Abstract
Previous studies revealed that examples of the non-naturally occurring microtubule (MT)-stabilizing triazolopyrimidines are both brain penetrant and orally bioavailable, indicating that this class of compounds may be potentially attractive in the development of MT-stabilizing therapies for the central nervous system (CNS). We now report on the pharmacokinetics (PK), pharmacodynamics (PD), and metabolism of a selected triazolopyrimidine congener, (S)-3-(4-(5-Chloro-7-((1,1,1-trifluoropropan-2-yl)amino)-[1,2,4]triazolo[1,5-a]pyrimidin-6-yl)-3,5-difluorophenoxy)-propan-1-ol (4). These studies revealed that 4 exhibits longer brain than plasma half-life that may be exploited to achieve a selective accumulation of the compound within the CNS. Furthermore, compound metabolism studies suggest that in plasma 4 is rapidly oxidized at the terminal hydroxyl group to form a comparatively inactive carboxylic acid metabolite. Peripheral administration of relatively low doses of 4 to normal mice was found to produce a significant elevation in acetylated α-tubulin, a marker of stable MTs, in the brain. Collectively, these results indicate that 4 may effectively target brain MTs at doses that produce minimal peripheral exposure.
Keywords: Triazolopyrimidine, Microtubule stabilization, Pharmacokinetic, Pharmacodynamic, CNS drug discovery
Graphical Abstract
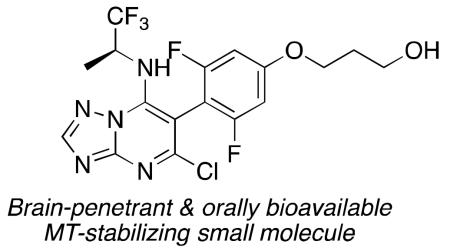
Microtubule (MT)-stabilizing agents constitute a relatively large and diverse group of molecules that for the most part comprise structurally complex natural products or derivatives thereof.1 However, in recent years an increasing number of synthetically accessible, non-naturally occurring MT-stabilizing agents have been described.1 Among these, the triazolopyrimidines, such as cevipabulin2 (1, Figure 1) and related heterocycles,3-7 attracted considerable attention as potential candidates for agrochemical and anti-cancer purposes. Furthermore, we have recently shown that selected members of this class of heterocycles (e.g., 2, Figure 1) are both orally bioavailable as well as brain penetrant and can increase markers of stable MTs in the brain of wild-type (WT) mice,8 suggesting that molecules from this class of heterocycles may be particularly promising in the development of CNS-directed MT-stabilizing therapies.
Figure 1.
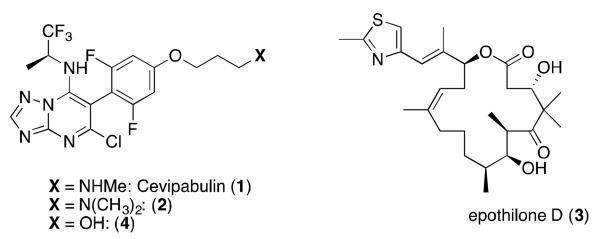
Structures of epothilone D and selected triazolopyrimidines MT-stabilizing compounds.
Given that MT-stabilizing compounds can trigger apoptosis in rapidly dividing cells, one of the challenges in developing MT-stabilizing therapies to treat CNS diseases relates to the potential for undesired side effects that might arise from the exposure of peripheral tissues to the MT-stabilizing drug. Thus, MT-stabilizing candidates for CNS indications should ideally show preferential distribution into the brain. Targeting of MT-stabilizing agents to the brain has been attempted by exploiting receptor-mediated uptake mechanisms at the level of the blood-brain barrier (BBB).9 This and related approaches,10 however, typically require the construction of prodrugs or bi-functional conjugates, or the encapsulation of the drug into nanoparticles, all of which may pose significant challenges. An alternative approach is to screen for analogues that are BBB-permeable and which exhibit longer brain than plasma half-life, such that the active drug may be retained in the CNS for prolonged periods of time. This passive targeting of the brain is generally due to greater non-specific binding of compound to brain proteins or lipids relative to plasma proteins (i.e., the unbound compound fraction is greater in plasma than in brain). However, this phenomenon can ultimately lead to a sustained brain pharmacodynamic effect through a depot-like release of drug, while plasma compound concentrations drop relatively quickly.
