Abstract
Infections caused by gram-negative bacteria (GNB) resistant to multiple classes of antibiotics are increasing in many hospitals. Extended-spectrum β-lactamase (ESBL)-producing and carbapenem-resistant Enterobacteriaceae in particular are now endemic in many parts of the world and represent a serious public health threat. In this era, antimicrobial stewardship programs are essential as targeted and responsible use of antibiotics improves patient outcomes and hopefully limits the selective pressure that drives the further emergence of resistance. However, some stewardship strategies aimed at promoting carbapenem-sparing regimens remain controversial and are difficult to implement when resistance rates to non-carbapenem antibiotics are increasing. Coordinated efforts between stewardship programs and infection control are essential for reversing conditions that favor the emergence and dissemination of multidrug-resistant GNB within the hospital and identifying extra-institutional “feeder reservoirs” of resistant strains such as long-term care facilities, where colonization is common despite limited numbers of serious infections. In settings where ESBL resistance is endemic, the cost-effectiveness of expanded infection control efforts and antimicrobial stewardship is still unknown. Once a patient has been colonized, selective oral or digestive decontamination may be considered, but evidence supporting its effectiveness is limited in patients who are already colonized or in centers with high rates of resistance. Moreover, temporary success at decolonization may be associated with a higher risk of relapse with strains that are resistant to the decolonizing antibiotics.
Keywords: Antimicrobial stewardship, Carbapenem-resistant Enterobacteriaceae, Extended-spectrum β-lactamase-producing Enterobacteriaceae, Infection control, Rapid diagnostics, Selective digestive decolonization
Introduction
Treating infections caused by gram-negative bacteria (GNB) resistant to multiple classes of antibiotics is an increasing challenge in many hospitals. Extended-spectrum β-lactamase (ESBL)-producing and carbapenem-resistant Enterobacteriaceae (CRE) in particular are now endemic in many parts of the world and represent one of the most serious public health threats [1, 2]. In this context, antimicrobial stewardship (AMS) programs play a critical role in improving appropriate antibiotic use to improve patient outcomes and reduce the antibiotic selective pressure that drives the emergence of further resistance. However, both the efficacy and collateral impact of many stewardship strategies are not well documented, starting from the battle against the overuse of carbapenems in non-critically ill patients to the use of carbapenem-sparing regimens also in critically ill patients in institutions with endemically high levels (>20%) of ESBL-producing Enterobacteriaceae.
Herein, we discuss the leading stewardship-related controversies and infection control (IC) measures needed to control infections caused by ESBL- and CR-producing Enterobacteriaceae. We also discuss recent efforts from our large teaching hospital to contain carbapenem resistance and its impact on other ESBL resistance rates. This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
ESBL-Producing Enterobacteriaceae
ESBL-producing GNBs are now a universal health concern [2]. ESBL production is frequently accompanied by other resistance mechanisms that impart cross-resistance to many other anti-GNB antibiotics, including aminoglycosides and fluoroquinolones. Consequently, carbapenems have long been regarded as the drugs of choice for suspected or proven serious infections caused by ESBL-producing organisms [3–6]. Nevertheless, the increased use of carbapenems driven by the spread of ESBL-producing Enterobacteriaceae has contributed to the emergence of carbapenem-resistant strains, which currently represent one of the main concerns in the management of patients with gram-negative infections [7, 8]. Avoidance of carbapenems for the treatment of ESBL-producing organisms is conceptually appealing from the standpoint of an AMS program, but could increase the risk of inappropriate treatment of relapse for patients with serious infections.
β-Lactam/β-lactamase inhibitor combinations (BLBLIs) such as piperacillin-tazobactam, have been recommended as an alternative to carbapenems for ESBL-producing Enterobacteriaceae if in vitro susceptibility is documented. Indeed, case-control studies from several countries have reported that frontline protocols that utilized piperacillin-tazobactam instead of cephalosporines or carbapenems were associated with a reduction in the risk of isolation of ESBL-producing and CRE, without clear increases in piperacillin-tazobactam resistance [8, 9].
However, there are some misgivings about the role of BLBLIs for the treatment of ESBL Enterobacteriaceae in clinical practice. First, BLBLIs may have diminished efficacy against ESBL-producing Enterobacteriaceae in the presence of a high bacterial load, known as the inoculum effect, which has been observed specifically with piperacillin-tazobactam [10]. Second, pharmacokinetic/pharmacodynamic (PK/PD) models may support the use of high doses of piperacillin/tazobactam for ESBL-producing Escherichia coli, but are much less encouraging for Klebsiella pneumoniae with higher minimum inhibitory concentrations (MICs) [11]. Third, increasing resistance to BLBLIs in ESBL producers (especially K. pneumoniae), overexpression of β-lactamases or complex coresistance mechanisms, including expression of other enzymes, such as plasmid-derived AmpC, may compromise the use of BLBLIs in empirical therapy.
Several cohort studies and a meta-analysis have suggested BLBLIs are safe and effective for ESBL-producing Enterobacteriaceae [12–14], even though prospective clinical studies are scarce [15, 16]. The most relevant studies are two observational, well-designed studies on patients with bloodstream infections (BSIs) caused by ESBL-producing GNB, which reached opposite conclusions: according to Rodríguez-Baño et al. [12], BLBLIs were equivalent to carbapenems for treating ESBL-producing E. coli, while Tamma et al. [17] reported that empiric treatment with piperacillin-tazobactam versus carbapenem was associated with increased risk of mortality in BSI caused by ESBL-producing E.coli, Klebsiella spp. or Proteus spp. These contrasting findings are probably due to the differences in terms of etiological agents of BSI (E.coli only versus E.coli, Klebsiella spp. or Proteus spp.), administered dosages of piperacillin/tazobactam (higher doses were used in the study from Spain, 4.5 versus 3.375 g qid) and infection sources (>70% of BSIs in the Rodríguez-Baño et al. [12] study originated from the urinary tract versus much more variable infection sources in the study of Tamma et al. [17]). The impact of the infectious source on mortality has been highlighted by a further analysis of the Spanish cohort: all the patients with BSI arising from the urinary tract survived irrespective of the piperacillin-tazobactam MIC, while, in case of different BSI sources, the outcome was poorer if the piperacillin-tazobactam MIC was >2 mg/l [18].
Ideally, the role of BLBLIs versus carbapenems for the treatment of bloodstream infection caused by ESBL producers should be assessed in a large randomized controlled trial. Such a study is now recruiting patients (the ‘MERINO’ trial; NCT02176122) and aims to be completed by 2018.
Based on the data described to date, BLBLIs can represent a reasonable carbapenem-sparing treatment option for urinary tract infections, including those causing bacteremia, caused by ESBL-producing E. coli or, in case of non-severe infections, from other sites in which the isolate is susceptible at a low MIC (≤2 µg/ml in the case of piperacillin-tazobactam). If piperacillin-tazobactam is used, it should be administered at high doses and using infusion strategies that maximize the PK/PD parameters (i.e., t > MIC; loading dose followed by maintenance doses administered by extended or continuous infusion) to guarantee an adequate exposure [19].
Carbapenems would remain the drug of choice for non-urinary or non-easily drainable infections (e.g., pneumonia) caused by ESBL-producing bacteria with complex resistance patterns, including K. pneumoniae and multiple β-lactamases. However, de-escalation of carbapenems, recently described to be safe and feasible [20], probably now represents the most relevant and cost-effective intervention of any AMS program aimed to reduce carbapenem exposure. The actual “high-speed” microbiological turn around could be a cornerstone for these strategies, as further described.
