Abstract
Identifying genes that are differentially expressed in response to social interactions is informative for understanding the molecular basis of social behavior. To address this question, we described changes in gene expression as a result of differences in the extent of social interactions. We housed threespine stickleback (Gasterosteus aculeatus) females in either group conditions or individually for one week, then measured levels of gene expression in three brain regions using RNA-sequencing. We found that numerous genes in the hindbrain/cerebellum had altered expression in response to group or individual housing. However, relatively few genes were differentially expressed in either the diencephalon or telencephalon. The list of genes upregulated in fish from social groups included many genes related to neural development and cell adhesion as well as genes with functions in sensory signaling, stress, and social and reproductive behavior. The list of genes expressed at higher levels in individually-housed fish included several genes previously identified as regulated by social interactions in other animals. The identified genes are interesting targets for future research on the molecular mechanisms of normal social interactions.
Introduction
Social interactions with conspecifics are found across all animal taxa, and the fundamental processes that govern social behavior are highly conserved. Among vertebrates, the core brain circuitry and key neuropeptides and neuromodulators that mediate social behavior are shared ([1,2]; but see [3]). Furthermore, recent work has shown that gene networks that regulate social behavior are even conserved across invertebrates and mammals [4].
To identify genes and molecular pathways involved in social behavior, previous studies have examined animals with different social experiences to determine which genes show changes in expression [5–7]. These studies have either examined the expression of candidate genes or have employed expression arrays or transcriptome sequencing to more globally sample gene expression changes [5–8]. Global expression studies in vertebrates have identified numerous genes that are socially regulated, highlighting genes not previously associated with social behavior [4,8–15]. These studies have been informative for dissecting the molecular mechanisms of sociality [4,5].
Here we sought to identify the genes that play a role in normal interactions among fish in a social group. We used threespine sticklebacks (Gasterosteus aculeatus), which are a longstanding model for studies of social behavior and have a wealth of genomic resources available, which facilitates transcriptomic analyses [16,17]. Marine sticklebacks are highly social, and are typically found in social groups [17,18]. We modulated the extent of social interactions of individual fish by housing fish either in social groups or individually for a one-week period. This manipulation should permit detection of a state change that is not due to the process of isolation (i.e. not within several hours), but also avoids the detrimental effects of long-term isolation on increasing stress and anxiety [19]. We then used RNA-sequencing (RNA-seq) to compare gene expression in brains of group- or individually-housed fish.
Materials and Methods
Fish and sample collection
Fish were from a lab-reared population of Japanese Pacific Ocean marine fish originally derived from the Bekanbeushi River in Japan. Fish were reared in 110-L tanks in 3.5 ppt seawater (Instant Ocean, United Pet Group, Blacksburg, VA) at 16 C, and under 16 h light / 8 h dark lighting conditions. Fish were fed Artemia nauplii and mysis shrimp. All fish were treated in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Fred Hutchinson Cancer Research Center (FHCRC), protocol number 1575.
For social housing manipulation, fish from a single community tank were caught and transferred to four new 38-L tanks. Fish were either housed individually (n = 2 tanks) or in groups of eight mixed sex fish (n = 2 tanks). After one week of individual or group housing, we removed a single fish from each tank for analysis such that we had two individually-housed and two group-housed fish. We replicated this experiment with a second tank of fish so that we had a total of four biological replicates for both individually- and group-housed fish, from two original home tanks. Gonads were visually inspected to identify sex and maturity. Only pre-reproductive females were included in the experiment. Fish were euthanized with MS-222 and their brains were removed into RNA-later (Life Technologies, Carlsbad, CA) and stored at -20 C. Brains of individual fish were then dissected into three portions: 1) the telencephalon, 2) the diencephalon, pituitary, and rostral midbrain, and 3) the caudal midbrain, hindbrain, and cerebellum. We will refer to these portions as telencephalon, diencephalon, and hindbrain/cerebellum for simplicity. Tissue was homogenized using a pellet pestle (Kimble-Chase, Vineland, NJ) and total RNA was isolated using Trizol (Life Technologies, Carlsbad, CA). We performed the dissection and RNA isolation in separate batches on two different days, such that fish from one experimental replicate (i.e. home tank of origin) were processed on the same day.
RNA-seq
Barcoded RNA libraries from 24 samples (eight fish each with three brain regions) were generated in the FHCRC Genomics facility using Illumina’s TruSeq RNA Sample Prep Kit v2 (Illumina, San Diego, CA) and a Sciclone NGS Workstation (PerkinElmer, Waltham, MA). Libraries were multiplexed, split across three lanes, and 50-bp paired-end sequences were generated on an Illumina HiSeq 2500 (Illumina, San Diego, CA). Demultiplexing was performed using Illumina's CASAVA v1.8.2 software, allowing for a single mismatch in the index read. Fastq files have been deposited to the Sequence Read Archive (Study Accession SRP056943). We used a local instance of Galaxy [20–22] to perform alignment and to quantify reads aligning to genes. Reads were first aligned to the stickleback genome (BroadS1 [16]) using the default parameters in tophat2 (version 2.0.9, Galaxy tool version 0.6 [23]). Next, reads that fell within predicted genes (Ensembl genes 76) were counted using htseq-count (“Count reads in features with htseq-count” Galaxy tool v1.0 [24]). In htseq-count, we used the following parameters:-q-m intersection-nonempty-s no-a 0-t exon-i gene_id. The resulting matrix was exported from Galaxy and imported into R (http://r-project.org) where we used edgeR, version 3.8.6, [25] to identify differentially expressed genes. A multidimensional scaling (MDS) plot was generated in edgeR. We also calculated the biological coefficient of variation (BCV) of samples using edgeR.
We first analyzed expression differences as a function of brain region, independent of social environment, by performing three analyses: telencephalon vs. diencephalon and hindbrain/cerebellum; diencephalon vs. telencephalon and hindbrain/cerebellum; and hindbrain/cerebellum vs. telencephalon and diencephalon. We filtered out genes that did not have at least 1 count per million reads in at least two samples. We present and discuss the top 10 differentially expressed genes for each brain region, all of which were significant at a False Discovery Rate (FDR) of P < 0.05.
To identify genes differentially expressed as a function of social environment, we next performed a General Linear Model (GLM) analysis separately for each brain region by comparing read counts in group- and individually-housed fish. We included experimental replicate (1 or 2) as a factor in the model to control for home tank of origin and RNA isolation-batch effects. We filtered out genes that did not have at least 1 count per million reads in at least two samples. Differentially expressed genes were those that had FDR of 0.05. We present and discuss the genes upregulated in group- and individually-housed fish separately, so for simplicity we report the log 2 fold change (log2FC) as positive for both comparisons.
