Abstract
Adenosquamous carcinoma of the pancreas (ASCP) is a rare entity. Like adenocarcinoma of the pancreas, overall survival is poor. Characteristics of ASCP include central tumor necrosis, along with osteoclasts and hypercalcemia. Various theories exist as to why this histological subtype exists, as normal pancreas tissue has no benign squamous epithelium. Due to the rarity of this disease, limited molecular analysis has been performed, and those reports indicate unique molecular features of ASCP. In this paper, we characterize 23 patients diagnosed with ASCP through molecular profiling using immunohistochemistry staining, fluorescent in situ hybridization, chromogenic in situ hybridization, and gene sequencing, Additionally, we provide a comprehensive literature review of what is known to date of ASCP. Molecular characterization revealed overexpression in MRP1 (80%), MGMT (79%), TOP2A (75), RRM1 (42%), TOPO1 (42%), PTEN (45%), CMET (40%), and C-KIT (10%) among others. One hundred percent of samples tested were positive for KRAS mutations. This analysis shows heretofore unsuspected leads to be considered for treatments of this rare type of exocrine pancreas cancer. Molecular profiling may be appropriate to provide maximum information regarding the patient’s tumor. Further work should be pursued to better characterize this disease.
Keywords: Adenosquamous carcinoma of the pancreas, Molecular profiling, Review
Core tip: This analysis of 23 adenosquamous carcinoma of the pancreas in light of the reviewed literature highlights the potential to identify novel treatments when using a personalized medicine approach to patient tumor characterization.
INTRODUCTION
Pancreas cancer remains a deadly disease. In 2014 it is estimated that 46420 new cases will occur, along with 39590 deaths, making it the fourth leading cause of cancer deaths in the United States[1]. The most commonly diagnosed pancreas cancer histology is adenocarcinoma, with an incidence of 85% of pancreas malignancies[2]. As shown in Table 1, other pancreas cancer histological subtypes include mucinous cyst adenocarcinoma, adenosquamous, undifferentiated/anaplastic, papillary mucinous, acinar cell, spindle cell, and pancreatoblastoma[2-4].
Table 1.
Frequency of malignant exocrine pancreatic neoplasms
| Frequency of malignant exocrine pancreatic neoplasms | |
|
Histological subtype |
Frequency |
| Adenocarcinoma | 85% |
| Mucinous cyst adenocarcinoma | 2% |
| Adenosquamous | 0.38%-10% |
| Undifferentiated/anaplastic carcinoma | < 1% |
| Intraductal papillary mucinous carcinoma | 3% |
| Acinar cell carcinoma | < 1% |
| Rare subtypes | 4% |
Rare subtypes include signet ring cell carcinoma, giant cell tumor, cystadenocarcinoma, pancreatoblastoma, spindle cell carcinoma.
Adenosquamous carcinoma of the pancreas (ASCP) is a rare entity. Its estimated incidence in the literature is between 0.38% to 10% of all exocrine pancreatic tumors (Table 2)[2,5-19]. ASCP has also been referred to as adenoacanthoma, mixed squamous and adenocarcinoma, and mucoepidermoid carcinoma[20]. The entity was first described in 1907 by Gotthold Herheimer, who referred it as cancroide[20]. Adenosquamous histology is seen in cancers of other organ systems such as lung, esophagus, colon, stomach, salivary glands, and the female reproductive system[20]. Compared to pancreatic adenocarcinoma, which has a poor 5-year overall survival, survival is worse in patients with ASCP[12-15].
Table 2.
Incidence of adenosquamous carcinoma of the pancreas
| Pancreatic cancer specimens | No. (%) of ASCP | Ref. |
| 15185 | 81 (0.05) | [2] |
| 5075 | 46 (0.9) | [6] |
| 264 | 10 (3.8) | [8] |
| 391 | 13 (3.4) | [9] |
| 80 | 8 (10) | [10] |
| 202 | 6 (3) | [11] |
| 3651 | 45 (1.2) | [12] |
| 45693 | 415 (0.9) | [13] |
| 237 | 7 (2.9) | [14] |
| 406 | 14 (4) | [15] |
| 1025 | 46 (4.5) | [16] |
| 24604 | 95 (0.38) | [17] |
| 635 | 20 (3.1) | [18] |
| 8372 | 25 (0.3) | [19] |
| 234 | 7 (2.9) | [20] |
ASCP: Adenosquamous carcinoma of the pancreas.
The etiology of ASCP is unknown. Most literature reports of this disease have come from Asia. The largest known case study showed that 79% of 415 patients with ASCP were Caucasian[12]. It is unknown if risk factors for the development of pancreatic adenocarcinoma such as chronic pancreatitis, ABO blood group, alcohol use, tobacco use, germline mutations such as BRCA2, PALB2, ATM, and p53 are also risk factors for the development of ASCP[12,21].
In this review, we will discuss the current understanding of ASCP. We have profiled 23 patients with ASCP and will present our findings, along with other molecular analyses reported in the literature. We will also discuss potential treatment strategies specifically targeting ASCP.
PATHOLOGY
Normal pancreas tissue has no benign squamous epithelial components[9,15,22]. Various hypotheses have been proposed regarding the histogenesis of ASCP. One theory hypothesizes that squamous metaplasia occurs as a result of ductal inflammation due to chronic pancreatitis or obstruction by an adenomatous tumor, and this process leads to ASCP[5,23]. Another theory, termed the collision theory, suggests that two histologically distinct tumors arise independently in the pancreas and are joined together leading to ASCP[20,23,24]. Finally, the third theory, the differentiation theory, suggests that a primitive pancreatic stem cell differentiates into either squamous or adenocarcinoma or becomes a combination of both[14,22,23]. Despite different hypotheses, there has been no study to elucidate the mechanism of origination of ASCP.