The scenario described above will likely change in the future, owing to the introduction of novel BLBLIs in clinical practice [21]. Ceftazidime-avibactam, an established antipseudomonal cephalosporin combined with a new β-lactamase inhibitor, and ceftolozane (a new cephalosporine) combined with tazobactam have been approved by the Food and Drug Administration (FDA) for the treatment of complicated intraabdominal and urinary tract infections [22–24]. These drugs could represent a further alternative to carbapenems to treat serious infections caused by ESBL-producing organisms, and this option for placement in therapy should be considered in the future hospital formularies. However, resistance to these newer cephalosporins or advanced spectrum β-lactamase inhibitors is already being reported among Enterobacteriaceae [25]; therefore, their judicious use is a key consideration for stewardship programs.
Carbapenem-Resistant Enterobacteriaceae
Over the last decade, the prevalence of infections with CRE has increased worldwide [1], and given the limited therapeutic options for these infections, they are associated with high morbidity and mortality. The crude mortality among patients with CRE infection is approximately 40% [26, 27]. The most relevant observational studies on risk factors for poor outcome in patients with CRE infection are summarized in Table 1. Predictors of poor outcome may be divided in three groups: (1) patient-related factors that included age, comorbidities and APACHE score; (2) infection-related factors such as the pulmonary source, BSI, severe sepsis or septic shock at infection onset, and isolation of a colistin-resistant strain; (3) treatment-related factors with prompt source control and combination therapy both associated with better outcome.
Table 1.
Observational studies analyzing risk factors for poor outcome among patients with carbapenem-resistant Enterobacteriaceae infection
| References | Number of patients and type of infection | Microbiological characteristics | Definition of outcome and observed rates | Unmodifiable conditions associated with outcome | Treatment factors associated with outcome |
|---|---|---|---|---|---|
| Nguyen et al. [96] | 48 (all BSI) | CR-KP |
30-day mortality 42% |
Persistent bacteremia at day 7 | Source control (protective factor) |
| Neuner et al. [97] | 60 (all BSI) | KPC |
14-day mortality 42% |
High Pitt score | No relationship was found between therapy and outcome |
| Zarkotou et al. [98] | 53 (all BSI) | KPC |
Infection mortality 34% |
Age APACHE II score at infection onset |
Appropriate antimicrobial treatment (protective factor) |
| Qureshi et al. [33] | 41 (all BSI) |
21 KPC-1 20 KPC-3 |
28-day mortality 39% |
Pulmonary source Cardiovascular disease Chronic liver disease |
Combination therapy (protective factor) |
| Tumbarello et al. [37] | 125 (all BSI) |
98 KPC-3 27 KPC-2 |
30-day mortality 41.6% |
Septic shock High APACHE III |
Inadequate initial antibiotic therapy Combination therapy with tigecycline, colistin and meropenem |
| Capone et al. [42] | 97 (34 BSI) |
89 KPC-3 5 CTX-M-15 3 VIM-1 |
In-hospital mortality 26% for all the infections 47% for BSI |
Charlson index ICU stay at infection onset BSI Colistin resistant strain |
No relationship was found between therapy and outcome |
| Daikos et al. [29] | 205 (all BSI) |
163 KPC (36 plus VIM) 42 VIM |
28-day mortality 40% |
Severity of underlying disease Septic shock Monotherapy |
Combination therapy (combination including carbapenem was associated with better outcome, mainly if carbapenem MIC was ≤8 mg/l) |
| Gonzalez-Padilla et al. [41] | 50 (24 pneumonia, 18 BSI) | Outbreak of highly resistant KPC clone. All isolates were resistant to colistin and had meropenem MIC >32 mg/l |
30-day mortality 38% |
Age Severe sepsis/septic shock Neoplasia |
Optimal targeted treatment with gentamicin |
| Tumbarello et al. [36] | 661 (447 BSI) |
497 KPC-3 164 KPC-2 |
14-day mortality 34% |
BSI Septic shock APACHE III score Chronic renal failure Colistin-resistant isolate Inadequate empirical treatment |
Combination therapy (combination including carbapenem was associated with better outcome at univariate analysis, mainly if meropenem MIC was ≤8 mg/l) |
BSI bloodstream infection, CR-KP Carbapenem-resistant Klebsiella pneumoniae, ICU intensive care unit, MIC minimum inhibitory concentration, KPC K. pneumoniae carbapenemase, VIM Verona integron-encoded metallo-β-lactamase
Given the limited number of effective and safe agents, several strategies have been proposed to treat CRE infections [28–37]. The currently recommended strategy is combination antimicrobial regimens for CRE with the hope that synergistic interactions between agents will improve bactericidal activity, suppress the emergence of resistance and overcome the PK weaknesses of individual agents. Indeed, several studies have reported lower mortality rates (0–40%) among patients who received combination therapy versus patients receiving meropenem, colistin or tigecycline monotherapy (40–80%) [29, 33, 36–38].
Unfortunately, the question of which combination is superior remains unresolved. In a meta-analysis of 12 retrospective cohort studies and case series, an advantage for combination therapy was identified only in patients classified as receiving “any combination” [39]. Among the different combinations, those that included high-dose carbapenems (i.e., 4–6 g meropenem administered by extended infusion) were associated with better outcome in some studies [29, 36, 37]. In the latest Italian study [36], a propensity score adjusted model to assess the impact on outcome of combination therapy revealed significantly improved survival among patients receiving a combination regimen that included a carbapenem. However, the protective role of carbapenems was maintained only for strains with MIC values ≤8 mg/l, representing around 30% of isolates in some settings with endemic carbapenem resistance.
The impact of carbapenem-sparing regimens on the outcome of CRE has only been investigated in a few small cohort studies. Combination regimens with high-dose tigecycline (100 mg every 12 h) have been proposed for patients with pulmonary infections and were associated with better outcomes than conventional doses of tigecycline in the treatment of ventilator-associated pneumonia caused by multidrug-resistant (MDR) GNB [30]. However, tigecycline use has been clearly associated with the emergence of reduced susceptibility to this drug [40]. In a recent report of 50 patients with highly resistant CR-K. pneumoniae (all strains resistant to colistin and with meropenem MIC >32 mg/l), regimens that included gentamicin, mostly in combination with other drugs, were the only combination associated with lower mortality [41].
The increasing prevalence of colistin-resistant strains, nearly 40% in some geographical areas, has required growing use of unconventional antibiotic combinations for CR-K. pneumoniae [42, 43]. Colistin plus rifampin, double carbapenem therapy and colistin plus double carbapenems have been proposed based on in vitro studies, animal models, and case reports or case series [28, 35, 44, 45]. However, the optimal regimen for such extremely drug-resistant/pan-drug-resistant (XDR/PDR) strains is still unknown.
Several new antibiotics, recently approved (i.e., ceftazidime-avibactam) or in development (RPX7009, plazomycin), have in vitro activity against CR-K. pneumoniae. Avibactam inhibits the activities of Ambler class A and C and some Ambler class D enzymes, restoring the activity of ceftazidime against ESBL, K. pneumoniae carbapenemases (KPC) and/or AmpC β-lactamases and P. aeruginosa. However, avibactam does not restore the spectrum of ceftazidime against metallo-β-lactamases (MBL), Acinetobacter baumannii and most gram-negative anaerobes. In a recent surveillance study of 124 CREs (KPC, n = 87; MBL, n = 13; OXA-48, n = 7), susceptibility rates to ceftazidime-avibactam for each group were 100, 0 and 85.7%, respectively [46]. Some authors also reported the compassionate use of ceftazidime-avibactam in four patients with infections due to Klebsiella oxytoca KPC (1) and K. pneumoniae OXA-48 (3); clinical cure was achieved in 2/4 (50%) [47].
Rapid Diagnostic Tests Used by AMS Programs
As previously affirmed, early discontinuation of broad-spectrum empirical antimicrobial regimens is one of the main goals of AMS programs. Tests for rapid identification of microorganisms represent a key tool for this purpose.
