Google Scholar]
- 15.Savin VJ, Sharma M, McCarthy ET, et al. Cardiotrophin like cytokine-1: Candidate for the focal glomerular sclerosis permeability factor. J Am Soc Neph. 2008;19:59A. (F-FC-260) [Google Scholar]
- 16.Shi Y, Wang W, Yourey PA, et al. Computational EST database analysis identifies a novel member of the neuropoietic cytokine family. Biochem Biophys Res Commun. 1999;262:132–138. doi: 10.1006/bbrc.1999.1181. [DOI] [PubMed] [Google Scholar]
- 17.Senaldi G, Varnum BC, Sarmiento U, et al. Novel neurotrophin-1/B cell-stimulating factor-3: a cytokine of the IL-6 family. Proc Natl Acad Sci U SA. 1999;96:11458–11463. doi: 10.1073/pnas.96.20.11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elson GC, Lelièvre E, Guillet C, et al. CLF associates with CLC to form a functional heteromeric ligand for the CNTF receptor complex. Nat Neurosci. 2000;3:867–872. doi: 10.1038/78765. [DOI] [PubMed] [Google Scholar]
- 19.Plun-Favreau H, Elson G, Chabbert M, et al. The ciliary neurotrophic factor receptor alpha component induces the secretion of and is required for functional responses to cardiotrophin-like cytokine. EMBO J. 2001;20:1692–1703. doi: 10.1093/emboj/20.7.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uemura A, Takizawa T, Ochiai W, Yanagisawa M, Nakashima K, Taga T. Cardiotrophin-like cytokine induces astrocyte differentiation of fetal neuroepithelial cells via activation of STAT3. Cytokine. 2002;18:1–7. doi: 10.1006/cyto.2002.1006. [DOI] [PubMed] [Google Scholar]
- 21.Senaldi G, Stolina M, Guo J, et al. Regulatory effects of novel neurotrophin-1/b cell-stimulating factor-3 (cardiotrophin-like cytokine) on B cell function. J Immunol. 2002;168(11):5690–5698. doi: 10.4049/jimmunol.168.11.5690. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt-Ott KM, Yang J, Chen X, et al. Novel regulators of kidney development from the tips of the ureteric bud. J Am Soc Nephrol. 2005;16:1993–2002. doi: 10.1681/ASN.2004121127. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt-Ott KM, Lan D, Hirsh BJ, Barasch J. Dissecting stages of mesenchymal-to-epithelial conversion during kidney development. Nephron Physiol. 2006;104:56–60. doi: 10.1159/000093287. [DOI] [PubMed] [Google Scholar]
- 24.Brosius FC., 3rd New insights into the mechanisms of fibrosis and sclerosis in diabetic nephropathy. Rev Endocr Metab Disord. 2008;9:245–254. doi: 10.1007/s11154-008-9100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savin VJ, Sharma R, Lovell HB, Welling DJ. Measurement of albumin reflection coefficient with isolated rat glomeruli. J Am Soc Nephrol. 1992;3:1260–1269. doi: 10.1681/ASN.V361260. [DOI] [PubMed] [Google Scholar]
- 26.Bravo J, Heath JK. Receptor recognition by gp130 cytokines. EMBO J. 2000;19:2399–2411. doi: 10.1093/emboj/19.11.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Purandare AV, McDevitt TM, Wan H, et al. Characterization of BMS-911543, a functionally selective small-molecule inhibitor of JAK2. Leukemia. 2012;26:280–288. doi: 10.1038/leu.2011.292. [DOI] [PubMed] [Google Scholar]
- 28.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol. 2006;13:1235–1242. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Shalhoub RJ. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet. 1974;2:556–560. doi: 10.1016/s0140-6736(74)91880-7. [DOI] [PubMed] [Google Scholar]
- 30.Dall’Aglio P, Chizzolini C, Brigati C. Minimal change glomerulonephritis and focal glomerulosclerosis markers and ‘in vitro’ activity of peripheral blood mononuclear cell. Proc Eur Dial Transplant Assoc. 1983;19:673–678. [PubMed] [Google Scholar]
- 31.Dall’Aglio P, Meroni PL, Barcellini W, et al. Altered expression of B lymphocyte surface immunoglobulins in minimal change nephrotic syndrome and focal glomerulosclerosis. Nephron. 1984;37:224–228. doi: 10.1159/000183253. [DOI] [PubMed] [Google Scholar]