The pathology of ASCP includes the typical squamous carcinoma pattern that is characterized by epithelium with whorls, keratohyalin, or pearls[14,16], as seen in Figure 1. Compared to nuclei of benign squamous cells, the nuclei of malignant squamous cells are hyperchromic and pleomorphic[15,22]. The squamous carcinoma component of ASCP appears to be more focal in the tumor. An interesting histological feature is the finding in several case series of ASCP that the squamous cell carcinoma component is located in the periphery of the tumor, while the adenocarcinoma component is in the center[9,22]. There is a transitional zone where the glandular structure blends into the squamous component[22]. There is an entity descriptive of pure squamous cell carcinoma of the pancreas, but this classification has been debated and is considered to be more secondary to metastasis to the pancreas from a non-pancreas primary carcinoma[15,25,26].
Figure 1.
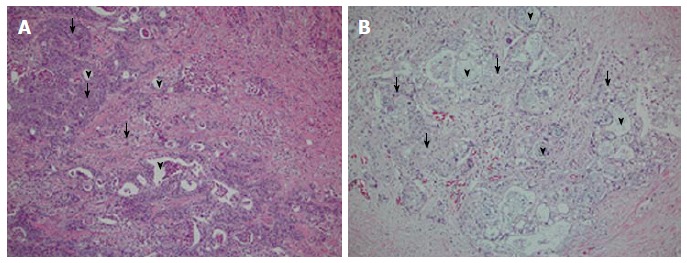
Typical pathology of adenosquamous carcinoma of the pancreas. H and E slides of two patient’s tissues, showing the adeno vs squamous component (arrowheads = adeno; arrows = squamous component). A: Tissue from head of pancreas; B: Tissue from tail of pancreas; both are G2, moderately differentiated cancers.
Tumor cell necrosis is frequently seen in patients with ASCP, along with high tumor grade[25,27]. Other unusual reported pathology has included the presence of clear cell and rhabdoid components[27,28]. One pathology case report noted the presence of both osteoclast and giant tumor cells which were scattered individually within the stroma[3]. The presence of osteoclasts is not unique to ASCP, as osteoclasts have been seen in adenosquamous carcinoma of other organs, including the esophagus, gallbladder, and kidney[3,13]. Acantholysis has also been noted[3]. The squamous component of the cancer has been shown to be more likely to demonstrate vascular invasion, but less likely to metastasize to the lymph nodes[16]. One study found that pancreatic adenosquamous carcinoma grows at twice the rate of pancreatic adenocarcinoma[29].
The current guideline to diagnose adenosquamous pancreatic cancer requires the presence of at least 30% of squamous component in the pancreas tumor tissue[18,20,30]. However, this classification system is being debated, due to both the subjective nature of estimating percentage composition and the sampling method of a patient’s tumor at biopsy through fine needle aspiration (FNA) vs surgical resection. The clinical relevance of the degree of squamous cell differentiation in adenosquamous pancreas cancer is unknown[16,18]. The proportion of squamous differentiation in ASCP did not influence survival in one case series of 38 patients[22]. Some have proposed that presence of any squamous cell carcinoma component in a pancreatic tumor should classify the cancer as adenosquamous[16,26,31].
Prior immunohistochemistry (IHC) analysis on patients with adenosquamous carcinoma have shown the cancer to be positive for cytokeratin (CK) 5/6, CK 7, and p63 and negative for CK 20, p16, and p53[18,32]. IHC positivity for pancreatic adenocarcinoma includes CK7, CK20, mesothelin, cancer antigen 125 (CA-125), and lysozyme[18,33]. The KI-67 index for one patient with ASCP with approximately a 70%-80% squamous carcinoma component was 33%[32].
As in pancreatic adenocarcinoma, KRAS mutations have also been observed in ASCP[18,27,31,34]. A molecular study involving analysis for p53, Dpc4/SMAD4, p16, E-cadherin, EGFR protein expression levels, KRAS mutational analysis; p16/CDKN2a amplification, and HPV DNA detection was carried out on 8 patients with ASCP[27]. The KRAS mutations only screened for mutations in codons 12 and 13, which were present in 5/8 of the squamous component of the cancer samples. A homozygous deletion of the p16 gene was present in 3/8 squamous components. Regarding protein expression in the same patient samples, DPC4 was lost in 5/8 samples, p53 was positive in 5/8 samples, p16 was universally lost, E-cadherin was either reduced or lost in 7/8 samples, and P63 and EGFR were positive in all 8 samples[27]. The lack of protein expression of p16 was particularly interesting since the gene was present in 5/8 patient samples, suggesting other causes of loss of protein expression, such as gene silencing like DNA methylation. There was no HPV DNA detectable in the eight patients tested[27]. HPV status was looked at another analysis of 7 patients, and none of these patients were positive[13]. The lack of positivity of HPV is noteworthy due to its influence in the development of other squamous histology cancers such as the cervix, head and neck, and anus[13].
We have conducted a molecular characterization using a commercially available assay[35]. Twenty-three patients with ASCP were identified and the results of the profiling are presented (Table 3, Table 4 and Table 5). The median age was 60 years old (range 41 to 86 years old), and 17/23 patients were male. Evaluation of protein expression by IHC analysis revealed the following: DNA topoisomerase2 (TOPO2A) overexpression was prevalent in 78% of the samples, which in some studies of other histologic types indicates sensitivity to agents such as doxorubicin or etoposide. Low expression of ribonucleotide reductase M1, which can indicate sensitivity to gemcitabine, was low in 57% of the patient samples. Low thymidylate synthase expression, found in 62% of patient samples, correlates to sensitivity in some tumor types to fluoropyrimidines such as 5-FU, capecitabine, and pemetrexed. Low expression of excision repair cross-complementation group 1, or ERCC1, is associated with sensitivity to platinum-based therapies in some tumor types and was found to be low in 69% of patient samples. Other positive findings included 10% (1 in 10) positivity of c-KIT, and TOPO1 overexpression in 38% of patient samples. These biomarkers are correlated to sensitivities to imatinib and topotecan/irinotecan, respectively, in some tumor types. The high expression of both MRP1 and BCRP1 at 80% highlights the difficulty of treating ASCP, as these proteins are involved in drug resistance to chemotherapy. FISH/CISH analysis revealed an 11% overexpression of c-MET, an oncoprotein that is increasingly targeted in new drug development. Also, one sample had a mutation in c-MET. Of note, mutation analysis revealed KRAS mutations in all sixteen patient samples tested, but none had EGFR mutations.
