Abstract
Radiotherapy is commonly offered to patients with pancreatic malignancies although its ultimate utility is compromised since the pancreas is surrounded by exquisitely radiosensitive normal tissues, such as the duodenum, stomach, jejunum, liver, and kidneys. Proton radiotherapy can be used to create dose distributions that conform to tumor targets with significant normal tissue sparing. Because of this, protons appear to represent a superior modality for radiotherapy delivery to patients with unresectable tumors and those receiving postoperative radiotherapy. A particularly exciting opportunity for protons also exists for patients with resectable and marginally resectable disease. In this paper, we review the current literature on proton therapy for pancreatic cancer and discuss scenarios wherein the improvement in the therapeutic index with protons may have the potential to change the management paradigm for this malignancy.
Keywords: Proton therapy, Pancreatic cancer, Review
Core tip: Radiotherapy is commonly offered to patients with pancreatic malignancies although its ultimate utility is compromised since the pancreas is surrounded by exquisitely radiosensitive normal tissues, such as the duodenum, stomach, jejunum, liver, and kidneys. Proton radiotherapy can be used to create dose distributions that conform to tumor targets with significant normal tissue sparing. Because of this, protons appear to represent a superior modality for radiotherapy delivery to patients with unresectable tumors and those receiving postoperative radiotherapy. A particularly exciting opportunity for protons also exists for patients with resectable and marginally resectable disease.
INTRODUCTION
Radiotherapy is commonly offered to patients with pancreatic malignancies although its ultimate utility is compromised since the pancreas is surrounded by exquisitely radiosensitive normal tissues, such as the duodenum, stomach, jejunum, liver, and kidneys. Proton radiotherapy can be used to create dose distributions that conform to tumor targets with significant normal-tissue sparing. Because of this, protons appear to represent a superior modality for radiotherapy delivery to patients with unresectable tumors and those receiving postoperative radiotherapy. A particularly exciting opportunity for protons also exists for patients with resectable and marginally resectable disease. While many surgeons are hesitant to perform major pancreatic operations on patients who have received preoperative X-ray-based radiotherapy, it is possible that the normal tissue-sparing characteristics of protons will allow for more wide-spread adoption of preoperative radiotherapy in the setting of resectable potentially curable disease.
PHYSICS OF PARTICLE THERAPY
Charged particles such as protons travel a finite distance into tissue, determined by their energy, and then release most of that energy in a tightly defined region called the “Bragg peak”. By delivering a range of energies directed toward the tumor target, a summation of these Bragg peaks allow for the creation of a “spread-out Bragg peak”, which conforms to the depth and position of the tumor target (Figure 1). This process stands in contrast to X-rays for which the highest dose is near the point of beam entry into the patient. With X-rays, the tumor dose is significantly less than the entry dose and exit dose is delivered beyond the tumor target (Figure 2).
Figure 1.
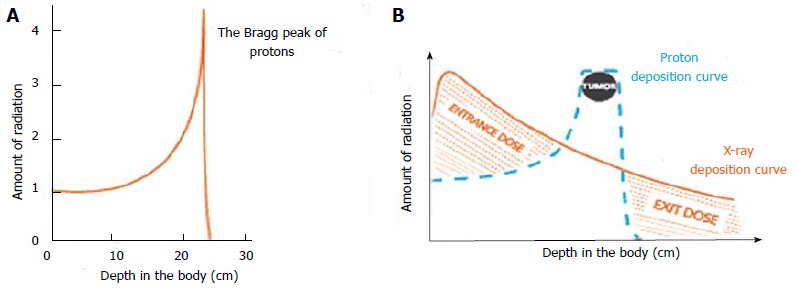
Charged particles like protons travel a finite distance into tissue, as determined by their energy, and then release that energy in a tightly defined region called “Bragg peak” (A). By delivering a range of energies toward the tumor target, a summation of these Bragg peaks allow for the creation of a “spread-out Bragg peak”, which conforms to the depth and position of the tumor target (B). Image borrowed from the University of Florida Health Proton Therapy Institute.
Figure 2.