- 32.Yokoyama H, Kida H, Abe T, Koshino Y, Yoshimura M, Hattori N. Impaired immunoglobulin G production in minimal change nephrotic syndrome in adults. Clin Exp Immunol. 1987;70:110–115. [PMC free article] [PubMed] [Google Scholar]
- 33.Spinale JM, Mariani LH, Kapoor S. A reassessment of soluble urokinase-type plasminogen activator receptor in glomerular disease. Kidney Int. 2014 Oct 29; doi: 10.1038/ki.2014.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meijers BK, Reiser J. Reassessing the Reassessment of suPAR in Glomerular Disease. Front Med (Lausanne) 2015 Jan 14;1:59. doi: 10.3389/fmed.2014.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eugen-Olsen J, Andersen O, Linneberg A, et al. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J Intern Med. 2010;268:296–308. doi: 10.1111/j.1365-2796.2010.02252.x. [DOI] [PubMed] [Google Scholar]
- 36.Savin VJ, Sharma R, Sharma M, et al. Circulating factor in recurrent focal segmental glomerularsclerosis. New Eng J Med. 1996;334:878–883. doi: 10.1056/NEJM199604043341402. [DOI] [PubMed] [Google Scholar]
- 37.Dahly-Vernon AJ, Sharma M, McCarthy ET, et al. Transforming Growth Factor-b, 20-HETE Interaction, and Glomerular Injury in Dahl Salt-Sensitive Rats. Hypetension. 2005;45:1–6. doi: 10.1161/01.HYP.0000153791.89776.43. [DOI] [PubMed] [Google Scholar]
- 38.Sharma M, Sharma R, Ge XL, et al. Early detection of radiation-induced glomerular injury by albumin permeability assay. Radiation Research. 2001;155:474–480. doi: 10.1667/0033-7587(2001)155[0474:edorig]2.0.co;2. 2001. [DOI] [PubMed] [Google Scholar]
- 39.McCarthy ET, Sharma M. Savin VJ: Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2010;5:2115–2121. doi: 10.2215/CJN.03800609. [DOI] [PubMed] [Google Scholar]
- 40.Elson GC, Graber P, Losberger C, et al. Cytokine-like factor-1, a novel soluble protein, shares homology with members of the cytokine type I receptor family. J Immunol. 1998;161:1371–1379. [PubMed] [Google Scholar]
- 41.Lesser SS, Lo DC. CNTF II, I presume? Nat Neurosci. 2000;3:851–852. doi: 10.1038/78738. [DOI] [PubMed] [Google Scholar]
- 42.Alexander WS, Rakar S, Robb L. Suckling defect in mice lacking the soluble haemopoietin receptor NR6. Curr Biol. 1999;9:605–608. doi: 10.1016/s0960-9822(99)80266-8. [DOI] [PubMed] [Google Scholar]
- 43.Forger NG, Prevette D, deLapeyrière O, et al. Cardiotrophin-like cytokine/cytokine-like factor 1 is an essential trophic factor for lumbar and facial motoneurons in vivo. J Neurosci. 2003;23:8854–8858. doi: 10.1523/JNEUROSCI.23-26-08854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sohar E, Shoenfeld Y, Udassin R, Magazanik A. Cold-induced profuse sweating on back and chest. A new genetic entity? Lancet. 1978;2:1073–1074. doi: 10.1016/s0140-6736(78)91805-6. [DOI] [PubMed] [Google Scholar]
- 45.Rousseau F, Gauchat JF, McLeod JG, et al. Inactivation of cardiotrophin-like cytokine, a second ligand for ciliary neurotrophic factor receptor, leads to cold-induced sweating syndrome in a patient. Proc Natl Acad Sci U S A. 2006;103:10068–10073. doi: 10.1073/pnas.0509598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crisponi L, Crisponi G, Meloni A, et al. Crisponi syndrome is caused by mutations in the CRLF1 gene and is allelic to cold-induced sweating syndrome type 1. Am J Hum Genet. 2007;80:971–981. doi: 10.1086/516843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aljabari S, Howard E, Bell T, Vasylyeva TL. Cold induced sweating syndrome with urinary system anomaly association. Case Rep Pediatr. 2013;2013:173890. doi: 10.1155/2013/173890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li R, Yang N, Zhang L. Inhibition of Jak/STAT signaling ameliorates mice experimental nephrotic syndrome. Am J Nephrol. 2007;27:580–589. doi: 10.1159/000108102. [DOI] [PubMed] [Google Scholar]