Table 3.
Molecular profiling of patients with adenosquamous carcinoma of the pancreas: Immunohistochemistry analysis
| IHC analysis percent positive expression (positive/number examined) | ||||||||||||||||||
|
MRP1 |
BCRP |
MGMT |
TOP2A |
TUBB3 |
PTEN |
SPARC |
TOPO1 |
RRM1 |
cMET |
TLE3 |
TS |
ERCC1 |
PGP |
C-kit |
PR |
AR |
ER |
Her2 |
| 80 (8/10) | 80 (4/5) | 76 (16/21) | 78 (14/18) | 38 (3/8) | 41 (9/22) | 39 (9/23) | 38 (8/21) | 43 (9/21) | 33 (4/12) | 42 (5/12) | 38 (8/21) | 31 (4/13) | 11 (2/18) | 10 (1/10) | 5 (1/21) | 0 (0/21) | 5 (1/21) | 0 (0/22) |
IHC: Immunohistochemistry.
Table 4.
Molecular profiling of patients with adenosquamous carcinoma of the pancreas: Fluorescence in situ hybridization/chromogenic in situ hybridization analysis
| FISH/CISH percent positive expression (positive/number examined) | ||||
|
cMET |
EGFR |
Her2 |
TOP2A |
ALK |
| 9 (1/11) | 0 (0/6) | 0 (0/12) | 0 (0/2) | 0 (0/1) |
FISH: Fluorescence in situ hybridization; CISH: Chromogenic in situ hybridization.
Table 5.
Molecular profiling of patients with adenosquamous carcinoma of the pancreas: Mutated gene analysis (either sanger or next generation sequencing)
| Mutated genes percent positive (number found/examined) | ||||||||
|
cMET |
KRAS |
TP53 |
BRAF |
NRAS |
SMAD4 |
cKIT |
PIK3CA |
EGFR |
| 13 (1/13) | 100 (16/16) | 50 (4/8) | 0 (0/9) | 0 (0/9) | 25 (2/8) | 0 (0/9) | 0 (0/11) | 0 (0/10) |
Very little genomic sequencing data is available in the literature on adenosquamous pancreatic cancers. However, a study published examining whole genomic sequencing in eleven patients with advanced cancer included one patient with ASCP[36]. This patient’s sequencing included single nucleotide variations (SNV), whole genome sequencing (WGS), and whole transcriptome sequencing (WTS). Some of the variations found included the upregulation of two ligands, transforming growth factor (TGF)-β 1 and TGF-β 2 along with their accompanying receptor, TGF-β receptor type II. These growth factors are involved in the epithelial to mesenchymal transition (EMT). Other members of a shared pathway, Lef-1, TCF8, and E2A, were also found to be upregulated. E-cadherin was found to be down-regulated, which is a hallmark of the EMT phenotype[33]. The EMT phenotype has been shown to play a crucial role in cancer cell metastasis along with resistance to chemotherapy and contributing to the formation of cancer stem cells[36]. This patient’s tumor did have a mutation in KRAS at codon 12 along with a mutation in PI3KCA. The patient’s sequencing was done during therapy and upon progression on gemcitabine and cisplatin. The patient was then enrolled on a phase I trial involving a combination PI3K and MEK inhibitor, and experienced a clinical benefit in the form of a dramatic decrease in his pain, along with tumor response[36].
Another genetic analysis done recently looked at 23 patients with ASCP through genomic sequencing and showed a mutation of the UPF1 gene, which encodes a RNA helicase essential for the highly conserved RNA degradation pathway, nonsense-mediated RNA decay[37]. This mutation was not seen in the adjacent normal tissue of these patient samples. The pathways that UPF1 is implicated in are not all known but appear to be involved in the normal splicing of RNA, affecting such genes as p53[37].
IMAGING
While there is not a definitive characteristic appearance of ASCP on computed tomography (CT) imaging, they are usually not well circumscribed[38]. CT imaging of ASCP lesions commonly show the presence of central necrosis within the tumor mass[31,38,39], which is rarely seen in pancreatic ductal adenocarcinoma or in endocrine tumors of the pancreas[40,41]. Another common imaging finding is the propensity for vascular and nerve encasement[38].
A large series looking at ASCP through CT and magnetic resonance imaging showed the presence of frequent intra-tumor necrosis, increased enhancement, and exophytic growth[42]. It is theorized that this phenomenon may reflect the presence of the squamous component causing rapid proliferation, as these characteristics are not seen as often in adenocarcinoma of the pancreas[43]. Other unique features noted in imaging evaluation with ASCP are the lack of pancreatic atrophy and mild duct dilatation, which are more common features of pancreatic adenocarcinoma[42]. Like adenocarcinoma of the pancreas, adenosquamous cancers of the pancreas may exhibit the double duct sign[38], which consists of simultaneous dilatation of the common bile and pancreatic ducts[44].
Gallium-67 is an older radioactive tracer that is taken up by some malignancies and infections and has been replaced by PET scans in relation to tumor staging[45]. Intense Gallium-67 uptake, which rarely is detected in pancreatic adenocarcinoma, has been observed in ASCP[45,46]. PET-CT imaging has been reported in a limited number of case reports. One case report noted a patient with localized ASCP to have a standardized uptake value (SUV) of 15.8, which was over 3 times higher than the SUV average for patients with pancreatic adenocarcinoma at their institution[47].
Figure 2 highlights several key imaging findings from patients we have treated with ASCP, including necrosis and mixed morphology. Of note is that in looking at one of our recent ASCP patients, the hypermetabolism that has been previously reported in patients with ASCP was not seen[47].
Figure 2.