Several rapid molecular assays are commercially available [48–50]. Peptide nucleic acid fluorescent in situ hybridization (PNA FISH; AdvanDx, Woburn, MA, USA), which uses synthetic oligonucleotide fluorescence-labeled probes that rapidly hybridize to species-specific ribosomal RNA, was one of the first. It can identify E. coli, P. aeruginosa and K. pneumoniae, but its inability to identify resistance genes in gram-negative organisms limits its usefulness. Indeed, no studies have evaluated the clinical impact of the use of this assay in patients with gram-negative infections.
Some clinical data are available about the implementation of another technique, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS), as an adjunctive tool to AMS programs. MALDI-TOF MS is a fast, reliable and cost-effective test using the technology of mass spectrometry (ionization and disintegration of a target molecule). The mass/charge ratio of the resulting molecular fragments is compared with those of well-characterized organisms in a database [51]. MALDI-TOF MS does not provide antimicrobial susceptibility data, but its integration in AMS programs is associated with a reduction in time to both active and optimal therapy, length of stay and hospital costs [52–54]. Also in a challenging population constituted of 153 patients with BSIs caused by MDR and/or ESBL-producing GNB, integrating rapid diagnostics with AMS improved outcome. Time to effective and optimal antibiotic therapy was reduced from 89.7 to 32 h (P < 0.001) and from 80.9 to 23.2 h (P < 0.001), respectively. Total and intensive care unit length of stay decreased from 23.3 to 15.3 days (P = 0.0001) and from 16 to 10.7 days (P = 0.008), respectively. Mortality was lower (21% versus 8.9%, P = 0.01) during the intervention period, and the study intervention was identified as a predictor of survival [odds ratio (OR), 0.3; 95% confidence interval (CI) 0.12–0.79] at multivariate logistic regression [55]. Interestingly, the impact of MALDI-TOF MS appears to be higher when a stewardship team manages the results rather than when the same results are just provided to the patient’s attending physicians (reduced time to appropriate therapy in 28.8% and 44.6% of patients, according to the physician’s choices and stewardship team recommendations, respectively; P = 0.001) [56].
Multiplex polymerase chain reaction (PCR) can be used for simultaneous detection of multiple organisms and resistance markers. BioFire Diagnostics’ FilmArray blood culture identification (BCID) panel tests for 24 organisms, including Enterobacter cloacae complex, E. coli, Klebsiella species, Proteus species, Serratia marcescens, and antimicrobial resistance genes, including carbapenem resistance, are also used for detection.
Nanoparticle Probe Technology (Nucleic Acid Extraction and PCR Amplification) Nanosphere’s Verigene blood culture gram-negative (BC-GN) assay uses nucleic acid extraction and PCR amplification followed by hybridization of target DNA to capture oligonucleotides on a microarray. After hybridization, signal amplification of hybridized probes provides an automated qualitative analysis of results. This test identifies genus, species and genetic resistance determinants [KPC, New Deli metallo-β-lactamases (NDM), CTX-M, Verona integron-encoded metallo-β-lactamase (VIM), IMP and OXA β-lactamases genes] of the most common gram-negative organisms, with an approximate turnaround time of 2 h from blood culture positivity [57, 58]. Although there are no published clinical studies on this topic, potential advantages of the use of BC-GN in combination with an AMS approach have recently been described by Bork et al. [59]. In their interesting simulation, they evaluated 132 GN-BSI episodes, performed a theoretical evaluation of the time to effective and optimal antibiotic therapy based on BC-GN reporting and AMS team review (intervention) and compared them with actual antibiotic administration times obtained from chart review (controls). Effective and optimal antibiotic therapy could be achieved an average of 3.7 h (95% CI 1.3–6.2; P < 0.01) and 18.3 h earlier (95% CI 13.3–23.4; P < 0.01) in the intervention group than in the controls [59].
In settings with epidemic ESBL-producing and CR-producing Enterobacteriaceae, rapid microbiologic tests, in particular novel techniques that allow early identification of antimicrobial resistance patterns, can represent a key tool to improve patient outcomes and antimicrobial use. Microbiologic technologies must be integrated in AMS programs, as their clinical impact can be weak without a stewardship-driven educational intervention [56, 60].
Infection Control Challenges
In consideration of the steady increase of occurrence of infections caused by ESBL and carbapenemase-producing GNB, AMS programs have been incorporated into IC strategies designed to prevent the spread of MDR GNB. IC practitioners similarly support AMS efforts through monitoring of resistance trends and outbreaks in the institution, promoting compliance with hand hygiene, contact and transmission precautions, supporting education efforts and assessing adherence to care bundles. Effective prevention strategies can reduce the rate of hospital-acquired infections, decrease the use of additional antibiotics and, in turn, reduce the rate of MDR GNB.
Guidelines proposed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) to prevent the diffusion of MDR GNB infection and colonization [61] identify four key aims for IC programs: (1) reduction of direct-contact transmission involving skin-to-skin contact and physical transfer of microorganisms to a susceptible host from an infected or colonized person via hand washing and wearing contact precautions; (2) early identification of colonized patients with active screening (culture) protocols at hospital admission followed by contact precautions and physical separation of colonized patients; (3) monitoring of cleaning performance and use of dedicated medical equipment for personal involved in the care of colonized or infected patients; (4) reduction in antimicrobial pressure favoring the selection of MDR GNB through AMS programs.
Similar measures are suggested for containing the spread of ESBL-producing strains in healthcare environments. However, data supporting the effectiveness of these recommendations, especially active screening protocols in institutions with endemically high resistance rates, are more controversial [61]. First, the epidemiology of ESBL-resistance is generally more complex than carbapenem-resistance in Enterobacteriacae. This complexity is compounded by the sometimes more sporadic nature of ESBL resistance. Each of these situations will need to be managed in different ways, depending on the risk to the patients involved.
When ESBL resistance is endemic to the institution, intensive IC efforts in the hospital alone and AMS may not be sufficient to reduce resistance. In contrast with other MDR bacteria, ESBL-mediated resistance is suspected to be primarily spread through the community and long-term care facilities and less so within within healthcare-related institutions [62]. Several reports have confirmed the environmental persistence of ESBL-producing E. coli in superficial water, wastewaters and farms [63, 64]. Antibiotic use in livestock as a growth-promoter appears to be a major contributing factor, yet controls on the prescription and use of antibiotics are almost nonexistent. Additionally, fecal carriage of ESBLs has been reported in healthy individuals residing in the community [65, 66]. As a result of this situation, a considerable proportion of patients infected with ESBL-producing strain in many European and US hospitals come from the community [67, 68].
Another controversial aspect of the circulation of the ESBL-producing strain is the method of transmission. The role of healthcare diffusion via the hands of healthcare workers during hospital outbreaks is predominant for K. pneumoniae strains but less clear for E. coli strains. In fact, several reports have shown the failure of contact precaution measures alone in preventing the diffusion of ESBL-producing E. coli during outbreaks [61, 69]. Moreover, in settings where contact precautions are not commonly used for containing the spread of ESBL strains, the rate of cross-transmission from healthcare workers' hands appeared to be low [70].
Several studies showed a possible spread of ESBL- and/or AmpC-producing bacterial strains transmitted via the food chain or food animal production environment [71–73]. In this situation, it is reasonable to assume that a significant proportion of hospitalized patients may be colonized without detection before admission to the hospital and therefore are not assigned to isolation precautions. Further studies are needed to assess the cost effectiveness of a massive implementation of IC measures to contain the diffusion of ESBLs in endemic settings.
A clearer understanding of essential IC strategies has been described for limiting the spread of CRE. In a seminal study describing the containment of a country-wide outbreak of carbapenem-resistant K. pneumoniae in Israeli hospitals, Schwaber and colleagues [74] analyzed the impact of guidelines instituted by the Israeli Ministry of Health in 2007 that mandated physical separation and dedicated staffing for hospitalized carriers of CRE. Adherence to the guidelines was ensured by a dedicated task force that reviewed all aspects of IC and new CRE cases, and it intervened with additional measures when necessary. With the nationally implemented intervention, the rates of CRE acquisition were reduced by nearly fivefold, and the incidence of new infection relative to carrier prevalence declined significantly.