Functional annotation and enrichment analysis
We used DAVID to perform functional annotation and enrichment analysis [26]. DAVID tests enrichment of Gene Ontology (GO) terms, as well as other annotation categories including Interpro domains, KEGG pathways, and SMART protein domains. Ensembl gene identifiers were first converted to zfin identifiers specifically for these analyses. Fold-enrichment of all significant up- or down-regulated genes was calculated over the background gene list, which included all genes expressed in the hindbrain/cerebellum. Functional annotation terms that were significantly enriched are reported, and are organized into clusters based on DAVID’s functional annotation clustering.
We also tested for enrichment of glutamate receptors in genes upregulated in group-housed fish. We counted the number of glutamate receptor and GABA receptor genes in the upregulated list and the list of all genes expressed in the hindbrain/cerebellum. We then used the test of equal proportions in R to determine whether there was significant enrichment of these gene classes.
Results and Discussion
Sequencing generated an average of 42 ± 2 million total reads per sample, of which 88 ± 1% aligned to the genome. Of the aligned reads, 40 ± 2% fell within a predicted gene, thus were counted by htseq-count, and included in the analysis. Genes expressed at low levels were not included, leaving a total of 17,095 genes for the telencephalon, 17,553 for the diencephalon, and 17,081 for the hindbrain/cerebellum.
Differential expression as a function of brain region
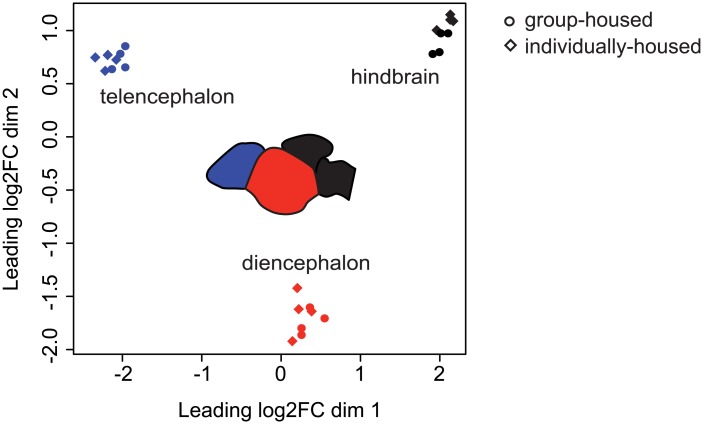
We first compared gene expression as a function of brain region, independent of social housing condition. A multidimensional scaling plot showed clear separation of samples based on brain region (Fig 1). The top ten differentially expressed genes in each brain area based on log2FC included genes with known functions in these parts of the brain (Table 1). For example, the top ten genes enriched in the hindbrain/cerebellum were all known or predicted homeobox (Hox) transcription factors (Table 1). Hox genes are involved in hindbrain patterning during development and are expressed in the adult brain [27]. Eight of the top ten differentially expressed genes in the diencephalon encode pituitary hormones (Table 1), which was expected as this portion of the brain contained the pituitary. The other two diencephalon-enriched genes were Nr5a1a, which is expressed in the diencephalon of zebrafish [28] and Mibp, whose function in the brain has not been studied. In the telencephalon, the top ten differentially expressed genes included: 1) genes that are known to be involved in forebrain patterning and/or used as forebrain markers (Eomesa and Emx3 [29], Tbr1b [30], Scgn [31]), 2) a gene expressed in the forebrain of zebrafish (Rtn4rl2b [32]), and 3) genes with unclear function in the brain (Apod, Ctrb1). The genes identified as being highly enriched in specific brain regions may prove to be useful markers of different neuronal populations in future neuroanatomy studies in sticklebacks and other fish.
Fig 1. Multidimensional scaling plot reveals separation of samples based on brain region.
Multidimensional scaling plot shows leading log2 fold-change (log2FC) differences between samples. Brain regions are colored as follows: blue = telencephalon; red = diencephalon; black = hindbrain/cerebellum. Inset shows schematic of brain with the same colors representing dissected brain regions. Circles = group-housed samples; diamonds = individually-housed samples.
Table 1. Top ten genes enriched in each brain region.
| Ensembl Gene ID | Log2FC | FDR | Symbol | Description |
|---|---|---|---|---|
| Telencephalon | ||||
| ENSGACG00000017663 | 8.7 | 1.57E-110 | Ctrb1 | Chymotrypsinogen B1 |
| ENSGACG00000016370 | 8.5 | 2.37E-108 | Emx3 | Empty spiracles homeobox 3 |
| ENSGACG00000016991 | 7.2 | 8.62E-38 | Apod | Apolipoprotein D |
| ENSGACG00000005648 | 6.6 | 2.31E-87 | Tbr1b | T-box, brain, 1b |
| ENSGACG00000003160 | 6.5 | 0 | Eomesa | Eomesodermin homolog a |
| ENSGACG00000018955 | 6.4 | 1.44E-23 | NA | Protein family: Solute Carrier Family 12 |
| ENSGACG00000003159 | 6.4 | 2.07E-142 | NA | Novel protein |
| ENSGACG00000009609 | 6.2 | 8.14E-73 | Scgn | Secretagogin |
| ENSGACG00000013917 | 6.0 | 1.49E-24 | NA | Novel protein |
| ENSGACG00000017836 | 6.0 | 5.99E-56 | Rtn4rl2b | Reticulon 4 receptor-like 2b |
| Diencephalon | ||||
| ENSGACG00000009153 | 9.0 | 2.57E-133 | Cga | Glycoprotein hormones, alpha polypeptide |
| ENSGACG00000006561 | 9.0 | 5.05E-248 | Prl | Prolactin |
| ENSGACG00000014829 | 8.9 | 2.75E-153 | Gh1 | Growth hormone 1 |
| ENSGACG00000009521 | 8.7 | 1.07E-239 | Pomca | Proopiomelanocortin a |
| ENSGACG00000018017 | 8.4 | 2.54E-284 | Pmchl | Pro-melanin-concentrating hormone, like |
| ENSGACG00000018317 | 8.4 | 1.05E-76 | Nr5a1a | Nuclear receptor subfamily 5, grp A, mbr 1b |
| ENSGACG00000006593 | 8.0 | 1.76E-126 | Smtla | Somatolactin alpha |
| ENSGACG00000005276 | 7.9 | 6.85E-70 | Tshb | Thyroid stimulating hormone, beta subunit |
| ENSGACG00000011475 | 7.7 | 1.80E-18 | Lhb | Luteinizing hormone, beta polypeptide |
| ENSGACG00000015226 | 7.5 | 8.56E-37 | Mipb | Major intrinsic protein of lens fiber b |
| Hindbrain/Cerebellum | ||||
| ENSGACG00000009421 | 9.7 | 2.69E-47 | Hoxc4a | Homeobox c4a |
| ENSGACG00000007108 | 9.0 | 1.48E-27 | Hoxa5a | Homeobox a5a |
| ENSGACG00000007100 | 8.1 | 3.51E-33 | Hoxa4 | Homeobox a4 |
| ENSGACG00000004548 | 7.5 | 3.86E-64 | Hoxd3a | Homeobox d3a |
| ENSGACG00000004551 | 7.2 | 1.10E-28 | Hoxd4a | Homeobox d4a |
| ENSGACG00000009416 | 7.2 | 3.84E-17 | Hoxc5a | Homeobox c5a |
| ENSGACG00000005631 | 7.0 | 3.11E-43 | Hoxb3a | Homeobox b3a |
| ENSGACG00000005626 | 6.9 | 1.54E-10 | NA | Protein family: Homeobox |
| ENSGACG00000003945 | 6.7 | 2.24E-12 | Hoxb5b | Homeobox b5b |
| ENSGACG00000005633 | 6.6 | 6.06E-42 | Hoxb2a | Homeobox b2a |
Log2FC = log2 fold-change, FDR = false discovery rate, Symbol = gene name, NA = novel gene with no associated name.