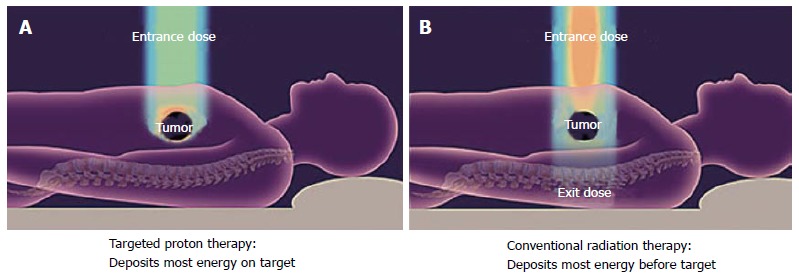
With X-rays, the tumor dose is significantly less than the entry dose and exit dose is delivered beyond the tumor target. With conventional radiotherapy (A) using X-rays (photons), the highest dose is near the point of beam entry into the patient. The tumor dose is significantly less than the entry dose. Also, an exit dose is delivered beyond the tumor target. With protons (B) and other particle therapies, such as carbon ions, the entry dose is low. The highest dose is at the depth of the tumor target and there is no exit dose beyond the target. Image borrowed from the University of Florida Health Proton Therapy Institute.
With X-ray-based therapies such as intensity-modulated radiotherapy (IMRT), the conformality of the dose distribution around a tumor target is achieved by delivering multiple treatment beams from multiple angles which intersect to create a central high-dose volume. This necessarily results in radiation exposure to virtually the entire cylinder of the abdomen. With protons, because the radiation dose deposition can be modulated along the beam path, fewer beam angles are required to create a conformal dose distribution. As a result, radiation exposure to large volumes of normal tissues is either minimized or eliminated (Figure 3)[1].
Figure 3.
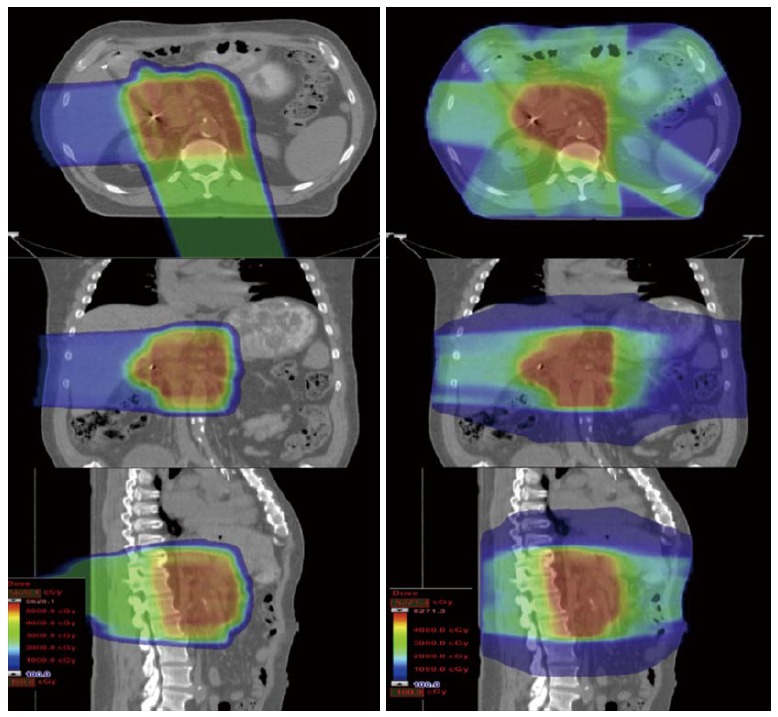
A passively scattered proton plan is shown on the left and an intensity-modulated X-ray therapy plan is shown on the right for a typical patient receiving postoperative radiotherapy for pancreatic cancer. To achieve a conformal dose distribution, the intensity-modulated X-ray therapy plan delivers beams from multiple angles and necessarily irradiates the entire cylinder of the abdomen. With protons, however, because the dose distribution can be modulated along the beam path, significant sparing of sensitive gastrointestinal structures (small bowel and stomach) can be achieved. In the proton plan, 75% of the dose is delivered via a posterior field that irradiates the tumor bed but does not exit into the small bowel. The remaining dose is delivered through a right lateral field that also irradiates the tumor bed but does not exit into the stomach.
