Abstract
Background
Despite their importance in animal and human health, the epidemiology of species of the Leishmania enriettii complex remains poorly understood, including the identity of their biological vectors. Biting midges of the genus Forcipomyia (Lasiohelea) have been implicated in the transmission of a member of the L. enriettii complex in Australia, but the far larger and more widespread genus Culicoides has not been investigated for the potential to include vectors to date.
Methodology/Principal Findings
Females from colonies of the midges Culicoides nubeculosus Meigen and C. sonorensis Wirth & Jones and the sand fly Lutzomyia longipalpis Lutz & Nevia (Diptera: Psychodidae) were experimentally infected with two different species of Leishmania, originating from Australia (Leishmania sp. AM-2004) and Brazil (Leishmania enriettii). In addition, the infectivity of L. enriettii infections generated in guinea pigs and golden hamsters for Lu. longipalpis and C. sonorensis was tested by xenodiagnosis. Development of L. enriettii in Lu. longipalpis was relatively poor compared to other Leishmania species in this permissive vector. Culicoides nubeculosus was not susceptible to infection by parasites from the L. enriettii complex. In contrast, C. sonorensis developed late stage infections with colonization of the thoracic midgut and the stomodeal valve. In hamsters, experimental infection with L. enriettii led only to mild symptoms, while in guinea pigs L. enriettii grew aggressively, producing large, ulcerated, tumour-like lesions. A high proportion of C. sonorensis (up to 80%) feeding on the ears and nose of these guinea pigs became infected.
Conclusions/Significance
We demonstrate that L. enriettii can develop late stage infections in the biting midge Culicoides sonorensis. This midge was found to be susceptible to L. enriettii to a similar degree as Lutzomyia longipalpis, the vector of Leishmania infantum in South America. Our results support the hypothesis that some biting midges could be natural vectors of the L. enriettii complex because of their vector competence, although not Culicoides sonorensis itself, which is not sympatric, and midges should be assessed in the field while searching for vectors of related Leishmania species including L. martiniquensis and "L. siamensis".
Author Summary
This study investigates the laboratory infection of two species of Culicoides biting midges (Diptera: Ceratopogonidae) and one species of sand fly (Diptera: Psychodidae) with two species of Leishmania. These members of the L. enriettii complex were demonstrated to colonize the stomodeal valve of Culicoides sonorensis following membrane feeding on blood-parasite mixtures or direct feeding on guinea pigs that demonstrated clinical signs of infection. In contrast, three other species of Leishmania that are known to be transmitted by sand flies failed to successfully develop in C. sonorensis. A sand fly species which is highly permissive to Leishmania infection, Lu. longipalpis, a widespread vector of L. infantum in Latin America, was found to support only moderate infections of L. enriettii from Brazil and Leishmania sp. AM-2004 from Australia. In addition to establishing a suitable laboratory model for infection of Culicoides with L. enriettii, successful infection of C. sonorensis highlights that vectors other than sand flies should be considered as part of epidemiological studies on parasites belonging to the L. enriettii complex.
Introduction
The leishmaniases are widespread protozoan diseases with dermal or visceral clinical symptoms that affect humans and animals worldwide. Members of the genus Leishmania (Trypanosomatidae: Kinetoplastida) follow a digenetic life cycle, alternating between a vertebrate host and insect vector. To date, phlebotomine sand flies are considered the only proven vectors responsible for maintenance of the life cycle and transmission of these parasites. The Leishmania species infecting humans comprise about 20 species, mostly belonging to the subgenera L. (Leishmania) and L. (Viannia) [1].
Reservoir hosts may be human in some cases (anthroponotic transmission), but for the majority of Leishmania species infecting humans the reservoirs are domestic or wild animals (zoonotic transmission). Most experts studying sand fly-Leishmania interactions accept six classical criteria for vector incrimination [1,2] that would ideally be satisfied to fully prove vector status: 1, there is a strong ecological association between the vector and the reservoir host; 2, parasites are isolated and/or typed from wild caught vectors not containing recent blood meals and are shown to be identical to those in the reservoir host; 3, infections in such wild caught vectors exhibit parasites in the anterior midgut, on the stomodeal valve and the presence of metacyclic promastigotes, or such development beyond the blood meal can be demonstrated by experimental infection of the vector using laboratory colonies; 4, the vector is attracted to and bites the reservoir host; 5, the vector can be infected by biting and feeding on the reservoir host or an equivalent laboratory model (xenodiagnosis); 6, experimental transmission by bite is achieved to the reservoir or an equivalent laboratory model. However, whilst desirable, rarely are all these criteria satisfied before conclusions are drawn about the identity of Leishmania vectors. Outbreaks of known species in new foci and newly discovered species are particularly problematic as in such cases the reservoir may be uncertain or completely unknown, making the testing of many of these criteria difficult.
The epidemiology of leishmaniases caused by a group of species called the L. enriettii complex is poorly understood, but is becoming increasingly important to human health. According to phylogenetic studies the L. enriettii complex occupies a position basal to all other euleishmania species, but falls outside the established subgenera Leishmania and Viannia [3–7]. The first described species within the complex, L. enriettii, was isolated from the skin of domestic guinea pigs (Cavia porcellus) in Paraná State, Brazil [8–10] and a second species (currently unnamed, here termed Leishmania sp. AM-2004) was more recently isolated from red kangaroos in Australia [11]. These sporadic infections of guinea pigs and kangaroos were characterized by occurrence of tumour-like skin lesions on the ears, nose, feet and testicles in animals [8–10], but both species appear to be non-pathogenic to humans. However, the L. enriettii complex was also recently extended to include three species known to cause clinical disease in humans: L. martiniquensis from Martinique (Caribbean island) and Thailand [7,12]; a second species from Thailand recorded as "L. siamensis" [13]; and another new species from Ghana [14]. ("L. siamensis" has not been formally described so is used in quotation marks). In addition, DNA samples from cutaneous lesions in horses and cattle in Central Europe [15,16] and the USA [17] appear to be identical to L. martiniquensis [7]. Human infections with L. martiniquensis manifest clinically as cutaneous [12,18] or visceral disease [7,19], "L. siamensis" presented as mixed cutaneous and visceral disease [13], and in Ghana the disease has only been found in the cutaneous form [14].