Differential expression as a function of social housing
We next identified genes that were differentially expressed as a result of social experience. There were numerous genes that were differentially expressed in the hindbrain/cerebellum (985 higher in group and 401 higher in isolate; all significant genes are shown in S1 File; the top 25 are shown in Tables 2 and 3). However, few genes were differentially expressed in either the diencephalon (5 higher in group) or telencephalon (1 higher in isolate). Four of the five differentially expressed genes in the diencephalon (Table 2) were also upregulated in the hindbrain/cerebellum of group-housed fish (S1 File; hindbrain/cerebellum values: Cyr61: log2FC = 2.4; FDR < 0.008, Tgm8: log2FC = 1.5; FDR = 0.008, Etv5a: log2FC = 0.9; FDR < 0.001, and Fam46d: log2FC = 1; FDR < 0.012). The fifth gene, novel gene ENSGACG00000012907, was not differentially expressed in the hindbrain/cerebellum (log2FC = 0.3; FDR = 0.13). Etv5a is a transcription factor involved in specification of dopaminergic cells in C. elegans, and has been shown to co-localize with diencephalic dopaminergic cell populations in fish [33]. Cyr61 is expressed at the midbrain-hindbrain boundary in developing zebrafish, but its function is unknown [34]. Tgm8 shares distant homology with the transglutaminase family, which are enzymes involved in protein cross-linking [35]. Tgm8 was highly differentially expressed in all three brain regions, although it did not reach an FDR threshold of p < 0.05 in the telencephalon (higher in group; log2FC = 1.9; FDR = 0.13). Fam46d has an unknown neural function but is known to be expressed at higher levels in a mouse model of autism [36]. The single gene that was differentially expressed in the telencephalon is Proca1, whose function is unknown other than it is found in a protein complex with the cell division gene cyclin A1 (Table 3).
Table 2. Genes significantly upregulated in group-housed fish.
| Ensembl Gene ID | Log2FC | FDR | Symbol | Description |
|---|---|---|---|---|
| Diencephalon | ||||
| ENSGACG00000017235 | 3.0 | 0.001 | Cyr61 | Cysteine-rich, angiogenic inducer, 61 |
| ENSGACG00000003741 | 1.8 | 0.000 | Tgm8 | Transglutaminase 8 |
| ENSGACG00000008646 | 1.3 | 0.000 | Etv5a | Ets variant 5a |
| ENSGACG00000018558 | 0.8 | 0.041 | Fam46d | Family with sequence similarity 46, member D |
| ENSGACG00000012907 | 0.8 | 0.041 | NA | Novel protein |
| Hindbrain/Cerebellum | ||||
| ENSGACG00000007463 | 3.9 | 0.025 | Syne2a | Spectrin repeat containing, nuclear envelope 2a |
| ENSGACG00000005626 | 3.4 | 0.001 | Hoxb5 | Homeobox B5 |
| ENSGACG00000001172 | 3.2 | 0.002 | NA | Protein family: Histone lysine N methyltransferase |
| ENSGACG00000005716 | 3.1 | 0.017 | NA | Protein family: Hyaluronidase |
| ENSGACG00000018064 | 3.1 | 0.008 | NA | Novel protein |
| ENSGACG00000003170 | 3.0 | 0.047 | NA | Protein family: Multiple PDZ domain |
| ENSGACG00000002950 | 3.0 | 0.044 | Szt2 | Seizure threshold 2 homolog |
| ENSGACG00000017590 | 2.9 | 0.007 | Crema | cAMP responsive element modulator a |
| ENSGACG00000003945 | 2.9 | 0.003 | Hoxb5b | Homeo box B5b |
| ENSGACG00000002005 | 2.9 | 0.015 | NA | Novel protein |
| ENSGACG00000013776 | 2.7 | 0.005 | Herc2 | Hect domain and RLD 2 |
| ENSGACG00000001636 | 2.7 | 0.044 | NA | Novel pseudogene |
| ENSGACG00000011127 | 2.7 | 0.048 | Stard9 | StAR-related lipid transfer domain containing 9 |
| ENSGACG00000009610 | 2.6 | 0.008 | NA | Novel protein |
| ENSGACG00000008919 | 2.5 | 0.013 | Kcnk9 | Potassium channel, subfamily K, member 9 |
| ENSGACG00000018488 | 2.5 | 0.004 | NA | Protein family: High affinity choline transporter 1 |
| ENSGACG00000007108 | 2.4 | 0.002 | Hoxa5a | Homeo box A5a |
| ENSGACG00000009416 | 2.4 | 0.020 | Hoxc5a | Homeo box C5a |
| ENSGACG00000014677 | 2.4 | 0.009 | Prrc2c | Proline-rich coiled-coil 2C |
| ENSGACG00000011293 | 2.4 | 0.015 | Hectd4 | HECT domain containing E3 ubiquitin ligase 4 |
| ENSGACG00000004479 | 2.4 | 0.008 | Sst1.1 | Somatostatin 1, tandem duplicate 1 |
| ENSGACG00000011057 | 2.4 | 0.004 | NA | Novel protein |
| ENSGACG00000004861 | 2.4 | 0.012 | Agrn | Agrin |
| ENSGACG00000007999 | 2.4 | 0.038 | Rarb | Retinoic acid receptor, beta |
| ENSGACG00000004506 | 2.3 | 0.008 | S100u | S100 calcium binding protein U |
All five significant genes from diencephalon and top 25 from hindbrain/cerebellum are shown; no genes were significantly upregulated in the telencephalon. Log2FC = log2 fold-change, FDR = false discovery rate, Symbol = gene name, NA = novel gene with no associated name.
