Abstract
Hereditary diffuse gastric cancer (HDGC) is an inherited autosomal dominant syndrome with a penetrance of up to 80% affecting diverse geographic populations. While it has been shown to be caused mainly by germline alterations in the E-cadherin gene (CDH1), problematically, the genetic diagnosis remains unknown in up to 60% of patients. Given the important knowledge gaps regarding the syndrome, asymptomatic carriers of CDH1 mutations are advised for a prophylactic total gastrectomy. Intensive annual endoscopic surveillance is the alternative for carriers who decline gastrectomy. As HDGCs have a prolonged indolent phase, this provides a window of opportunity for surveillance and treatment. Recent findings of other gene defects in CTNNA1 and MAP3K6, as well as further characterization of CDH1 mutations and their pathogenicity will change the way HDGC patients are counselled for screening, surveillance and treatment. This review will bring the reader up to date with these changes and discuss future directions for research; namely more accurate risk stratification and surveillance methods to improve clinical care of HDGC patients.
Keywords: Hereditary diffuse gastric cancer, CDH1, CTNNA1, MAP3K6, Gastrectomy
Core tip: While the incidence of hereditary diffuse gastric cancer remains low, it is an important clinical entity to recognize due to its high pathogenicity and penetrance. The International Gastric Cancer Linkage Consortium has outlined CDH1 testing criteria and developed clinical utility gene cards to help clinicians manage such patients. Significant progress has been made in recent years and in future, testing of other genes is likely for CDH1-negative families. The mainstay of treatment for asymptomatic carriers of CDH1 pathogenic mutations remains prophylactic total gastrectomy. Future research should focus on better risk stratification and surveillance methods.
INTRODUCTION
Gastric cancer (GC) is currently the fourth most common cancer and the second leading cause of cancer associated death worldwide[1]. Based on the Lauren classification, at least two main histological types of GC have been identified: intestinal and diffuse[2]. Both histological types have different clinical features and molecular mechanisms[3-8]. Hereditary GCs account for only 1%-3% of GC cases[9], but are important for clinicians to identify as potentially curative interventions are available. One well-characterized syndrome is Hereditary diffuse gastric cancer (HDGC), which was attributed to germline mutations of the E-cadherin gene (CDH1) in 1998[10]. The International Gastric Cancer Linkage Consortium (IGCLC) has since established the latest set of clinical criteria in 2010 (listed in Table 1) to guide genetic screening[11].
Table 1.
Clinical criteria for CDH1 genetic testing (adapted from Fitzgerald et al[11])
| ≥ 2 diffuse GC cases in 1st or 2nd degree relatives with one < 50 yr of age |
| ≥ 3 diffuse GC cases in 1st or 2nd degree relatives independent of age |
| Diffuse GC < 40 yr of age, without a family history |
| Personal or family history of diffuse GC and lobular breast cancer with one < 50 yr of age |
GC: Gastric cancer.
Only about 40% of probands meeting the 2010 criteria carry CDH1 germline alterations (often point or small frameshift mutations)[9,12]. Of the remaining 60%, a small percentage is due to CDH1 deletions not detected by conventional DNA sequencing. More intriguingly, mutations in other genes like CTNNA1[13], MAP3K6[14], INSR, FBXO24 and DOT1L[15] are starting to be identified. However, pathogenicity and penetrance of many newer mutations remain unanswered, creating management dilemmas. These non-CDH1 mutations published thus far have been summarized in Table 2. Most studies are small and will require validation in consortium-led efforts for us to better understand the longitudinal impact.
Table 2.
Summary of non-CDH1 germline mutations in hereditary diffuse gastric cancer
| Gene | Mutation | Location | Mutation type | Ethnicity | Ref. | Study type | Frequency | Remarks |
| CTNNA1 | c.76delGA | Chr 5: 138117693 | Nonsense | No data | [13] | Family study | 1/1 family | Results in a framshift after Arg27 (p.Arg27Thr.fs*17) |
| MAP3K6 | c.598G>T | Chr 1: 27690792 | Missense | Canada | [14] | Family study and case series | 1/1 family 1/115 cases | Likely pathogenic |
| MAP3K6 | c.620T>G | Chr 1: 27690770 | Missense | No data | [14] | No data | ||
| MAP3K6 | c.2837C>T | Chr 1: 27684750 | Silent | No data | [14] | No data | Single nucleotide variant also in Canadian family, likely pathogenic | |
| MAP3K6 | c.2872C>A | Chr 1: 27684715 | Missense | No data | [14] | No data | ||
| MAP3K6 | c.2544delC | Chr 1: 27685238 - 27685239 | Nonsense | Portugese | [14] | 1/115 cases | ||
| INSR | c.3937 G>A | Chr 19: 7117279 | Missense | Finland | [15] | Family study | 1/1 family | |
| FBXO24 | c.242G>C | Chr 7: 100187900 | Missense | Finland | [15] | 1/1 family | ||
| DOT1L | c.3437C>T | Chr 19: 2223326 | Missense | Finland | [15] | 1/1 family |
CLINICAL HISTORY
Presentation
Similar to other gastric carcinomas, patients with HDGC are often asymptomatic in the early stages and tend to present late with symptoms such as weight loss, abdominal pain, nausea, anorexia, dysphagia, melaena and early satiety. The median age at diagnosis is 38 years, with the range varying greatly from 14-82 years[10,16].