Notably, in the realm of MT-stabilizing natural products, selected epothilones, such as epothilone B11 and D12, 13 (3, Figure 1), have been found to be both brain penetrant and exhibit a large differential between brain and plasma half-life. Furthermore, in the case of epothilone D, prolonged brain retention may have played an important role in the favorable therapeutic window that was observed in different efficacy studies involving tau transgenic mouse models.12, 14, 15
In the context of the non-naturally occurring triazolopyrimidines and related heterocyclic MT-stabilizing compounds, none of the brain penetrant and orally bioavailable examples that were initially examined were found to have a significantly different brain and plasma half-life.8 This led us to screen for alternative congeners that might exhibit epothilone D-like prolonged brain retention and thus be more suitable for in vivo efficacy studies. These studies resulted in the identification of triazolopyrimidine 4, a previously known MT-stabilizing compound2 and a close analogue of the anti-cancer clinical candidate 1.
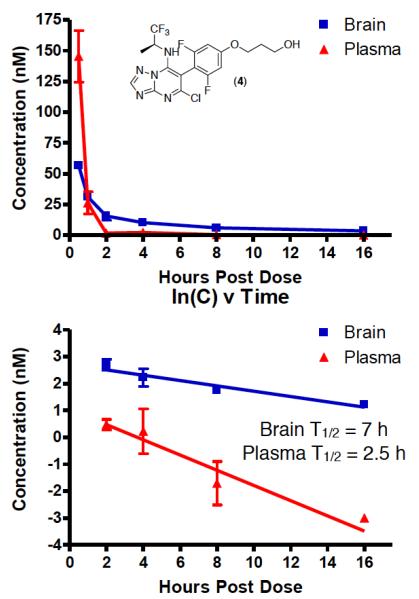
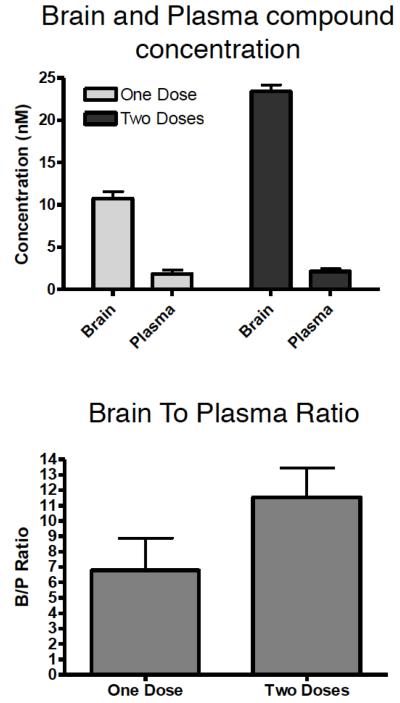
Evaluation of the PK properties of 4 revealed that this triazolopyrimidine exhibits relatively prolonged brain half-life (7 h), which combined with a comparatively short plasma half-life (2.5 h) leads to a widening of the brain-to-plasma concentration ratio (B/P) over time (Figure 2). Although the observed differential in brain and plasma half-life is not nearly as wide as the one described for epothilone D, a progressive build-up of 4 in the brain relative to plasma could be reached upon repeated dosing of the compound in mice (see Figure 3). In humans, this might translate to twice daily dosing, based on allometric scaling.16
Figure 2.
Brain and plasma levels of 4 after ip administration of 5 mg/kg (top); rate of elimination from brain and plasma (bottom).
Figure 3.
Brain and plasma levels of 4 4 h after a single dose of 5 mg/Kg ip or 4 h after two repeated doses separated by a 4 h interval (top); brain-to-plasma concentrations of 4 after one and two doses that were separated by 4 h (bottom).
Structurally, the only difference of triazolopyrimidine 4 relative to 1 is the presence of a primary hydroxyl at the side chain, rather than a secondary amine. Given that 1, unlike 4, exhibits relatively long plasma half-life (i.e., 13 h),2 we hypothesized that the limited metabolic stability of 4 may involve a relatively rapid metabolism of the primary hydroxyl to the corresponding carboxylic acid (5, Scheme 1), followed possibly by a spontaneous elimination of phenol 6 (Scheme 1) with formation of acrylic acid. To evaluate this possibility, we conducted in vitro microsomal stability studies in which compound-treated mixtures were analyzed by LC/MS/MS to monitor for both the disappearance of parent compound and the formation of these possible metabolites. Interestingly, the ionization and fragmentation of the two most abundant in vitro metabolites were found to be consistent with proposed structures 5 and 6 (see Supporting Information).