Table 3. Genes significantly upregulated in individually-housed fish.
| Ensembl Gene ID | Log2FC | FDR | Symbol | Description |
|---|---|---|---|---|
| Telencephalon | ||||
| ENSGACG00000011223 | 2.3 | 0.003 | Proca1 | Protein interacting with cyclin A1 |
| Hindbrain/Cerebellum | ||||
| ENSGACG00000001322 | 2.5 | 0.029 | NA | Novel protein |
| ENSGACG00000005350 | 2.2 | 0.037 | Slc16a1 | Solute carrier family 16 member 1 |
| ENSGACG00000017681 | 2.0 | 0.043 | Pmt | Phosphoethanolamine methyltransferase |
| ENSGACG00000004653 | 2.0 | 0.045 | NA | Novel protein |
| ENSGACG00000001231 | 1.9 | 0.019 | NA | Novel protein |
| ENSGACG00000021449 | 1.9 | 0.006 | NA | Novel miRNA |
| ENSGACG00000002911 | 1.9 | 0.004 | Tcf24 | Transcription factor 24 |
| ENSGACG00000001910 | 1.6 | 0.027 | NA | Protein family: MHC class I antigen |
| ENSGACG00000007674 | 1.5 | 0.047 | NA | Protein family: Glutathione S transferase |
| ENSGACG00000008596 | 1.4 | 0.011 | Ddit4 | DNA-damage-inducible transcript 4 |
| ENSGACG00000004576 | 1.4 | 0.028 | Mad2l1bp | Mad2l1 binding protein |
| ENSGACG00000022181 | 1.3 | 0.003 | NA | Novel miRNA |
| ENSGACG00000007379 | 1.3 | 0.000 | Stmn1b | Stathmin 1b |
| ENSGACG00000015933 | 1.3 | 0.025 | Clec18b | C-type lectin domain family 18, member B |
| ENSGACG00000012872 | 1.2 | 0.019 | Eps8l1 | Eps8-like1 |
| ENSGACG00000011011 | 1.2 | 0.015 | NA | Novel protein |
| ENSGACG00000018331 | 1.2 | 0.009 | Mxd3 | MAX dimerization protein 3 |
| ENSGACG00000002889 | 1.2 | 0.044 | Sox1b | SRY-box containing gene 1b |
| ENSGACG00000021538 | 1.2 | 0.040 | NA | Novel miRNA |
| ENSGACG00000017065 | 1.1 | 0.048 | Clul1 | Clusterin-like 1 (retinal) |
| ENSGACG00000006502 | 1.1 | 0.048 | Parp6b | Poly (ADP-ribose) polymerase, member 6b |
| ENSGACG00000019774 | 1.1 | 0.020 | NA | Novel protein |
| ENSGACG00000015028 | 1.1 | 0.048 | Gatm | Glycine amidinotransferase |
| ENSGACG00000015636 | 1.1 | 0.000 | Cdk2ap1 | Cyclin-dependent kinase 2 associated protein 1 |
| ENSGACG00000015171 | 1.1 | 0.002 | NA | Novel protein |
One significant gene from telencephalon and top 25 from hindbrain/cerebellum are shown. Log2FC = log2 fold-change, FDR = false discovery rate, Symbol = gene name, NA = novel gene with no associated name.
It was interesting that many genes were differentially expressed in the hindbrain/cerebellum compared with few in either the telencephalon and diencephalon, which both contain nuclei known to be involved in the control of social behavior [37]. There are several possible explanations for this result. First, the hindbrain and cerebellum may indeed show a greater response to this alteration in social housing than the rest of the brain. Social interactions are associated with sensory stimulation, and this is reduced in individually-housed fish. The hindbrain serves as a primary sensory relay for several senses, and thus may show an increased transcriptional response to this manipulation. Alternatively, lack of detection of differentially expressed genes in the telencephalon and diencephalon could theoretically result from increased heterogeneity of these regions compared with the hindbrain/cerebellum. However, the coefficient of variation is similar across all brain regions (telencephalon BCV = 0.212; diencephalon BCV = 0.206; hindbrain/cerebellum BCV = 0.201), suggesting that this is not the cause in this case. Moreover, another study of stickleback gene expression differences that dissected the brain into similar portions did detect gene expression differences in all regions 30 min after social stimulation [14]. In that study, the diencephalon had the largest number of differentially expressed genes, whereas the telencephalon had the fewest. Thus, it is likely that there are differences in which brain regions respond to different stimuli. In addition, timing of stimulus exposure likely has an important impact on differential gene expression; this should be tested more thoroughly in future studies.
Genes upregulated in the hindbrain of group-housed fish
The 25 genes that were higher in the hindbrain of group-housed fish, based on fold-change, are shown in Table 2. Many of these genes were involved in developmental processes. The Hox genes and retinoic acid receptor (Rarb) are specifically involved in hindbrain development [38]. Several additional genes are otherwise implicated in neural development (Agrn [39] and Syne2a [40]) or intellectual disability (Herc2 [41] and Kcnk9 [42]), and Stard9 is involved in cell division [43]. Functional annotation and enrichment analysis echoed the finding that developmental genes are strongly enriched in the list of genes upregulated in group-housed fish (Table 4). All of the significantly enriched functional clusters were related to development, including cell morphogenesis and neural development, cell adhesion, plexin/semaphorin signaling, and EGF signaling (Table 4). Semaphorins and EGF signaling are involved in neural development [44,45]. Increased activity of developmental processes is suggestive of more arborization and neurogenesis in group-housed fish. There is ongoing neurogenesis in the hindbrain/cerebellum of sticklebacks [46], and previous work has shown that sensory stimulation, including social housing, can alter levels of neurogenesis in other fish [47]. It is possible that the upregulated gene expression of developmental genes in the hindbrain/cerebellum of group-housed fish is related to increased sensory function due to higher levels of sensory stimulation.
