Abstract
BACKGROUND
Data from randomized trials are lacking on the benefits and risks of initiating antiretroviral therapy in patients with asymptomatic human immunodeficiency virus (HIV) infection who have a CD4+ count of more than 350 cells per cubic millimeter.
METHODS
We randomly assigned HIV-positive adults who had a CD4+ count of more than 500 cells per cubic millimeter to start antiretroviral therapy immediately (immediate-initiation group) or to defer it until the CD4+ count decreased to 350 cells per cubic millimeter or until the development of the acquired immunodeficiency syndrome (AIDS) or another condition that dictated the use of antiretroviral therapy (deferred-initiation group). The primary composite end point was any serious AIDS-related event, serious non–AIDS-related event, or death from any cause.
RESULTS
A total of 4685 patients were followed for a mean of 3.0 years. At study entry, the median HIV viral load was 12,759 copies per milliliter, and the median CD4+ count was 651 cells per cubic millimeter. On May 15, 2015, on the basis of an interim analysis, the data and safety monitoring board determined that the study question had been answered and recommended that patients in the deferred-initiation group be offered antiretroviral therapy. The primary end point occurred in 42 patients in the immediate-initiation group (1.8%; 0.60 events per 100 person-years), as compared with 96 patients in the deferred-initiation group (4.1%; 1.38 events per 100 person-years), for a hazard ratio of 0.43 (95% confidence interval [CI], 0.30 to 0.62; P<0.001). Hazard ratios for serious AIDS-related and serious non–AIDS-related events were 0.28 (95% CI, 0.15 to 0.50; P<0.001) and 0.61 (95% CI, 0.38 to 0.97; P = 0.04), respectively. More than two thirds of the primary end points (68%) occurred in patients with a CD4+ count of more than 500 cells per cubic millimeter. The risks of a grade 4 event were similar in the two groups, as were the risks of unscheduled hospital admissions.
CONCLUSIONS
The initiation of antiretroviral therapy in HIV-positive adults with a CD4+ count of more than 500 cells per cubic millimeter provided net benefits over starting such therapy in patients after the CD4+ count had declined to 350 cells per cubic millimeter. (Funded by the National Institute of Allergy and Infectious Diseases and others; START ClinicalTrials.gov number, NCT00867048.)
The immune compromise caused by the human immunodeficiency virus (HIV) is characterized by a loss of CD4+ T cells. Rates of HIV-associated complications and death increase as the number of these cells in peripheral blood (CD4+ count) declines.1-3 It has been general practice to defer the initiation of antiretroviral therapy in asymptomatic patients with a CD4+ count above a certain threshold level. The applicable threshold has changed over time, and recommendations remain inconsistent across various guidelines.4
Randomized studies that have assessed the benefits and risks of treating patients with HIV infection sooner rather than later have largely enrolled patients with a CD4+ count of less than 500 cells per cubic millimeter. In such studies, “later” has been defined as a CD4+ count of 200 or 250 cells per cubic millimeter.5-8 These data, along with observational studies, provide strong evidence for the initiation of antiretroviral therapy in patients with a CD4+ count of 350 cells per cubic millimeter.
Evidence for initiating antiretroviral therapy in patients with a CD4+ count of more than 350 cells per cubic millimeter comes mainly from the results of observational studies.9-13 However, the findings of these studies are inconsistent and are subject to residual confounding.14-16 Furthermore, most studies have focused only on the risks of the acquired immunodeficiency syndrome (AIDS) and death and have not fully addressed the risks and benefits of initiating antiretroviral therapy in patients with a high CD4+ count, in whom complications and death are largely attributed to non–AIDS-related events.17,18 Some studies have raised concern about the adverse effects of antiretroviral therapy on cardiovascular and renal disease,19,20 particularly in an aging HIV-positive population. However, in an earlier large, randomized study, the continuous use of anti-retroviral therapy, as compared with intermittent therapy, reduced these risks.17,18
Given the small absolute risk of AIDS among patients with a high CD4+ count, it is important to establish whether it is safe and beneficial to initiate antiretroviral therapy in asymptomatic patients who have a CD4+ count that is much higher than 350 cells per cubic millimeter. This information is particularly important given the known benefits of antiretroviral therapy in reducing infectivity.7,21-24
In response to this gap in evidence, we designed a multicontinental randomized study, Strategic Timing of Antiretroviral Therapy (START), to determine the risks and benefits of the immediate initiation of antiretroviral therapy in asymptomatic HIV-positive patients who have a CD4+ count of more than 500 cells per cubic millimeter, as compared with deferring initiation until the CD4+ count is 350 cells per cubic millimeter.
METHODS
The START trial was designed and conducted by the International Network for Strategic Initiatives in Global HIV Trials (INSIGHT). A description of the contributions of the study members is provided in Section 2 in the Supplementary Appendix, available with the full text of this article at NEJM.org.
STUDY PATIENTS
HIV-positive patients who were 18 years of age or older and who had not yet initiated antiretroviral therapy, had no history of AIDS, and were in generally good health were eligible for the study if they had had two CD4+ counts of more than 500 cells per cubic millimeter at least 2 weeks apart within 60 days before enrollment. Women who were pregnant or breast-feeding at screening were not eligible; women who became pregnant during follow-up remained in the study. (For full details regarding the study design, see the study protocol, available at NEJM.org.) The study was approved by the institutional review board or ethics committee at each participating site, and written informed consent was obtained from all patients.
STUDY DESIGN
We evaluated two strategies for initiating anti-retroviral therapy: immediate initiation and deferred initiation until the CD4+ count declined to 350 cells per cubic millimeter or the development of an AIDS-related event or another condition that dictated the use of antiretroviral therapy (e.g., pregnancy).25,26 When the study was initiated in 2009, the deferral strategy was consistent with recommendations in most treatment guidelines.27-30 During the course of the study, some treatment guidelines changed. Because the evidence prompting these changes was limited, we did not revise the deferred-initiation strategy in our study.