CONTROVERSIES REGARDING THE ROLE OF RADIOTHERAPY FOR PANCREATIC CANCER
While radiotherapy has historically been offered to patients with unresectable disease or postoperatively to patients with resected disease, several recent studies have questioned its value, suggesting that its toxicity outweighs its potential benefit. The ESPAC-1 trial, using a complicated randomization scheme[2,3], concluded that postoperative radiotherapy was associated with a nominal, but statistically insignificant, survival decrement as irradiated patients demonstrated a 15.5-mo median survival vs 16.1 mo for patients receiving chemotherapy alone. While valid criticisms of the ESPAC-1 study have been published[4], chemotherapy alone, without radiotherapy, has been adopted as a standard postoperative approach for resected patients in many centers. For patients with unresectable disease, the recent report of the LAP 07 study (of patients with locally advanced pancreatic cancer) showing a 16.4-mo median overall survival for patients receiving chemotherapy alone vs 15.2 mo for the chemoradiation arm[5] has led to further doubts about the utility of radiotherapy in this group of patients. Finally, while some institutions have advocated preoperative X-ray-based radiotherapy for patients with marginally resectable or resectable disease, many surgeons are reluctant to operate on previously irradiated patients, citing concerns about radiotherapy toxicities complicating what is already a complicated operation.
CAN PROTONS IMPROVE THE THERAPEUTIC RATIO?
Considering the above concerns regarding the toxicity-efficacy tradeoffs for X-ray-based radiotherapy, numerous dosimetric and clinical studies have explored the possibility that protons might offer an improved therapeutic index for pancreatic cancer patients receiving radiotherapy.
Dosimetric studies
Hsiung-Stripp et al[6] demonstrated the ability of 130-180 MeV protons to effectively treat unresectable pancreatic cancers. Compared with similarly effective X-ray plans, proton plans significantly reduced doses to the spinal cord (P = 0.003), left kidney (P = 0.025), right kidney (P = 0.057), and liver (P = 0.061). The authors argued that this reduction in normal tissue exposure might allow for radiotherapy dose escalation.
Kozak et al[7] demonstrated the dosimetric feasibility of hypo-fractionated proton therapy for neoadjuvant pancreatic cancer treatment using anatomical data from 9 patients. Compared with IMRT, protons offered a significant reduction of dose to the liver, kidneys and small bowel-particularly in the low-dose regions.
Bouchard et al[8] compared 3-dimensional (3D) conformal photon radiotherapy with IMRT and protons in the delivery of 72 Gy (RBE) to unresectable tumors. The authors concluded that protons were superior to photons for tumors with anteriorly located small bowel.
Nichols et al[9] compared passively scattered protons with intensity-modulated X-ray therapy for 8 patients in the postoperative setting. Patients were treated with a planning target volume dose of 50.4 Gy (RBE). Proton plans offered significantly reduced normal tissue exposure over the IMRT plans with respect to median small bowel V20Gy (RBE) (P = 0.0157), median gastric V20Gy (RBE) (P = 0.0313), and median right kidney V18Gy (RBE) (P = 0.0156). The authors argued that, by reducing small bowel and gastric exposure, protons have the potential to reduce acute and late toxicities of postoperative chemoradiation.
Lee et al[10] explored the feasibility of using proton therapy in the neoadjuvant setting to cover a planning target volume including gross disease and regional lymph nodes. Utilizing a field arrangement heavily weighted to a posterior field, the investigators demonstrated the feasibility of expanding the target volume to cover nodal targets without significantly increasing critical normal tissue exposure. The authors argued that treating a similar increase in target volume would be substantially more difficult with X-rays due to normal tissue exposure issues.
Ding et al[11] compared passively scattered and modulated scanning proton therapy to a number of X-ray-based strategies including 3D conformal radiation therapy (3DCRT), 5-field IMRT, and 2-arc volumetric modulated radiation therapy. Proton plans demonstrated lower doses to the kidneys, stomach, liver, and bowel.