Table 4. Functional annotation and clustering of genes expressed at higher levels in group-housed fish.
| Cluster | Term | Description | Fold Enrichment |
|---|---|---|---|
| 1 | GO:0000904 | Cell morphogenesis involved in differentiation | 4.2 |
| 1 | GO:0007409 | Axonogenesis | 4.1 |
| 1 | GO:0048667 | Cell morphogenesis involved in neuron differentiation | 4.1 |
| 1 | GO:0032989 | Cellular component morphogenesis | 2.9 |
| 1 | GO:0048812 | Neuron projection morphogenesis | 4.1 |
| 1 | GO:0000902 | Cell morphogenesis | 3.1 |
| 1 | GO:0031175 | Neuron projection development | 4.0 |
| 1 | GO:0007411 | Axon guidance | 5.2 |
| 1 | GO:0048666 | Neuron development | 3.2 |
| 1 | GO:0048858 | Cell projection morphogenesis | 3.1 |
| 1 | GO:0030030 | Cell projection organization | 2.9 |
| 2 | GO:0007155 | Cell adhesion | 2.9 |
| 2 | GO:0022610 | Biological adhesion | 2.9 |
| 3 | IPR002165 | Plexin | 7.9 |
| 3 | IPR003659 | Plexin/semaphorin/integrin | 6.4 |
| 3 | SM00423 | Domain found in Plexins, Semaphorins and Integrins | 5.7 |
| 3 | IPR001627 | Semaphorin/CD100 antigen | 6.9 |
| 3 | SM00630 | Sema | 6.2 |
| 4 | IPR013032 | EGF-like region, conserved site | 3.0 |
| 4 | IPR006210 | EGF-like | 3.5 |
| 4 | SM00181 | EGF | 3.2 |
| 4 | IPR000742 | EGF-like, type 3 | 3.4 |
| 4 | IPR002049 | EGF-like, laminin | 7.7 |
| 4 | SM00180 | Laminin-type epidermal growth factor-like domain | 7.0 |
| 4 | IPR003961 | Fibronectin, type III | 2.9 |
| 5 | IPR002909 | Cell surface receptor IPT/TIG | 8.4 |
| 5 | SM00429 | Ig-like, plexin, transcription factor domain | 7.6 |
Terms beginning with: GO = Gene Ontology term; IPR = interpro; SM = SMART protein domain.
Other genes in the top 25 upregulated genes included Szt2 and Sst1.1. Szt2 mutant mice have a lower seizure threshold [48]. Somatostatin (Sst1.1) has previously been implicated in decreasing growth as well as decreasing aggressive behavior in fish [49,50]. Social isolation can lead to increased aggression in fish [51]. It would be interesting to determine whether group housed sticklebacks have slower growth and reduced aggression than individually-housed fish. In addition, it would be interesting to manipulate somatostatin levels [49] and determine whether there was an impact on growth and gene expression.
The list of 985 genes upregulated in the hindbrain/cerebellum as a result of group housing included many other interesting genes in addition to those presented in Table 2. We will highlight a few here, although the entire list can be found in S1 File. Many enriched genes were in neurotransmitter or neuromodulator pathways. First, several genes related to acetylcholine synthesis and signaling were higher in group-housed fish: acetylcholinesterase (Ache, ENSGACG00000000728; log2FC = 0.9; FDR = 0.009), choline o-acetyltransferase (Chat, ENSGACG00000002482; log2FC = 0.8; FDR = 0.008), and the muscarinic acetylcholine receptor, Chrm2a (ENSGACG00000019948; log2FC = 1.2; FDR = 0.04) (S1 File). Acetylcholinergic cells are found in cranial sensory and motor nuclei and throughout the reticular formation of the hindbrain [52]. Chrm2a also expressed in cranial nuclei [53]. Given these expression patterns, we speculate that increased acetylcholine signaling is related to higher levels of sensory processing due to more sensory stimulation in the group-housing environment.
In addition, galanin receptor (Galr1; log2FC = 1.4; FDR = 0.014) and several insulin signaling genes were regulated as a function of social status. Specifically, an insulin receptor (Insr, ENSGACG00000010475, log2FC = 1.1; FDR = 0.0003), insulin-like growth factor 2 receptor (Igf2r, ENSGACG00000005960; log2FC = 1.1; FDR = 0.016), and two insulin receptor substrate 2 orthologs (Irs2: ENSGACG00000014133; log2FC = 0.8; FDR = 0.003; ENSGACG00000003564; log2FC = 0.7; FDR = 0.01) were all significantly higher in the hindbrain/cerebellum of group-housed fish (S1 File). Both galanin and insulin have been implicated in fish feeding [54], so perhaps upregulation of these genes is related to increased competition for food in group housing conditions. In addition, several insulin-related genes are regulated in response to social conditions: Igf2r was shown to be increased in brains of subordinate rats [55], and insulin signaling alters social behavior in honeybees [56].
Another signaling pathway gene that was differentially expressed was prostaglandin F2 receptor inhibitor (Ptgfrn; ENSGACG00000014419; log2FC = 0.8; FDR = 0.013). Prostaglandin F2α signaling increases fish reproductive physiology [57] and behavior [58]. The females in social groups were exposed to males but isolated females were not, so it may be that mixed-sex housing facilitates reproduction. Investigating levels of reproductive hormones would directly address this question.
Opiate signaling pathway genes were also regulated as a function of social status. Prepronociceptin a (Pnoca, ENSGACG00000014805; log2FC = 1.7; FDR = 0.003) and its receptor, opiate receptor-like 1 (Oprl1; ENSGACG00000010479; log2FC = 1.1; FDR = 0.02), were both expressed at higher levels in fish in social housing. Interestingly, Pnoc and Oprl1 (aka NOP) were also found to be higher in brains of mice housed in groups than in mice housed in isolation [59]. Nociceptin signaling decreases stress and anxiety in mammals [60]. It may be that social interactions in group-housed fish lead to increased nociceptin signaling, which results in reduced stress and anxiety. Alternatively, individually-housed fish might have decreased levels of nociception signaling.
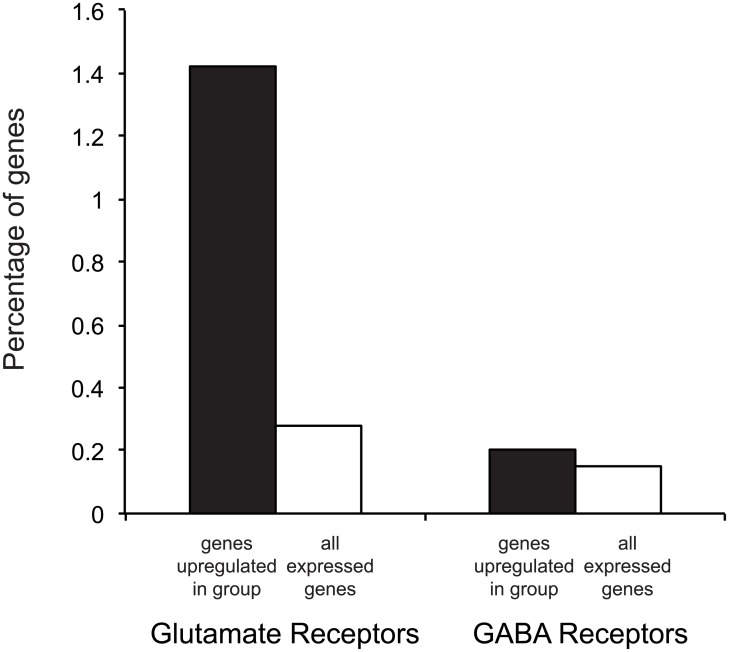
Finally, 14 glutamate receptor subtypes were found in the list of significantly upregulated genes in socially housed fish (S1 File; Gria1a, Gria4b, Grik2, Grik3, Grik5, Grin2ab, Grin2b, Grin2bb, Grin2ca, Grin2db, Grip2b, Grm3, Grm5, Grm8). Because the glutamate receptor family is quite large, we tested to see whether this was a specific enrichment or was simply a result of there being a large number of glutamate receptor genes in the entire gene list. We also compared the level of enrichment of another large neurotransmitter receptor family, the GABA receptors. This analysis showed that glutamate but not GABA receptors were significantly enriched in fish housed in social groups (Χ2 = 43, P < 0.00001; Fig 2).