Suspected vectors of the L. enriettii complex include a variety of sand fly and non-sand fly Diptera. In Brazil, Lutzomyia monticola was suggested as a possible vector for L. enriettii [9], although no definitive studies have been conducted [20]. Candidate vectors of L. martiniquensis include Lutzomyia atroclavatus and Lu. cayennensis, since these are the only known sand fly species on Martinique island [12]. In Thailand, Leishmania DNA was found in Sergentomyia species, namely Sergentomyia gemmea [21,22] and S. barraudi [22], although Sergentomyia species are not usually regarded as vectors for human-infective Leishmania. In contrast, in Australia, day-feeding biting midges of the genus Forcipomyia (Lasiohelea) were implicated as vectors of cutaneous leishmaniasis caused by Leishmania sp. AM-2004 in red kangaroos and other macropods [5,11,23]. Microscopical examination revealed that Forcipomyia produced late stage Leishmania infection of high intensities including colonization of the stomodeal valve, the presence of material resembling promastigote secretory gel (PSG) [24] and promastigotes with morphology of infectious metacyclic stages [5]. This evidence for midge-transmission of Leishmania sp. AM-2004 is compelling, and the strongest vector incrimination for any member of the L. enriettii complex, but is not conclusive as a number of the criteria set out above are yet to be satisfied or tested.
The aim of this study was to evaluate the possibility that L. enriettii is also midge-transmitted, as indicated for the related species Leishmania sp AM-2004. Direct testing of this hypothesis using wild caught midges from Brazil is currently not feasible, as there is no information on likely midge vectors or colonised insects from Brazil. Therefore, the vector competences of two species of midge available in established colonies were assessed, Culicoides (Monoculicoides) sonorensis and C. (M.) nubeculosus. Neither of these can be the true vector of L. enriettii as they are not sympatric, C. sonorensis is a north American species [25] and C. nubeculosus is European [26], but both are model systems that have been used to study a wide variety of arbovirus strains and species [25–27]. Infections of L. enriettii in these two midge species were generated by membrane feeding and compared with those produced in the neotropic sand fly Lutzomyia longipalpis, which is highly permissive for all Leishmania species tested to date [28]. To provide a parasite control, parallel infections in all three insects were also performed with Leishmania sp. AM-2004. These experiments were complemented by the use of guinea pigs (Cavia porcellus) and golden hamsters (Mesocricetus auratus) experimentally infected with L. enriettii in xenodiagnosis experiments with C. sonorensis and Lu. longipalpis, testing the ability of these insects to acquire infections by feeding on these mammalian hosts.
Materials and Methods
Ethical statement
Animals were maintained and handled in the animal facility of Charles University in Prague in accordance with institutional guidelines and Czech legislation (Act No. 246/1992 and 359/2012 coll. on Protection of Animals against Cruelty in present statutes at large), which complies with all relevant European Union and international guidelines for experimental animals. All the experiments were approved by the Committee on the Ethics of Laboratory Experiments of the Charles University in Prague and were performed under permission no. MSMT-31114/2013-13 of the Ministry of the Environment of the Czech Republic. Investigators are certificated for experimentation with animals by the Ministry of Agriculture of the Czech Republic.
Parasites and vectors
Leishmania enriettii LV90 (MCAV/BR/45/LV90) and Leishmania sp. AM-2004 (MMAC/AU/2004/AM-2004; Roo1; LV756), and three human infecting Leishmania strains, L. major FVI (MHOM/IL/81/Friedlin/FVI), L. infantum CUK3 (TOB/TR/2005/CUK3) and L. donovani GR374 (MHOM/ET/2010/GR374), were used. Parasites were maintained at 23°C in M199 medium supplemented with 10% fetal calf serum (Gibco), 1% BME vitamins (Sigma), 2% sterile urine and 250 μg/ml amikin (Amikin, Bristol-Myers Squibb), and were in culture for about 10 subpassages from an animal host before use. Before experimental feeding, parasites were washed by centrifugation and resuspended in saline solution.
Lutzomyia longipalpis (Jacobina colony) was maintained at Charles University in Prague under standard conditions [29]. Females from the colonies of Culicoides nubeculosus and C. sonorensis (both belonging to subgenus Monoculicoides) were sent to Charles University from the Pirbright Institute, UK and kept at 20°C before exposure to feeding. All insects were initially given free access to 50% sucrose supplemented with penicillin (5000 U/ml), which was replaced with sugar solution alone 3 days before experimental feeding.
Membrane feeding on infected blood
All infection experiments were performed at Charles University in Prague. In each experiment, approximately 150 Lu. longipalpis or Culicoides females (5–7 days old) were fed through a chick-skin membrane on heat-inactivated rabbit blood containing 107 promastigotes/ml from one of the strains described above. Engorged females were separated, maintained at 26°C or 20°C, according to experimental design, and dissected at days 1–2, 3, 5–6 and 9–10 post-blood meal (PBM). The localization and intensity of Leishmania infection in guts were evaluated in situ under a light microscope, by scoring the proportions of flies with low (<100 parasites/gut), moderate (100–1000 parasites/gut) or heavy (>1000 parasites/gut) infections [30]. All experiments were repeated at least twice.
Morphological analysis
Smears from midguts of C. sonorensis (7 and 10 days PBM) and Lu. longipalpis (10 days PBM) infected with L. enriettii were fixed with methanol, stained with Giemsa, examined under the light microscope with an oil-immersion objective and measured using ImageJ program. Body length, flagellar length and body width of parasites were measured for determination of morphological forms according to the criteria of Walters et al. [31] and Cihakova and Volf [32]. The following morphological forms were distinguished: (i) short nectomonads: body length <14 μm and flagellar length < 2 times body length; (ii) long nectomonads: body length ≥ 14 μm and (iii) metacyclic promastigotes: body length <14 μm and flagellar length ≥ 2 times body length, as summarized in Sadlova et al. [33].
Infection and xenodiagnoses of guinea pigs and hamsters
Two guinea pigs (Cavia porcellus) and two golden hamsters (Mesocricetus auratus), anaesthetized with ketamin/xylazin (150 mg/kg and 15 mg/kg, respectively), were injected with 107 late-log stage promastigotes intradermally into the ear pinnae and nose. The course of infection was recorded weekly.
Xenodiagnoses were performed on animals 3, 4, 7, 9, 12 weeks post-infection (PI) using Lu. longipalpis (5–6 days old) and C. sonorensis (5 days old). Female Lu. longipalpis or C. sonorensis were placed into plastic vials covered by fine nylon mesh and allowed to feed on the inoculated site of anaesthetized animals. Successfully blood-fed individuals were then maintained for two days at 20°C and then stored in Elution Buffer at -20°C for subsequent quantitative PCR (Q-PCR). After the last xenodiagnosis, the hosts (golden hamsters and guinea pigs) were euthanized, dissected and tissues from ears, draining lymph nodes, noses, spleens, livers and blood were stored at -20°C for subsequent Q-PCR.
Quantitative PCR
Extractions of DNA from vectors and animal tissues were performed using a High Pure PCR Template Preparation Kit (Roche) according to the manufacturer´s instructions. The total DNA was used as a template for Q-PCR amplification with the primers described by Mary et al. [34] in Bio-Rad iCycler and iQ Real-Time PCR Systems using the SYBR Green detection method (iQ SYBR Green Supermix, Bio-Rad).