Scheme 1.
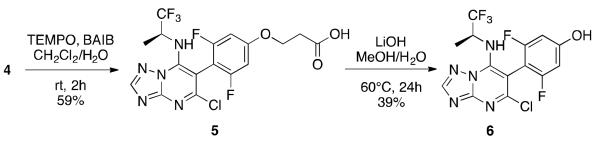
Synthesis of proposed metabolites of 5 and 6.
To confirm the identity of these two metabolites, compounds 5 and 6 were synthesized from 4, as highlighted in Scheme 1 (see Supporting Infromation), and compared by LC/MS/MS with the metabolites generated in the microsomal stability studies.
Consistent with the proposed structures, the synthetic compounds 5 and 6 were found to exhibit identical retention time and fragmentation pathways as the two main metabolites detected in the microsomal stability studies (see Supporting Information).
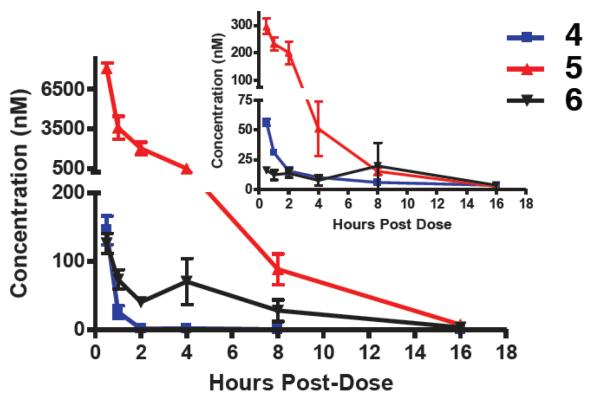
Furthermore, analysis of brain and plasma samples of mice that were collected after intraperitoneal (ip) injection of 5 mg/kg of 4 revealed that both metabolites 5 and 6 were detected predominantly in plasma, with the former compound found at much larger concentrations than the latter (Figure 4).
Figure 4.
Plasma concentration of 4 and metabolites 5 and 6. The inset shows brain concentrations of the parent compound and metabolites.
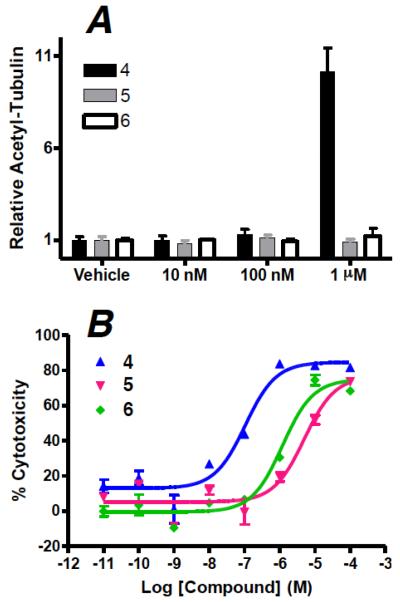
An evaluation of the biological activity of both metabolites 5 and 6 in a 4 hour cell-based assay of MT-stabilization revealed that both 5 and 6 were significantly less potent than 4, with little to no activity up to 1 μM compound concentration (Figure 5A). Consistent with these results, 24 hour cytotoxicity studies in rapidly dividing QBI cells that are typically sensitive to MT-stabilizing agents confirmed that the IC50 values of 5 and 6 are at least 10–50 times higher than that of 4 (Figure 5B). Thus, the observation that the MT-stabilizing agent 4 is rapidly metabolized in plasma to comparatively less active, less cytotoxic metabolites 5 and 6, coupled with the relatively long brain half-life of 4, suggests that peripheral administration of this triazolopyrimidine may produce significant stabilization of MTs in the brain at doses that result in comparatively low peripheral exposure.
Figure 5.
(A) compound-induced elevation in acetylated α-tubulin in QBI cells treated for 4 hours, relative to vehicle treated cells; (B) comparison of cytotoxicity curves of compounds 4–6 in QBI cells treated for 24 hours.