Majority of HDGCs are inherited in an autosomal dominant pattern. It exhibits high penetrance and invasive disease often manifests before age 40. Therefore, one should have a high clinical suspicion when a family history reveals two or more cases of gastric cancer in first or second degree relatives, especially with one case diagnosed before age 50. The lifetime cumulative risk for diffuse GC reaches > 80% in men and women by age 80 years[11].
Other features seen in HDGC familes
There is an association of HDGC with lobular breast cancer (LBC) and it can be the presentating pathology[17]. Data based on 11 HDGC families, estimated the cumulative risk for LBC for female CDH1 mutation carriers to be 39% (95%CI: 12%-84%) by 80 years of age[18]. Thus, personal or family history of multiple LBCs at a young age should also prompt CDH1 screening even if there is no HDGC. There have also been case reports of colorectal, prostate and ovarian carcinomas in HDGC families although these are rare and of uncertain significance[19-22]. Interestingly, cleft-lip, with or without cleft-palate malformations have been reported in several HDGC families, some of whom have specific CDH1 splice site mutations[23,24].
Other relevant hereditary cancer syndromes
It should be remembered that GC can develop in the setting of other hereditary cancer syndromes aside from HDGC. One example would be Lynch syndrome which more often presents with intestinal-type gastric cancers and also has a high lifetime risk of colorectal and endometrial cancer. Other examples include Familial adenomatous polyposis, Li-Fraumeni syndrome, Peutz-Jegher’s syndrome (PJS) and Juvenile Polyposis Syndrome (JPS) (Table 3). The lifetime risk of GC in these syndromes varies considerably but is generally lower than that in HDGC.
Table 3.
Comparison of hereditary cancer syndromes
| Condition | Genetic pathology | Lifetime risk of gastric cancer | Histological subtype | Other clinical features |
| Hereditary diffuse gastric cancer | CDH1 germline and other gene mutations | 80% | Diffuse | Association with lobular breast cancer and cleft-lip malformations |
| Lynch syndrome | Mutations in mismatch repair genes | 4.8% in MLH1 carrier 9% in MLH2 carrier[58] | Mainly intestinal-type | Lifetime risk of colon cancer 31%-38%, endometrial cancer 34% and ovarian cancer 20%[59] |
| Familial adenomatous polyposis | APC germline mutations | Population risk[60] | No data | Malignant extraintestinal tumours rare < 3% (thyroid, pancreas, medulloblastoma)[61] |
| Li-Fraumeni syndrome | TP53 mutations | 14.9%[62] | No predominant subtype | Associated with wide range of early-onset cancers. Includes haematological and solid organ cancers: sarcomas, breast, brain, adrenal and lung cancers |
| Peutz-Jegher’s syndrome | STK11 mutations | 29%[63] | No data | Characteristic mucocutaneous pigmentation commonly around mouth and nose High cumulative lifetime risk of any cancer (85%), most commonly colorectal (50%)[58] |
| Juvenile polyposis syndrome | SMAD4 or BMPR1A mutations | 121%[64] | No data | Also at increased |
Frequency based on cross-sectional sample rather than lifetime risk from cohort study.
PATHOPHYSIOLOGY
Genetic susceptibility
E-cadherin is a cell adhesion protein that is required for development, cell differentiation and maintenance of epithelial architecture[6]. Since the E-cadherin gene CDH1 was identified as a genetic basis for HDGC in 1998, more than 120 CDH1 germline mutations have been published[25]. The most common germline alterations are small frameshifts, splice-site and nonsense mutations[9]. Of note, only two de novo mutations have been reported to date[26,27].
However, newer HDGC-susceptibility genes have been identified (Table 2). In 2012, an alpha-E-catenin (CTNNA1) germline truncating mutation was been found in a large Dutch HDGC pedigree[14] although the evidence presented was not definitive given a number of carriers remained cancer-free and other studies have failed to replicate findings[28]. At time of writing, MAP3K6[15], INSR, FBXO24 and DOT1L[16] have also identified as candidate genes although they remain reports from single families. The insulin receptor (INSR) gene mutation is of special interest given insulin signaling has been reported to affect tumour cell invasion capability by modulating E-cadherin glycosylation[29] and is known to play a role in a variety of cancers[30]. There has also been a reported possibility of an association of early onset gastric cancer with IL12RB1 mutation carriers[31] although this is mainly of the intestinal-type.