Results
Development of L. enriettii and Leishmania sp. AM-2004 in Lu. longipalpis, C. nubeculosus and C. sonorensis
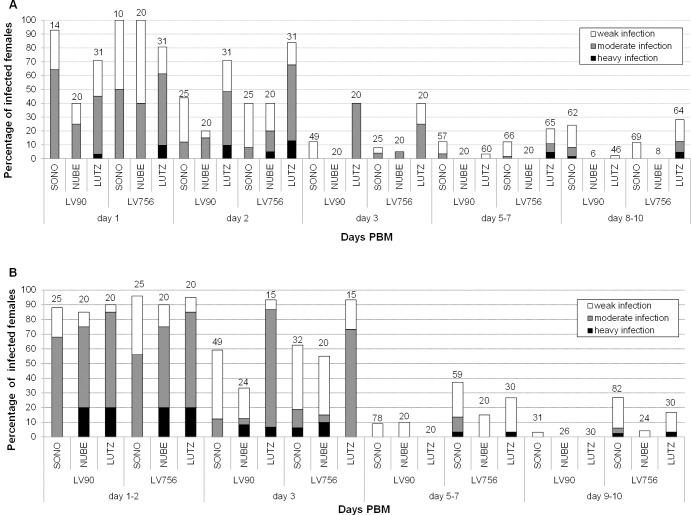
Infection of Lu. longipalpis was attempted with two species of Leishmania, L. enriettii (LV90 strain from Brazil) and Leishmania sp. AM-2004 (LV756 strain from Australia), by membrane feeding in flies maintained at two different temperatures (26°C and 20°C). At 26°C (Fig 1A), a high infection rate (70–80%) was observed for both parasite species on days 1–2 PBM, all parasites being located in the abdominal midgut (AMG). Then, due to defecation of the blood meal remnants, the infection rate was reduced to 40% on day 3 PBM. In late stage infections (days 5–10 PBM), L. enriettii was observed only at low numbers, all being located in the AMG with no colonisation of the stomodeal valve (SV). Leishmania sp. AM-2004 generated somewhat better infections, producing moderate or heavy infections in 12% of infected females and colonization of the SV in 15–20% of them. At 20°C parasite development was similar (Fig 1B), but no L. enriettii and very few Leishmania sp. AM-2004 infections developed to a late-stage in Lu. longipalpis.
Fig 1. Development of Leishmania from the L. enriettii complex in vectors.
Experimental infection of the sand fly Lutzomyia longipalpis (LUTZ) and two biting midges Culicoides nubeculosus (NUBE) and C. sonorensis (SONO) with Leishmania enriettii (LV90) and Leishmania sp. AM-2004 (LV756). Insects were maintained either at 26°C (A) or 20°C (B). Intensities of infection were estimated as light (˂100 promastigotes/gut), moderate (100–1,000 promastigotes/gut) or heavy (˃1,000 promastigotes/gut). Numbers above each bar indicate the number of dissected females.
In C. nubeculosus, L. enriettii and Leishmania sp. AM-2004 parasites were present only in the AMG before and immediately after defecation. On days 6 and 10 PBM, all 54 examined females maintained at 26°C were negative (Fig 1A), while in those maintained at 20°C very few parasites were occasionally found in the abdominal midgut (Fig 1B). Neither Leishmania species were able to establish late stage infections in C. nubeculosus or any colonization of the SV.
In C. sonorensis, L. enriettii and Leishmania sp. AM-2004 both developed early stage infections at high rates (in approximately 90% of midges), producing mostly moderate infections (Fig 1A and 1B). Immediately after defecation (2 days PBM at 26°C and 3 days PBM at 20°C), again the parasite numbers were reduced, but moderate or heavy infections were still observed in some females. However, a striking contrast to C. nubeculosus was observed in parasite development on days 5–7 PBM and onwards. The infection rates observed were comparable to Lu. longipalpis, however, in C. sonorensis, Leishmania promastigotes migrated to the thoracic midgut (TMG), forming typical rosettes, and then colonized the SV in 20–25% and 20–38% of midges, for L. enriettii and Leishmania sp. AM2004, respectively, which are significantly higher percentages than in Lu. longipalpis for both parasite species. Parasite development was similar and the rate of SV colonization was comparable at both temperatures tested (Fig 1A and 1B).
Light microscopy was used to examine L. enriettii parasites in the region of the SV (Fig 2). Large masses of parasites could be seen attached to the cuticular surface of the SV, potentially partially obstructing the opening of the SV. Morphological analysis was performed on L. enriettii parasites recovered from Lu. longipalpis and C. sonorensis at 10 days PBM. The majority of parasites were short nectomonads, 80% in Lu. longipalpis and 72% in C. sonorensis, and many of these were in rosettes. There were also long nectomonads present, 13% and 23%, respectively, and metacyclic promastigotes at 7% and 5%, respectively (Fig 3).
Fig 2. Midgut dissected from C. sonorensis females with infection of L. enriettii colonizing the stomodeal valve.
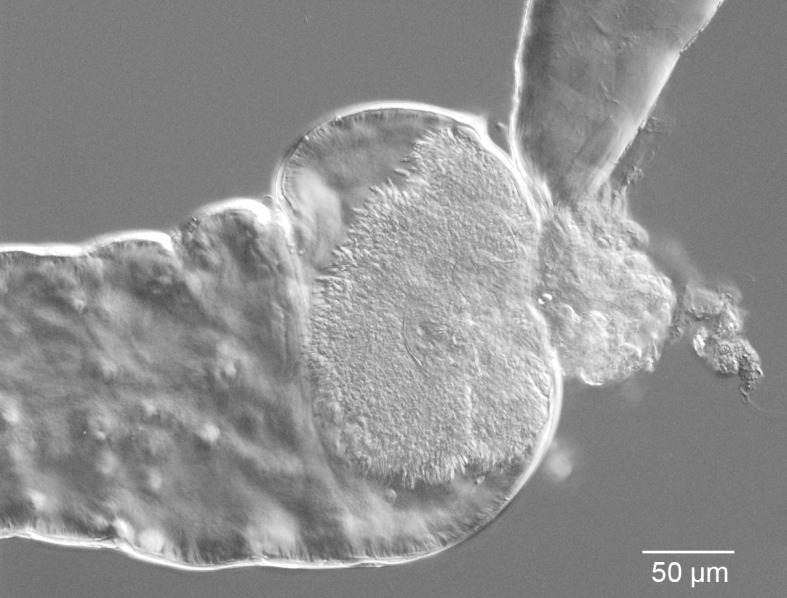
Phase contrast light microscopy showing a mass of promastigotes attached to the stomodeal valve. Bar represents 50 μm.