At an institutional level, Ciabotaro et al. [75] described the practical implementation and impact of the 2007 Israeli guidelines in their hospital. In the 40 months following implementation of the CRE patient screening, cohorting, cleaning, and education program, the investigators reported a 16-fold reduction in carbapenemase-resistant K. pneumoniae that was sustained for 30 months.
We recently performed a quasi-experimental study from 2010 to 2014 with 30-month follow-up of a multifaceted IC program combined with a stewardship initiative to reduce CRE BSIs in our 1420-bed university-affiliated hospital. The program, managed by a dedicated team, required CRE rectal culture screening for any patient transferred or admitted to high-risk units, cohorting or isolating positive patients, and using dedicated medical equipment and transport pathways within the hospital, education programs for patients, staff and caregivers, and an intensive hygiene program with a particular focus on hand washing and room cleaning. In terms of AMS, a coordinated program promoting carbapenem-sparing regimens was implemented to reduce selective antimicrobial pressure. Any prescription of the above-mentioned drugs was recorded by pharmacists, who notified the infectious disease consultants (IDCs) daily through an ad-hoc alert system. IDCs reviewed each prescription within 48 h with a bedside patient evaluation and recommended modifications to the prescribers whenever indicated.
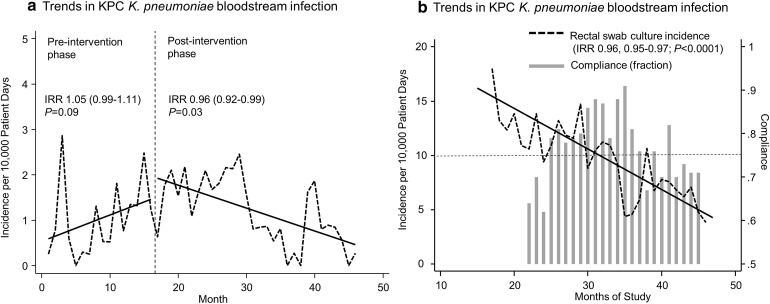
Following the IC and AMS intervention, the incidence rate of CRE BSI (risk reduction 0.96, 0.92–0.99, P = 0.03) and CRE colonization (risk reduction 0.96, 0.95–0.97; P < 0.0001) decreased over 30 months (Fig. 1). Our analysis showed this reduction was not coincidental, as CRE-screening compliance rates were associated with the observed reduction in the incidence of CRE carriage in the hospitalized population, as detected by monthly rectal swab cultures. Moreover, when average compliance rates with the screening protocols waned after 20 months of the program (Fig. 1b), rates of CRE BSI and positive-rectal swabs rebounded and only decreased once average screening compliance rates had been restored above 75% [76].
Fig. 1.
Monthly incidence trends in KPC-carbapenemase producing K. pneumoniae bloodstream infections (a) and colonization (b) detected by rectal swab cultures following introduction of a comprehensive antimicrobial stewardship and infection control program to limit carbapenem resistance. Dotted lines represent the monthly incidence per 10,000 patient days. Solid lines represent the incidence trends. Bars in panel b indicate monthly compliance rates of the fraction of patients who were appropriately screened with rectal cultures for KPC-carbapenemase producing K. pneumoniae. IRR calculated by segmented Poisson regression. Despite declining CRE rates, the IRRs of ESBL-positive Enterobacteriaceae were stable during the study period (IRR 1.02, 95% CI 0.99–1.04; P = 0.06). Likewise, we did not observe marked changes in the rates of MDR among MDR non-fermenting GNB (IRR 1.04, 95% CI 0.22–1.22, P = 0.132) or vancomycin-resistant Enterococcus (IRR 0.98, 0.92–1.04; P = 0.57). IRR Incidence rate ratio, KPC K. pneumoniae carbapenemases
Support of the regional health authority [77] and our hospital administration as well as the strict application of the IC policy were key elements in the success of the program and the AMS initiatives. Interestingly, during the intervention period, we did not observe a significant decrease in the incidence of BSI due to ESBL-producing Enterobacteriaceae or other MDR GNR organisms, highlighting that in the absence of specific screening for ESBLs, strictly applied IC procedures for CRE are unlikely to blindly decrease other endemic resistance problems.
Decolonization Strategies for MDR GNB
Nearly 30 years of selective decontamination studies that included more than 50 randomized studies and 10 meta-analyses have suggested that, in general, selective oral decontamination (SOD) or selective digestive decontamination (SDD) has a favorable effect on mortality from serious infections in adult patients admitted to intensive care units (ICUs) [68, 78]. However, the largest studies were performed in ICUs with relatively low rates of antibiotic resistance in The Netherlands, and the efficacy of these approaches is less certain when used in patients who are already documented to be colonized with MDR organisms. Whether these results translate to settings with higher rates of endemic MDR GNB is debatable. Indeed, several observational studies [79–82] and a small randomized trial [83] from ICUs with higher resistance rates have reported contradictory results regarding the benefits of SDD for decolonization of MDR GNB.
In the last few years, the efficacy and safety of SDD with non-absorbable antibiotics for the eradication of CRE carriage were evaluated in populations with various underlying conditions [82, 84–87].
The use of oral regimens of colistin and/or gentamicin has been mainly proposed for some specific settings such as patients with hematological malignancies or candidates to major surgery including solid organ transplantation (SOT). However, there are several misgivings about the use of CRE decolonization. First, the need for decolonizing SOT candidates is under debate, mainly for liver transplantation; indeed, the risk for CRE infection has been shown to be similar between CRE colonized and noncolonized candidates [88, 89]. Second, the efficacy of decolonization was short term [84–86]. Third, it has been associated with the emergence of further resistance [82]. In a large randomized controlled trial performed in The Netherlands, routine use of SDD protocols of colistin, tobramycin and amphotericin B prior to detected colonization with resistant organisms was associated with a 7% increase in the recovery of aminoglycoside-resistant organisms per month [90]. In a retrospective cohort study performed in surgical ICUs in Germany, Lubbert and colleagues [82] reported that gentamicin and colistin SDD achieved decolonization rates of KPC-2 carbapenemase-producing K. pneumoniae in 6/14 patients (43%), but was associated with a rapid increase in secondary bacterial resistance to both antibiotics. Selection of colistin-resistant strains in particular is a major concern because it may predispose patients to infections with pathogens that are untreatable with currently available antibiotics [91]. Therefore, the potential ecological impact of SDD and limited benefits suggest that SDD can only be used with careful microbiological monitoring for resistance development.
Clearly, more data are needed to define the role of preemptive versus targeted SOD and SDD in the management of MDR GNB and its potential collateral impact on resistance rates in the ICU or hospital.
Conclusions
AMS has been defined as “a marriage of IC and antimicrobial management finalized to share the principles of the optimized treatment between the bench to bedside point of view and the hospital-wide vision” [92]. According to this definition, in our opinion, the main aims of stewardship should be: promoting the appropriate use of antimicrobials to decrease the spread of infections caused by MDR organisms without forgetting the patient outcome.
There are two principal approaches: restrictive and persuasive. The oldest strategy is antibiotic restriction or pre-prescription authorization, which consists of the requirement for approval of the antibiotic from an infectious disease specialist. In endemic settings for ESBL infections, the restriction of some key antimicrobials may reduce the antibiotic pressure and rate of resistant pathogens. For example, a fluoroquinolone restriction strategy was demonstrated to increase fluoroquinolone susceptibility within a short span of 4 months and reduce the overall rate of ESBL and Clostridium difficile infection cases [93, 94].
The persuasive strategy usually consists of a post-prescription review by an infectious disease specialist of some of the antimicrobials prescribed by the attending physicians, providing advice if necessary. The advantage of this strategy may also comprise the improvement of the overall appropriateness of prescription and the opportunity for early de-escalation [95]. In a study evaluating the efficacy of an AMS program based on early carbapenem de-escalation, the patients enrolled in the program had a comparable clinical success, fewer adverse effects and a lower incidence of the development of resistance compared to controls [20].