Somatic events
Guilford et al[10] has suggested HDGC develops from multiple foci of signet ring cell carcinomas (SRCC) in mutation carriers before 30 years of age. These SRCC, which have been termed “early HDGC”[32], develop after loss of the second CDH1 allele via a 2nd-hit mechanism[33-36]. The same patient may present with distinct 2nd hit mechanisms in different lesions. Promoter methylation is the most common 2nd-hit mechanism in primary HDGC tumours although loss of heterozygosity was found to be the most prevalent in lymph node metastases[37].
Interestingly, other studies are starting to look at oncogenic pathways involved in metastatic progression in HDGC and have found one such candidate driver in a transforming growth factor beta receptor 2 loss-of-function mutation[38].
MANAGEMENT
Diagnosis
The identification of germline mutations in families fulfilling the criteria for HDGC relies on information from pathology reports from at least one proband. A report by Hebbard et al[39] on 23 patients who underwent prophylactic total gastrectomy showed 21 of them had evidence of diffuse/signet-ring carcinoma on final standardized pathological evaluation which was not picked up by preoperative endoscopic screening. Thus, for adequate pathological sampling, IGCLC recommends targeting any endoscopically visible lesions as well as random sampling of six biopsies for each of the following anatomical zones: antrum, transitional zone, body, fundus, cardia. This would give a minimum of 30 biopsies[11].
Treatment
Probands often present with advanced stage GC and treatment consists of palliative chemotherapy (often taxanes, platinum agents or irinotecan), targeted radiotherapy and bypass surgery. While research looks into E-cadherin pathway regulators to increase chemosensitivity to epidermal growth factor receptor inhibitors and cytotoxics[40-42], there are currently no specific targeted therapies for diffuse GCs although there is an ongoing Phase I clinical trial studying everolimus in combination with chemotherapy[43].
As personalized therapy becomes increasingly prominent in cancer care, management of patients with HDGC should involve a multidisciplinary team of geneticists, surgeons and pathologists to address the following aspects of care: (1) genetic counselling and screening for both CDH1 positive and negative patients. This should include a three-generation family pedigree, analysis of CDH1/other candidate gene mutation and translation into lifetime risks of diffuse GC and LBC[11]; and (2) discussion of prophylactic gastrectomy vs surveillance.
Guidelines for the clinical management of CDH1 mutation carriers have been reviewed by the IGCLC (2010) and are outlined in clinical utility cards for HDGC[44]. Figure 1 summarises the management algorithm.
Figure 1.
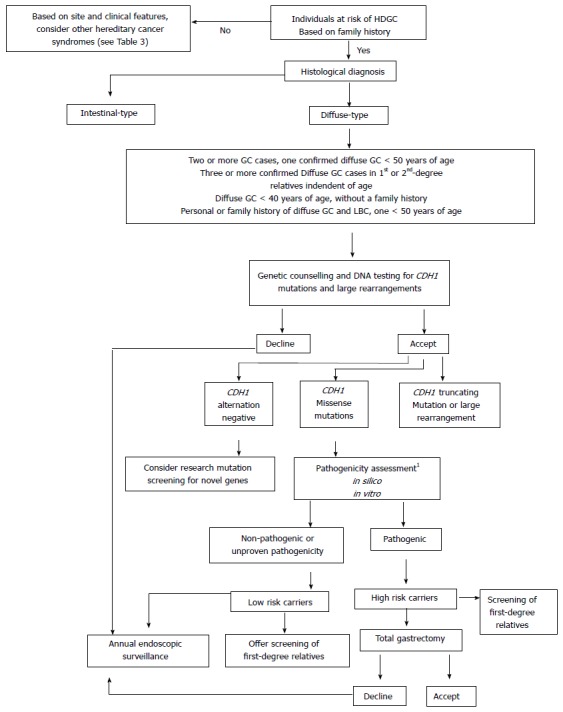
Clinical management of individuals suspected to have hereditary diffuse gastric cancer. Adapted from Pinheiro et al[9]. 1Analyses recommended include: mutation frequency in healthy control population, co-segregation of mutation within pedigree, recurrence of mutation in independent families, in-silico predictions and in vitro functional assays[45,65-68].
CDH1 missense mutation carriers
It is suggested that these individuals go on to have their mutations assessed for pathogenicity via functional in-vitro testing (aggregation and invasion assays) and in-silico models that have been developed[45]. These techniques have found a significant number of pathogenic missense variants and should be carried out by molecular diagnostic laboratories with appropriate expertise.
CDH1-negative individuals
Mutation screening in the research setting of HDGC families without CDH1 mutations can be considered. Approaches needed would include high density single-nucleotide polymorphism (SNP) genotyping, non-parametric and parametric linkage analysis, whole exome sequencing as well as aforementioned pathogenicity assessments[14,15].