Thompson et al[12] compared proton and IMRT plans in 13 patients with unresectable cancer of the pancreatic head. Both the double-scattered and pencil-beam plans decreased gastric, duodenal, and small bowel dose in the low-dose regions compared to IMRT; however, protons were associated with increased dose in the mid- to high-dose regions.
Clinical studies
Three groups (Massachusetts General Hospital in Boston, Hyogo Ion Beam Center in Japan, and University of Florida) have published preliminary clinical data on the treatment of pancreatic cancer patients with protons.
The group from Massachusetts General Hospital completed a phase 1 study of preoperative short-course chemoradiation confirming the safety of a preoperative dose of 25 Gy (RBE) in 5 fractions over 1 wk with concomitant oral capecitabine at 825 mg/m² twice a day, Monday through Friday, for 10 d followed by surgery. No dose-limiting toxicities were observed. Grade 3 toxicity was noted in 4 of 15 patients. Eleven patients underwent resection. Mean postsurgical length of stay was 6 d with no unexpected 30-d postoperative complications[13]. Of note, a corresponding study of hypofractionated preoperative X-ray-based radiotherapy using the same dose with X-rays was closed early due to toxicities that included intraoperative fibrosis and increased operating room time[14]. A phase II trial of proton therapy using the above dose regimen enrolled 50 patients, of whom 47 were eligible for analysis and 37 underwent pancreaticoduodenectomy. Of this cohort, 81% had positive nodes. Local regional failures occurred in 6 of 37 resected patients and distant metastases in 35 of 48. With a median follow-up of 38 mo, the median progression-free survival for the entire group was 10 mo and overall survival was 17 mo. The grade 3 toxicity rate was 4.1%.
Investigators at the Hyogo Ion Beam Center in Japan published the results of an aggressive phase I/II study of chemoradiation for patients with locally advanced pancreatic cancer. All patients received gemcitabine at 800 mg/m² weekly for 3 wk concurrent with proton therapy. Most of the patients received a dose of 67.5 Gy (RBE) in 25 fractions. The initial report suggested tolerability of this regimen[15]; however, a subsequent publication in the gastroenterology literature reported a high rate of upper gastrointestinal complications[16]. Post-treatment endoscopic examinations in 45 of 91 patients revealed radiation-induced ulcers in the stomach and duodenum. While the authors of the second publication suggested that proton therapy for inoperable pancreatic cancer was associated with a high rate of gastric and duodenal ulceration, a subsequent criticism of this study[17] pointed out that the severe toxicity exhibited was more likely due to the extremely aggressive radiotherapy dose offered with full-dose gemcitabine rather than any toxicity unique to proton therapy.
Researchers at the University of Florida published a preliminary report on the outcomes of 22 patients treated with proton therapy and concomitant capecitabine (1000 mg by mouth twice a day) for resected (n = 5), marginally resectable (n = 5), and unresectable/inoperable (n = 12) biopsy-proven pancreatic and ampullary adenocarcinoma[18]. Proton doses ranged from 50.4 Gy (RBE) to 59.4 Gy (RBE). No patient demonstrated any grade 3 toxicity during treatment or during follow-up. Three patients experienced grade 2 gastrointestinal toxicity; all 3 of these patients were treated early in the series with fields that included anterior and left lateral components. When field design was modified to deliver the majority of the dose through the posterior field with a lightly weighted right-lateral field, grade 2 gastrointestinal toxicity was eliminated. The median weight loss during treatment was 1.3 kg. Chemotherapy was well-tolerated with a median of 99% of the prescribed doses delivered.
A subsequent publication by the same group reported the outcomes of a phase II clinical trial for patients with unresectable pancreatic cancer[19]. A total of 11 patients were reported. All patients received 59.4 Gy (RBE) at 1.8 Gy (RBE) per fraction over 7 wk with concomitant oral capecitabine at 1000 mg by mouth twice a day on radiation treatment days only. The median follow-up for surviving patients was 23 mo. The 2-year overall survival rate was 31%, the median survival rate was 18.4 mo, and the 2-year freedom from local progression rate was 69% (Figure 4). No patient experienced grade 2 or higher gastrointestinal toxicity. Four patients had an adequate radiographic response to radiation therapy to justify surgical exploration.