- 50.Seavey MM, Dobrzanski P. The many faces of Janus kinase. Biochem Pharmacol. 2012;83:1136–1145. doi: 10.1016/j.bcp.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 51.Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 52.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aggarwal BB, Kunnumakkara AB, Harikumar KB, et al. Signal transducer and activator of transcription-3, inflammation, and cancer: How intimate is the relationship? Ann N Y Acad Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He G, Karin M. NF-κB and STAT3: Key players in liver inflammation and cancer. Cell Res. 2011;21:159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19:2628–2637. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- 56.Wegrzyn J, Potla R, Chwae YJ, et al. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323:793–797. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gough DJ, Corlett A, Schlessinger K, et al. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science. 2009;324:1713–1716. doi: 10.1126/science.1171721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reich NC. STAT3 revs up the powerhouse. Sci Signal. 2009;2:pe61. doi: 10.1126/scisignal.290pe61. [DOI] [PubMed] [Google Scholar]
- 59.Liu R, Zhong Y, Li X, et al. Role of transcription factor acetylation in diabetic kidney disease. Diabetes. 2014;63:2440–2453. doi: 10.2337/db13-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pietrocola F, Izzo V, Niso-Santano M, et al. Regulation of autophagy by stress-responsive transcription factors. Semin Cancer Biol. 2013;23:310–322. doi: 10.1016/j.semcancer.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 61.Gong J, Muñoz AR, Chan D, et al. STAT3 down regulates LC3 to inhibit autophagy and pancreatic cancer cell growth. Oncotarget. 2014;5:2529–2541. doi: 10.18632/oncotarget.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu L, McBride KM, Reich NC. STAT3 nuclear import is independent of tyrosine phosphorylation and mediated by importin-alpha3. Proc Natl Acad Sci USA. 2005;102:8150–8155. doi: 10.1073/pnas.0501643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Timofeeva OA, Chasovskikh S, Lonskaya I, et al. Mechanisms of unphosphorylated STAT3 transcription factor binding to DNA. J Biol Chem. 2012;287:14192–14200. doi: 10.1074/jbc.M111.323899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Germain D, Frank DA. Targeting the cytoplasmic and nuclear functions of signal transducers and activators of transcription 3 for cancer therapy. Clin Cancer Res. 2007;13:5665–5669. doi: 10.1158/1078-0432.CCR-06-2491. [DOI] [PubMed] [Google Scholar]
- 65.Sehgal PB. Paradigm shifts in the cell biology of STAT signaling. Semin Cell Dev Biol. 2008;19:329–340. doi: 10.1016/j.semcdb.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dai Y, Gu L, Yuan W, et al. Podocyte-specific deletion of signal transducer and activator of transcription 3 attenuates nephrotoxic serum-induced glomerulonephritis. Kidney Int. 2013;84:950–961. doi: 10.1038/ki.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gu L, Dai Y, Xu J, et al. Deletion of podocyte STAT3 mitigates the entire spectrum of HIV-1-associated nephropathy. AIDS. 2013;27:1091–1098. doi: 10.1097/QAD.0b013e32835f1ea1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fagard R, Metelev V, Souissi I, Baran-Marszak F. STAT3 inhibitors for cancer therapy: Have all roads been explored? JAKSTAT. 2013;2:e22882. doi: 10.4161/jkst.22882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miklossy G, Hilliard TS, Turkson J. Therapeutic modulators of STAT signalling for human diseases. Nat Rev Drug Discov. 2013;12:611–629. doi: 10.1038/nrd4088. [DOI] [PMC free article] [PubMed] [Google Scholar]