The study protocol required the use of an approved drug combination derived from the guidelines of the Department of Health and Human Services as the first antiretroviral regimen in the two study groups (Section 3 in the Supplementary Appendix).27
STUDY END POINTS
The primary end point was a composite outcome that included two major components. The first was any serious AIDS-related event, which included death from AIDS or any AIDS-defining event (as outlined in the 1993 expanded surveillance document of the Centers for Disease Control and Prevention),31 with the exception of nonfatal herpes simplex virus infection and esophageal candidiasis, which were not counted because of their lesser severity. Hodgkin's lymphoma was also counted as a serious AIDS-related event. The second component was any serious non–AIDS-related event, including death from causes other than AIDS. Serious non–AIDS-related events consisted of the following conditions: cardiovascular disease (myocardial infarction, stroke, or coronary revascularization) or death from cardiovascular disease, end-stage renal disease (initiation of dialysis or renal transplantation) or death from renal disease, liver disease (decompensated liver disease) or death from liver disease, non– AIDS-defining cancer (except for basal-cell or squamous-cell skin cancer) or death from cancer, and any death not attributable to AIDS.
An end-point review committee whose members were unaware of study-group assignments reviewed all reported serious AIDS-related and serious non–AIDS-related events and deaths using preestablished criteria.32 Events that the committee considered to be confirmed or probable were counted as end points.33,34
Secondary end points included serious AIDS-related events, serious non–AIDS-related events, death from any cause, grade 4 events, and un-scheduled hospitalizations for reasons other than AIDS. Grade 4 events were defined as potentially life-threatening symptomatic events not attributable to AIDS that required a medical intervention.35 Grade 4 events and unscheduled hospitalizations (also referred to as “other serious clinical events”) were reported regardless of antiretroviral-therapy use or of any perceived association with antiretroviral therapy and were categorized according to codes used in the Medical Dictionary for Regulatory Activities, version 18.0.
The design and data collection plan for START have been reported previously.25 Key features, including a summary of a planned sample-size reestimation in February 2013, are provided in Section 4 in the Supplementary Appendix.
INTERIM MONITORING
Study investigators were unaware of interim summary results throughout the study. An independent data and safety monitoring board reviewed the results of interim analyses. An O'Brien–Fleming boundary and the Lan–DeMets spending function based on information time (fraction of primary events accrued) were used to adjust for a type I error in the analysis of the primary end point.36,37 The data and safety monitoring board was also asked to consider the consistency of findings for the two major components of the primary end point, serious AIDS-related and serious non–AIDS-related events, before making a recommendation to stop or modify the study. Consistency was specified as treatment hazard ratios for each component favoring the same treatment group, with a z statistic of more than 1.5.
On May 15, 2015, the board informed the National Institute of Allergy and Infectious Diseases (NIAID) and the study leadership that the primary question of the study had been answered and recommended that the findings be immediately disseminated. The board also recommended offering antiretroviral therapy to patients in the deferred-initiation group who were not receiving antiretroviral therapy and continuing to follow the study patients. By the time of this review, approximately 60% of the planned 213 primary events had occurred. On May 27, 2015, the study team leaders and NIAID notified investigators and patients of the findings.
STATISTICAL ANALYSIS
We compared the two study groups according to the intention-to-treat principle. We used time-to-event methods, including Kaplan–Meier survival curves and Cox proportional-hazards models, to compare the two groups for the primary end point, its two major components, death from any cause, and other serious clinical events. Follow-up was censored on May 26, 2015, or the date of last study contact.
For the primary end point, hazard ratios and 95% confidence intervals were estimated from a Cox model stratified according to six geographic regions (Africa, Europe and Israel, North America, South America and Mexico, Australia, and Asia) with a single binary indicator (immediate vs. deferred therapy). The proportional-hazards assumption was tested by including an interaction term between the randomized treatment indicator and log-transformed follow-up time.
We performed sensitivity analyses to assess the effects of the diagnostic certainty of events and of missing data (Section 5 in the Supplementary Appendix). The primary end point was also summarized for subgroups that were categorized according to eight predefined baseline characteristics. Heterogeneity of the treatment effect across subgroups was assessed by including terms for interactions between treatment and subgroup variables in expanded Cox models. Results of subgroup analyses should be interpreted with caution because there was no adjustment made for the type I error for the number of subgroups examined, and the statistical power was limited for subgroup analyses. Kaplan– Meier estimates were used to describe the time until the initiation of antiretroviral therapy. We compared the two study groups for changes in the CD4+ count from study entry through follow-up using longitudinal mixed models with random intercepts. Descriptive analyses (number and rate of primary end point) were performed for accumulated person-years within strata of specific, time-updated (latest) CD4+ counts during follow-up. Statistical analyses were performed with the use of SAS software, version 9.3 (SAS Institute). All P values are two-sided.
RESULTS
STUDY PATIENTS
From April 2009 through December 2013, we randomly assigned 4685 patients to receive immediate antiretroviral therapy (2326 patients) or deferred antiretroviral therapy (2359 patients) at 215 sites in 35 countries38 (Section 1 in the Supplementary Appendix). The two study groups were well balanced at baseline (Table 1). The median age was 36 years, and 27% of the patients were women. The median CD4+ count was 651 cells per cubic millimeter, with counts as high as 2296 cells per cubic millimeter. The median HIV RNA viral load was 12,759 copies per milliliter.
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | Immediate-Initiation Group (N = 2326) | Deferred-Initiation Group (N = 2359) | All Patients (N =4685) |
|---|---|---|---|
| Median age (IQR) — yr | 36 (29–44) | 36 (29–44) | 36 (29–44) |
| Female sex — no. (%) | 624 (26.8) | 633 (26.8) | 1,257 (26.8) |
| Race or ethnic group — no. (%)† | |||
| Asian | 198 (8.5) | 190 (8.1) | 388 (8.3) |
| Black | 702 (30.2) | 708 (30.0) | 1,410 (30.1) |
| Latino or Hispanic | 320 (13.8) | 318 (13.5) | 638 (13.6) |
| White | 1,015 (43.6) | 1,071 (45.4) | 2,086 (44.5) |
| Other | 91 (3.9) | 72 (3.1) | 163 (3.5) |
| Geographical region — no. (%) | |||
| Africa | 499 (21.5) | 501 (21.2) | 1,000 (21.3) |
| Asia | 179 (7.7) | 177 (7.5) | 356 (7.6) |
| Australia | 56 (2.4) | 53 (2.2) | 109 (2.3) |
| Europe and Israel | 763 (32.8) | 776 (32.9) | 1,539 (32.8) |
| North America | 248 (10.7) | 259 (11.0) | 507 (10.8) |
| South America and Mexico | 581 (25.0) | 593 (25.1) | 1,174 (25.1) |
| Mode of infection with HIV — no. (%) | |||
| Sexual contact | |||
| Men having sex with men | 1,300 (55.9) | 1,286 (54.5) | 2,586 (55.2) |
| With person of opposite sex | 873 (37.5) | 917 (38.9) | 1,790 (38.2) |
| Injection-drug use | 37 (1.6) | 27 (1.1) | 64 (1.4) |
| Blood products, other, or unknown | 116 (5.0) | 129 (5.5) | 245 (5.2) |
| Median time since HIV diagnosis (IQR) — yr | 1.0 (0.4–3.0) | 1.1 (0.4–3.1) | 1.0 (0.4–3.1) |
| Median CD4+ count (IQR) — cells/mm3‡ | 651 (585–765) | 651 (582–764) | 651 (584–765) |
| Median HIV RNA (IQR) – copies/ml | 13,000 (3133–43,808) | 12,550 (2963–42,567) | 12,759 (3019–43,391) |
| Current smoker — no. (%) | 730 (31.4) | 766 (32.5) | 1,496 (31.9) |
| Median CHD risk at 10 yr (IQR) — %§ | 1.9 (0.5–5.0) | 1.9 (0.5–5.3) | 1.9 (0.5–5.1) |
There were no significant differences between the two groups at baseline. HIV denotes human immunodeficiency virus, and IQR interquartile range.