Fig 2. Glutamate receptors are enriched in the list of upregulated genes from group-housed fish.
The percentage of genes in the significantly upregulated and total gene list is shown for glutamate and GABA receptors. There is a significant enrichment in glutamate but not GABA receptors in the list of genes upregulated in group-housed fish.
Genes upregulated in the hindbrain of individually-housed fish
We next examined genes that were higher in the hindbrain of individually-housed fish (Table 3). The list of the top 25 genes with the highest fold-change contained genes with diverse functions. For example, Slc16a1 has been implicated in neurogenesis in zebrafish [61]. Ddit4 may play a role in development through interactions with Wnt/beta catenin signaling [62]. There were several transcription factors with varied functions (Tcf24, Mxd3, Sox1b). Gatm is involved in creatine synthesis. Interestingly, Mad2l1bp, which has homology to a gene involved in cell division and the spindle checkpoint pathway, was also found to be regulated by social interactions in other populations of sticklebacks. Specifically, it was higher in males following a territorial intrusion [14]. Finally, novel gene ENSGACG00000001910 has homology to the MHC class 1 antigen family. A gene from this family was previously shown to be expressed at higher levels in brains of female than male cichlids [11].
The entire list of 401 genes upregulated in individually-housed fish included several other genes with interesting functions, and is shown in S1 File. One of these was an enzyme involved in steroid biosynthesis, hydroxysteroid (17-beta) dehydrogenase 7, which was expressed at higher levels (Hsd17b7; ENSGACG00000016134; log2FC = 0.6; FDR = 0.02). Hsd17b7 is involved in the biosynthesis of cholesterol and sex steroids, and thus may play a role in regulating steroid hormone abundance in the brain. Another gene upregulated in individually-housed fish, MAD2 mitotic arrest deficient-like 1, was also shown to be higher in brains of isolated rats (Mad2l1; ENSGACG00000001594; log2FC = 0.8; FDR = 0.007) [13].
We next performed functional annotation and enrichment analysis of the list of genes upregulated in individually-housed fish. Relatively few categories were enriched, and they included genes related to RNA processing (Table 5).
Table 5. Functional annotation and clustering of genes expressed at higher levels in individually-housed fish.
| Cluster | Term | Description | Fold Enrichment |
|---|---|---|---|
| 1 | dre03040 | Spliceosome | 5.4 |
| 1 | SM00651 | Small nuclear ribonucleoprotein involved in pre-mRNA splicing | 33.5 |
| 1 | IPR006649 | Like-Sm ribonucleoprotein, eukaryotic and archaea-type, core | 19.5 |
| 1 | IPR001163 | Like-Sm ribonucleoprotein, core | 18.0 |
| 2 | GO:0030529 | Ribonucleoprotein complex | 3.4 |
Terms beginning with: GO = Gene Ontology term; IPR = Interpro protein domain; SM = SMART protein domain; dre = KEGG pathway.
Conclusions
In summary, we found that manipulating social housing impacted the expression of genes predominantly in the hindbrain/cerebellum. In group-housed fish, many of the upregulated genes were in developmental signaling pathways, and functional annotation reinforced the conclusion that there was enrichment of development-related genes in this dataset. These results suggest that fish in group-housing environments experience more neurogenesis or more axon and dendrite outgrowth. Alternatively, because many developmental genes act as repressors, it may be that upregulated expression of these genes is actually associated with decreased neurogenesis. It would be interesting to distinguish between these possibilities by directly by comparing levels of cell division and differentiation on a cellular level. Other differentially expressed genes were involved in stress/anxiety, social behavior, and possibly sensory processing. These findings suggest interesting directions for future research on the molecular control of normal social interactions in sticklebacks and other systems. In the future it could also be interesting to evaluate different timescales of experimental manipulation, for instance social isolation for an entire lifetime or across evolutionary timescales [63].
Supporting Information
File contains a list of all hindbrain genes that were significantly upregulated (FDR < 0.05) in group- and individually-housed fish, on two separate worksheets.
(XLSX)
Acknowledgments
We thank Shaugnessy McCann for fish care; Jeff Delrow, Andy Marty, Ryan Basom and the FHCRC Genomics Shared Resource for RNA-seq library preparation and sequencing; and Brian Claywell for help with local Galaxy setup.
Data Availability
All RNA-seq fastq files are available from the NCBI Sequence Read Archive (Study Accession SRP056943).