Surveillance
There is currently no reliable screening test for early diagnosis of diffuse GCs in mutation carriers. While IGCLC guidelines suggest annual endoscopic surveillance in specific settings, it should be known that direct visualization with endoscopy tends to detect lesions late in the disease process[46] and multiple random endoscopic samples often returns false negatives[39]. Other screening methods like chromoendoscopy and positron emission tomography have not been deemed to be consistently effective[47,48].
Prophylactic gastrectomy
Due to the lack of reliably sensitive surveillance methods, prophylactic total gastrectomy should be considered in the early 20s and is usually advised before age 40 for those carrying CDH1 mutations. Some authors suggest consideration of gastrectomies in CDH1 mutation carriers at an age 5 years younger than the youngest family member who developed gastric cancer[49].
There are currently no recommendations with regards to prophylactic gastrectomy in CDH1-negative individuals. Prospective studies evaluating prophylactic gastrectomy in HDGC have offered the surgery only to CDH1 positive individuals[50], while a systematic retrospective review of 28 articles on prophylactic gastrectomy found a small sample of 11 CDH1-negative individuals who had undergone the gastrectomy before CDH1 testing all had negative histopathology results for cancer[51].
Patients may refuse or decide to postpone the procedure due to young age, fertility concerns or fear of surgical complications. Fortunately, there have been reports of successful pregnancies post-prophylactic gastrectomy[52] and the youngest known carrier to date to undergo gastrectomy was 16 years of age[53].
ONGOING CHALLENGES
Risk stratification for CDH1-negative individuals
A significant proportion of HDGC families are likely to be CDH1 negative. Further study to identify other genetic causes is needed before their risk and therefore management measures such as prophylactic gastrectomy can be assessed. As more cases of HDGC are identified, two lines of study are especially valuable. First, pathogenicity and penetrance of new germline mutations need to be documented to improve genetic counselling and decision-making. This is especially so for missense mutations. Second, prophylactic gastrectomy specimens provide material to identify molecular mechanisms that may predict progression from SRCC lesions to HDGC. In particular, elucidating epigenetic mechanisms, such as analysis of hypermethylation of cell cycle or DNA repair genes[54-57], may provide useful insights into possible environmental or pharmaceutical chemoprevention strategies.
Surveillance methods
Better surveillance methods could reduce morbidity by picking up target lesions earlier such that they are amenable to endoscopic therapies. While detection of diffuse GCs has proven difficult and surveillance frequency remains challenging, one paradigm to guide further research would be to assume that microfoci of SRCC will be present in all adult mutation carriers. Thus, rather than trying to detect all microfoci, the aim of surveillance should be geared towards detecting “high risk” SRCC. While this will require further elucidation of mechanisms of carcinogensis, it is plausible to imagine current surveillance methods, combined with genetic data, as a reliable alternative to prophylactic total gastrectomy.
CONCLUSION
While the incidence of HDGC remains low, it is an important clinical entity to recognize because of its high pathogenicity and penetrance. The IGCLC 2010 has outlined CDH1 testing criteria and developed clinical utility gene cards to help clinicians manage such patients. Significant progress has been made in recent years and in future, testing of other genes is likely for CDH1-negative families. The mainstay of treatment for asymptomatic carriers of CDH1 pathogenic mutations remains prophylactic total gastrectomy. However, it is hoped future research will lead to better risk stratification and surveillance methods to improve clinical care for patients in terms of screening, prevention and treatment.
Footnotes
Supported by National Medical Research Council Transition Award (to Joanne Ngeow).