Race or ethnic group was self-reported.
The median CD4+ count is based on an average of two CD4+ counts for each patient obtained at screening.
The 10-year risk of coronary heart disease (CHD) was calculated with the use of the risk-assessment tool from the Framingham Heart Study.
FOLLOW-UP
The mean follow-up time was 3.0 years, and the median was 2.8 years (interquartile range, 2.1 to 3.9); 23% of the patients were followed for more than 4 years. On May 26, 2015, the status with regard to the primary end point was unknown (which was defined as a lack of contact for at least 10 months) for 93 patients (4.0%) in the immediate-initiation group and 119 (5.0%) in the deferred-initiation group (Fig. S1 in the Supplementary Appendix).
USE OF ANTIRETROVIRAL THERAPY, HIV RNA LEVELS, AND CD4+ COUNTS
As of May 26, 2015, antiretroviral therapy had been started in 98% of patients in the immediate-initiation group and in 48% of those in the deferred-initiation group. The median CD4+ count at the time of the initiation of antiretroviral therapy in the deferred-initiation group was 408 cells per cubic millimeter. Reasons for starting antiretroviral therapy in the deferred-initiation group are provided in Table S1 in the Supplementary Appendix.
Patients received antiretroviral therapy for 94% of the total follow-up time accrued in the immediate-initiation group and for 28% in the deferred-initiation group. The median time until the initiation of antiretroviral therapy in the deferred-initiation group was 3 years (Fig. 1A, and Fig. S2 in the Supplementary Appendix). Drugs that were primarily used for initial treatment in the immediate-initiation group and the deferred-initiation group were tenofovir (89% in the two groups), emtricitabine (89% and 88%, respectively), and efavirenz (73% and 51%, respectively) (Table S2 in the Supplementary Appendix).
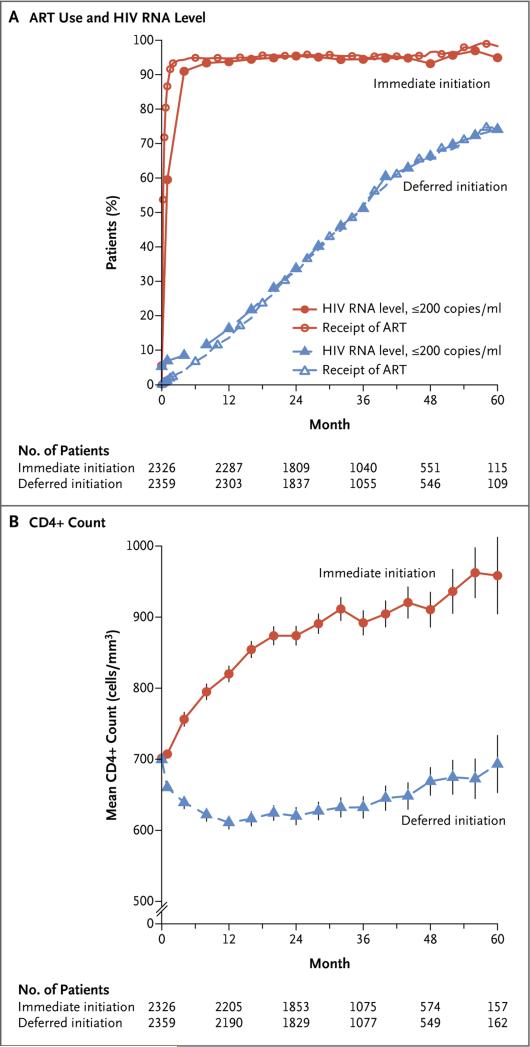
Figure 1. Antiretroviral Therapy, HIV RNA Suppression, and CD4+ Count.
Shown are the percentages of patients who were receiving antiretroviral therapy (ART) and the percentages of patients who had an HIV RNA level of less than 200 copies per milliliter (Panel A) and the mean CD4+ count (Panel B) in the immediate-initiation group and the deferred-initiation group over a 5-year period. The vertical lines around the data points in Panel B indi cate 95% confidence intervals. Once ART was initiated, appropriate changes in regimens were mandated in cases of treatment-limiting adverse drug reactions or if the existing regimen did not fully suppress viral replication. Additional details regarding study-specified initial regimens of antiretroviral therapy and the frequency of specific antiretroviral drugs that were used in the initial regimens are provided in Section 3 and Table S2 in the Supplementary Appendix.
The percentage of patients who had an HIV RNA level of 200 copies per milliliter or less during follow-up mirrored the percentage who were receiving antiretroviral therapy (Fig. 1A). The median HIV RNA level at time of the initiation of antiretroviral therapy was lower in the immediate-initiation group than in the deferred-initiation group (13,462 and 41,525 copies per milliliter, respectively), but the percentages of patients with full viral suppression by 12 months after initiation were similar (98% vs. 97%).
Average CD4+ counts increased markedly during the first year after randomization in the immediate-initiation group and continued to gradually increase thereafter (Fig. 1B). Conversely, in the deferred-initiation group, average CD4+ counts decreased during the first year and then stabilized and subsequently increased slightly as more patients started to receive antiretroviral therapy. During the follow-up period, the average CD4+ count was 194 cells per cubic millimeter higher in the immediate-initiation group than in the deferred-initiation group (Fig. S3 in the Supplementary Appendix).