Funding Statement
This research was funded by the National Science Foundation Division of Integrative Organismal Systems grant IOS 1145866 to AKG and CLP (http://www.nsf.gov/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Goodson JL. The vertebrate social behavior network: Evolutionary themes and variations. Horm Behav. 2005;48: 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Connell LA, Hofmann HA. Evolution of a vertebrate social decision-making network. Science. 2012;336: 1154–1157. 10.1126/science.1218889 [DOI] [PubMed] [Google Scholar]
- 3. Goodson JL, Kingsbury MA. What's in a name? Considerations of homologies and nomenclature for vertebrate social behavior networks. Horm Behav. 2013; 64: 103–112. 10.1016/j.yhbeh.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rittschof CC, Bukhari SA, Sloofman LG, Troy JM, Caetano-Anolle D, Cash-Ahmed A, et al. Neuromolecular responses to social challenge: Common mechanisms across mouse, stickleback fish, and honey bee. Proc Natl Acad Sci USA. 2014;111: 17929–17934. 10.1073/pnas.1420369111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robinson GE, Grozinger CM, Whitfield CW. Sociogenomics: Social life in molecular terms. Nat Rev Genet. 2005;6: 257–271. [DOI] [PubMed] [Google Scholar]
- 6. Robinson GE, Fernald RD, Clayton DF. Genes and social behavior. Science. 2008;322: 896–900. 10.1126/science.1159277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Donaldson ZR, Young LJ,.Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322: 900–904. 10.1126/science.1158668 [DOI] [PubMed] [Google Scholar]
- 8. Hitzemann R, Bottomly D, Darakjian P, Walter N, Iancu O, Searles R, et al. Genes, behavior and next-generation sequencing. Genes Brain Behav. 2013;12: 1–12. 10.1111/gbb.12007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aubin-Horth N, Landry CR, Letcher BH, Hofmann HA. Alternative life histories shape brain gene expression profiles in males of the same population. Proc Biol Sci. 2005;272: 1655–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schunter C, Vollmer SV, Macpherson E, Pascual M. Transcriptome analyses and differential gene expression in a non-model fish species with alternative mating tactics. BMC Genomics. 2014;15: 167 10.1186/1471-2164-15-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Renn SCP, Aubin-Horth N, Hofmann HA. Fish and chips: functional genomics of social plasticity in an African cichlid fish. J Exp Biol. 2008;211: 3041–3056. 10.1242/jeb.018242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cummings ME, Larkins-Ford J, Reilly CRL, Wong RY, Ramsey M, Hofmann HA. Sexual and social stimuli elicit rapid and contrasting genomic responses. Proc Biol Sci. 2008;275: 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levine JB, Youngs RM, MacDonald ML, Chu M, Leeder AD, Berthiaume F, et al. Isolation rearing and hyperlocomotion are associated with reduced immediate early gene expression levels in the medial prefrontal cortex. Neuroscience. 2007;145: 42–55. [DOI] [PubMed] [Google Scholar]
- 14. Sanogo YO, Band M, Blatti C, Sinha S, Bell AM. Transcriptional regulation of brain gene expression in response to a territorial intrusion. Proc Biol Sci. 2012;279: 4929–4938. 10.1098/rspb.2012.2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sanogo YO, Hankison S, Band M, Obregon A, Bell AM. Brain transcriptomic response of threespine sticklebacks to cues of a predator. Brain Behav Evol. 2011;77: 270–285. 10.1159/000328221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones FC, Grabherr MG, Chan YF, Russell P, Mauceli E, Johnson J, et al. The genomic basis of adaptive evolution in threespine sticklebacks. Nature. 2012;484: 55–61. 10.1038/nature10944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wootton RJ. The Biology of Sticklebacks. London: Academic Press; 1976. [Google Scholar]
- 18. Wark AR, Greenwood AK, Taylor EM, Yoshida K, Peichel CL. Heritable differences in schooling behavior among threespine sticklebacks revealed by a novel assay. PLoS ONE. 2011;6: e18316 10.1371/journal.pone.0018316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fone KCF, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents—Relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32: 1087–1102. 10.1016/j.neubiorev.2008.03.003 [DOI] [PubMed] [Google Scholar]
- 20. Blankenberg D, Von Kuster G, Coraor N, Ananda G, Lazarus R, Mangan M, et al. Galaxy: a web-based genome analysis tool for experimentalists. Curr Protoc Mol Biol. 2010; 89: 19.10.11–19.10.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giardine B, Riemer C, Hardison RC, Burhans R, Elnitski L, Shah P, et al. Galaxy: A platform for interactive large-scale genome analysis. Genome Res. 2005;15: 1451–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goecks J, Nekrutenko A, Taylor J, Galaxy Team. Galaxy: a comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol. 2010;11: R86 10.1186/gb-2010-11-8-r86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14: R36 10.1186/gb-2013-14-4-r36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anders S, Pyl PT, Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31: 166–169. 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26: 139–140. 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dennis G, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4: R60. [PubMed] [Google Scholar]
- 27. Zapala MA, Hovatta I, Ellison JA, Wodicka L, Del Rio JA, Tennant R, et al. Adult mouse brain gene expression patterns bear an embryologic imprint. Proc Natl Acad Sci USA. 2005;102: 10357–10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kurrasch DM, Cheung CC, Lee FY, Tran PV, Hata K, Ingraham HA. The neonatal ventromedial hypothalamus transcriptome reveals novel markers with spatially distinct patterning. J Neurosci. 2007;27: 13624–13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ganz J, Kroehne V, Freudenreich D, Machate A, Geffarth M, Braasch I, et al. Subdivisions of the adult zebrafish pallium based on molecular marker analysis. F1000Res. 2014;3: 308 10.12688/f1000research.5595.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Puelles L, Kuwana E, Puelles E, Bulfone A, Shimamura K, Keleher J, et al. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1 . J Comp Neurol. 2000;424: 409–438. [DOI] [PubMed] [Google Scholar]
- 31. Mulder J, Spence L, Tortoriello G, DiNieri JA, Uhlen M, Shui B, et al. Secretagogin is a Ca2+-binding protein identifying prospective extended amygdala neurons in the developing mammalian telencephalon. Eur J Neurosci. 2010;31: 2166–2177. 10.1111/j.1460-9568.2010.07275.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thisse B, Wright GJ, Thisse C. Embronic and larval expression patterns from a large scale screening for novel low affinity extracellular protein interactions; 2008. Database: ZFIN: The Zebrafish Model Organism Database. Available: http://zfin.org.