Conflict-of-interest statement: None.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 12, 2015
First decision: May 18, 2015
Article in press: July 10, 2015
P- Reviewer: Fan YM, Heitzer E, Petridis C, Wang Y S- Editor: Ma YJ L- Editor: A E- Editor: Wu HL
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 3.Matysiak-Budnik T, Mégraud F. Helicobacter pylori infection and gastric cancer. Eur J Cancer. 2006;42:708–716. doi: 10.1016/j.ejca.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 5.Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, Realpe JL, Malcom GT, Li D, Johnson WD, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-helicobacter pylori therapy. J Natl Cancer Inst. 2000;92:1881–1888. doi: 10.1093/jnci/92.23.1881. [DOI] [PubMed] [Google Scholar]
- 6.Jang BG, Kim WH. Molecular pathology of gastric carcinoma. Pathobiology. 2011;78:302–310. doi: 10.1159/000321703. [DOI] [PubMed] [Google Scholar]
- 7.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 8.Henson DE, Dittus C, Younes M, Nguyen H, Albores-Saavedra J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973-2000: increase in the signet ring cell type. Arch Pathol Lab Med. 2004;128:765–770. doi: 10.5858/2004-128-765-DTITIA. [DOI] [PubMed] [Google Scholar]
- 9.Pinheiro H, Oliveira C, Seruca R, Carneiro F. Hereditary diffuse gastric cancer - pathophysiology and clinical management. Best Pract Res Clin Gastroenterol. 2014;28:1055–1068. doi: 10.1016/j.bpg.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A, Reeve AE. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402–405. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald RC, Hardwick R, Huntsman D, Carneiro F, Guilford P, Blair V, Chung DC, Norton J, Ragunath K, Van Krieken JH, et al. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet. 2010;47:436–444. doi: 10.1136/jmg.2009.074237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliveira C, Senz J, Kaurah P, Pinheiro H, Sanges R, Haegert A, Corso G, Schouten J, Fitzgerald R, Vogelsang H, et al. Germline CDH1 deletions in hereditary diffuse gastric cancer families. Hum Mol Genet. 2009;18:1545–1555. doi: 10.1093/hmg/ddp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majewski IJ, Kluijt I, Cats A, Scerri TS, de Jong D, Kluin RJ, Hansford S, Hogervorst FB, Bosma AJ, Hofland I, et al. An α-E-catenin (CTNNA1) mutation in hereditary diffuse gastric cancer. J Pathol. 2013;229:621–629. doi: 10.1002/path.4152. [DOI] [PubMed] [Google Scholar]
- 14.Gaston D, Hansford S, Oliveira C, Nightingale M, Pinheiro H, Macgillivray C, Kaurah P, Rideout AL, Steele P, Soares G, et al. Germline mutations in MAP3K6 are associated with familial gastric cancer. PLoS Genet. 2014;10:e1004669. doi: 10.1371/journal.pgen.1004669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donner I, Kiviluoto T, Ristimäki A, Aaltonen LA, Vahteristo P. Exome sequencing reveals three novel candidate predisposition genes for diffuse gastric cancer. Fam Cancer. 2015;14:241–246. doi: 10.1007/s10689-015-9778-z. [DOI] [PubMed] [Google Scholar]
- 16.Wirtzfeld D, Goldberg RM, Savarese DMF. Hereditary diffuse gastric cancer. UpToDate, Post TW (Ed), UpToDate, Waltham, MA. Cited 11-11-14. Available from: http://www.uptodate.com/contents/hereditary-diffuse-gastric-cancer.
- 17.Masciari S, Larsson N, Senz J, Boyd N, Kaurah P, Kandel MJ, Harris LN, Pinheiro HC, Troussard A, Miron P, et al. Germline E-cadherin mutations in familial lobular breast cancer. J Med Genet. 2007;44:726–731. doi: 10.1136/jmg.2007.051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pharoah PD, Guilford P, Caldas C. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology. 2001;121:1348–1353. doi: 10.1053/gast.2001.29611. [DOI] [PubMed] [Google Scholar]
- 19.Brooks-Wilson AR, Kaurah P, Suriano G, Leach S, Senz J, Grehan N, Butterfield YS, Jeyes J, Schinas J, Bacani J, et al. Germline E-cadherin mutations in hereditary diffuse gastric cancer: assessment of 42 new families and review of genetic screening criteria. J Med Genet. 2004;41:508–517. doi: 10.1136/jmg.2004.018275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caldas C, Carneiro F, Lynch HT, Yokota J, Wiesner GL, Powell SM, Lewis FR, Huntsman DG, Pharoah PD, Jankowski JA, et al. Familial gastric cancer: overview and guidelines for management. J Med Genet. 1999;36:873–880. [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveira C, Bordin MC, Grehan N, Huntsman D, Suriano G, Machado JC, Kiviluoto T, Aaltonen L, Jackson CE, Seruca R, et al. Screening E-cadherin in gastric cancer families reveals germline mutations only in hereditary diffuse gastric cancer kindred. Hum Mutat. 2002;19:510–517. doi: 10.1002/humu.10068. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira C, Seruca R, Caldas C. Genetic screening for hereditary diffuse gastric cancer. Expert Rev Mol Diagn. 2003;3:201–215. doi: 10.1586/14737159.3.2.201. [DOI] [PubMed] [Google Scholar]