STUDY END POINTS
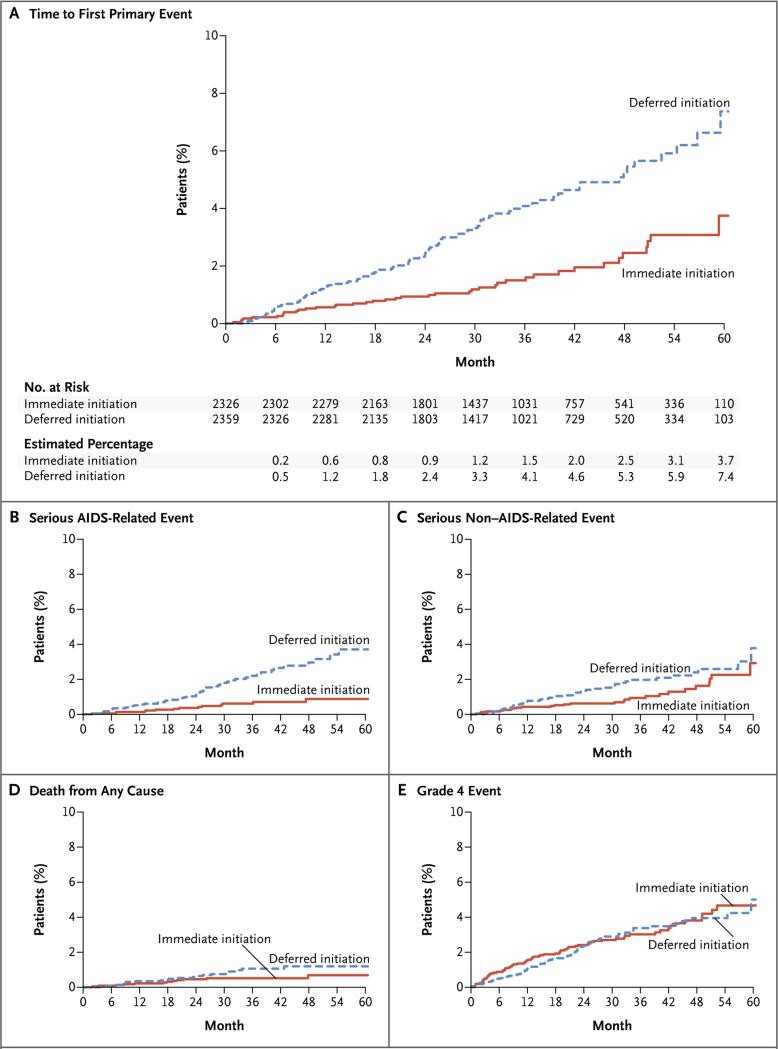
The composite primary end point was reported in 42 patients in the immediate-initiation group and in 96 in the deferred-initiation group (Table 2 and Fig. 2A). The estimated hazard ratio in the immediate-initiation group, as compared with the deferred-initiation group, was 0.43 (95% confidence interval [CI], 0.30 to 0.62; P<0.001) and did not vary significantly during the follow-up period (P = 0.77 by proportional-hazards testing). Sensitivity analyses showed similar estimates for the hazard ratio (Section 5 in the Supplementary Appendix).
Table 2.
Primary and Secondary End Points.*
| End Point | Immediate-Initiation Group (N = 2326) | Deferred-Initiation Group (N = 2359) | Hazard Ratio (95% CI)† | P Value | ||
|---|---|---|---|---|---|---|
| no. | no./100 person-yr | no. | no./100 person-yr | |||
| Composite primary end point | 42 | 0.60 | 96 | 1.38 | 0.43 (0.30–0.62) | <0.001 |
| Components of the primary end point | ||||||
| Serious AIDS-related event | 14 | 0.20 | 50 | 0.72 | 0.28 (0.15–0.50) | <0.001 |
| Serious non–AIDS-related event | 29 | 0.42 | 47 | 0.67 | 0.61 (0.38–0.97) | 0.04 |
| Death from any cause | 12 | 0.17 | 21 | 0.30 | 0.58 (0.28–1.17) | 0.13 |
| Tuberculosis | 6 | 0.09 | 20 | 0.28 | 0.29 (0.12–0.73) | 0.008 |
| Kaposi's sarcoma | 1 | 0.01 | 11 | 0.16 | 0.09 (0.01–0.71) | 0.02 |
| Malignant lymphoma | 3 | 0.04 | 10 | 0.14 | 0.30 (0.08–1.10) | 0.07 |
| Cancer not related to AIDS | 9 | 0.13 | 18 | 0.26 | 0.50 (0.22–1.11) | 0.09 |
| Cardiovascular disease | 12 | 0.17 | 14 | 0.20 | 0.84 (0.39–1.81) | 0.65 |
| Other secondary end points | ||||||
| Grade 4 event‡ | 73 | 1.06 | 73 | 1.05 | 1.01 (0.73–1.39) | 0.97 |
| Unscheduled hospitalization§ | 262 | 4.02 | 287 | 4.40 | 0.91 (0.77–1.08) | 0.28 |
| Grade 4 event, unscheduled hospitalization, or death from any cause | 283 | 4.36 | 311 | 4.78 | 0.91 (0.77–1.07) | 0.25 |
| Most common grade 4 events, unscheduled hospitalization, or death from any cause¶ | ||||||
| Bacterial infectious disorder | 14 | 0.20 | 36 | 0.52 | 0.38 (0.20–0.70) | 0.002 |
| Bone or joint injury | 17 | 0.24 | 11 | 0.16 | 1.55 (0.73–3.31) | 0.26 |
| Depressed mood disorder or disturbance | 12 | 0.17 | 9 | 0.13 | 1.34 (0.57–3.19) | 0.50 |
| Infection with unspecified pathogen | 64 | 0.93 | 65 | 0.94 | 0.99 (0.70–1.40) | 0.96 |
| Injury not elsewhere classified | 11 | 0.16 | 22 | 0.31 | 0.50 (0.24–1.03) | 0.06 |
| Suicidal or self-injurious behavior not elsewhere classified | 27 | 0.39 | 24 | 0.34 | 1.15 (0.66–1.99) | 0.63 |
| Viral infectious disorder | 12 | 0.17 | 15 | 0.21 | 0.81 (0.38–1.72) | 0.58 |
| Grade 4 event, unscheduled hospitalization, or primary end point | 295 | 4.56 | 355 | 5.52 | 0.82 (0.71–0.96) | 0.01 |
The primary end point was a composite of serious AIDS-related and serious non–AIDS-related events, including death from any cause. Person-years differ for each row since the “time to first event” differs slightly according to event type. The numbers of serious AIDS-related and serious non–AIDS-related events do not sum to the total number of primary events because one patient in each group had a serious AIDS-related event first and later died from causes other than AIDS (a serious non–AIDS-related event). Additional details regarding the full composition of the primary end point are provided in Table S3 in the Supplementary Appendix.