To investigate whether repeated administrations of a relatively low 5 mg/kg dose of 4 resulted in MT-stabilization in the brain, we assessed whether the compound induced an increase in acetylated α-tubulin, a known marker of stable MTs,17 within brain homogenates. As shown in Figure 6, a significant elevation of this marker of stable MTs was observed 1 day after administration of two 5 mg/kg doses (ip).
Figure 6.
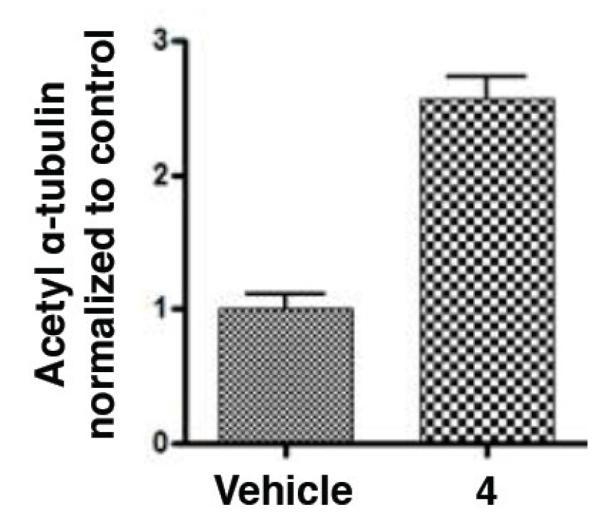
Relative increase in acetylated α-tubulin in the brain of normal mice 1 day after treatment with 5 mg/Kg of 4.
Finally, to estimate the oral bioavailability of 4, we compared the compound concentration in plasma after ip and oral administration. These studies confirmed that, like 1 and other related congeners, 4 is orally bioavailable (see Supporting Information). Thus in summary, the PK, PD, and metabolism data suggest that triazolopyrimidine 4 is an orally bioavailable and brain retentive MT-stabilizing compound. These results indicate that 4 holds promise as a CNS-directed MT-stabilizing agent.
Supplementary Material
Acknowledgments
Financial support for this work has been provided by NIH/NIA Grant AG044332, and the Marian S. Ware Alzheimer Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Ballatore C, Brunden KR, Huryn DM, Trojanowski JQ, Lee VM-Y, Smith AB., III Microtubule Stabilizing Agents as Potential Treatment for Alzheimer’s Disease and Related Neurodegenerative Tauopathies. J. Med. Chem. 2012;55:8979–8996. doi: 10.1021/jm301079z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang N, Ayral-Kaloustian S, Nguyen T, Afragola J, Hernandez R, Lucas J, Gibbons J, Beyer C. Synthesis and SAR of [1,2,4]triazolo[1,5-a]pyrimidines, a class of anticancer agents with a unique mechanism of tubulin inhibition. J. Med. Chem. 2007;50:319–327. doi: 10.1021/jm060717i. [DOI] [PubMed] [Google Scholar]
- 3.Crowley PJ, Lamberth C, Müller U, Wendeborn S, Nebel K, Williams J, Sageot O-A, Carter N, Mathie T, Kempf H-J, Godwin J, Schneiter P, Dobler MR. Synthesis and fungicidal activity of tubulin polymerisation promoters. Part 1: pyrido[2,3-b]pyrazines. Pest. Manag. Sci. 2010;66:178–185. doi: 10.1002/ps.1852. [DOI] [PubMed] [Google Scholar]
- 4.Lamberth C, Trah S, Wendeborn S, Dumeunier R, Courbot M, Godwin J, Schneiter P. Synthesis and fungicidal activity of tubulin polymerisation promoters. Part 2: Pyridazines. Bioorg. Med. Chem. 2012;20:2803–2810. doi: 10.1016/j.bmc.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 5.Lamberth C, Dumeunier R, Trah S, Wendeborn S, Godwin J, Schneiter P, Corran A. Synthesis and fungicidal activity of tubulin polymerisation promoters. Part 3: imidazoles. Bioorg. Med. Chem. 2013;21:127–134. doi: 10.1016/j.bmc.2012.10.052. [DOI] [PubMed] [Google Scholar]
- 6.Zhang N, Ayral-Kaloustian S, Nguyen T, Hernandez R, Beyer C. 2-cyanoaminopyrimidines as a class of antitumor agents that promote tubulin polymerization. Bioorg. Med. Chem. Lett. 2007;17:3003–3005. doi: 10.1016/j.bmcl.2007.03.070. [DOI] [PubMed] [Google Scholar]