- 23.Frebourg T, Oliveira C, Hochain P, Karam R, Manouvrier S, Graziadio C, Vekemans M, Hartmann A, Baert-Desurmont S, Alexandre C, et al. Cleft lip/palate and CDH1/E-cadherin mutations in families with hereditary diffuse gastric cancer. J Med Genet. 2006;43:138–142. doi: 10.1136/jmg.2005.031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kluijt I, Siemerink EJ, Ausems MG, van Os TA, de Jong D, Simões-Correia J, van Krieken JH, Ligtenberg MJ, Figueiredo J, van Riel E, et al. CDH1-related hereditary diffuse gastric cancer syndrome: clinical variations and implications for counseling. Int J Cancer. 2012;131:367–376. doi: 10.1002/ijc.26398. [DOI] [PubMed] [Google Scholar]
- 25.Corso G, Marrelli D, Pascale V, Vindigni C, Roviello F. Frequency of CDH1 germline mutations in gastric carcinoma coming from high- and low-risk areas: metanalysis and systematic review of the literature. BMC Cancer. 2012;12:8. doi: 10.1186/1471-2407-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah MA, Salo-Mullen E, Stadler Z, Ruggeri JM, Mirander M, Pristyazhnyuk Y, Zhang L. De novo CDH1 mutation in a family presenting with early-onset diffuse gastric cancer. Clin Genet. 2012;82:283–287. doi: 10.1111/j.1399-0004.2011.01744.x. [DOI] [PubMed] [Google Scholar]
- 27.Sugimoto S, Yamada H, Takahashi M, Morohoshi Y, Yamaguchi N, Tsunoda Y, Hayashi H, Sugimura H, Komatsu H. Early-onset diffuse gastric cancer associated with a de novo large genomic deletion of CDH1 gene. Gastric Cancer. 2014;17:745–749. doi: 10.1007/s10120-013-0278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuetz JM, Leach S, Kaurah P, Jeyes J, Butterfield Y, Huntsman D, Brooks-Wilson AR. Catenin family genes are not commonly mutated in hereditary diffuse gastric cancer. Cancer Epidemiol Biomarkers Prev. 2012;21:2272–2274. doi: 10.1158/1055-9965.EPI-12-1110. [DOI] [PubMed] [Google Scholar]
- 29.de-Freitas-Junior JC, Carvalho S, Dias AM, Oliveira P, Cabral J, Seruca R, Oliveira C, Morgado-Díaz JA, Reis CA, Pinho SS. Insulin/IGF-I signaling pathways enhances tumor cell invasion through bisecting GlcNAc N-glycans modulation. an interplay with E-cadherin. PLoS One. 2013;8:e81579. doi: 10.1371/journal.pone.0081579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 31.Vogelaar IP, van der Post RS, van de Vosse E, van Krieken JH, Hoogerbrugge N, Ligtenberg MJ, Gómez García E. Gastric cancer in three relatives of a patient with a biallelic IL12RB1 mutation. Fam Cancer. 2015;14:89–94. doi: 10.1007/s10689-014-9764-x. [DOI] [PubMed] [Google Scholar]
- 32.Huntsman DG, Carneiro F, Lewis FR, MacLeod PM, Hayashi A, Monaghan KG, Maung R, Seruca R, Jackson CE, Caldas C. Early gastric cancer in young, asymptomatic carriers of germ-line E-cadherin mutations. N Engl J Med. 2001;344:1904–1909. doi: 10.1056/NEJM200106213442504. [DOI] [PubMed] [Google Scholar]
- 33.Grady WM, Willis J, Guilford PJ, Dunbier AK, Toro TT, Lynch H, Wiesner G, Ferguson K, Eng C, Park JG, et al. Methylation of the CDH1 promoter as the second genetic hit in hereditary diffuse gastric cancer. Nat Genet. 2000;26:16–17. doi: 10.1038/79120. [DOI] [PubMed] [Google Scholar]
- 34.Oliveira C, de Bruin J, Nabais S, Ligtenberg M, Moutinho C, Nagengast FM, Seruca R, van Krieken H, Carneiro F. Intragenic deletion of CDH1 as the inactivating mechanism of the wild-type allele in an HDGC tumour. Oncogene. 2004;23:2236–2240. doi: 10.1038/sj.onc.1207335. [DOI] [PubMed] [Google Scholar]
- 35.Becker KF, Höfler H. Frequent somatic allelic inactivation of the E-cadherin gene in gastric carcinomas. J Natl Cancer Inst. 1995;87:1082–1084. doi: 10.1093/jnci/87.14.1082. [DOI] [PubMed] [Google Scholar]
- 36.Barber M, Murrell A, Ito Y, Maia AT, Hyland S, Oliveira C, Save V, Carneiro F, Paterson AL, Grehan N, et al. Mechanisms and sequelae of E-cadherin silencing in hereditary diffuse gastric cancer. J Pathol. 2008;216:295–306. doi: 10.1002/path.2426. [DOI] [PubMed] [Google Scholar]