Fig 3. Morphological forms determined in midgut smears.
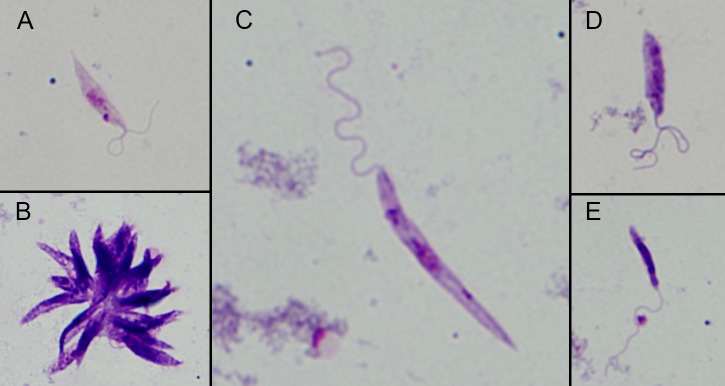
Leishmania parasites distinguished in the midgut of C. sonorensis infected with L. enriettii 10 days PBM. (A) Short nectomonads, (B) short nectomonads forming in rossetes, (C) long nectomonads and (D and E) metacyclic forms.
Development of L. major, L. infantum and L. donovani in Culicoides sonorensis
To evaluate the significance of the above results with L. enriettii complex parasites in C. sonorensis, by membrane feeding we tested the susceptibility of this biting midge to three Leishmania species from the subgenus L. (Leishmania) capable of infecting humans, all of which have proven sand fly vectors. None of these Leishmania species were able to develop successfully into late stage infections in C. sonorensis (Fig 4). Before defecation of blood meal remnants, high numbers of procyclic promastigotes were found in the abdominal midgut in more than 90% of females on days 1–2 PBM. However, after defecation (day 3 PBM and onward) the majority of C. sonorensis females were negative and the rest had only very few parasites in the AMG. On days 6–10, no L. major or L. infantum were present in the midges, although three out of 60 females infected by L. donovani displayed long nectomonads in the AMG, but without any parasites in the TMG or SV (Fig 4). No metacyclic promastigotes were observed in C. sonorensis infected with L. major, L. infantum or L. donovani.
Fig 4. Development of human-infecting Leishmania species in C. sonorensis.
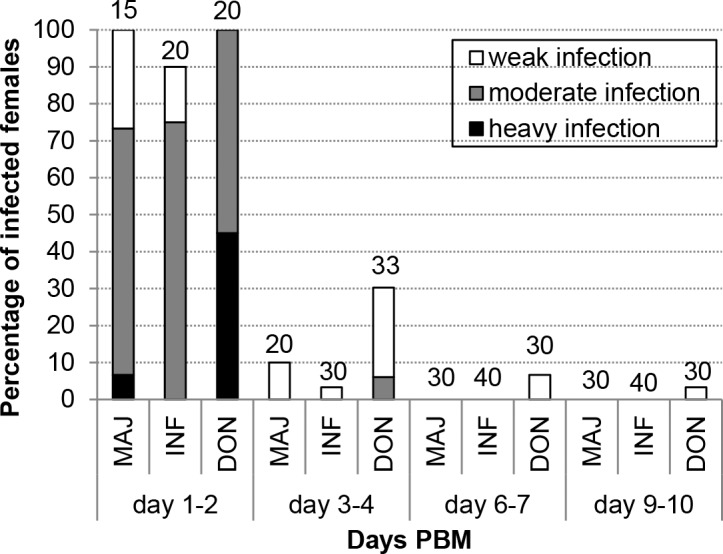
Experimental infection of C. sonorensis with L. major FVI (MAJ), L. infantum CUK3 (INF) and L. donovani GR374 (DON) (at 20°C). Intensities of infection were estimated as in Fig 1.
Course of infection in guinea pigs and xenodiagnosis
The first clinical signs of infection with L. enriettii in guinea pigs were redness and swelling on the inoculated ear 3–4 weeks PI. In the following two weeks (5–6 weeks PI), the swelling developed into small cutaneous lesions (~4×3 mm), which later grew rapidly to become large and ulcerated (~14×10 mm) by weeks 9–12 PI. In addition, a secondary dermal lesion appeared in one guinea pig on the skin between the eyes and nose (4.1×4.7 mm). In the animal inoculated via a nasal route, the first clinical manifestation of infection was observed in week 5 PI (two weeks later than on the the ear), but then the signs increased in severity more rapidly and later resembled a necrotic tumour-like ulcer by the end of experiment (12 weeks PI) (Fig 5).
Fig 5. Skin symptoms in rodent hosts infected by L. enriettii.
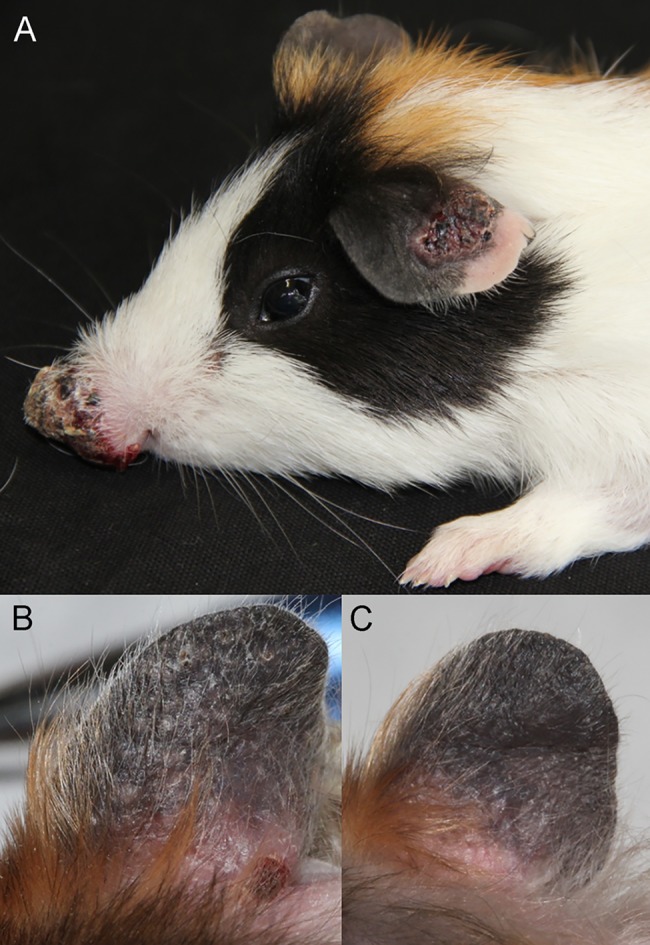
Manifestation of infection on the ear and on the nose of the guinea pig 12 weeks post infection (A). Same ear of the golden hamster 6 weeks post-infection (B) and 12 weeks post-infection (C).