The hazard ratio is for the immediate-initiation group as compared with the deferred-initiation group.
Listed are symptomatic grade 4 events, according to the Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events.35
This category excludes hospitalizations for AIDS-related illnesses.
The cited subcategories of grade 4 events, unscheduled hospitalizations, or deaths from any cause are high-level group terms from the Medical Dictionary for Regulatory Activities, version 18.0, that were reported in at least 20 patients in the two study groups.
Figure 2. Primary and Secondary End Points.
Shown are Kaplan–Meier estimates of the cumulative percentages of patients with the composite primary end point (a serious AIDS-related or serious non–AIDS-related event, including death) in the two study groups (Panel A). Secondary end points included serious AIDS-related events (Panel B), serious non–AIDS-related events (Panel C), death from any cause (Panel D), and grade 4 events (Panel E). Grade 4 events were defined as potentially life-threatening symptomatic events that were not attributable to AIDS and that required medical intervention.
Among the individual events included in the composite primary end point, the three most common events in the immediate-initiation group and the deferred-initiation group were cardiovascular disease (29% and 15%, respectively), non–AIDS-defining cancer (21% and 19%, respectively), and tuberculosis (14% and 20%, respectively) (Table S3 in the Supplementary Appendix). Of the 42 primary end points, only 4 (10%) occurred before the initiation of antiretroviral therapy in the immediate-initiation group, as compared with 68 of 96 (71%) in the deferred-initiation group.
In the comparison between the immediate-initiation group and the deferred-initiation group, the estimated hazard ratio was 0.28 (95% CI, 0.15 to 0.50; P<0.001) for a serious AIDS-related event, 0.61 (95% CI, 0.38 to 0.97; P = 0.04) for a serious non–AIDS-related event, and 0.58 (95% CI, 0.28 to 1.17; P = 0.13) for death from any cause (Table 2 and Fig. 2B, 2C, and 2D). Of the 33 deaths, 20 (61%) were attributable to causes other than AIDS, cardiovascular disease, renal disease, liver disease, or cancer (Table S4 in the Supplementary Appendix).
Hazard ratios for the three most frequent serious AIDS-related events (tuberculosis, Kaposi's sarcoma, and malignant lymphomas) are provide in Table 2. Most of the tuberculosis events (16 of 26) occurred in patients living in Africa. For the two most frequent serious non–AIDS- related events (non-AIDS cancer and cardiovascular disease), the estimated hazard ratios were 0.50 (95% CI, 0.22 to 1.11; P = 0.09) and 0.84 (95% CI, 0.39 to 1.81; P = 0.65), respectively. Most of the cancers and cardiovascular events occurred in patients from Australia, Europe, Israel, and the United States (22 of 27 and 19 of 26, respectively). The hazard ratio for cancer (combining AIDS and non-AIDS cancers) was 0.36 (95% CI, 0.19 to 0.66; P = 0.001). (Table S3 in the Supplementary Appendix summarizes the types of cancer.)
OTHER SERIOUS CLINICAL EVENTS
Symptomatic grade 4 events occurred in 73 patients in each study group (hazard ratio, 1.01; 95% CI, 0.73 to 1.39; P = 0.97) (Table 2 and Fig. 2E). Unscheduled hospitalizations for reasons other than events associated with AIDS progression occurred in 262 patients in the immediate-initiation group and 287 in the deferred-initiation group (hazard ratio, 0.91; 95% CI, 0.77 to 1.08; P = 0.28) (Table 2). In an analysis of the most common types of events in a composite end point of grade 4 events, unscheduled hospitalizations, or death, the rate of bacterial infections was significantly reduced in the immediate-initiation group (P=0.002); rates of other common events did not differ significantly between the groups. For a composite end point that included grade 4 events, unscheduled hospitalizations, and the primary end point as an overall measure of clinical benefit, there were 295 patients in the immediate-initiation group and 355 in the deferred-initiation group who had an event (hazard ratio, 0.82; 95% CI, 0.71 to 0.96; P = 0.01). Sixteen suspected, unexpected serious adverse reactions were reported among patients receiving antiretroviral therapy (Table S5 in the Supplementary Appendix).
SUBGROUP ANALYSES
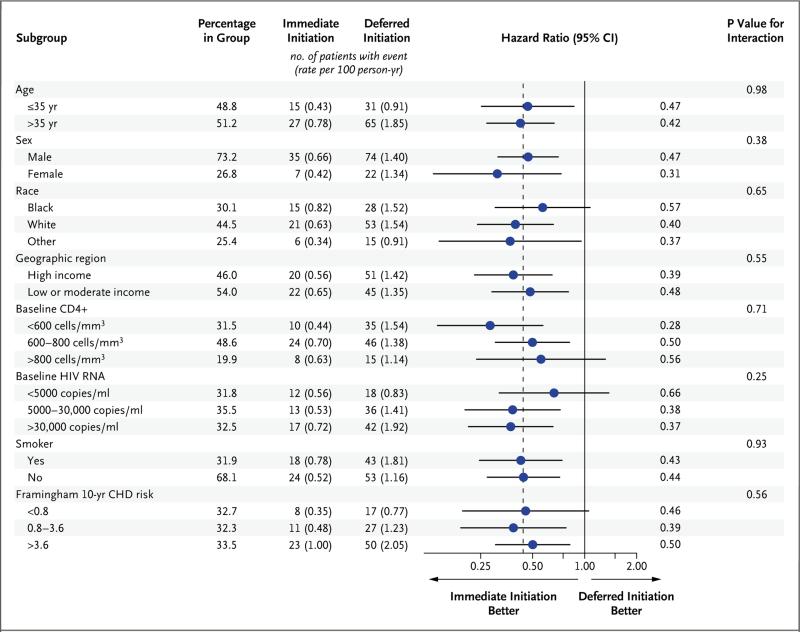
The study population was divided into subgroups according to characteristics at study entry, including demographic measures and other risk factors for serious AIDS-related and serious non–AIDS-related disease. Across all subgroups, hazard ratios consistently favored the immediate-initiation group (Fig. 3).