Figure 4.
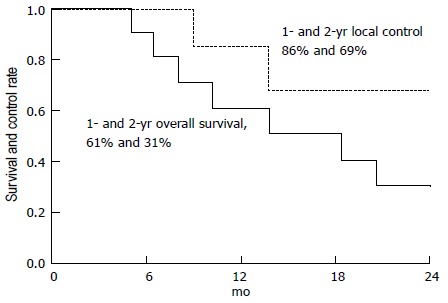
Overall survival and freedom from local progression at 2 years for 11 patients accrued to a phase II clinical trial for unresectable pancreatic cancer. Image borrowed from Ref. [19].
RATIONALE FOR PREOPERATIVE RADIOTHERAPY
Of the approximately 49000 cases of pancreatic cancer diagnosed annually in the United States, only 20% of these patients can be considered resectable or “curable”[20]. Unfortunately, the “cure” rate for these patients is only approximately 20%[21]. While many of these patients fail exclusively with distant metastatic disease, a substantial number experience local recurrence after surgery. Published data suggest that the local failure rate after surgery, even with negative margins, is in the range of 50%-80% if these patients do not receive radiotherapy[22,23]. Postoperative radiation therapy, however, has intrinsic limitations in this disease site. For example, postoperative convalescence generally necessitates a 10- to 12-wk window between surgery and initiation of postoperative radiation therapy. In reality, many patients are unable to receive postoperative radiation therapy within a clinically meaningful time frame. Additionally, the dose of postoperative radiation therapy is limited by the fact that a large volume of transposed small bowel is located in the radiotherapy field, making it unlikely that doses above 50 Gy can be safely delivered to these patients - a dose that is unlikely to control anything larger than the smallest microscopic adenocarcinoma deposits. In fact, published studies on patients receiving postoperative radiation therapy after surgery indicate local-regional failure rates ranging from 25%-36%[24,25]. Additionally, published data from respected high-volume centers suggest that patients undergoing extirpative surgery in the modern era for pancreas cancer have a high rate of margin and lymph node positivity. The series published by investigators at Johns Hopkins Medicine (Baltimore, MD) on 905 patients undergoing pancreaticoduodenectomy between 1995 and 2005 indicated a 41% margin positivity rate and a 79% node positivity rate[26]. The series from Memorial Sloan-Kettering Cancer Center (New York, NY) on 625 resections between 2000 and 2009 indicated a 16% margin positivity rate and a 70% node positivity rate[27]. Based on these data it is reasonable to believe that even “resectable” patients would be likely to benefit from preoperative radiotherapy - perhaps even with fields that could cover regional lymph nodes.
PLANNED PREOPERATIVE PROTON THERAPY FOR RESECTABLE OR MARGINALLY RESECTABLE DISEASE
It is possible that proton therapy in the postoperative setting will offer reduced toxicity compared to X-ray-based therapy and thereby improve local control and offer a positive impact on survival. While the results of proton therapy for patients with unresectable pancreatic cancer are encouraging, it is unlikely that this therapy, without meaningful improvements in systemic therapy, can be viewed as a potentially curative intervention.
It may be argued, however, that the best use of particle therapy would be in the preoperative setting for patients with resectable or marginally resectable disease. Preoperative radiotherapy is well-established in the treatment of other gastrointestinal disease sites (such as the esophagus and rectum) and improves local disease control and survival. It is reasonable to infer that a similar benefit could be achieved in the setting of pancreatic malignancy. As stated earlier, the main resistance to the use of preoperative radiotherapy involves concerns about radiotherapy toxicity and its potential to complicate what is already a complicated operation. If proton therapy can be delivered with negligible toxicity so that it does not compromise the performance of extirpative surgery, proton therapy would represent more than a “kinder/gentler” form of radiotherapy; proton therapy would have the potential to alter the management paradigm for this group of potentially curable patients.