- 37.Oliveira C, Sousa S, Pinheiro H, Karam R, Bordeira-Carriço R, Senz J, Kaurah P, Carvalho J, Pereira R, Gusmão L, et al. Quantification of epigenetic and genetic 2nd hits in CDH1 during hereditary diffuse gastric cancer syndrome progression. Gastroenterology. 2009;136:2137–2148. doi: 10.1053/j.gastro.2009.02.065. [DOI] [PubMed] [Google Scholar]
- 38.Nadauld LD, Garcia S, Natsoulis G, Bell JM, Miotke L, Hopmans ES, Xu H, Pai RK, Palm C, Regan JF, et al. Metastatic tumor evolution and organoid modeling implicate TGFBR2 as a cancer driver in diffuse gastric cancer. Genome Biol. 2014;15:428. doi: 10.1186/s13059-014-0428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hebbard PC, Macmillan A, Huntsman D, Kaurah P, Carneiro F, Wen X, Kwan A, Boone D, Bursey F, Green J, et al. Prophylactic total gastrectomy (PTG) for hereditary diffuse gastric cancer (HDGC): the Newfoundland experience with 23 patients. Ann Surg Oncol. 2009;16:1890–1895. doi: 10.1245/s10434-009-0471-z. [DOI] [PubMed] [Google Scholar]
- 40.Nam JS, Ino Y, Kanai Y, Sakamoto M, Hirohashi S. 5-aza-2’-deoxycytidine restores the E-cadherin system in E-cadherin-silenced cancer cells and reduces cancer metastasis. Clin Exp Metastasis. 2004;21:49–56. doi: 10.1023/b:clin.0000017180.19881.c1. [DOI] [PubMed] [Google Scholar]
- 41.Peng G, Wargovich MJ, Dixon DA. Anti-proliferative effects of green tea polyphenol EGCG on Ha-Ras-induced transformation of intestinal epithelial cells. Cancer Lett. 2006;238:260–270. doi: 10.1016/j.canlet.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 42.Chu Q, Ling MT, Feng H, Cheung HW, Tsao SW, Wang X, Wong YC. A novel anticancer effect of garlic derivatives: inhibition of cancer cell invasion through restoration of E-cadherin expression. Carcinogenesis. 2006;27:2180–2189. doi: 10.1093/carcin/bgl054. [DOI] [PubMed] [Google Scholar]
- 43.Everolimus and Combination Chemotherapy in Treating Patients With Metastatic Stomach or Esophageal Cancer In Clinical Trials. gov; 2014; Cited 04-03-15. Available from: http://clinicaltrials.gov/ct2/show/NCT01231399?term=diffuse gastric cancer&rank=22.
- 44.Oliveira C, Seruca R, Hoogerbrugge N, Ligtenberg M, Carneiro F. Clinical utility gene card for: Hereditary diffuse gastric cancer (HDGC) Eur J Hum Genet. 2013;21 doi: 10.1038/ejhg.2012.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suriano G, Seixas S, Rocha J, Seruca R. A model to infer the pathogenic significance of CDH1 germline missense variants. J Mol Med (Berl) 2006;84:1023–1031. doi: 10.1007/s00109-006-0091-z. [DOI] [PubMed] [Google Scholar]
- 46.Carneiro F, Huntsman DG, Smyrk TC, Owen DA, Seruca R, Pharoah P, Caldas C, Sobrinho-Simões M. Model of the early development of diffuse gastric cancer in E-cadherin mutation carriers and its implications for patient screening. J Pathol. 2004;203:681–687. doi: 10.1002/path.1564. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y, Kingham K, Ford JM, Rosing J, Van Dam J, Jeffrey RB, Longacre TA, Chun N, Kurian A, Norton JA. A prospective study of total gastrectomy for CDH1-positive hereditary diffuse gastric cancer. Ann Surg Oncol. 2011;18:2594–2598. doi: 10.1245/s10434-011-1648-9. [DOI] [PubMed] [Google Scholar]
- 48.De Potter T, Flamen P, Van Cutsem E, Penninckx F, Filez L, Bormans G, Maes A, Mortelmans L. Whole-body PET with FDG for the diagnosis of recurrent gastric cancer. Eur J Nucl Med Mol Imaging. 2002;29:525–529. doi: 10.1007/s00259-001-0743-8. [DOI] [PubMed] [Google Scholar]
- 49.Cisco RM, Ford JM, Norton JA. Hereditary diffuse gastric cancer: implications of genetic testing for screening and prophylactic surgery. Cancer. 2008;113:1850–1856. doi: 10.1002/cncr.23650. [DOI] [PubMed] [Google Scholar]
- 50.Worster E, Liu X, Richardson S, Hardwick RH, Dwerryhouse S, Caldas C, Fitzgerald RC. The impact of prophylactic total gastrectomy on health-related quality of life: a prospective cohort study. Ann Surg. 2014;260:87–93. doi: 10.1097/SLA.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 51.Seevaratnam R, Coburn N, Cardoso R, Dixon M, Bocicariu A, Helyer L. A systematic review of the indications for genetic testing and prophylactic gastrectomy among patients with hereditary diffuse gastric cancer. Gastric Cancer. 2012;15 Suppl 1:S153–S163. doi: 10.1007/s10120-011-0116-3. [DOI] [PubMed] [Google Scholar]