Both of these AMS strategies have pros and cons; when compared in systematic reviews, the restrictive policy seems to allow more rapid results, mainly in terms of reducing antimicrobial consumptions, but these results are usually limited in the time because of the general low level of acceptance of this strategy by prescribers. The second one seems to be less effective in terms of reducing antimicrobial consumption, but has the advantage of improving the prescription appropriateness with a potential advantage on patient outcome.
Beyond the classical question about the best strategy between persuasive or restrictive actions, an important question remains unanswered: should stewardship programs be planned in a whole hospital vision or can we also think about setting-related, disease-related, drug-related projects? Another advocated limit of stewardship programs is the relative paucity, if not the absence, of multicenter studies across large healthcare systems; however, because the inter-center variability of clinical settings and missions is a distinctive feature of any healthcare system, it seems logical to ask ourselves which is the primary goal of any stewardship program: reproducibility or attainability in relation to the specific unmet needs of any single institution or organization?
Acknowledgments
This supplement was not sponsored by outside commercial interests. No source of funding was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Conflict of interest
PV has been a consultant for Merck Sharpe and Dhome and Achaogen. MG, MB, ST and RL have nothing to disclose.
Compliance with ethics guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Contributor Information
Pierluigi Viale, Email: pierluigi.viale@unibo.it.
Maddalena Giannella, Email: maddalena.giannella@libero.it.
References
- 1.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13(9):785–796. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8(3):159–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 3.Tumbarello M, Sanguinetti M, Montuori E, Trecarichi EM, Posteraro B, Fiori B, et al. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother. 2007;51(6):1987–1994. doi: 10.1128/AAC.01509-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paterson DL, Ko WC, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, et al. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum beta-lactamases. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2004;39(1):31–37. doi: 10.1086/420816. [DOI] [PubMed] [Google Scholar]
- 5.Endimiani A, Luzzaro F, Brigante G, Perilli M, Lombardi G, Amicosante G, et al. Proteus mirabilis bloodstream infections: risk factors and treatment outcome related to the expression of extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 2005;49(7):2598–2605. doi: 10.1128/AAC.49.7.2598-2605.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paterson DL. Recommendation for treatment of severe infections caused by Enterobacteriaceae producing extended-spectrum beta-lactamases (ESBLs) Clin Microbiol Infect. 2000;6(9):460–463. doi: 10.1046/j.1469-0691.2000.00107.x. [DOI] [PubMed] [Google Scholar]
- 7.Canton R, Akova M, Carmeli Y, Giske CG, Glupczynski Y, Gniadkowski M, et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect: Off Publ Eur Soc Clin Microbiol Infect Dis. 2012;18(5):413–431. doi: 10.1111/j.1469-0691.2012.03821.x. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin M, Advincula MR, Malczynski M, Qi C, Bolon M, Scheetz MH. Correlations of antibiotic use and carbapenem resistance in enterobacteriaceae. Antimicrob Agents Chemother. 2013;57(10):5131–5133. doi: 10.1128/AAC.00607-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Livermore DM, Hope R, Reynolds R, Blackburn R, Johnson AP, Woodford N. Declining cephalosporin and fluoroquinolone non-susceptibility among bloodstream Enterobacteriaceae from the UK: links to prescribing change? J Antimicrob Chemother. 2013;68(11):2667–2674. doi: 10.1093/jac/dkt212. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Cerero L, Picon E, Morillo C, Hernandez JR, Docobo F, Pachon J, et al. Comparative assessment of inoculum effects on the antimicrobial activity of amoxycillin-clavulanate and piperacillin-tazobactam with extended-spectrum beta-lactamase-producing and extended-spectrum beta-lactamase-non-producing Escherichia coli isolates. Clin Microbiol Infect. 2010;16(2):132–136. doi: 10.1111/j.1469-0691.2009.02893.x. [DOI] [PubMed] [Google Scholar]
- 11.Ambrose PG, Bhavnani SM, Jones RN. Pharmacokinetics-pharmacodynamics of cefepime and piperacillin-tazobactam against Escherichia coli and Klebsiella pneumoniae strains producing extended-spectrum beta-lactamases: report from the ARREST program. Antimicrob Agents Chemother. 2003;47(5):1643–1646. doi: 10.1128/AAC.47.5.1643-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Bano J, Navarro MD, Retamar P, Picon E, Pascual A. Beta-lactam/beta-lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli: a post hoc analysis of prospective cohorts. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2012;54(2):167–174. doi: 10.1093/cid/cir790. [DOI] [PubMed] [Google Scholar]
- 13.Harris PN, Peleg AY, Iredell J, Ingram PR, Miyakis S, Stewardson AJ, et al. Meropenem versus piperacillin-tazobactam for definitive treatment of bloodstream infections due to ceftriaxone non-susceptible Escherichia coli and Klebsiella spp. (the MERINO trial): study protocol for a randomised controlled trial. Trials. 2015;16(1):24. doi: 10.1186/s13063-014-0541-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vardakas KZ, Tansarli GS, Rafailidis PI, Falagas ME. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum beta-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother. 2012;67(12):2793–2803. doi: 10.1093/jac/dks301. [DOI] [PubMed] [Google Scholar]
- 15.Harris PN, Tambyah PA, Paterson DL. beta-lactam and beta-lactamase inhibitor combinations in the treatment of extended-spectrum beta-lactamase producing Enterobacteriaceae: time for a reappraisal in the era of few antibiotic options? Lancet Infect Dis. 2015;15(4):475–485. doi: 10.1016/S1473-3099(14)70950-8. [DOI] [PubMed] [Google Scholar]
- 16.Perez F, Bonomo RA. Editorial commentary: bloodstream infection caused by extended-spectrum beta-lactamase-producing Gram-negative bacteria: how to define the best treatment regimen? Clin Infect Dis: Off Publ Infect Dis Soc Am. 2015;60(9):1326–1329. doi: 10.1093/cid/civ007. [DOI] [PubMed] [Google Scholar]
- 17.Tamma PD, Han JH, Rock C, Harris AD, Lautenbach E, Hsu AJ, et al. carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum beta-lactamase bacteremia. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2015;60(9):1319–1325. doi: 10.1093/cid/civ003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Retamar P, Lopez-Cerero L, Muniain MA, Pascual A, Rodriguez-Bano J. Impact of the MIC of piperacillin-tazobactam on the outcome of patients with bacteremia due to extended-spectrum-beta-lactamase-producing Escherichia coli. Antimicrob Agents Chemother. 2013;57(7):3402–3404. doi: 10.1128/AAC.00135-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felton TW, Hope WW, Lomaestro BM, Butterfield JM, Kwa AL, Drusano GL, et al. Population pharmacokinetics of extended-infusion piperacillin-tazobactam in hospitalized patients with nosocomial infections. Antimicrob Agents Chemother. 2012;56(8):4087–4094. doi: 10.1128/AAC.00521-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lew KY, Ng TM, Tan M, Tan SH, Lew EL, Ling LM, et al. Safety and clinical outcomes of carbapenem de-escalation as part of an antimicrobial stewardship programme in an ESBL-endemic setting. J Antimicrob Chemother. 2015;70(4):1219–1225. doi: 10.1093/jac/dku479. [DOI] [PubMed] [Google Scholar]