CLINICAL DATA SUPPORTING THE FEASIBILITY OF PREOPERATIVE PARTICLE THERAPY
In addition to the data published by Massachusetts General Hospital regarding the feasibility of surgery after preoperative hypofractionated proton therapy, a report from the University of Florida analyzed the outcomes of 5 patients with initially unresectable disease who unexpectedly achieved enough of a tumor response to justify surgical resection after high-dose conventionally fractionated proton therapy[28]. All patients received 59.4 Gy (RBE) in 33 fractions with concomitant oral capecitabine. Three patients subsequently underwent a laparoscopic standard pancreaticoduodenectomy, 1 underwent open pylorus-sparing pancreaticoduodenectomy, and 1 underwent an open distal pancreatectomy with irreversible electroporation after biopsies of the pancreatic head were negative. Duration of surgery, blood loss, intensive care unit stay, total hospital stay, and readmissions were consistent with historical benchmarks. None of the operating surgeons described fibrosis, anastomotic leaks, or perception that the proton therapy complicated the operation. The fact that surgery could be performed without significant complications after high-dose radiotherapy for patients who are initially unresectable suggests that lower doses of preoperative proton therapy in the range of 50 Gy (RBE) or even higher should not complicate surgery for patients with resectable or borderline resectable disease.
CONCLUSION
Dosimetric studies and early clinical outcomes suggest that particle therapy improves the therapeutic index for pancreatic cancer patients receiving radiotherapy. By reducing or eliminating the gastrointestinal toxicity historically associated with X-ray-based radiotherapy, proton therapy should address the concerns of clinicians who are hesitant to employ radiotherapy in the postoperative setting (based on the ESPAC-1 data) and those who are reluctant to offer radiotherapy to patients with unresectable disease (based on the LAP-07 data).
Arguably, the most exciting potential role for particle therapy is in the neoadjuvant treatment of patients with resectable and marginally resectable disease. These patients are well recognized to suffer a high risk of local and regional failure after surgery - a risk that is only marginally reduced with postoperative X-ray-based radiotherapy. Based on the treatment of other gastrointestinal disease sites (such as the esophagus and rectum) it is reasonable to believe that preoperative radiotherapy would have a greater impact on securing local and regional control than chemotherapy or postoperative radiotherapy. Recognizing that the primary barrier to the adoption of preoperative radiotherapy in this setting is the concern of operating surgeons that the gastrointestinal toxicity of radiotherapy will complicate the procedure, it is possible that the favorable toxicity profile associated with proton therapy will make the oncologically rational intervention (preoperative radiation therapy) technically feasible. If this is the case, proton therapy would indeed result in a change in the management paradigm for patients with resectable and potentially curable pancreatic cancer.
Footnotes
Conflict-of-interest statement: The authors have no conflict of interest related to the manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 2, 2015
First decision: April 23, 2015
Article in press: July 22, 2015
P- Reviewer: Amedei A, Chen YC S- Editor: Ma YJ L- Editor: A E- Editor: Wu HL
References
- 1.Paganetti H. Proton Therapy Physics. Boca Raton, FL: CRC Press;; 2011. [Google Scholar]
- 2.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 3.Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, Bassi C, Falconi M, Pederzoli P, Dervenis C, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576–1585. doi: 10.1016/s0140-6736(01)06651-x. [DOI] [PubMed] [Google Scholar]