- 33. O'Connell LA, Fontenot MR, Hofmann HA. Neurochemical profiling of dopaminergic neurons in the forebrain of a cichlid fish, Astatotilapia burtoni . J Chem Neuroanat. 2013;47: 106–115. 10.1016/j.jchemneu.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 34. Fernando CA, Conrad PA, Bartels CF, Marques T, To M, Balow SA, et al. Temporal and spatial expression of CCN genes in zebrafish. Dev Dyn. 2010;239: 1755–1767. 10.1002/dvdy.22279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Deasey S, Grichenko O, Du S, Nurminskaya M. Characterization of the transglutaminase gene family in zebrafish and in vivo analysis of transglutaminase-dependent bone mineralization. Amino Acids. 2012;42: 1065–1075. 10.1007/s00726-011-1021-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hamilton SM, Spencer CM, Harrison WR, Yuva-Paylor LA, Graham DF, Daza RA, et al. Multiple autism-like behaviors in a novel transgenic mouse model. Behav Brain Res. 2011;218: 29–41. 10.1016/j.bbr.2010.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O'Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. J Comp Neurol. 2011;519: 3599–3639. 10.1002/cne.22735 [DOI] [PubMed] [Google Scholar]
- 38. Moens CB, Prince VE. Constructing the hindbrain: Insights from the zebrafish. Dev Dyn. 2002;224: 1–17. [DOI] [PubMed] [Google Scholar]
- 39. Kim MJ, Liu IH, Song YQ, Lee JA, Halfter W, Balice-Gordon RJ, et al. Agrin is required for posterior development and motor axon outgrowth and branching in embryonic zebrafish. Glycobiology. 2007;17: 231–247. [DOI] [PubMed] [Google Scholar]
- 40. Del Bene F, Wehman AM, Link BA, Baier H. Regulation of neurogenesis by interkinetic nuclear migration through an apical-basal Notch gradient. Cell. 2008;134: 1055–1065. 10.1016/j.cell.2008.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Puffenberger EG, Jinks RN, Wang H, Xin BZ, Fiorentini C, Sherman EA, et al. A homozygous missense mutation in HERC2 associated with global developmental delay and autism spectrum disorder. Human Mutat. 2012;33: 1639–1646. [DOI] [PubMed] [Google Scholar]
- 42. Barel O, Shalev SA, Ofir R, Cohen A, Zlotogora J, Shorer Z, et al. Maternally inherited Birk Barel mental retardation dysmorphism syndrome caused by a mutation in the genomically imprinted potassium channel KCNK9. Am J Hum Genet. 2008;83: 193–199. 10.1016/j.ajhg.2008.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Torres JZ, Summers MK, Peterson D, Brauer MJ, Lee J, Senese S, et al. The STARD9/Kif16a kinesin associates with mitotic microtubules and regulates spindle pole assembly. Cell. 2011;147: 1309–1323. 10.1016/j.cell.2011.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol. 2005;6: 789–800. [DOI] [PubMed] [Google Scholar]
- 45. Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36: 1021–1034. [DOI] [PubMed] [Google Scholar]
- 46. Ekstrom P, Johnsson CM, Ohlin LM. Ventricular proliferation zones in the brain of an adult teleost fish and their relation to neuromeres and migration (secondary matrix) zones. J Comp Neurol. 2001;436: 92–110. [PubMed] [Google Scholar]
- 47. Dunlap KD, McCarthy EA, Jashari D. Electrocommunication signals alone are sufficient to increase neurogenesis in the brain of adult electric fish, Apteronotus leptorhynchus . Dev Neurobiol. 2008;68: 1420–1428. 10.1002/dneu.20673 [DOI] [PubMed] [Google Scholar]
- 48. Frankel WN, Yang Y, Mahaffey CL, Beyer BJ, O'Brien TP. Szt2, a novel gene for seizure threshold in mice. Genes Brain Behav. 2009;8: 568–576. 10.1111/j.1601-183X.2009.00509.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Klein SE, Sheridan MA. Somatostatin signaling and the regulation of growth and metabolism in fish. Mol Cell Endocrinol. 2008;286: 148–154. [DOI] [PubMed] [Google Scholar]
- 50. Trainor BC, Hofmann HA. Somatostatin regulates aggressive behavior in an African cichlid fish. Endocrinology. 2006;147: 5119–5125. [DOI] [PubMed] [Google Scholar]
- 51. Earley RL, Edwards JT, Aseem O, Felton K, Blumer LS, Karom M, et al. Social interactions tune aggression and stress responsiveness in a territorial cichlid fish (Archocentrus nigrofasciatus). Physiol Behav. 2006;88: 353–363. [DOI] [PubMed] [Google Scholar]
- 52. Rodriguez-Moldes I, Molist P, Adrio F, Pombal MA, Yanez SE, Mandado M, et al. Organization of cholinergic systems in the brain of different fish groups: A comparative analysis. Brain Res Bull. 2002;57: 331–334. [DOI] [PubMed] [Google Scholar]
- 53. Thisse B, Heyer V, Lux A, Alunni V, Degrave A, Seiliez I, et al. Spatial and temporal expression of the zebrafish genome by large-scale in situ hybridization screening. Methods Cell Biol. 2004;77: 505–519. [DOI] [PubMed] [Google Scholar]
- 54. Volkoff H, Peter RE. Feeding behavior of fish and its control. Zebrafish. 2006;3: 131–140. 10.1089/zeb.2006.3.131 [DOI] [PubMed] [Google Scholar]
- 55. Kroes RA, Panksepp J, Burgdorf J, Otto NJ, Moskal JR. Modeling depression: Social dominance-submission gene expression patterns in rat neocortex. Neuroscience. 2006;137: 37–49. [DOI] [PubMed] [Google Scholar]
- 56. Ament SA, Corona M, Pollock HS, Robinson GE. Insulin signaling is involved in the regulation of worker division of labor in honey bee colonies. Proc Natl Acad Sci USA. 2008;105: 4226–4231. 10.1073/pnas.0800630105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stacey NE, Goetz FW. Role of prostaglandins in fish reproduction. Can J Fish Aquat Sci. 1982;39: 92–98. [Google Scholar]
- 58. Kidd MR, Dijkstra PD, Alcott C, Lavee D, Ma J, O'Connell LA, et al. Prostaglandin F2 alpha facilitates female mating behavior based on male performance. Behav Ecol Sociobiol. 2013;67: 1307–1315. [Google Scholar]
- 59. Reiss D, Wolter-Sutter A, Krezel W, Ouagazzal AM. Effects of social crowding on emotionality and expression of hippocampal nociceptin/orphanin FQ system transcripts in mice. Behav Brain Res. 2007;184: 167–173. [DOI] [PubMed] [Google Scholar]
- 60. Koster A, Montkowski A, Schulz S, Stube EM, Knaudt K, Jenck F, et al. Targeted disruption of the orphanin FQ/nociceptin gene increases stress susceptibility and impairs stress adaptation in mice. Proc Natl Acad Sci USA. 1999;96: 10444–10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tseng YC, Kao ZJ, Liu ST, Chen RD, Hwang PP. Spatial expression and functional flexibility of monocarboxylate transporter isoforms in the zebrafish brain. Comp Biochem Physiol A Mol Integr Physiol 2013;165: 106–118. 10.1016/j.cbpa.2013.01.025 [DOI] [PubMed] [Google Scholar]
- 62. Feng Q, Zou X, Lu L, Li Y, Liu YZ, Zhou J, et al. The stress-response gene redd1 regulates dorsoventral patterning by antagonizing wnt/beta-catenin activity in zebrafish. PLoS ONE. 2012;7: e52674 10.1371/journal.pone.0052674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Snell-Rood EC. An overview of the evolutionary causes and consequences of behavioural plasticity. Anim Behav. 2013;2013: 1004–1011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File contains a list of all hindbrain genes that were significantly upregulated (FDR < 0.05) in group- and individually-housed fish, on two separate worksheets.
(XLSX)
Data Availability Statement
All RNA-seq fastq files are available from the NCBI Sequence Read Archive (Study Accession SRP056943).