- 7.Zhang N, Ayral-Kaloustian S, Nguyen T, Hernandez R, Lucas J, Discafani C, Beyer C. Synthesis and SAR of 6-chloro-4-fluoroalkylamino-2-heteroaryl-5-(substituted)phenylpyrimidines as anti-cancer agents. Bioorg. Med. Chem. 2009;17:111–118. doi: 10.1016/j.bmc.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Lou K, Yao Y, Hoye AT, James MJ, Cornec AS, Hyde E, Gay B, Lee VM-Y, Trojanowski JQ, Smith AB, III, Brunden KR, Ballatore C. Brain-penetrant, orally bioavailable microtubule-stabilizing small molecules are potential candidate therapeutics for Alzheimer’s disease and related tauopathies. J. Med. Chem. 2014;57:6116–6127. doi: 10.1021/jm5005623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Régina A, Demeule M, Ché C, Lavallée I, Poirier J, Gabathuler R, Béliveau R, Castaigne JP. Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2. Br. J. Pharmacol. 2008;155:185–197. doi: 10.1038/bjp.2008.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pardridge WM. Drug targeting to the brain. Pharm. Res. 2007;24:1733–1744. doi: 10.1007/s11095-007-9324-2. [DOI] [PubMed] [Google Scholar]
- 11.O’Reilly T, Wartmann M, Maira SM, Hattenberger M, Vaxelaire J, Muller M, Ferretti S, Buchdunger E, Altmann KH, McSheehy PM. Patupilone (epothilone B, EPO906) and imatinib (STI571, Glivec) in combination display enhanced antitumour activity in vivo against experimental rat C6 glioma. Cancer Chemother. Pharmacol. 2005;55:307–317. doi: 10.1007/s00280-004-0913-z. [DOI] [PubMed] [Google Scholar]
- 12.Brunden KR, Zhang B, Carroll J, Yao Y, Potuzak JS, Hogan AM, Iba M, James MJ, Xie SX, Ballatore C, Smith AB, III, Lee VM-Y, Trojanowski JQ. Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. J. Neurosci. 2010;30:13861–13866. doi: 10.1523/JNEUROSCI.3059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunden KR, Yao Y, Potuzak JS, Ferrer NI, Ballatore C, James MJ, Hogan AM, Trojanowski JQ, Smith AB, III, Lee VM-Y. The characterization of microtubule-stabilizing drugs as possible therapeutic agents for Alzheimer’s disease and related tauopathies. Pharmacol. Res. 2011;63:341–351. doi: 10.1016/j.phrs.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barten DM, Fanara P, Andorfer C, Hoque N, Wong PYA, Husted KH, Cadelina GW, DeCarr LB, Yang L, Liu L, Fessler C, Protassio J, Riff T, Turner H, Janus CG, Sankaranarayanan S, Polson C, Meredith JE, Gray G, Hanna A, Olson RE, Kim SH, Vite GD, Lee FY, Albright CF. Hyperdynamic microtubules, cognitive deficits, and pathology are improved in tau transgenic mice with low doses of the microtubule-stabilizing agent BMS-241027. J. Neurosci. 2012;32:7137–7145. doi: 10.1523/JNEUROSCI.0188-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang B, Carroll J, Trojanowski JQ, Yao Y, Iba M, Potuzak JS, Hogan AM, Xie SX, Ballatore C, Smith AB, III, Lee VM-Y, Brunden KR. The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J. Neurosci. 2012;32:3601–3611. doi: 10.1523/JNEUROSCI.4922-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caldwell GW, Masucci JA, Yan Z, Hageman W. Allometric scaling of pharmacokinetic parameters in drug discovery: can human CL, Vss and t1/2 be predicted from in-vivo rat data? Eur. J. Drug Metab. Pharmacokinet. 2004;29:133–143. doi: 10.1007/BF03190588. [DOI] [PubMed] [Google Scholar]
- 17.Black MM, Baas PW, Humphries S. Dynamics of alpha-tubulin deacetylation in intact neurons. J. Neurosci. 1989;9:358–368. doi: 10.1523/JNEUROSCI.09-01-00358.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.