- 4.Abrams RA, Lillemoe KD, Piantadosi S. Continuing controversy over adjuvant therapy of pancreatic cancer. Lancet. 2001;358:1565–1566. doi: 10.1016/S0140-6736(01)06666-1. [DOI] [PubMed] [Google Scholar]
- 5.Hammel P, Huguet F, Van Laethem JL. Comparison of chemoradiotherapy (CRT) and chemotherapy (CT) in patients with a locally advanced pancreatic cancer (LAPC) controlled after 4 months of gemcitabine with or without erlotinib: Final results of the international phase III LAP 07 study [abstract] J Clin Oncol. 2013;31:LBA4003a. [Google Scholar]
- 6.Hsiung-Stripp DC, McDonough J, Masters HM, Levin WP, Hahn SM, Jones HA, Metz JM. Comparative treatment planning between proton and X-ray therapy in pancreatic cancer. Med Dosim. 2001;26:255–259. doi: 10.1016/s0958-3947(01)00072-3. [DOI] [PubMed] [Google Scholar]
- 7.Kozak KR, Kachnic LA, Adams J, Crowley EM, Alexander BM, Mamon HJ, Fernandez-Del Castillo C, Ryan DP, DeLaney TF, Hong TS. Dosimetric feasibility of hypofractionated proton radiotherapy for neoadjuvant pancreatic cancer treatment. Int J Radiat Oncol Biol Phys. 2007;68:1557–1566. doi: 10.1016/j.ijrobp.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 8.Bouchard M, Amos RA, Briere TM, Beddar S, Crane CH. Dose escalation with proton or photon radiation treatment for pancreatic cancer. Radiother Oncol. 2009;92:238–243. doi: 10.1016/j.radonc.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Nichols RC, Huh SN, Prado KL, Yi BY, Sharma NK, Ho MW, Hoppe BS, Mendenhall NP, Li Z, Regine WF. Protons offer reduced normal-tissue exposure for patients receiving postoperative radiotherapy for resected pancreatic head cancer. Int J Radiat Oncol Biol Phys. 2012;83:158–163. doi: 10.1016/j.ijrobp.2011.05.045. [DOI] [PubMed] [Google Scholar]
- 10.Lee RY, Nichols RC, Huh SN, Ho MW, Li Z, Zaiden R, Awad ZT, Ahmed B, Hoppe BS. Proton therapy may allow for comprehensive elective nodal coverage for patients receiving neoadjuvant radiotherapy for localized pancreatic head cancers. J Gastrointest Oncol. 2013;4:374–379. doi: 10.3978/j.issn.2078-6891.2013.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding X, Dionisi F, Tang S, Ingram M, Hung CY, Prionas E, Lichtenwalner P, Butterwick I, Zhai H, Yin L, et al. A comprehensive dosimetric study of pancreatic cancer treatment using three-dimensional conformal radiation therapy (3DCRT), intensity-modulated radiation therapy (IMRT), volumetric-modulated radiation therapy (VMAT), and passive-scattering and modulated-scanning proton therapy (PT) Med Dosim. 2014;39:139–145. doi: 10.1016/j.meddos.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Thompson RF, Mayekar SU, Zhai H, Both S, Apisarnthanarax S, Metz JM, Plastaras JP, Ben-Josef E. A dosimetric comparison of proton and photon therapy in unresectable cancers of the head of pancreas. Med Phys. 2014;41:081711. doi: 10.1118/1.4887797. [DOI] [PubMed] [Google Scholar]
- 13.Hong TS, Ryan DP, Blaszkowsky LS, Mamon HJ, Kwak EL, Mino-Kenudson M, Adams J, Yeap B, Winrich B, DeLaney TF, et al. Phase I study of preoperative short-course chemoradiation with proton beam therapy and capecitabine for resectable pancreatic ductal adenocarcinoma of the head. Int J Radiat Oncol Biol Phys. 2011;79:151–157. doi: 10.1016/j.ijrobp.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 14.Wo JY, Mamon HJ, Ferrone CR, Ryan DP, Blaszkowsky LS, Kwak EL, Tseng YD, Napolitano BN, Ancukiewicz M, Swanson RS, et al. Phase I study of neoadjuvant accelerated short course radiation therapy with photons and capecitabine for resectable pancreatic cancer. Radiother Oncol. 