- 52.Kaurah P, Fitzgerald R, Dwerryhouse S, Huntsman DG. Pregnancy after prophylactic total gastrectomy. Fam Cancer. 2010;9:331–334. doi: 10.1007/s10689-009-9316-y. [DOI] [PubMed] [Google Scholar]
- 53.Wickremeratne T, Lee CH, Kirk J, Charlton A, Thomas G, Gaskin KJ. Prophylactic gastrectomy in a 16-year-old. Eur J Gastroenterol Hepatol. 2014;26:353–356. doi: 10.1097/MEG.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 54.Issa JP. CpG island methylator phenotype in cancer. Nat Rev Cancer. 2004;4:988–993. doi: 10.1038/nrc1507. [DOI] [PubMed] [Google Scholar]
- 55.Kim TY, Jong HS, Jung Y, Kim TY, Kang GH, Bang YJ. DNA hypermethylation in gastric cancer. Aliment Pharmacol Ther. 2004;20 Suppl 1:131–142. doi: 10.1111/j.1365-2036.2004.01984.x. [DOI] [PubMed] [Google Scholar]
- 56.Jee CD, Kim MA, Jung EJ, Kim J, Kim WH. Identification of genes epigenetically silenced by CpG methylation in human gastric carcinoma. Eur J Cancer. 2009;45:1282–1293. doi: 10.1016/j.ejca.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 57.Yamashita S, Tsujino Y, Moriguchi K, Tatematsu M, Ushijima T. Chemical genomic screening for methylation-silenced genes in gastric cancer cell lines using 5-aza-2’-deoxycytidine treatment and oligonucleotide microarray. Cancer Sci. 2006;97:64–71. doi: 10.1111/j.1349-7006.2006.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Capelle LG, Van Grieken NC, Lingsma HF, Steyerberg EW, Klokman WJ, Bruno MJ, Vasen HF, Kuipers EJ. Risk and epidemiological time trends of gastric cancer in Lynch syndrome carriers in the Netherlands. Gastroenterology. 2010;138:487–492. doi: 10.1053/j.gastro.2009.10.051. [DOI] [PubMed] [Google Scholar]
- 59.Patel SG, Ahnen DJ. Familial colon cancer syndromes: an update of a rapidly evolving field. Curr Gastroenterol Rep. 2012;14:428–438. doi: 10.1007/s11894-012-0280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arnason T, Liang WY, Alfaro E, Kelly P, Chung DC, Odze RD, Lauwers GY. Morphology and natural history of familial adenomatous polyposis-associated dysplastic fundic gland polyps. Histopathology. 2014;65:353–362. doi: 10.1111/his.12393. [DOI] [PubMed] [Google Scholar]
- 61.Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044–2058. doi: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Masciari S, Dewanwala A, Stoffel EM, Lauwers GY, Zheng H, Achatz MI, Riegert-Johnson D, Foretova L, Silva EM, Digianni L, et al. Gastric cancer in individuals with Li-Fraumeni syndrome. Genet Med. 2011;13:651–657. doi: 10.1097/GIM.0b013e31821628b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Lier MG, Wagner A, Mathus-Vliegen EM, Kuipers EJ, Steyerberg EW, van Leerdam ME. High cancer risk in Peutz-Jeghers syndrome: a systematic review and surveillance recommendations. Am J Gastroenterol. 2010;105:1258–1264; author reply 1265. doi: 10.1038/ajg.2009.725. [DOI] [PubMed] [Google Scholar]
- 64.Howe JR, Sayed MG, Ahmed AF, Ringold J, Larsen-Haidle J, Merg A, Mitros FA, Vaccaro CA, Petersen GM, Giardiello FM, et al. The prevalence of MADH4 and BMPR1A mutations in juvenile polyposis and absence of BMPR2, BMPR1B, and ACVR1 mutations. J Med Genet. 2004;41:484–491. doi: 10.1136/jmg.2004.018598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suriano G, Oliveira C, Ferreira P, Machado JC, Bordin MC, De Wever O, Bruyneel EA, Moguilevsky N, Grehan N, Porter TR, et al. Identification of CDH1 germline missense mutations associated with functional inactivation of the E-cadherin protein in young gastric cancer probands. Hum Mol Genet. 2003;12:575–582. doi: 10.1093/hmg/ddg048. [DOI] [PubMed] [Google Scholar]
- 66.Fitzgerald RC, Caldas C. Clinical implications of E-cadherin associated hereditary diffuse gastric cancer. Gut. 2004;53:775–778. doi: 10.1136/gut.2003.022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simões-Correia J, Figueiredo J, Lopes R, Stricher F, Oliveira C, Serrano L, Seruca R. E-cadherin destabilization accounts for the pathogenicity of missense mutations in hereditary diffuse gastric cancer. PLoS One. 2012;7:e33783. doi: 10.1371/journal.pone.0033783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Figueiredo J, Söderberg O, Simões-Correia J, Grannas K, Suriano G, Seruca R. The importance of E-cadherin binding partners to evaluate the pathogenicity of E-cadherin missense mutations associated to HDGC. Eur J Hum Genet. 2013;21:301–309. doi: 10.1038/ejhg.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]