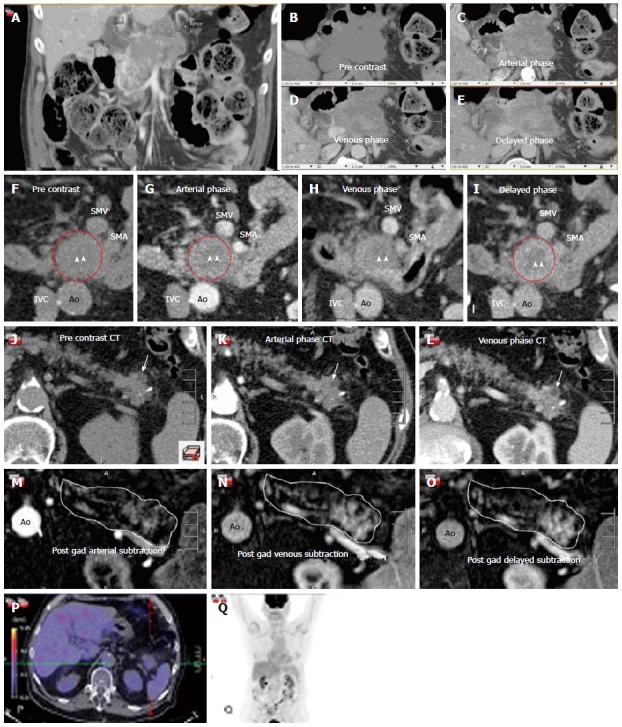
Collection of images from three separate patients with adenosquamous carcinoma of the pancreas. The typical complex enhancing mass and mixed morphology of necrosis and enhancing tissue is demonstrated in this figure. A-E are taken from a four phase contrast enhanced CT (pre-contrast, arterial, venous and delayed images). This type of scanning technique can be helpful to define the tumor and its invasion into surrounding structrures. A-E represent a coronal (A) and axial (B-E) images through a large, infiltrating, necrotic tumor with islands of slow enhancement (B-E). Note the islands of soft tissue enhancement increasing from arterial to delayed phase contrast enhanced CTs. These features are usually signs of very aggressive tumors. In another subject (F-I) there is again a central area of necrosis (arrowheads) surrounded by a ring of slowly enhancing tumor (red circle). Note the relative lack of surrounding soft tissue infiltration compared to the tumor on Panels A-E. Panels J-O are taken from a third subject and are an example of an atypical adenosquamous carcinoma involving the pancreas tail with a slowly enhancing, non-infiltrating lesion both on CT (J-L) and post gadolinium subtraction MRI (M-O). The white outline in Panels M-O outlines the contour of the pancreas with the enhancing lesion seen towards the tail of the pancreas. There is a small focus of necrosis present (arrow), a feature typical of adenosquamous carcinoma of the pancreas. The corresponding FDG PET/CT (P and Q) is unusual in that it shows that this mass is not hypermetabolic unlike most adenosquamous pancreas carcinomas. Ao: Aorta; IVC: Inferior vena caval; SMA: Superior mesenteric artery; SMV: Superior mesenteric vein.
CLINICAL CHARACTERISTICS
The characteristics of patients with ASCP tend to favor more aggressive features with more node positive disease, more poorly differentiated disease, and more perineural invasion present compared to patients with pancreatic adenocarcinoma[16]. Patients with ASCP present with symptoms similar in nature to patients with pancreatic adenocarcinoma, with abdominal pain, weight loss, back pain, nausea, vomiting, anorexia, and jaundice being the most common presenting symptoms[19,20,38]. As with pancreatic adenocarcinoma, there appears to be an increased risk of deep vein thrombosis[25]. In larger case series, patients are typically male, white, present in their sixth decade of life, and the tumor is located in the head of the pancreas[12,13,20]. Serum lab abnormalities may include elevated bilirubin, elevated alkaline phosphatase, anemia, and elevated carbohydrate antigen 19-9 (CA 19-9)[19,20,25]. Occasionally, patients may also have elevated levels of carcinoembryonic antigen (CEA) or CA-125[38].
Long term survival overall is poor for ASCP. Survival, despite surgical resection, is slightly poorer for patients with ASCP than those with pancreatic adenocarcinoma. Those with ASCP have a 3-year survival rate of 14% with surgery, as opposed to 19% 3-year survival of resected pancreatic adenocarcinoma patients[29,48]. Like patients with pancreatic adenocarcinoma, patients with ASCP tend to present more commonly in advanced stage, with one large analysis through the California Cancer Registry database (CCR) indicating over 50% of ASCP patients presenting in advanced stage[11]. The mean tumor diameter in one series of resected ASCP patients was 46.3 mm vs 33.5 mm of adenocarcinoma pancreas patients (P value 0.0001)[11]. Comparisons between patients at single institutions and matching for stage have yielded an overall median survival of 6.51 mo vs 11.0 mo for ASCP vs adenocarcinoma[15]. In another large single institution analysis of patients with ASCP, the median survival of patients with resection was 10.9 mo, which was worse than those with pancreatic adenocarcinoma who underwent resection, which was 17.9 mo[16].
In an analysis of Surveillance, Epidemiology, and End Results (SEER) patients that identified 415 patients with ASCP, the mean age was found to be 66 years old and the tumor more likely to be in the head of the pancreas. Compared to patients with adenocarcinoma of the pancreas, patients with ASCP were more likely to be poorly differentiated (71.4% vs 45%), larger (5.7 cm vs 4.3 cm), and more likely to have positive lymph nodes (52.8% vs 47.1%)[12]. In patients with ASCP, overall 1 and 2-year survival was 21.2% and 10.8% compared to 24.7% and 10.9% in patients with pancreatic adenocarcinoma[12]. Patients with ASCP were found to have a median survival of 4 mo compared to 5 mo in patients with pancreatic adenocarcinoma[12]. Patients with ASCP who underwent resection had worse survival rates than those with adenocarcinoma pancreas cancer who underwent resection. One year and 2-year survival rates of 50.7% and 29% and median survival was 12 mo in patients with ASCP as opposed to 60.1% and 35.8% and median survival of 16 mo in those with adenocarcinoma of the pancreas[12]. After primary resection, recurrence may occur in a number of sites. Common sites of metastases include the liver, lung, retroperitoneum, and development of malignant ascites[16,38].