2014;110:160–164. doi: 10.1016/j.radonc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Terashima K, Demizu Y, Hashimoto N, Jin D, Mima M, Fujii O, Niwa Y, Takatori K, Kitajima N, Sirakawa S, et al. A phase I/II study of gemcitabine-concurrent proton radiotherapy for locally advanced pancreatic cancer without distant metastasis. Radiother Oncol. 2012;103:25–31. doi: 10.1016/j.radonc.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 16.Takatori K, Terashima K, Yoshida R, Horai A, Satake S, Ose T, Kitajima N, Kinoshita Y, Demizu Y, Fuwa N. Upper gastrointestinal complications associated with gemcitabine-concurrent proton radiotherapy for inoperable pancreatic cancer. J Gastroenterol. 2014;49:1074–1080. doi: 10.1007/s00535-013-0857-3. [DOI] [PubMed] [Google Scholar]
- 17.Nichols RC, Hoppe BS. RE: Takatori K, Terashima K, Yoshida R, Horai A, Satake S, Ose T, Kitajima N, Kinoshita Y, Demizu Y, Fuwa N. Upper gastrointestinal complications associated with gemcitabine-concurrent proton radiotherapy for inoperable pancreatic cancer. J Gastroenterol. 2013; (E-pub only) J Gastrointest Oncol. 2013;4:E33–E34. doi: 10.3978/j.issn.2078-6891.2013.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichols RC, George TJ, Zaiden RA, Awad ZT, Asbun HJ, Huh S, Ho MW, Mendenhall NP, Morris CG, Hoppe BS. Proton therapy with concomitant capecitabine for pancreatic and ampullary cancers is associated with a low incidence of gastrointestinal toxicity. Acta Oncol. 2013;52:498–505. doi: 10.3109/0284186X.2012.762997. [DOI] [PubMed] [Google Scholar]
- 19.Sachsman S, Nichols RS, Morris CG, Zaiden R, Johnson EA, Awad Z, Bose D, Ho MW, Huh SN, Li Z, et al. Proton Therapy and Concomitant Capecitabine for Non-Metastatic Unresectable Pancreatic Adenocarcinoma. Int J Particle Ther. 2014;1:692–701. [Google Scholar]
- 20.National Cancer Institute. Pancreatic Cancer Treatment (PDQ®): Incidence and Mortality. Available from: http://www.cancer.gov/cancertopics/pdq/treatment/pancreatic/HealthProfessional/page1#_7_toc.
- 21.National Cancer Institute. Pancreatic Cancer Treatment (PDQ®): Prognosis and Survival. Available from: http://www.cancer.gov/cancertopics/pdq/treatment/pancreatic/HealthProfessional/page1#_49_toc.
- 22.Tepper J, Nardi G, Sutt H. Carcinoma of the pancreas: review of MGH experience from 1963 to 1973. Analysis of surgical failure and implications for radiation therapy. Cancer. 1976;37:1519–1524. doi: 10.1002/1097-0142(197603)37:3<1519::aid-cncr2820370340>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Gudjonsson B. Cancer of the pancreas. 50 years of surgery. Cancer. 1987;60:2284–2303. doi: 10.1002/1097-0142(19871101)60:9<2284::aid-cncr2820600930>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 24.Regine WF, Winter KA, Abrams R, Safran H, Hoffman JP, Konski A, Benson AB, Macdonald JS, Rich TA, Willett CG. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol. 2011;18:1319–1326. doi: 10.1245/s10434-011-1630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hattangadi JA, Hong TS, Yeap BY, Mamon HJ. Results and patterns of failure in patients treated with adjuvant combined chemoradiation therapy for resected pancreatic adenocarcinoma. Cancer. 2009;115:3640–3650. doi: 10.1002/cncr.24410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, Wolfgang C, Hruban RH, Schulick RD, Yeo CJ, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141:610–618. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Winter JM, Brennan MF, Tang LH, D’Angelica MI, Dematteo RP, Fong Y, Klimstra DS, Jarnagin WR, Allen PJ. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol. 2012;19:169–175. doi: 10.1245/s10434-011-1900-3. [DOI] [PubMed] [Google Scholar]
- 28.Nichols RC, Morris CG, Bose D, Hughes SJ, Stauffer JA, Celinski SA, Martin RC, Johnson EA, Zaiden RA, Rutenberg MS. Feasibility of pancreatectomy after high dose proton therapy for unresectable pancreatic cancer. Int J Particle Ther. 2014;1:767–768. [Google Scholar]