- 21.Toussaint KA, Gallagher JC. Beta-lactam/beta-lactamase inhibitor combinations: from then to now. Ann Pharmacother. 2015;49(1):86–98. doi: 10.1177/1060028014556652. [DOI] [PubMed] [Google Scholar]
- 22.Lucasti C, Popescu I, Ramesh MK, Lipka J, Sable C. Comparative study of the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infections in hospitalized adults: results of a randomized, double-blind, phase II trial. J Antimicrob Chemother. 2013;68(5):1183–1192. doi: 10.1093/jac/dks523. [DOI] [PubMed] [Google Scholar]
- 23.Vazquez JA, Gonzalez Patzan LD, Stricklin D, Duttaroy DD, Kreidly Z, Lipka J, et al. Efficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: results of a prospective, investigator-blinded, randomized study. Curr Med Res Opin. 2012;28(12):1921–1931. doi: 10.1185/03007995.2012.748653. [DOI] [PubMed] [Google Scholar]
- 24.Bulik CC, Tessier PR, Keel RA, Sutherland CA, Nicolau DP. In vivo comparison of CXA-101 (FR264205) with and without tazobactam versus piperacillin-tazobactam using human simulated exposures against phenotypically diverse gram-negative organisms. Antimicrob Agents Chemother. 2012;56(1):544–549. doi: 10.1128/AAC.01752-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Humphries RM, Yang S, Hemarajata P, Ward KW, Hindler JA, Miller SA, et al. First report of ceftazidime-avibactam resistance in a KPC-3 expressing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2015. doi:10.1128/AAC.01165-15 [DOI] [PMC free article] [PubMed]
- 26.Viale P, Giannella M, Lewis R, Trecarichi EM, Petrosillo N, Tumbarello M. Predictors of mortality in multidrug-resistant Klebsiella pneumoniae bloodstream infections. Expert Rev Anti-infect Ther. 2013;11(10):1053–1063. doi: 10.1586/14787210.2013.836057. [DOI] [PubMed] [Google Scholar]
- 27.Petrosillo N, Giannella M, Lewis R, Viale P. Treatment of carbapenem-resistant Klebsiella pneumoniae: the state of the art. Expert Rev Anti-infect Ther. 2013;11(2):159–177. doi: 10.1586/eri.12.162. [DOI] [PubMed] [Google Scholar]
- 28.Bulik CC, Nicolau DP. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2011;55(6):3002–3004. doi: 10.1128/AAC.01420-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother. 2014;58(4):2322–2328. doi: 10.1128/AAC.02166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Pascale G, Montini L, Pennisi M, Bernini V, Maviglia R, Bello G, et al. High dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit Care. 2014;18(3):R90. doi: 10.1186/cc13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirsch EB, Tam VH. Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. J Antimicrob Chemother. 2011;65(6):1119–1125. doi: 10.1093/jac/dkq108. [DOI] [PubMed] [Google Scholar]
- 32.Jernigan MG, Press EG, Nguyen MH, Clancy CJ, Shields RK. The combination of doripenem and colistin is bactericidal and synergistic against colistin-resistant, carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2012;56(6):3395–3398. doi: 10.1128/AAC.06364-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56(4):2108–2113. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sbrana F, Malacarne P, Viaggi B, Costanzo S, Leonetti P, Leonildi A, et al. Carbapenem-sparing antibiotic regimens for infections caused by Klebsiella pneumoniae Carbapenemase-producing K. pneumoniae in Intensive Care Unit. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2012;56(5):697–700. [DOI] [PubMed]
- 35.Tascini C, Tagliaferri E, Giani T, Leonildi A, Flammini S, Casini B, et al. Synergistic activity of colistin plus rifampin against colistin-resistant KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57(8):3990–3993. doi: 10.1128/AAC.00179-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, et al. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother. 2015;70(7):2133–43 (Epub 2015/04/23). [DOI] [PubMed]
- 37.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2012;55(7):943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 38.Girometti N, Lewis RE, Giannella M, Ambretti S, Bartoletti M, Tedeschi S, et al. Klebsiella pneumoniae bloodstream infection: epidemiology and impact of inappropriate empirical therapy. Medicine. 2014;93(17):298–309. doi: 10.1097/MD.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paul M, Carmeli Y, Durante-Mangoni E, Mouton JW, Tacconelli E, Theuretzbacher U, et al. Combination therapy for carbapenem-resistant Gram-negative bacteria. J Antimicrob Chemother. 2014;69(9):2305–2309. doi: 10.1093/jac/dku168. [DOI] [PubMed] [Google Scholar]
- 40.van Duin D, Cober ED, Richter SS, Perez F, Cline M, Kaye KS, et al. Tigecycline therapy for carbapenem-resistant Klebsiella pneumoniae (CRKP) bacteriuria leads to tigecycline resistance. Clin Microbiol Infect: Off Publ Eur Soc Clin Microbiol Infect Dis. 2014;20(12):O1117–O1120. doi: 10.1111/1469-0691.12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez-Padilla M, Torre-Cisneros J, Rivera-Espinar F, Pontes-Moreno A, Lopez-Cerero L, Pascual A, et al. Gentamicin therapy for sepsis due to carbapenem-resistant and colistin-resistant Klebsiella pneumoniae. J Antimicrob Chemother. 2015;70(3):905–913. doi: 10.1093/jac/dku432. [DOI] [PubMed] [Google Scholar]
- 42.Capone A, Giannella M, Fortini D, Giordano A, Meledandri M, Ballardini M, et al. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin Microbiol Infect: Off Publ Eur Soc Clin Microbiol Infect Dis. 2013;19(1):E23–E30. doi: 10.1111/1469-0691.12070. [DOI] [PubMed] [Google Scholar]
- 43.Monaco M, Giani T, Raffone M, Arena F, Garcia-Fernandez A, Pollini S, et al. Colistin resistance superimposed to endemic carbapenem-resistant Klebsiella pneumoniae: a rapidly evolving problem in Italy, November 2013 to April 2014. Euro Surveill. 2014;19(42) (Epub 2014/10/31). [DOI] [PubMed]
- 44.Giamarellou H, Galani L, Baziaka F, Karaiskos I. Effectiveness of a double-carbapenem regimen for infections in humans due to carbapenemase-producing pandrug-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57(5):2388–2390. doi: 10.1128/AAC.02399-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shields RK, Nguyen MH, Potoski BA, Press EG, Chen L, Kreiswirth BN, et al. Doripenem MICs and ompK36 porin genotypes of sequence type 258, KPC-producing Klebsiella pneumoniae may predict responses to carbapenem-colistin combination therapy among patients with bacteremia. Antimicrob Agents Chemother. 2015;59(3):1797–1801. doi: 10.1128/AAC.03894-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Biedenbach D, deJonge B, Nichols W, Sahm D. Activity of ceftazidime-avibactam and comparators against carbapenemase-producing Enterobacteriaceae isolated in sampled countries of the European Union: 2013 INFORM surveillance programme. In: 25th European congress of clinical microbriology and infectious diseases (ECCMID 2015). 2015. p. P1293.
- 47.Loeches-Yague B, Mora-Rillo M, Paño-Pardo J, Diaz-Pollan B, Ramos J, Rico A, et al. Compassionate use of ceftazidime-avibactam for carbapenemase-producing Enterobacteriaceae (CPE): a single-hospital experience. In: 25th European congress of clinical microbriology and infectious diseases (ECCMID 2015). 2015. p. P1297.