In total, 195 Lu. longipalpis and 125 C. sonorensis female adults were fed on the ears, and 93 Lu. longipalpis and 69 C. sonorensis fed on the noses of two guinea pigs infected with L. enriettii. Preliminary experiments confirmed that Leishmania in such insect guts transformed from amastigote to short and long nectomonads, metacyclic promastigote forms and that in C. sonorensis these proliferated vigorously and colonized the stomodeal valve. The development pattern was similar as observed during experimental feeding. Therefore, Q-PCR was an appropriate method for quantification of xenodiagnosis.
Both infected ears and noses appeared to be a good source of parasites for the insects. In C. sonorensis the infection rates were about 50% and 80% for ears and nose, while in Lu. longipalpis the infection rates were a little lower at about 30% and 50%, respectively. In both vectors, the highest infection rates were observed using animals between 4–7 weeks PI, afterwards the infectivity of guinea pigs for both vectors decreased. The xenodiagnosis results in guinea pigs are summarized in Fig 6.
Fig 6. Infectiousness of guinea pigs for Lu. longipalpis and C. sonorensis feeding on L. enriettii inoculated ears or noses.

Xenodiagnoses were performed at three intervals (3, 4, 7 weeks post-infection) on ears of guinea pigs, and five times (3, 4, 7, 9 and 12 weeks post-infection) on noses of guinea pigs. The fed females were tested for presence of Leishmania parasites 2 days PBM using Q-PCR.
After the last xenodiagnosis experiment (12 weeks PI), the guinea pigs were euthanized and Q-PCR showed high numbers of parasites present in inoculated ears and noses (ranging from 8.8×106–3.8×107 parasites in each organ). In guinea pigs L. enriettii also visceralized to the spleen, and parasites were also detected in draining lymph nodes and co-lateral ears (20–500 parasites in each organ).
Course of infection in hamsters and xenodiagnosis
In golden hamsters the first signs of disease (redness and swelling) were also observed 3 weeks PI on the inoculated ears. Then, however, the course of infections strikingly differed from those in guinea pigs. In hamsters, multiple small nodules appeared on the inoculated ears in weeks 4–7 PI (Fig 5B), but all these nodules self-healed after 7–9 weeks PI (Fig 5C). Similarly, on the nose the infection was manifested only by redness, small sores and oedema, which was reabsorbed by 7 weeks PI. At the end of the experiment neither hamster presented clinical signs of infection.
In total, 159 Lu. longipalpis and 112 C. sonorensis were fed on ears and noses of two hamsters infected with L. enriettii. Generally, the vectors were less willing to feed on hamsters than on guinea pigs and their infectivity rate was much lower. Leishmania were detected only in two groups of Lu. longipalpis females fed on hamster ears 4 and 9 weeks PI (infection rates were about 30% and 10%, respectively). No positivity was found in 80 C. sonorensis females fed on the hamster´s ears. All 85 Lu. longipalpis and 31 C. sonorensis fed on inoculated noses were negative. In hamsters euthanized 12 weeks PI the Q-PCR results demonstrated very low parasite numbers in inoculated ears (<50 parasites). Other organs tested (nose, co-lateral ear, draining lymph node, blood, spleen and liver) were negative in both hamsters.
Discussion
To date phlebotomine sand flies are the only proven vectors of Leishmania species, however, based on the discovery of Leishmania sp. AM-2004 in Australian biting midges [5], we assessed the possibility that L. enriettii may also have a midge vector. Based on the examination of vector competence presented here, we conclude that it is more likely that L. enriettii is transmitted by biting midges than by sand flies. However, several important aspects of vector incrimination need to be tested in future work such as their ecological associations with reservoir hosts and transmission dynamics, which may either provide further support for midge-transmision or lead to rejection of this hypothesis. There are many neotropical species of midges and sand flies, and resolution of this will require careful fieldwork and laboratory testing of any new proposed midge or sand fly vector. In the meantime we recommend that vector studies on members of the L. enriettii complex consider both midges and sand flies as potential vectors.
Our conclusion that L. enriettii is most likely to be midge-transmitted is based on several lines of evidence presented here: L. enriettii developed in the three insects in a similar way to Leishmania sp. AM-2004 and in marked contrast to L. major, L. infantum and L. donovani; the best insect host for L. enriettii was C. sonorensis, showing a similar pattern of development to that seen in Lu. longipalpis but with a higher percentage of stomodeal valve infections, and surviving beyond the blood meal to a "late stage" infection; L. major, L. infantum and L. donovani did not survive after the bloodmeal in C. sonorensis but previous work has shown these to develop mature transmissible infections in Lu. longipalpis; and both C. sonorensis and Lu. longipalpis were infected after feeding on infected guinea pigs, but to a greater extent in C. sonorensis. These data are consistent with midge-transmission of L. enriettii, but do not prove it, and each is discussed in more detail below.
Colonization of biting midges is regarded as extremely challenging, as only a very small number of species possess life cycles traits suitable for laboratory maintanance and the vast majority will not take blood meals under laboratory conditions [25]. The Nearctic species C. sonorensis was demonstrated to be susceptible to infection and our experiments showed that L. enriettii developed late stage infections in 10–30% of C. sonorensis females. We define these as "late stage" infections, meaning that they have progressed beyond the early blood meal phase and become established in the midges. The development seen is remarkable and similar to that seen with Leishmania sp. AM-2004, but in marked contrast to L. major, L. infantum or L. donovani. Moreover, 20% of infected midges with such late stage L. enriettii infections exhibited heavy colonization of the SV. Short and long nectomonads were observed during the late-stage infection in C. sonorensis gut. The short nectomonads are responsible for forward migration and colonization of the stomodeal valve including production of promastigote secretory gel (PSG), which together with sand fly saliva are critical components for disease outcome and progress [35,36]. One area of interest for future investigation would be to see if midge saliva had disease exacerbating properties similar to those of sand fly saliva [37]. Localization of parasite masses on the SV and presence of metacyclic promastigotes is associated with Leishmania transmission in sand flies [38,39] and has been observed in Forcipomyia midges naturally infected with Leishmania sp. AM-2004 [5]. It should be noted that these experiments were performed by membrane feeding, where high doses of parasites can be ingested.