Figure 3. Subgroup Analyses for the Primary End Point.
For subgroups that were defined according to age, CD4+ count, HIV RNA level, and risk of coronary heart disease (CHD), the continuous variables were used for interaction tests. For 71 patients (1.5%), the Framingham Heart Study risk of CHD could not be calculated because of missing data. Of the patients with missing data, the primary end point occurred in 2 in the deferred-initiation group.
PRIMARY END POINT ACCORDING TO LATEST CD4+ COUNT
As intended, the percentage of follow-up time that patients spent in various categories of latest CD4+ count differed markedly according to study group (Fig. S4 in the Supplementary Appendix). In the two groups, the majority of primary events occurred when the CD4+ count was greater than 500 cells per cubic millimeter, in 37 of 42 patients (88%; rate of 0.6 per 100 person-years) in the immediate-initiation group and in 57 of 96 (59%; rate of 1.1 per 100 person-years) in the deferred-initiation group. The percentage of follow-up time in which patients had a CD4+ count of more than 500 cells per cubic millimeter was 94% in the immediate-initiation group and 72% in the deferred-initiation group. During this time with high latest CD4+ counts, antiretroviral therapy was administered for 95% of the person-years accrued in the immediate-initiation group and for 27% of the person-years in the deferred-initiation group.
Consistent with the planned deferral strategy, latest CD4+ counts were below 350 cells per cubic millimeter for only 4% of follow-up time in the deferred-initiation group. During this time, 5 primary events occurred (Fig. S4 in the Supplementary Appendix).
DISCUSSION
In this large, international, randomized study involving previously untreated HIV-positive adults with a CD4+ count of more than 500 cells per cubic millimeter, the immediate initiation of antiretroviral therapy was superior to deferral of such therapy until the CD4+ count declined to 350 cells per cubic millimeter. A beneficial effect of immediate antiretroviral therapy was evident for both serious AIDS-related and serious non– AIDS-related events, and no increased rate of adverse effects associated with this strategy was observed. There was no evidence that the beneficial effect of immediate antiretroviral therapy differed according to age, sex, race, region of the world, CD4+ count, viral load, or risk factors for serious non-AIDS diseases.
In contrast to our study, previous randomized studies enrolled patients with lower CD4+ counts and compared immediate antiretroviral therapy with deferred therapy when counts reached 250 or 200 cells per cubic millimeter.5-8 These trials established that antiretroviral therapy should be started before the CD4+ count declined to 250 cells per cubic millimeter. Since rates of AIDS-related and non–AIDS-related events are low when CD4+ counts are more than 500 cells per cubic millimeter (as demonstrated in this study), it was important that the risks and benefits of antiretroviral therapy should be reliably established because even unusual drug-related adverse events could offset reductions in AIDS-related events. In our study, the benefit of immediate antiretroviral therapy over deferred therapy was quantified, and safety was assessed across various outcomes, including a diverse set of non-AIDS conditions. Our results indicate that antiretroviral therapy should be recommended for patients in whom HIV has been diagnosed regardless of the CD4+ count.
Coupled with previous studies showing that antiretroviral therapy reduces the risk of sexual transmission of HIV,7,21 the results of our study provide policymakers, clinicians, and HIV-positive patients with data to inform policies regarding the initiation of antiretroviral therapy. Patients may wish to consider and differently weigh multiple factors when making the decision with their clinician to initiate lifelong anti-retroviral therapy. Although the relative reduction in the risk of the primary end point was large in the immediate-initiation group, the absolute differences were fairly small for most subgroups, which may mean that some low-risk patients may still choose to defer antiretroviral therapy.39 Conversely, some patients may want to start antiretroviral therapy primarily to reduce transmission risk.39 The individual health benefit from antiretroviral therapy that is shown in this study should better inform such choices.
In designing this study, we hypothesized that immediate antiretroviral therapy would have a greater benefit for serious AIDS-related events than for serious non–AIDS-related events. The 72% relative reduction in serious AIDS-related events in the immediate-initiation group largely arose from reductions in rates of tuberculosis, Kaposi's sarcoma, and malignant lymphomas, whereas the 39% relative reduction in serious non–AIDS-related events was largely due to non–AIDS-defining cancers. The effects of immediate anti-retroviral therapy on AIDS and non-AIDS events were seen even though more patients in the deferred-initiation group than we anticipated started antiretroviral therapy when they had a CD4+ count of more than 350 per cubic millimeter.25
Our findings have global relevance, since the study was conducted in 215 clinics in 35 countries and represents a broad population of HIV-positive patients. Although specific outcomes differed according to geographic region, the benefits of immediate antiretroviral therapy were consistent. Tuberculosis was more frequent in Africa, where the disease remains endemic, whereas cardiovascular disease and cancer were more frequent in higher-income countries. The benefit of immediate antiretroviral therapy across regions of the world indicates that controlling viral replication and improving immune function have broad positive effects.
The primary end point incorporated potentially serious positive and negative effects of antiretroviral therapy, and no safety concerns in the immediate-initiation group were identified. Grade 4 events and unscheduled hospitalizations occurred more often than did the primary outcome, but rates of these events were similar in the two study groups.
Most of the AIDS-related and non–AIDS-related events occurred when patients had a high CD4+ count. Immediate antiretroviral therapy benefited even those with a latest CD4+ count of more than 500 cells per cubic millimeter, which may indicate that a substantial part of the beneficial effect of immediate treatment is due to changes induced by antiretroviral therapy in markers other than the CD4+ count. This finding is consistent with the results of studies that were used as a basis for our study11,25 and with other large observational studies conducted both in resource-rich and resource-poor settings.40,41
The risk of AIDS was not zero among patients receiving antiretroviral therapy, even among those who had full viral suppression while receiving antiretroviral drugs. This finding indicates that damage to the immune system may occur early in the course of HIV infection.42 It further supports the need for better markers of impaired immune function and research on treatments to use along with antiretroviral therapy to reduce disease among HIV-positive patients.