Several studies have examined various clinical features of survival in patients with ASCP. Lymph node status, tumor size, or resection in patients with ASCP does not impact survival when compared with patients with adenocarcinoma of the pancreas[12,16]. Not surprisingly, risk factors for poorer survival of patients with ASCP are those with distant disease, advanced age, and patients unable to undergo surgical resection[12]. In one study, only 40% of patients with ASCP were resectable[12]. A single institution case series from the Mayo clinic showed that patients with an R1 resection still benefited in survival compared to those who did not undergo surgery[49]. Patients from that study that had either an R0 or R1 resection had a median survival of 14.4 and 8 mo respectively, compared to 4.8 mo who received no surgical treatment[49]. Location of the tumor matters, with poorer survival noted if the location was in the body or tail as opposed to the head of the pancreas[48]. This was based on a chart analysis of 39 patients with ASCP and may be accounted for by size of these tumors by location as the ones located in the head were smaller (4.7 ± 1.9 cm) as opposed to the body/tail lesions (7.3 ± 1.8 cm)[48]. The likely reason for poorer survival is that patients with head of pancreas lesions tend to present with obstructive symptoms, which are clinically evident when the lesion is smaller in comparison to body/tail lesions of the pancreas.
It is unclear why patients with ASCP have such a poor prognosis. Due to the small sample size, data to shed light on this disease has been limited. One case series from Voong et al[16] looking at patients diagnosed with ASCP and who had undergone surgery showed via univariate analysis that only patients who received adjunct chemoradiation had a clinical significant improvement in survival[16]. The patients who received adjunct chemoradiation had a median survival of 13.6 mo as opposed to 8.6 mo for those that did not[16]. Other factors such as age, tumor size, differentiation, margin, node status, type of surgery were not shown to affect survival in this case series[16].
Malignancy associated hypercalcemia, which is a rare phenomenon of exocrine pancreatic carcinoma, has been described in ASCP[50,51]. Of note is that malignancy associated hypercalcemia is more commonly associated with squamous cell carcinomas of the head, neck, lung, and esophagus. Case reports have also described patients with adenosquamous pancreatic cancer having elevated levels of calcium due to elevated levels of parathyroid hormone related protein[50,51]. In both reported cases, hypercalcemia persisted despite bisphosphonate treatment[50,51]. Curiously, hyperglycemia has not been reported with great frequency in ASCP despite being reported in up to 80% of patients with pancreatic adenocarcinoma[52].
MANAGEMENT
Diagnosis of patients with ASCP requires biopsy along with pathology review using criteria of ASCP with at least 30% of the tumor positive for squamous histology. Staging is done in a similar manner as pancreatic adenocarcinoma with guidelines set forth by the American Joint Committee on Cancer (AJCC)/International Union Against Cancer (UICC). Unresectable disease is designated as Stage III and metastatic disease is designated as stage IV. One issue with diagnosis includes the current standard approach of using endoscopic ultrasound for diagnosing pancreatic cancer and using FNA. In a retrospective review of patients at John Hopkins University and Emory University it was noted that in patients who eventually had a diagnosis of ASCP after surgical resection, two thirds of them (67%) were initially diagnosed as being pancreatic adenocarcinoma only. It is possible for pathologists to misclassify or ignore the squamous cell compartment in pancreatic FNA specimens, which not only leads to underreporting of ASCP but may also miss the diagnosis of malignancy altogether[15].
There are currently no guidelines for treating patients with ASCP. Literature reports often cite treatment regimens similar to adenocarcinoma[48]. Due to its relative infrequency in incidence there have been no published randomized clinical trials specifically targeting patients with ASCP. Treatments in years past have focused on resection of local adenosquamous pancreatic carcinoma using the same guidelines for pancreatic adenocarcinoma. These include pancreaticoduodenectomy (PD), pylorus-preserving PD, distal pancreatectomy, and total pancreatectomy[48]. These techniques are not modified for ASCP and surgical resection remains the best opportunity to achieve long lasting survival[48].
The role of neoadjuvant and adjuvant chemotherapy is unclear, mimicking some questions that continue to be explored in pancreatic adenocarcinoma[48]. Most case reports in the literature have used 5-fluorouracil based therapies for treatment around surgical procedures and have not examined the role of gemcitabine or more robust regimens such as FOLFIRINOX or nab-paclitaxel/gemcitabine[49]. In a retrospective series of 62 patients identified with pancreatic adenosquamous carcinoma, 14 patients received platinum therapy in the adjuvant setting as opposed to 48 who did not[53]. The patients who received platinum therapy in the adjuvant setting had an overall median survival of 19.1 mo as opposed to 10.7 mo for those who did not (P = 0.011, hazard ratio of survival 0.48)[53].
The role of radiation therapy as an adjunct to resection of ASCP is also unclear[48,54,55]. Two retrospective studies examined adjuvant radiation therapy, but did not show a benefit in overall survival for those that received adjuvant therapy vs those who did not. In a previously published literature review of 30 patients who received radiation therapy either intra and/or postoperatively, the 2-year survival rate was 20% and median survival 13 mo[48]. In the patients who did not receive radiation therapy their 2-year survival rate was 9% and median survival period was 6 mo. Despite the differences in survival between the 2 groups, they did not reach statistical significance[48].
CONCLUSION
ASCP is an aggressive variation of carcinoma of the pancreas. Overall it carries a poor prognosis. A study to assess the percentage component of squamous carcinoma in ASCP and associating this with differences in clinical outcome is certainly warranted, but may be difficult to carry out due to the scarcity of this disease and the subjective evaluation needed by pathologists to determine percent squamous in a pancreas carcinoma specimen. Obtaining the proper amount of tissue makes diagnosis difficult and is akin to diagnosing patients with lymphoma by way of FNA: there may be diagnostic inaccuracies depending upon where the sample is biopsied. This role of subjective evaluation also makes interpreting retrospective analysis difficult, such as examining databases like SEER.