Lu. longipalpis, a widespread vector of L. infantum in Latin America, was capable of supporting L. enriettii to a similar extent as C. sonorensis, although fewer SV infections were observed. However, all other Leishmania species tested in Lu. longipalpis to date, including L. major, L. infantum and L. donovani produce mature infections with high precentages of metacyclic promastigotes and PSG [28]. The percentages and intensity of late stage infections observed here for L. enriettii are far lower than normally found for infection of Lu. longipalpis with other Leishmania species. The Palearctic species C. nubeculosus was not susceptible to Leishmania enriettii, but neither was it susceptible to Leishmania sp. AM-2004. This lack of vector competence is consistent with our previous findings that C. nubeculosus does not support development of L. infantum and L. major [40]. In fact this is the predicted outcome given that there are over 1400 known species of Culicoides known worldwide [25], so the chances of finding one that supports post-blood meal development of any Leishmania parasite must be quite low, and just further emphasises the potential significance of the results obtained with C. sonorensis.
Leishmania enriettii is known as a pathogen of guinea pigs causing tumour-like skin lesions. While some authors [41,42] reported metastatic spread of parasites to distant parts of the guinea pig body (eyelids, lips, feet and genitalia), others found parasites limited to the inoculation site [10,37]. The evolution of skin lesions caused by L. enriettii can be extremely fast within two weeks PI [42] and can be enhanced by addition of sand fly salivary gland extract to the inoculum [37]. In our study, disease manifestation differed between individuals and inoculation sites. Lesions developed 5 weeks PI on the ear (ulcerated between 7–9 weeks PI) and 7 weeks PI on the nose. However, parasites inoculated into the noses grew very quickly, producing large, ulcerated, tumour-like lesions. We did not observe a self-healing process, as previously reported [37,42,43]. According to recent studies, parasites of the L. enriettii complex do not only cause cutaneous forms of leishmaniasis, but can also produce visceral leishmaniasis [7,13,19]. These findings correlate with our results from Q-PCR, which detected L. enriettii parasites in the draining lymph nodes and spleen of infected guinea pigs.
In hamsters, L. enriettii is known to be less pathogenic than in guinea pigs and some studies suggested spontaneous self-healing [44]. Here, we demonstrated that experimental infection of hamsters led only to mild symptoms. On the ear, non-ulcerated multiple nodules appeared at four weeks PI, but had self-healed by eight weeks PI. No clear signs of disease were recorded on the nose during the entire experimental period. This is in accordance to results from xenodiagnosis showing that experimentally infected hamsters were less infectious, with a low proportion of infections found in female Lu. longipalpis fed on ears 4 and 9 weeks PI, but no infections were seen in C. sonorensis.
Xenodiagnosis is currently the gold standard method used to determine infectivity of naturally or experimentally infected hosts for insect vectors. It has been repeatedly used to prove infectivity of potential reservoirs to natural vectors of L. infantum [45–47] and L. tropica [48]. In the current study the infection rate recorded was up to 50% in Lu. longipalpis and up to 80% in C. sonorensis. This is a much higher infection rate than achieved using any rodent infected with L. major, L. tropica or L. donovani [48,49]. Similar high rates (around 60–80%), were obtained only using P. perniciosus and Lu. longipalpis fed on L. infantum-infected dogs [50,51]. It also demostrated that guinea pigs were most infective for Lu. longipalpis and C. sonorensis one month post-infection, despite more serious clinical manifestation of the disease being found later during the experiment. These results agree with previous findings using mouse models where no direct link was observed between host symptoms and infectivity to vectors [48,49].
In summary, we have demonstrated experimentally, for the first time, that two species of the L. enriettii complex, L. enriettii and Leishmania sp. AM-2004, can develop late-stage infections in the biting midge C. sonorensis. This species provides a readily manipulable experimental subject for study of the L. enriettii complex under laboratory conditions; it was found to be similarly susceptible to these parasites as a permissive sand fly species Lutzomyia longipalpis. Both promastigote and amastigote infection of C. sonorensis (performed by membrane feeding and xenodiagnoses, respectively) resulted in masses of parasites in thoracic midgut and colonization of the stomodeal valve, which was found twice as frequently in C. sonorensis as in Lu. longipalpis. These data support those of Dougall et al. [5] who reported mature infections of Leishmania sp. AM-2004 in field-collected biting midges of the genus Forcipomyia. Our results support the hypothesis that biting midges might be natural vectors of the L. enriettii complex, but more detailed studies especially focused on transmission potential and field collections need to be done. However, these results should be taken in consideration while searching for vectors of L. martiniquensis, "L. siamensis" and the recently reported species from Ghana, whose sand fly vectors are unknown.
Data Availability
All relevant data are contained in the paper.
Funding Statement
The study was supported by FP7-261504 EDENext, and the manuscript is cataloged as EDENext345. Culicoides used during experiments were provided by a National Capability grant from the Biotechnological and Biological Sciences Research Council. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Maroli M, Feliciangeli MD, Bichaud L, Charrel RN, Gradoni L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med Vet Entomol. 2012;27: 123–47. 10.1111/j.1365-2915.2012.01034.x [DOI] [PubMed] [Google Scholar]
- 2. Killick-Kendrick R. Phlebotomine vectors of the leishmaniases: a review. Med Vet Entomol. 1990;4: 1–24. http://www.ncbi.nlm.nih.gov/pubmed/2132963 [DOI] [PubMed] [Google Scholar]
- 3. Noyes H, Pratlong F, Chance M, Ellis J, Lanotte G, Dedet JP. A previously unclassified trypanosomatid responsible for human cutaneous lesions in Martinique (French West Indies) is the most divergent member of the genus Leishmania ss. Parasitology. 2002;124: 17–24. [DOI] [PubMed] [Google Scholar]