Our study has some limitations. The percentage of primary events that were attributable to serious non–AIDS-related conditions was lower than anticipated (54% vs. 77% projected), which, combined with the early termination of the deferred-therapy strategy, resulted in a low statistical power to precisely quantify benefit. This factor limits our understanding of the effects of immediate therapy on the risk of individual serious non-AIDS conditions such as cardiovascular disease, for which reductions might be anticipated because of reductions in coagulation and inflammatory markers.43,44 Further follow-up of this young cohort and analyses of intermediate markers45,46 may elucidate the effects of immediate antiretroviral therapy on arterial disease and other serious non-AIDS conditions. Finally, although we conducted a relatively long study of HIV treatment, 3 years is a rather short period for patients who will require antiretroviral therapy for the rest of their lives. It will be important to assess risks and benefits of long-term therapy.
In conclusion, we found a significant benefit in the immediate initiation of antiretroviral therapy in patients with HIV infection regardless of CD4+ count. These results align benefits for individual patients with the public health benefit of anti-retroviral therapy in reducing the risk of viral transmission. Our findings reinforce the need for health systems to improve programs to diagnose HIV infection and link such patients to care. The results support global goals set by the World Health Organization and the Joint United Nations Programme on HIV/AIDS to expand the use of antiretroviral therapy to all HIV-positive patients in order to improve their health and as part of efforts to reduce the future spread of HIV.22-24,47,48
Supplementary Material
Acknowledgments
Supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health Clinical Center, National Cancer Institute, National Heart, Lung, and Blood Institute, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Mental Health, National Institute of Neurological Disorders and Stroke, National Institute of Arthritis and Musculoskeletal and Skin Diseases, Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (France), National Health and Medical Research Council (Australia), National Research Foundation (Denmark), Bundes ministerium für Bildung und Forschung (Germany), European AIDS Treatment Network, Medical Research Council (United Kingdom), National Institute for Health Research, National Health Service (United Kingdom), and University of Minnesota. Antiretroviral drugs were donated to the central drug repository by AbbVie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmith-Kline/ViiV Healthcare, Janssen Scientific Affairs, and Merck.
We thank all the patients who participated in this study.
APPENDIX
The affiliations of the members of the writing group are as follows: Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (J.D.L.); Medical Research Council Clinical Trials Unit at University College London, London (A.G.B.); Washington Veterans Affairs Medical Center, George Washington University, Washington, DC (F.G.); Kirby Institute, University of New South Wales, Sydney, Australia (S.E., D.A.C.); University of Minnesota, Minneapolis (B.G., S.S.); Thai Red Cross AIDS Research Center, Chulalongkorn University, Bangkok, Thailand (A.A.); University Hospital of Cologne, German Center for Infection Research, partner site Bonn–Cologne, Germany (G.F.); University Hospital Germans Trias, Universitat Autònoma de Barcelona, Badalona, Spain (J.M.L.); University of Paris Diderot, Saint-Louis Hospital, Paris (J.-M.M.); Medical Research Council Research Unit on AIDS, Entebbe, Uganda (P.M.); Projeto Praça Onze, Rio de Janeiro (M.S.); Desmond Tutu HIV Center, University of Cape Town, Cape Town, South Africa (R.W.); National Institute of Allergy and Infectious Diseases, Bethesda, MD (K.L.K.); HIV i-Base, London (S.C.); National Institute of Allergy and Infectious Diseases, Bethesda, MD (H.C.L.); UCL, London (A.N.P.); and University of Minnesota, Minneapolis (J.D.N.).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Lane HC, Masur H, Gelmann EP, et al. Correlation between immunologic function and clinical subpopulations of patients with the acquired immune deficiency syndrome. Am J Med. 1985;78:417–22. doi: 10.1016/0002-9343(85)90332-8. [DOI] [PubMed] [Google Scholar]
- 2.Phillips AN, Lundgren JD. The CD4 lymphocyte count and risk of clinical progression. Curr Opin HIV AIDS. 2006;1:43–9. doi: 10.1097/01.COH.0000194106.12816.b1. [DOI] [PubMed] [Google Scholar]
- 3.Lundgren JD, Babiker A, El-Sadr W, et al. Inferior clinical outcome of the CD4+ cell count-guided antiretroviral treatment interruption strategy in the SMART study: role of CD4+ cell counts and HIV RNA levels during follow-up. J Infect Dis. 2008;197:1145–55. doi: 10.1086/529523. [DOI] [PubMed] [Google Scholar]
- 4.De Cock KM, El-Sadr WM. When to start ART in Africa — an urgent research priority. N Engl J Med. 2013;368:886–9. doi: 10.1056/NEJMp1300458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emery S, Neuhaus JA, Phillips AN, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133–44. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- 6.Severe P, Juste MA, Ambroise A, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363:257–65. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14:281–90. doi: 10.1016/S1473-3099(13)70692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sterne JA, May M, Costagliola D, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–63. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Writing Committee for the CASCADE Collaboration Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Arch Intern Med. 2011;171:1560–9. doi: 10.1001/archinternmed.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cain LE, Logan R, Robins JM, et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med. 2011;154:509–15. doi: 10.1059/0003-4819-154-8-201104190-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lodi S, Phillips A, Logan R, et al. Comparative effectiveness of immediate antiretroviral therapy versus CD4-based initiation in HIV-positive individuals in high-income countries: observational cohort study. Lancet HIV. 2015 Jul 7; doi: 10.1016/S2352-3018(15)00108-3. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabin CA, Cooper DA, Collins S, Schechter M. Rating evidence in treatment guidelines: a case example of when to initiate combination antiretroviral therapy (cART) in HIV-positive asymptomatic persons. AIDS. 2013;27:1839–46. doi: 10.1097/qad.0b013e328360d546. [DOI] [PubMed] [Google Scholar]
- 15.Lundgren JD, Babiker AG, Gordin FM, Borges ÁH, Neaton JD. When to start antiretroviral therapy: the need for an evidence base during early HIV infection. BMC Med. 2013;11:148. doi: 10.1186/1741-7015-11-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernán MA, Robins JM. Early versus deferred antiretroviral therapy for HIV. N Engl J Med. 2009;361:822–3. [PubMed] [Google Scholar]
- 17.El-Sadr WM, Lundgren J, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 18.El-Sadr WM, Grund B, Neuhaus J, et al. Risk for opportunistic disease and death after reinitiating continuous antiretroviral therapy in patients with HIV previously receiving episodic therapy: a randomized trial. Ann Intern Med. 2008;149:289–99. doi: 10.7326/0003-4819-149-5-200809020-00003. [DOI] [PubMed] [Google Scholar]
- 19.Friis-Møller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 20.Ryom L, Mocroft A, Kirk O, et al. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: the D:A:D study. J Infect Dis. 2013;207:1359–69. doi: 10.1093/infdis/jit043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodger A, Cambiano V, Bruun T, et al. HIV transmission risk through condomless sex if the HIV positive partner is on suppressive ART: PARTNER study.. Presented at the annual Conference on Retroviruses and Opportunistic Infections; Boston. March 3–6, 2014; abstract ( http://www.cphiv.dk/portals/0/files/CROI_2014_PARTNER_slides.pdf) [Google Scholar]
- 22.Eaton JW, Menzies NA, Stover J, et al. Health benefits, costs, and cost-effectiveness of earlier eligibility for adult anti-retroviral therapy and expanded treatment coverage: a combined analysis of 12 mathematical models. Lancet Glob Health. 2014;2(1):e23–e34. doi: 10.1016/S2214-109X(13)70172-4. [DOI] [PubMed] [Google Scholar]
- 23.UNAIDS 90-90-90: an ambitious treatment target to help end the AIDS epidemic. 2014 http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf.