- 48.Bauer KA, Perez KK, Forrest GN, Goff DA. Review of rapid diagnostic tests used by antimicrobial stewardship programs. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2014;59(Suppl 3):S134–S145. doi: 10.1093/cid/ciu547. [DOI] [PubMed] [Google Scholar]
- 49.Tuite N, Reddington K, Barry T, Zumla A, Enne V. Rapid nucleic acid diagnostics for the detection of antimicrobial resistance in Gram-negative bacteria: is it time for a paradigm shift? J Antimicrob Chemother. 2014;69(7):1729–1733. doi: 10.1093/jac/dku083. [DOI] [PubMed] [Google Scholar]
- 50.Kothari A, Morgan M, Haake DA. Emerging technologies for rapid identification of bloodstream pathogens. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2014;59(2):272–278. doi: 10.1093/cid/ciu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan KE, Ellis BC, Lee R, Stamper PD, Zhang SX, Carroll KC. Prospective evaluation of a matrix-assisted laser desorption ionization-time of flight mass spectrometry system in a hospital clinical microbiology laboratory for identification of bacteria and yeasts: a bench-by-bench study for assessing the impact on time to identification and cost-effectiveness. J Clin Microbiol. 2012;50(10):3301–3308. doi: 10.1128/JCM.01405-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang AM, Newton D, Kunapuli A, Gandhi TN, Washer LL, Isip J, et al. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2013;57(9):1237–1245. doi: 10.1093/cid/cit498. [DOI] [PubMed] [Google Scholar]
- 53.Perez KK, Olsen RJ, Musick WL, Cernoch PL, Davis JR, Land GA, et al. Integrating rapid pathogen identification and antimicrobial stewardship significantly decreases hospital costs. Arch Pathol Lab Med. 2013;137(9):1247–1254. doi: 10.5858/arpa.2012-0651-OA. [DOI] [PubMed] [Google Scholar]
- 54.Clerc O, Prod’hom G, Vogne C, Bizzini A, Calandra T, Greub G. Impact of matrix-assisted laser desorption ionization time-of-flight mass spectrometry on the clinical management of patients with Gram-negative bacteremia: a prospective observational study. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2013;56(8):1101–1107. doi: 10.1093/cid/cis1204. [DOI] [PubMed] [Google Scholar]
- 55.Perez KK, Olsen RJ, Musick WL, Cernoch PL, Davis JR, Peterson LE, et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect. 2014;69(3):216–225. doi: 10.1016/j.jinf.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 56.Tamma PD, Tan K, Nussenblatt VR, Turnbull AE, Carroll KC, Cosgrove SE. Can matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF) enhance antimicrobial stewardship efforts in the acute care setting? Infect Control Hosp Epidemiol. 2013;34(9):990–995. doi: 10.1086/671731. [DOI] [PubMed] [Google Scholar]
- 57.Mancini N, Infurnari L, Ghidoli N, Valzano G, Clementi N, Burioni R, et al. Potential impact of a microarray-based nucleic acid assay for rapid detection of Gram-negative bacteria and resistance markers in positive blood cultures. J Clin Microbiol. 2014;52(4):1242–1245. doi: 10.1128/JCM.00142-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hill JT, Tran KD, Barton KL, Labreche MJ, Sharp SE. Evaluation of the nanosphere Verigene BC-GN assay for direct identification of gram-negative bacilli and antibiotic resistance markers from positive blood cultures and potential impact for more-rapid antibiotic interventions. J Clin Microbiol. 2014;52(10):3805–3807. doi: 10.1128/JCM.01537-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bork JT, Leekha S, Heil EL, Zhao L, Badamas R, Johnson JK. Rapid testing using the Verigene Gram-negative blood culture nucleic acid test in combination with antimicrobial stewardship intervention against Gram-negative bacteremia. Antimicrob Agents Chemother. 2015;59(3):1588–1595. doi: 10.1128/AAC.04259-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holtzman C, Whitney D, Barlam T, Miller NS. Assessment of impact of peptide nucleic acid fluorescence in situ hybridization for rapid identification of coagulase-negative staphylococci in the absence of antimicrobial stewardship intervention. J Clin Microbiol. 2011;49(4):1581–1582. doi: 10.1128/JCM.02461-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tacconelli E, Cataldo MA, Dancer SJ, De Angelis G, Falcone M, Frank U, et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin Microbiol Infect: Off Publ Eur Soc Clin Microbiol Infect Dis. 2014;20(Suppl 1):1–55. doi: 10.1111/1469-0691.12427. [DOI] [PubMed] [Google Scholar]
- 62.Woerther PL, Burdet C, Chachaty E, Andremont A. Trends in human fecal carriage of extended-spectrum beta-lactamases in the community: toward the globalization of CTX-M. Clin Microbiol Rev. 2013;26(4):744–758. doi: 10.1128/CMR.00023-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blaak H, Lynch G, Italiaander R, Hamidjaja RA, Schets FM, de Roda Husman AM. Multidrug-resistant and extended spectrum beta-lactamase-producing Escherichia coli in Dutch surface water and wastewater. PLoS One. 2015;10(6):e0127752. doi: 10.1371/journal.pone.0127752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brinas L, Moreno MA, Zarazaga M, Porrero C, Saenz Y, Garcia M, et al. Detection of CMY-2, CTX-M-14, and SHV-12 beta-lactamases in Escherichia coli fecal-sample isolates from healthy chickens. Antimicrob Agents Chemother. 2003;47(6):2056–2058. doi: 10.1128/AAC.47.6.2056-2058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodriguez-Bano J, Alcala JC, Cisneros JM, Grill F, Oliver A, Horcajada JP, et al. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med. 2008;168(17):1897–1902. doi: 10.1001/archinte.168.17.1897. [DOI] [PubMed] [Google Scholar]
- 66.Canton R, Novais A, Valverde A, Machado E, Peixe L, Baquero F, et al. Prevalence and spread of extended-spectrum beta-lactamase-producing Enterobacteriaceae in Europe. Clin Microbiol Infect. 2008;14(Suppl 1):144–153. doi: 10.1111/j.1469-0691.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- 67.Leistner R, Meyer E, Gastmeier P, Pfeifer Y, Eller C, Dem P, et al. Risk factors associated with the community-acquired colonization of extended-spectrum beta-lactamase (ESBL) positive Escherichia Coli. an exploratory case-control study. PLoS One. 2013;8(9):e74323. doi: 10.1371/journal.pone.0074323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doi Y, Park YS, Rivera JI, Adams-Haduch JM, Hingwe A, Sordillo EM, et al. Community-associated extended-spectrum beta-lactamase-producing Escherichia coli infection in the United States. Clin Infect Dis. 2013;56(5):641–648. doi: 10.1093/cid/cis942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paterson DL, Singh N, Rihs JD, Squier C, Rihs BL, Muder RR. Control of an outbreak of infection due to extended-spectrum beta-lactamase-producing Escherichia coli in a liver transplantation unit. Clin Infect Dis. 2001;33(1):126–128. doi: 10.1086/320882. [DOI] [PubMed] [Google Scholar]
- 70.Tschudin-Sutter S, Frei R, Dangel M, Stranden A, Widmer AF. Rate of transmission of extended-spectrum beta-lactamase-producing enterobacteriaceae without contact isolation. Clin Infect Dis. 2012;55(11):1505–1511. doi: 10.1093/cid/cis770. [DOI] [PubMed] [Google Scholar]
- 71.Kojima A, Ishii Y, Ishihara K, Esaki H, Asai T, Oda C, et al. Extended-spectrum-beta-lactamase-producing Escherichia coli strains isolated from farm animals from 1999 to 2002: report from the Japanese Veterinary Antimicrobial Resistance Monitoring Program. Antimicrob Agents Chemother. 2005;49(8):3533–3537. doi: 10.1128/AAC.49.8.3533-3537.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kola A, Kohler C, Pfeifer Y, Schwab F, Kuhn K, Schulz K, et al. High prevalence of extended-spectrum-beta-lactamase-producing Enterobacteriaceae in organic and conventional retail chicken meat, Germany. J Antimicrob Chemother. 2012;67(11):2631–2634. doi: 10.1093/jac/dks295. [DOI] [PubMed] [Google Scholar]