There is a need to better characterize the disease beyond traditional pathology analysis. Doing further work characterizing this disease on a molecular level may further elucidate the requirements for classifying pancreatic carcinomas as adenosquamous or adeno. Our work in molecular characterization, while small in sample size, points to the use of novel therapeutic combinations in patients with ASCP, such as epirubicin/cisplatin/5-FU, which may be tested in small clinical trials. Targeting novel pathways such as those affecting the epithelial to mesenchymal change pathway, using agents that target APC, WNT, B-catenin, along with those targeting chromatin remodeling may be worth trying against this disease. Using cell lines derived from ASCP patients and studying them in growth assays and xenograft models may yield clues regarding their response to newer anti-cancer agents in development[54,55]. Understanding the key genetic drivers for this disease may lead to better treatment outcomes since it is clear traditional treatments for pancreatic adenocarcinoma do not translate well to ASCP.
Footnotes
Supported by In part the Lee T. Hanley Fund for Pancreatic Cancer Research.
Conflict-of-interest statement: Sherri Z Millis and Zoran Gatalica are employees with Caris and Daniel D Von Hoff also has a consulting role as Executive Director Caris Life Sciences, Scientific Advisory Board. Dr. Daniel D Von Hoff: Executive Director Caris Life Sciences, Scientific Advisory Board. Dr. Sherri Z Millis: Scientific Program Manager, Bioinformatics at Caris Life Sciences. Dr. Zoran Gatalica: medical director and pathologist at Caris Life Sciences. All remaining authors have declared no conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 3, 2015
First decision: April 27, 2015
Article in press: July 14, 2015
P- Reviewer: Mocellin S, Tsuchida A S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Fitzgerald TL, Hickner ZJ, Schmitz M, Kort EJ. Changing incidence of pancreatic neoplasms: a 16-year review of statewide tumor registry. Pancreas. 2008;37:134–138. doi: 10.1097/MPA.0b013e318163a329. [DOI] [PubMed] [Google Scholar]
- 3.Alwaheeb S, Chetty R. Adenosquamous carcinoma of the pancreas with an acantholytic pattern together with osteoclast-like and pleomorphic giant cells. J Clin Pathol. 2005;58:987–990. doi: 10.1136/jcp.2004.025221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Baithun SI. Morphological study of 391 cases of exocrine pancreatic tumours with special reference to the classification of exocrine pancreatic carcinoma. J Pathol. 1985;146:17–29. doi: 10.1002/path.1711460103. [DOI] [PubMed] [Google Scholar]
- 5.Cihak RW, Kawashima T, Steer A. Adenoacanthoma (adenosquamous carcinoma) of the pancreas. Cancer. 1972;29:1133–1140. doi: 10.1002/1097-0142(197205)29:5<1133::aid-cncr2820290503>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Baylor SM, Berg JW. Cross-classification and survival characteristics of 5,000 cases of cancer of the pancreas. J Surg Oncol. 1973;5:335–358. doi: 10.1002/jso.2930050410. [DOI] [PubMed] [Google Scholar]
- 7.Kissane JM. Carcinoma of the exocrine pancreas: pathologic aspects. J Surg Oncol. 1975;7:167–174. doi: 10.1002/jso.2930070213. [DOI] [PubMed] [Google Scholar]
- 8.Morohoshi T, Held G, Klöppel G. Exocrine pancreatic tumours and their histological classification. A study based on 167 autopsy and 97 surgical cases. Histopathology. 1983;7:645–661. doi: 10.1111/j.1365-2559.1983.tb02277.x. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi K, Enjoji M. Adenosquamous carcinoma of the pancreas: a clinicopathologic study. J Surg Oncol. 1991;47:109–116. doi: 10.1002/jso.2930470210. [DOI] [PubMed] [Google Scholar]
- 10.Motojima K, Tomioka T, Kohara N, Tsunoda T, Kanematsu T. Immunohistochemical characteristics of adenosquamous carcinoma of the pancreas. J Surg Oncol. 1992;49:58–62. doi: 10.1002/jso.2930490114. [DOI] [PubMed] [Google Scholar]
- 11.Katz MH, Taylor TH, Al-Refaie WB, Hanna MH, Imagawa DK, Anton-Culver H, Zell JA. Adenosquamous versus adenocarcinoma of the pancreas: a population-based outcomes analysis. J Gastrointest Surg. 2011;15:165–174. doi: 10.1007/s11605-010-1378-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyd CA, Benarroch-Gampel J, Sheffield KM, Cooksley CD, Riall TS. 415 patients with adenosquamous carcinoma of the pancreas: a population-based analysis of prognosis and survival. J Surg Res. 2012;174:12–19. doi: 10.1016/j.jss.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simone CG, Zuluaga Toro T, Chan E, Feely MM, Trevino JG, George TJ. Characteristics and outcomes of adenosquamous carcinoma of the pancreas. Gastrointest Cancer Res. 2013;6:75–79. [PMC free article] [PubMed] [Google Scholar]
- 14.Cubilla AL, Fitzgerald PJ. Morphological patterns of primary nonendocrine human pancreas carcinoma. Cancer Res. 1975;35:2234–2248. [PubMed] [Google Scholar]