- 24.HIV, universal health coverage and the post-2015 development agenda. World Health Organization; Geneva: 2014. http://www.who.int/hiv/pub/toolkits/universal-coverage2014/en. [Google Scholar]
- 25.Babiker AG, Emery S, Fätkenheuer G, et al. Considerations in the rationale, design and methods of the Strategic Timing of AntiRetroviral Treatment (START) study. Clin Trials. 2013;10(Suppl):S5–S36. doi: 10.1177/1740774512440342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The INSIGHT Strategic Timing of AntiRetroviral Treatment (START) Study Group The START trial characteristics at study entry. HIV Med. 2015;16(Suppl S1):1–146. [Google Scholar]
- 27.Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, Panel on Antiretroviral Guidelines for Adults and Adolescents; Washington, DC: 2015. https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. [Google Scholar]
- 28.Günthard HF, Aberg JA, Eron JJ, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA. 2014;312:410–25. doi: 10.1001/jama.2014.8722. [DOI] [PubMed] [Google Scholar]
- 29.European AIDS Clinical Society Antiretroviral therapy guidelines, version 7.1. 2014 http://www.eacsociety.org/files/guidelines-7.1-english.pdf.
- 30.Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. World Health Organization; Geneva: 2013. http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1. [PubMed] [Google Scholar]
- 31.1993 revised classification sytem for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 32.INSIGHT Criteria for event reporting in START. 2010 http://insight.ccbr.umn.edu/start/index.php?study=start&page=&menu=eventreport.
- 33.Lifson AR, Rhame FS, Belloso WH, et al. Reporting and evaluation of HIV-related clinical endpoints in two multicenter international clinical trials. HIV Clin Trials. 2006;7:125–41. doi: 10.1310/7MER-XFA7-1762-E2WR. [DOI] [PubMed] [Google Scholar]
- 34.Lifson AR, Belloso WH, Davey RT, et al. Development of diagnostic criteria for serious non-AIDS events in HIV clinical trials. HIV Clin Trials. 2010;11:205–19. doi: 10.1310/hct1104-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS Division of AIDS table for grading the severity of adult and pediatric adverse events, version 1.0. 2004 Dec; http://rsc.tech-res.com/Document/safetyandpharmacovigilance/DAIDS-AE-Grading-Table-v1.0-DEC2004.pdf.
- 36.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Bio-metrics. 1979;35:549–56. [PubMed] [Google Scholar]
- 37.Lan KG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–63. [Google Scholar]
- 38.Grarup J, Rappoport C, Engen NW, et al. Challenges, successes and patterns of enrolment in the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med. 2015;161(Suppl):14–23. doi: 10.1111/hiv.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodger AJ, Phillips A, Speakman A, et al. Attitudes of people in the UK with HIV who re Antiretroviral (ART) Naïve to starting ART at high CD4 counts for potential health benefit or to prevent HIV transmission. PLoS One. 2014;9(5):e97340. doi: 10.1371/journal.pone.0097340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips AN, Gazzard B, Gilson R, et al. Rate of AIDS diseases or death in HIV-infected antiretroviral therapy-naive individuals with high CD4 cell count. AIDS. 2007;21:1717–21. doi: 10.1097/QAD.0b013e32827038bf. [DOI] [PubMed] [Google Scholar]
- 41.Mocroft A, Furrer HJ, Miro JM, et al. The incidence of AIDS-defining illnesses at a current CD4 count ≥ 200 cells/μL in the post-combination antiretroviral therapy era. Clin Infect Dis. 2013;57:1038–47. doi: 10.1093/cid/cit423. [DOI] [PubMed] [Google Scholar]
- 42.Maduna PH, Dolan M, Kondlo L, et al. Morbidity and mortality according to latest CD4+ cell count among HIV positive individuals in South Africa who enrolled in project Phidisa. PLoS One. 2015;10(4):e0121843. doi: 10.1371/journal.pone.0121843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng M, Paiardini M, Engram JC, et al. Critical role of CD4 T cells in maintaining lymphoid tissue structure for immune cell homeostasis and reconstitution. Blood. 2012;120:1856–67. doi: 10.1182/blood-2012-03-418624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baker JV, Lundgren JD. Cardiovascular implications from untreated human immunodeficiency virus infection. Eur Heart J. 2011;32:945–51. doi: 10.1093/eurheartj/ehq483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker JV, Neuhaus J, Duprez D, et al. Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred anti-retroviral therapy in patients with HIV infection. J Acquir Immune Defic Syndr. 2011;56:36–43. doi: 10.1097/QAI.0b013e3181f7f61a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker JV, Engen NW, Huppler Hullsiek K, et al. Assessment of arterial elasticity among HIV-positive participants with high CD4 cell counts: a substudy of the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med. 2015;16(Suppl 1):109–18. doi: 10.1111/hiv.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soliman EZ, Sharma S, Arastéh K, et al. Baseline cardiovascular risk in the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med. 2015;16(Suppl 1):46–54. doi: 10.1111/hiv.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.UNAIDS Implications of the START study data: questions and answers. http://www.unaids.org/sites/default/files/media_asset/2015_Implications_of_the_START_study_data_en.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.