- 4. Asato Y, Oshiro M, Myint CK, Yamamoto Y, Kato H, Marco JD, et al. Phylogenic analysis of the genus Leishmania by cytochrome b gene sequencing. Exp Parasitol. 2009;121: 352–61. 10.1016/j.exppara.2008.12.013 [DOI] [PubMed] [Google Scholar]
- 5. Dougall AM, Alexander B, Holt DC, Harris T, Sultan AH, Bates PA, et al. Evidence incriminating midges (Diptera: Ceratopogonidae) as potential vectors of Leishmania in Australia. Int J Parasitol. 2011;41: 571–9. 10.1016/j.ijpara.2010.12.008 [DOI] [PubMed] [Google Scholar]
- 6. Marcili A, Sperança MA, da Costa AP, Madeira M de F, Soares HS, Sanches C de OCC, et al. Phylogenetic relationships of Leishmania species based on trypanosomatid barcode (SSU rDNA) and gGAPDH genes: Taxonomic revision of Leishmania (L.) infantum chagasi in South America. Infect Genet Evol. 2014;25: 44–51. 10.1016/j.meegid.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 7. Pothirat T, Tantiworawit A, Chaiwarith R, Jariyapan N, Wannasan A, Siriyasatien P, et al. First isolation of Leishmania from Northern Thailand: case report, identification as Leishmania martiniquensis and phylogenetic position within the Leishmania enriettii complex. PLoS Negl Trop Dis. 2014;8: e3339 10.1371/journal.pntd.0003339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muniz J, Medina H. Leishmaniose tegumentar do cobaio (Leishmania enriettii n.spp.). Hosp (Lond 1886). 1948;33: 7–25. [PubMed] [Google Scholar]
- 9. Luz E, Giovannoni M, Borba A. Infeccao de Lutzomyia monticola por Leishmania enriettii . An Fac Med Univ Fed Paraná. 1967;9–10: 121–128. [Google Scholar]
- 10. Machado MI, Milder R V, Pacheco RS, Silva M, Braga RR, Lainson R. Naturally acquired infections with Leishmania enriettii Muniz and Medina 1948 in guinea-pigs from São Paulo, Brazil. Parasitology. 1994;109 (Pt 2: 135–8. http://www.ncbi.nlm.nih.gov/pubmed/8084659 [DOI] [PubMed] [Google Scholar]
- 11. Rose K, Curtis J, Baldwin T, Mathis A, Kumar B, Sakthianandeswaren A, et al. Cutaneous leishmaniasis in red kangaroos: isolation and characterisation of the causative organisms. Int J Parasitol. 2004;34: 655–64. [DOI] [PubMed] [Google Scholar]
- 12. Desbois N, Pratlong F, Quist D, Dedet J-P. Leishmania (Leishmania) martiniquensis n. sp. (Kinetoplastida: Trypanosomatidae), description of the parasite responsible for cutaneous leishmaniasis in Martinique Island (French West Indies). Parasite. 2014;21: 12 10.1051/parasite/2014011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bualert L, Charungkiattikul W, Thongsuksai P, Mungthin M, Siripattanapipong S, Khositnithikul R, et al. Autochthonous disseminated dermal and visceral leishmaniasis in an AIDS patient, southern Thailand, caused by Leishmania siamensis . Am J Trop Med Hyg. 2012;86: 821–4. 10.4269/ajtmh.2012.11-0707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kwakye-Nuako G, Mosore MT, Duplessis C, Bates MD, Puplampu N, Mensah-Attipoe I, et al. First isolation of a new species of Leishmania responsible for human cutaneous leishmaniasis in Ghana and classification in the Leishmania enriettii complex. Int J Parasitol. 2015. [DOI] [PubMed] [Google Scholar]
- 15. Müller N, Welle M, Lobsiger L, Stoffel MH, Boghenbor KK, Hilbe M, et al. Occurrence of Leishmania sp. in cutaneous lesions of horses in Central Europe. Vet Parasitol. 2009;166: 346–51. 10.1016/j.vetpar.2009.09.001 [DOI] [PubMed] [Google Scholar]
- 16. Lobsiger L, Müller N, Schweizer T, Frey CF, Wiederkehr D, Zumkehr B, et al. An autochthonous case of cutaneous bovine leishmaniasis in Switzerland. Vet Parasitol. 2010;169: 408–14. 10.1016/j.vetpar.2010.01.022 [DOI] [PubMed] [Google Scholar]
- 17. Reuss SM, Dunbar MD, Calderwood Mays MB, Owen JL, Mallicote MF, Archer LL, et al. Autochthonous Leishmania siamensis in horse, Florida, USA. Emerg Infect Dis. 2012;18: 1545–7. 10.3201/eid1809.120184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chiewchanvit S, Tovanabutra N, Jariyapan N, Bates MD, Mahanupab P, Chuamanochan M, et al. Chronic generalized fibrotic skin lesions from disseminated leishmaniasis caused by Leishmania martiniquensis in two patients from northern Thailand infected with HIV. Br J Dermatol. 2015. [DOI] [PubMed] [Google Scholar]
- 19. Liautaud B, Vignier N, Miossec C, Plumelle Y, Kone M, Delta D, et al. First case of visceral leishmaniasis caused by Leishmania martiniquensis . Am J Trop Med Hyg. 2015;92: 317–9. 10.4269/ajtmh.14-0205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lainson R. On Leishmania enriettii and other enigmatic Leishmania species of the Neotropics. Mem Inst Oswaldo Cruz. 1997;92: 377–87. http://www.ncbi.nlm.nih.gov/pubmed/9332605 [DOI] [PubMed] [Google Scholar]
- 21. Kanjanopas K, Siripattanapipong S, Ninsaeng U, Hitakarun A, Jitkaew S, Kaewtaphaya P, et al. Sergentomyia (Neophlebotomus) gemmea, a potential vector of Leishmania siamensis in southern Thailand. BMC Infect Dis. 2013;13: 333 10.1186/1471-2334-13-333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chusri S, Thammapalo S, Silpapojakul K, Siriyasatien P. Animal reservoirs and potential vectors of Leishmania siamensis in southern Thailand. Southeast Asian J Trop Med Public Health. 2014;45: 13–9. http://www.ncbi.nlm.nih.gov/pubmed/24964648 [PubMed] [Google Scholar]
- 23. Dougall A, Shilton C, Low Choy J, Alexander B, Walton S. New reports of Australian cutaneous leishmaniasis in Northern Australian macropods. Epidemiol Infect. 2009;137: 1516–20. 10.1017/S0950268809002313 [DOI] [PubMed] [Google Scholar]
- 24. Rogers ME. The role of Leishmania proteophosphoglycans in sand fly transmission and infection of the mammalian host. Front Microbiol. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nayduch D, Cohnstaedt LW, Saski C, Lawson D, Kersey P, Fife M, et al. Studying Culicoides vectors of BTV in the post-genomic era: resources, bottlenecks to progress and future directions. Virus Res. 2014;182: 43–9. 10.1016/j.virusres.2013.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carpenter S, Groschup MH, Garros C, Felippe-Bauer ML, Purse BV. Culicoides biting midges, arboviruses and public health in Europe. Antiviral Res. 2013;100:102–13. 10.1016/j.antiviral.2013.07.020 [DOI] [PubMed] [Google Scholar]
- 27. Purse B V, Carpenter S, Venter GJ, Bellis G, Mullens BA. Bionomics of temperate and tropical culicoides midges: knowledge gaps and consequences for transmission of Culicoides-borne viruses. Annu Rev Entomol. 2015;60: 373–92. 10.1146/annurev-ento-010814-020614 [DOI] [PubMed] [Google Scholar]