- 73.Leverstein-van Hall MA, Dierikx CM, Cohen Stuart J, Voets GM, van den Munckhof MP, van Essen-Zandbergen A, et al. Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin Microbiol Infect. 2011;17(6):873–880. doi: 10.1111/j.1469-0691.2011.03497.x. [DOI] [PubMed] [Google Scholar]
- 74.Schwaber MJ, Lev B, Israeli A, Solter E, Smollan G, Rubinovitch B, et al. Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2011;52(7):848–55. [DOI] [PubMed]
- 75.Ciobotaro P, Oved M, Nadir E, Bardenstein R, Zimhony O. An effective intervention to limit the spread of an epidemic carbapenem-resistant Klebsiella pneumoniae strain in an acute care setting: from theory to practice. Am J Infect Control. 2011;39(8):671–677. doi: 10.1016/j.ajic.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 76.Viale P, Tumietto F, Giannella M, Bartoletti M, Tedeschi S, Ambretti S, et al. Impact of a hospital-wide multifaceted programme for reducing carbapenem-resistant Enterobacteriaceae infections in a large teaching hospital in northern Italy. Clin Microbiol Infect. 2015;21(3):242–247. doi: 10.1016/j.cmi.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 77.Gagliotti C, Cappelli V, Carretto E, Marchi M, Pan A, Ragni P, et al. Control of carbapenemase-producing Klebsiella pneumoniae: a region-wide intervention. Euro Surv: bulletin Europeen sur les maladies transmissibles. Eur Commun Dis Bull. 2014;19(43). doi:10.2807/1560-7917.ES2014.19.43.20943(Epub 2014/11/07). [DOI] [PubMed]
- 78.Price R, MacLennan G, Glen J. Selective digestive or oropharyngeal decontamination and topical oropharyngeal chlorhexidine for prevention of death in general intensive care: systematic review and network meta-analysis. BMJ. 2014;348:g2197. doi: 10.1136/bmj.g2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taylor ME, Oppenheim BA. Selective decontamination of the gastrointestinal tract as an infection control measure. J Hosp Infect. 1991;17(4):271–278. doi: 10.1016/0195-6701(91)90271-9. [DOI] [PubMed] [Google Scholar]
- 80.Decre D, Gachot B, Lucet JC, Arlet G, Bergogne-Berezin E, Regnier B. Clinical and bacteriologic epidemiology of extended-spectrum beta-lactamase-producing strains of Klebsiella pneumoniae in a medical intensive care unit. Clin Infect Dis. 1998;27(4):834–844. doi: 10.1086/514938. [DOI] [PubMed] [Google Scholar]
- 81.Agusti C, Pujol M, Argerich MJ, Ayats J, Badia M, Dominguez MA, et al. Short-term effect of the application of selective decontamination of the digestive tract on different body site reservoir ICU patients colonized by multi-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2002;49(1):205–208. doi: 10.1093/jac/49.1.205. [DOI] [PubMed] [Google Scholar]
- 82.Lubbert C, Faucheux S, Becker-Rux D, Laudi S, Durrbeck A, Busch T, et al. Rapid emergence of secondary resistance to gentamicin and colistin following selective digestive decontamination in patients with KPC-2-producing Klebsiella pneumoniae: a single-centre experience. Int J Antimicrob Agents. 2013;42(6):565–570. doi: 10.1016/j.ijantimicag.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 83.Brun-Buisson C, Legrand P, Rauss A, Richard C, Montravers F, Besbes M, et al. Intestinal decontamination for control of nosocomial multiresistant gram-negative bacilli. Study of an outbreak in an intensive care unit. Ann Intern Med. 1989;110(11):873–881. doi: 10.7326/0003-4819-110-11-873. [DOI] [PubMed] [Google Scholar]
- 84.Zuckerman T, Benyamini N, Sprecher H, Fineman R, Finkelstein R, Rowe JM, et al. SCT in patients with carbapenem resistant Klebsiella pneumoniae: a single center experience with oral gentamicin for the eradication of carrier state. Bone Marrow Transplant. 2011;46(9):1226–1230. doi: 10.1038/bmt.2010.279. [DOI] [PubMed] [Google Scholar]
- 85.Saidel-Odes L, Polachek H, Peled N, Riesenberg K, Schlaeffer F, Trabelsi Y, et al. A randomized, double-blind, placebo-controlled trial of selective digestive decontamination using oral gentamicin and oral polymyxin E for eradication of carbapenem-resistant Klebsiella pneumoniae carriage. Infect Control Hosp Epidemiol. 2012;33(1):14–19. doi: 10.1086/663206. [DOI] [PubMed] [Google Scholar]
- 86.Oren I, Sprecher H, Finkelstein R, Hadad S, Neuberger A, Hussein K, et al. Eradication of carbapenem-resistant Enterobacteriaceae gastrointestinal colonization with nonabsorbable oral antibiotic treatment: a prospective controlled trial. Am J Infect Control. 2013;41(12):1167–1172. doi: 10.1016/j.ajic.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 87.Tascini C, Sbrana F, Flammini S, Tagliaferri E, Arena F, Leonildi A, et al. Oral gentamicin gut decontamination for prevention of KPC-producing Klebsiella pneumoniae infections: relevance of concomitant systemic antibiotic therapy. Antimicrob Agents Chemother. 2014;58(4):1972–1976. doi: 10.1128/AAC.02283-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Giannella M, Bartoletti M, Morelli MC, Tedeschi S, Cristini F, Tumietto F, et al. Risk factors for infection with carbapenem-resistant Klebsiella pneumoniae after liver transplantation: the importance of pre- and posttransplant colonization. Am J Transplant. 2015;15(6):1708–1715. doi: 10.1111/ajt.13136. [DOI] [PubMed] [Google Scholar]
- 89.Giannella M, Morelli MC, Cristini F, Ercolani G, Cescon M, Bartoletti M, et al. Carbapenem-resistant Klebsiella pneumoniae colonization at liver transplantation: a management challenge. Liver Transpl. 2014;20(5):631–633. doi: 10.1002/lt.23857. [DOI] [PubMed] [Google Scholar]
- 90.Oostdijk EA, de Smet AM, Bonten MJ, Dutch SODSDDtg Effects of decontamination of the digestive tract and oropharynx in intensive care unit patients on 1-year survival. Am J Respir Crit Care Med. 2013;188(1):117–120. doi: 10.1164/rccm.201209-1733LE. [DOI] [PubMed] [Google Scholar]
- 91.Kollef MH, Micek ST. Rational use of antibiotics in the ICU: balancing stewardship and clinical outcomes. JAMA. 2014;312(14):1403–1404. doi: 10.1001/jama.2014.8427. [DOI] [PubMed] [Google Scholar]
- 92.Dellit TH, Owens RC, McGowan JE, Jr, Gerding DN, Weinstein RA, Burke JP, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2007;44(2):159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 93.Sarma JB, Marshall B, Cleeve V, Tate D, Oswald T, Woolfrey S. Effects of fluoroquinolone restriction (from 2007 to 2012) on Clostridium difficile infections: interrupted time-series analysis. J Hosp Infect. 2015;91(1):74–80. doi: 10.1016/j.jhin.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 94.Sarma JB, Marshall B, Cleeve V, Tate D, Oswald T, Woolfrey S. Effects of fluoroquinolone restriction (from 2007 to 2012) on resistance in Enterobacteriaceae: interrupted time-series analysis. J Hosp Infect. 2015;91(1):68–73. doi: 10.1016/j.jhin.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 95.Davey P, Brown E, Charani E, Fenelon L, Gould IM, Holmes A, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;4:CD003543. doi: 10.1002/14651858.CD003543.pub3. [DOI] [PubMed] [Google Scholar]
- 96.Nguyen M, Eschenauer GA, Bryan M, O’Neil K, Furuya EY, Della-Latta P, et al. Carbapenem-resistant Klebsiella pneumoniae bacteremia: factors correlated with clinical and microbiologic outcomes. Diagn Microbiol Infect Dis. 2010;67(2):180–184. doi: 10.1016/j.diagmicrobio.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 97.Neuner EA, Yeh JY, Hall GS, Sekeres J, Endimiani A, Bonomo RA, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn Microbiol Infect Dis. 2011;69(4):357–362. doi: 10.1016/j.diagmicrobio.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, Ranellou K, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect: Off Publ Eur Soc Clin Microbiol Infect Dis. 2011;17(12):1798–1803. doi: 10.1111/j.1469-0691.2011.03514.x. [DOI] [PubMed] [Google Scholar]