- 15.Olson MT, Siddiqui MT, Ali SZ. The differential diagnosis of squamous cells in pancreatic aspirates: from contamination to adenosquamous carcinoma. Acta Cytol. 2013;57:139–146. doi: 10.1159/000346326. [DOI] [PubMed] [Google Scholar]
- 16.Voong KR, Davison J, Pawlik TM, Uy MO, Hsu CC, Winter J, Hruban RH, Laheru D, Rudra S, Swartz MJ, et al. Resected pancreatic adenosquamous carcinoma: clinicopathologic review and evaluation of adjuvant chemotherapy and radiation in 38 patients. Hum Pathol. 2010;41:113–122. doi: 10.1016/j.humpath.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cubilla A, Fitzgerald P. Surgical pathology of tumors of the exocrine pancreas. In: Mooss AR, editor. Tumors of the Pancreas. Baltimore: Williams and Wilkins; 1980. pp. 159–193. [Google Scholar]
- 18.Kardon DE, Thompson LD, Przygodzki RM, Heffess CS. Adenosquamous carcinoma of the pancreas: a clinicopathologic series of 25 cases. Mod Pathol. 2001;14:443–451. doi: 10.1038/modpathol.3880332. [DOI] [PubMed] [Google Scholar]
- 19.Hsu JT, Yeh CN, Chen YR, Chen HM, Hwang TL, Jan YY, Chen MF. Adenosquamous carcinoma of the pancreas. Digestion. 2005;72:104–108. doi: 10.1159/000088364. [DOI] [PubMed] [Google Scholar]
- 20.Madura JA, Jarman BT, Doherty MG, Yum MN, Howard TJ. Adenosquamous carcinoma of the pancreas. Arch Surg. 1999;134:599–603. doi: 10.1001/archsurg.134.6.599. [DOI] [PubMed] [Google Scholar]
- 21.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa O, Matsui Y, Aoki I, Iwanaga T, Terasawa T, Wada A. Adenosquamous carcinoma of the pancreas: a clinicopathologic study and report of three cases. Cancer. 1980;46:1192–1196. doi: 10.1002/1097-0142(19800901)46:5<1192::aid-cncr2820460519>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 23.Trikudanathan G, Dasanu CA. Adenosquamous carcinoma of the pancreas: a distinct clinicopathologic entity. South Med J. 2010;103:903–910. doi: 10.1097/SMJ.0b013e3181ebadbd. [DOI] [PubMed] [Google Scholar]
- 24.Kovi J. Adenosquamous carcinoma of the pancreas: a light and electron microscopic study. Ultrastruct Pathol. 1982;3:17–23. doi: 10.3109/01913128209016621. [DOI] [PubMed] [Google Scholar]
- 25.Rahemtullah A, Misdraji J, Pitman MB. Adenosquamous carcinoma of the pancreas: cytologic features in 14 cases. Cancer. 2003;99:372–378. doi: 10.1002/cncr.11855. [DOI] [PubMed] [Google Scholar]
- 26.Adachi K. Primary squamous cell carcinoma of the pancreas: a case report. JOP. 2011;12:181–184. [PubMed] [Google Scholar]
- 27.Brody JR, Costantino CL, Potoczek M, Cozzitorto J, McCue P, Yeo CJ, Hruban RH, Witkiewicz AK. Adenosquamous carcinoma of the pancreas harbors KRAS2, DPC4 and TP53 molecular alterations similar to pancreatic ductal adenocarcinoma. Mod Pathol. 2009;22:651–659. doi: 10.1038/modpathol.2009.15. [DOI] [PubMed] [Google Scholar]
- 28.Jamali M, Serra S, Chetty R. Adenosquamous carcinoma of the pancreas with clear cell and rhabdoid components. A case report. JOP. 2007;8:330–334. [PubMed] [Google Scholar]
- 29.O’Connor JK, Sause WT, Hazard LJ, Belnap LP, Noyes RD. Survival after attempted surgical resection and intraoperative radiation therapy for pancreatic and periampullary adenocarcinoma. Int J Radiat Oncol Biol Phys. 2005;63:1060–1066. doi: 10.1016/j.ijrobp.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 30.Hruban RH, Pitman MB, Klimstra DS. Tumors of the pancreas. In: Silverberg SG, Sobin LH, editors. AFIP Atlas of Tumor Pathology, Series 4, Fascicle 6. Washington, DC: American Registry of Pathology; 2007. pp. 177–181. [Google Scholar]
- 31.Murakami Y, Yokoyama T, Yokoyama Y, Kanehiro T, Uemura K, Sasaki M, Morifuji M, Sueda T. Adenosquamous carcinoma of the pancreas: preoperative diagnosis and molecular alterations. J Gastroenterol. 2003;38:1171–1175. doi: 10.1007/s00535-003-1226-4. [DOI] [PubMed] [Google Scholar]
- 32.Matsubayashi H, Matsunaga K, Uesaka K, Kanemoto H, Ito I, Asakura H, Yagishita A, Ono H. Pancreatic adenosquamous carcinoma with 7-year survival: a case report and literature review. J Dig Dis. 2013;14:207–210. doi: 10.1111/1751-2980.12018. [DOI] [PubMed] [Google Scholar]
- 33.Dennis JL, Hvidsten TR, Wit EC, Komorowski J, Bell AK, Downie I, Mooney J, Verbeke C, Bellamy C, Keith WN, et al. Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clin Cancer Res. 2005;11:3766–3772. doi: 10.1158/1078-0432.CCR-04-2236. [DOI] [PubMed] [Google Scholar]
- 34.Uemura K, Hiyama E, Murakami Y, Kanehiro T, Ohge H, Sueda T, Yokoyama T. Comparative analysis of K-ras point mutation, telomerase activity, and p53 overexpression in pancreatic tumours. Oncol Rep. 2003;10:277–283. [PubMed] [Google Scholar]
- 35.Borazanci E, Millis SZ, Winkler J, Ramanathan RK, Von Hoff DD. Multiplatform profiling of rare exocrine pancreatic carcinoma subtypes to identify potential actionable targets. J Clin Oncol. 2015;32(suppl):abstr e15229). Available from: http://meetinglibrary.asco.org/content/135008-144. [Google Scholar]