- 28. Volf P, Myskova J. Sand flies and Leishmania: specific versus permissive vectors. Trends Parasitol. 2007;23: 91–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Volf P, Volfova V. Establishment and maintenance of sand fly colonies. J Vector Ecol. 2011;36 Suppl 1: S1–9. 10.1111/j.1948-7134.2011.00106.x [DOI] [PubMed] [Google Scholar]
- 30. Myskova J, Votypka J, Volf P. Leishmania in sand flies: comparison of quantitative polymerase chain reaction with other techniques to determine the intensity of infection. J Med Entomol. 2008;45: 133–8. http://www.ncbi.nlm.nih.gov/pubmed/18283954 [DOI] [PubMed] [Google Scholar]
- 31. Walters LL. Leishmania differentiation in natural and unnatural sand fly hosts. J Eukaryot Microbiol. 40: 196–206. http://www.ncbi.nlm.nih.gov/pubmed/8461893 [DOI] [PubMed] [Google Scholar]
- 32. Ciháková J, Volf P. Development of different Leishmania major strains in the vector sandflies Phlebotomus papatasi and P. duboscqi . Ann Trop Med Parasitol. 1997;91: 267–79. http://www.ncbi.nlm.nih.gov/pubmed/9229020 [DOI] [PubMed] [Google Scholar]
- 33. Sádlová J, Price HP, Smith BA, Votýpka J, Volf P, Smith DF. The stage-regulated HASPB and SHERP proteins are essential for differentiation of the protozoan parasite Leishmania major in its sand fly vector, Phlebotomus papatasi . Cell Microbiol. 2010;12: 1765–79. 10.1111/j.1462-5822.2010.01507.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mary C, Faraut F, Lascombe L, Dumon H. Quantification of Leishmania infantum DNA by a Real-Time PCR Assay with High Sensitivity. J Clin Microbiol. 2004;42: 5249–5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Titus RG, Ribeiro JM. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science. 1988;239: 1306–8. http://www.ncbi.nlm.nih.gov/pubmed/3344436 [DOI] [PubMed] [Google Scholar]
- 36. Rogers ME, Chance ML, Bates PA. The role of promastigote secretory gel in the origin and transmission of the infective stage of Leishmania mexicana by the sandfly Lutzomyia longipalpis . Parasitology. 2002;124: 495–507. http://www.ncbi.nlm.nih.gov/pubmed/12049412 [DOI] [PubMed] [Google Scholar]
- 37. Paranaíba L, de Assis R, Nogueira P, Torrecilhas A, Campos J, Silveira A, et al. Leishmania enriettii : biochemical characterisation of lipophosphoglycans (LPGs) and glycoinositolphospholipids (GIPLs) and infectivity to Cavia porcellus . Parasit Vectors. 2015;8: 31 10.1186/s13071-015-0633-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bates PA, Rogers ME. New insights into the developmental biology and transmission mechanisms of Leishmania . Curr Mol Med. 2004;4: 601–9. http://www.ncbi.nlm.nih.gov/pubmed/15357211 [DOI] [PubMed] [Google Scholar]
- 39. Bates PA. Transmission of Leishmania metacyclic promastigotes by phlebotomine sand flies. Int J Parasitol. 2007;37: 1097–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Seblova V, Sadlova J, Carpenter S, Volf P. Development of Leishmania parasites in Culicoides nubeculosus (Diptera: Ceratopogonidae) and implications for screening vector competence. J Med Entomol. 2012;49: 967–70.: http://www.ncbi.nlm.nih.gov/pubmed/23025175 [DOI] [PubMed] [Google Scholar]
- 41. Paraense WL. The spread of Leishmania enriettii through the body of the guineapig. Trans R Soc Trop Med Hyg. 1953;47: 556–60. http://www.ncbi.nlm.nih.gov/pubmed/13113665 [DOI] [PubMed] [Google Scholar]
- 42. Thomaz-Soccol V, Pratlong F, Langue R, Castro E, Luz E, Dedet JP. New isolation of Leishmania enriettii Muniz and Medina, 1948 in Paranástate, Brazil, 50 years after the first description, and isoenzymatic polymorphism of the L. enriettii taxon. Ann Trop Med Parasitol. 1996;90: 491–5. http://www.ncbi.nlm.nih.gov/pubmed/8915125 [DOI] [PubMed] [Google Scholar]
- 43. Bryceson AD, Bray RS, Wolstencroft RA, Dumonde DC. Immunity in cutaneous leishmaniasis of the guinea-pig. Clin Exp Immunol. 1970;7: 301–41. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1712737&tool=pmcentrez&rendertype=abstract [PMC free article] [PubMed] [Google Scholar]
- 44. Belehu A, Turk JL. Establishment of cutaneous Leishmania enriettii infection in hamsters. Infect Immun. 1976;13: 1235–41. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=420744&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Molina R, Amela C, Nieto J, San-Andrés M, González F, Castillo JA, et al. Infectivity of dogs naturally infected with Leishmania infantum to colonized Phlebotomus perniciosus . Trans R Soc Trop Med Hyg. 1994;88: 491–3. http://www.ncbi.nlm.nih.gov/pubmed/7570854 [DOI] [PubMed] [Google Scholar]
- 46. Maroli M, Pennisi MG, Di Muccio T, Khoury C, Gradoni L, Gramiccia M. Infection of sandflies by a cat naturally infected with Leishmania infantum . Vet Parasitol. 2007;145: 357–60. http://www.sciencedirect.com/science/article/pii/S0304401706006467 [DOI] [PubMed] [Google Scholar]
- 47. Jiménez M, González E, Martín-Martín I, Hernández S, Molina R. Could wild rabbits (Oryctolagus cuniculus) be reservoirs for Leishmania infantum in the focus of Madrid, Spain? Vet Parasitol. 2014;202: 296–300. 10.1016/j.vetpar.2014.03.027 [DOI] [PubMed] [Google Scholar]
- 48. Svobodová M, Votýpka J, Nicolas L, Volf P. Leishmania tropica in the black rat (Rattus rattus): persistence and transmission from asymptomatic host to sand fly vector Phlebotomus sergenti . Microbes Infect. 2003;5: 361–4. http://www.ncbi.nlm.nih.gov/pubmed/12737990 [DOI] [PubMed] [Google Scholar]
- 49. Sadlova J, Seblova V, Votypka J, Warburg A, Volf P. Xenodiagnosis of Leishmania donovani in BALB/c mice using Phlebotomus orientalis: a new laboratory model. Parasit Vectors. 2015;8: 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Guarga JL, Moreno J, Lucientes J, Gracia MJ, Peribáñez MA, Castillo JA. Evaluation of a specific immunochemotherapy for the treatment of canine visceral leishmaniasis. Vet Immunol Immunopathol. 2002;88: 13–20. http://www.ncbi.nlm.nih.gov/pubmed/12088640 [DOI] [PubMed] [Google Scholar]
- 51. Ribeiro RR, Moura EP, Pimentel VM, Sampaio WM, Silva SM, Schettini DA, et al. Reduced tissue parasitic load and infectivity to sand flies in dogs naturally infected by Leishmania (Leishmania) chagasi following treatment with a liposome formulation of meglumine antimoniate. Antimicrob Agents Chemother. 2008;52: 2564–72. 10.1128/AAC.00223-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are contained in the paper.