- 36.Weiss GJ, Liang WS, Demeure MJ, Kiefer JA, Hostetter G, Izatt T, Sinari S, Christoforides A, Aldrich J, Kurdoglu A, et al. A pilot study using next-generation sequencing in advanced cancers: feasibility and challenges. PLoS One. 2013;8:e76438. doi: 10.1371/journal.pone.0076438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu C, Karam R, Zhou Y, Su F, Ji Y, Li G, Xu G, Lu L, Wang C, Song M, et al. The UPF1 RNA surveillance gene is commonly mutated in pancreatic adenosquamous carcinoma. Nat Med. 2014;20:596–598. doi: 10.1038/nm.3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yin Q, Wang C, Wu Z, Wang M, Cheng K, Zhao X, Yuan F, Tang Y, Miao F. Adenosquamous carcinoma of the pancreas: multidetector-row computed tomographic manifestations and tumor characteristics. J Comput Assist Tomogr. 2013;37:125–133. doi: 10.1097/RCT.0b013e31827bc452. [DOI] [PubMed] [Google Scholar]
- 39.Nabae T, Yamaguchi K, Takahata S, Utsunomiya N, Matsunaga H, Sumiyoshi K, Chijiiwa K, Tanaka M. Adenosquamous carcinoma of the pancreas: report of two cases. Am J Gastroenterol. 1998;93:1167–1170. doi: 10.1111/j.1572-0241.1998.00299.x. [DOI] [PubMed] [Google Scholar]
- 40.Mergo PJ, Helmberger TK, Buetow PC, Helmberger RC, Ros PR. Pancreatic neoplasms: MR imaging and pathologic correlation. Radiographics. 1997;17:281–301. doi: 10.1148/radiographics.17.2.9084072. [DOI] [PubMed] [Google Scholar]
- 41.Buetow PC, Parrino TV, Buck JL, Pantongrag-Brown L, Ros PR, Dachman AH, Cruess DF. Islet cell tumors of the pancreas: pathologic-imaging correlation among size, necrosis and cysts, calcification, malignant behavior, and functional status. AJR Am J Roentgenol. 1995;165:1175–1179. doi: 10.2214/ajr.165.5.7572498. [DOI] [PubMed] [Google Scholar]
- 42.Ding Y, Zhou J, Sun H, He D, Zeng M, Rao S. Contrast-enhanced multiphasic CT and MRI findings of adenosquamous carcinoma of the pancreas. Clin Imaging. 2013;37:1054–1060. doi: 10.1016/j.clinimag.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Myung SJ, Kim MH, Lee SK, Seo DW, Kim YS, Min YI. Adenosquamous carcinoma of the pancreas: differentiation from pancreatic pseudocyst. Gastrointest Endosc. 1998;47:410–413. doi: 10.1016/s0016-5107(98)70230-5. [DOI] [PubMed] [Google Scholar]
- 44.Ahualli J. The double duct sign. Radiology. 2007;244:314–315. doi: 10.1148/radiol.2441041978. [DOI] [PubMed] [Google Scholar]
- 45.Silberstein EB. Cancer diagnosis. The role of tumor-imaging radiopharmaceuticals. Am J Med. 1976;60:226–237. doi: 10.1016/0002-9343(76)90432-0. [DOI] [PubMed] [Google Scholar]
- 46.Kuji I, Sumiya H, Taki J, Nakajima K, Yokoyama K, Kinuya S, Kinuya K, Ichikawa A, Konishi S, Michigishi T, et al. Intense Ga-67 uptake in adenosquamous carcinoma of the pancreas. Ann Nucl Med. 1997;11:41–43. doi: 10.1007/BF03164758. [DOI] [PubMed] [Google Scholar]
- 47.Izuishi K, Takebayashi R, Suzuki Y. Electronic image of the month. Adenosquamous carcinoma of the pancreas. Clin Gastroenterol Hepatol. 2010;8:e40. doi: 10.1016/j.cgh.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 48.Okabayashi T, Hanazaki K. Surgical outcome of adenosquamous carcinoma of the pancreas. World J Gastroenterol. 2008;14:6765–6770. doi: 10.3748/wjg.14.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smoot RL, Zhang L, Sebo TJ, Que FG. Adenosquamous carcinoma of the pancreas: a single-institution experience comparing resection and palliative care. J Am Coll Surg. 2008;207:368–370. doi: 10.1016/j.jamcollsurg.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi N, Higurashi T, Iida H, Mawatari H, Endo H, Nozaki Y, Tomimoto A, Yoneda K, Akiyama T, Fujita K, et al. Adenosquamous carcinoma of the pancreas associated with humoral hypercalcemia of malignancy (HHM) J Hepatobiliary Pancreat Surg. 2008;15:531–535. doi: 10.1007/s00534-007-1258-x. [DOI] [PubMed] [Google Scholar]
- 51.Inoue T, Nagao S, Tajima H, Okudaira K, Hashiguchi K, Miyazaki J, Matsuzaki K, Tsuzuki Y, Kawaguchi A, Itoh K, et al. Adenosquamous pancreatic cancer producing parathyroid hormone-related protein. J Gastroenterol. 2004;39:176–180. doi: 10.1007/s00535-003-1270-0. [DOI] [PubMed] [Google Scholar]
- 52.Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009;10:88–95. doi: 10.1016/S1470-2045(08)70337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wild AT, Dholakia AS, Fan KY, Kumar R, Moningi S, Rosati LM, Laheru DA, Zheng L, De Jesus-Acosta A, Ellsworth SG, et al. Efficacy of platinum chemotherapy agents in the adjuvant setting for adenosquamous carcinoma of the pancreas. J Gastrointest Oncol. 2015;6:115–125. doi: 10.3978/j.issn.2078-6891.2014.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ikeda Y, Ezaki M, Hayashi I, Yasuda D, Nakayama K, Kono A. Establishment and characterization of human pancreatic cancer cell lines in tissue culture and in nude mice. Jpn J Cancer Res. 1990;81:987–993. doi: 10.1111/j.1349-7006.1990.tb03336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meitner PA, Kajiji SM, LaPosta-Frazier N, Bogaars HA, Jolly GA, Dexter DL, Calabresi P, Turner MD. “COLO 357,” a human pancreatic adenosquamous carcinoma: growth in artificial capillary culture and in nude mice. Cancer Res. 1983;43:5978–5985. [PubMed] [Google Scholar]


