Abstract
In human cutaneous leishmaniasis (CL), the immune response is mainly mediated by T-cells. The role of CD8+ T-lymphocytes, which are related to healing or deleterious functions, in affecting clinical outcome is controversial. The aim of this study was to evaluate T-cell receptor diversity in late-differentiated effector (LDE) and memory CD8+ T-cell subsets in order to create a profile of specific clones engaged in deleterious or protective CL immune responses. Healthy subjects, patients with active disease (PAD) and clinically cured patients were enrolled in the study. Total CD8+ T-lymphocytes showed a disturbance in the expression of the Vβ2, Vβ9, Vβ13.2, Vβ18 and Vβ23 families. The analyses of CD8+T-lymphocyte subsets showed high frequencies of LDE CD8+T-lymphocytes expressing Vβ12 and Vβ22 in PAD, as well as effector-memory CD8+ T-cells expressing Vβ22. We also observed low frequencies of effector and central-memory CD8+ T-cells expressing Vβ2 in PAD, which correlated with a greater lesion size. Particular Vβ expansions point to CD8+ T-cell clones that are selected during CL immune responses, suggesting that CD8+ T-lymphocytes expressing Vβ12 or Vβ22 are involved in a LDE response and that Vβ2 contractions in memory CD8+T-cells are associated with larger lesions.
Keywords: Vβ repertoire, human cutaneous leishmaniasis, flow cytometry, CD8+ T-lymphocyte - TCR, Leishmania braziliensis
Leishmaniasis is ranked as the ninth major infectious disease in the world (Alvar et al. 2012). In Brazil, human cutaneous leishmaniasis (CL) is habitually registered in all states and is endemic in Rio de Janeiro (RJ), where it is caused mainly by Leishmania (Viannia) braziliensis, (de Oliveira-Neto et al. 2000, WHO 2010). A better understanding regarding the phenomena that occur during the T-cell immune response toLeishmania is crucial for prospecting the immunopathogenesis outcome (Reithinger et al. 2007). The course of disease is determined by the nature and magnitude of the immune response orchestrated by specific T-lymphocytes, which must be activated by antigen presentation and cytokines to completely perform their function (Silveira et al. 2009). Although CD4+ T-lymphocytes orchestrate the cellular immune response in CL, CD8+ T-lymphocytes seem to be directly related to the destruction of infected cells, acting not only via cytotoxic activity by secreting cytolytic granules, but also via interferon (IFN)-γ production, though there is controversy pertaining to the beneficial or deleterious role of these cells (Pirmez 1992, Da-Cruz et al. 1994, Barral-Netto et al. 1995, Mendonça et al. 1995, Coutinho et al. 1998, 2002, Bertho et al. 2000, Toledo et al. 2001, Bousoffara et al. 2004, Rostami et al. 2010b, Viola et al. 2010, Keesen et al. 2011, de Oliveira & Brodskyn 2012, Novais et al. 2013, Santos et al. 2013, Cardoso et al. 2015).
Because specific T-lymphocyte responses against Leishmania antigens are responsible for the induction of protective and pathogenic immune responses in human CL, an understanding of which CD8+ T-cell subsets are involved in this response will aid in understanding the immunological response to Leishmaniaparasites (Keesen et al. 2011).
T-lymphocytes are characterised by the expression of T-cell receptor (TCR), which is linked to the CD3 molecule. In this molecular complex (TCR-CD3), the TCR is the antigen recognition unit, whereas CD3 is the signal transduction driver of T-lymphocytes. The TCR is specialised to recognise complex molecular compounds by the intracellular peptides presented by the major histocompatibility complex molecules or CD1 and it is unable to recognise soluble antigens (Meuer et al. 1983). Different TCRs comprise the repertoire of T-lymphocytes, ensuring that a variety of nonself peptides are recognised, which allows for specifically selected clones to proliferate and participate in the adaptive immune response (Lennon et al. 2000, Viola et al. 2010). The TCR is a heterodimer consisting of two covalently linked glycoprotein chains. Most T-lymphocytes express αβ TCR type (90-99%), whereas a minority expressed the γδ TCR type. The requirement to recognise a variety of antigens by TCR diversity is generated during T-cell ontogeny in the thymus. The variability among different TCRs is determined by the variable domain of the α and β chains (Kimura et al. 1987, van der Merwe & Davis 2003). The ability of T-lymphocytes to detect, remove and remember a variety of antigens found in various pathogens is due to the extraordinary diversity of the receptors’ repertoire and its capacity to distinguish different peptide sequences, triggering a cascade of immunological events (Murre 2007).
The T-lymphocyte Vβ repertoire, which is usually determined by molecular biology assays (Pantaleo et al. 1994, Ria et al. 2001), has also been studied by flow cytometry, particularly to assess its ability to gather phenotypic profile information of TCR diversity, allowing the Vβ repertoire to be analysed from a small number of samples, optimising such a study (van den Beemd et al. 2000, Menezes et al. 2004, Giacoia-Gripp et al. 2005, Clarêncio et al. 2006, Keesen et al. 2011, Bretschneider et al. 2014). The phenotypic changes which characterise the process of cellular differentiation have also been exploited for the identification of lymphocyte subpopulations by flow cytometry, using combinations of monoclonal antibodies such as anti-CD45RA, anti-CD45RO, anti-CD27, anti-CD28, anti-CCR7 and anti-CD62L (Hamann et al. 1997, Sallusto et al. 1999,2000, Baars et al. 2000, Campbell et al. 2009, Libri et al. 2011, Bretschneider et al. 2014). In this scenario, flow cytometry has proven to be suitable and indispensable for the phenotypic/functional study of diverse cell populations, allowing for the determination of characteristics and immunological phenomena in samples obtained from humans and experimental models. Moreover, the TCR/Vβ repertoire analysis in blood can provide strong evidence of clonality, particularly when a single expanded Vß family is detected (Salameire et al. 2012).
Different distribution profiles in the TCR/Vβ repertoire have been linked to the immunopathogenesis of several diseases, such as acquired immune deficiency syndrome (Giacoia-Gripp et al. 2005), multiple sclerosis (Hong et al. 1999), rheumatoid arthritis (Plasilova et al. 2003) and Chagas disease (Costa et al. 2000, Menezes et al. 2004). In CL, few studies have been conducted to evaluate possible changes in the TCR/Vβ repertoire profile in T-lymphocytes (Uyemura et al. 1993, Clarêncio et al. 2006, Keesen et al. 2011). Moreover, there is no literature on CL that describes any profile of the TCR/Vβ repertoire in CD8+ T-lymphocyte subsets, allowing for the assessment of preferentially induced cell clones, which may participate in the immune response against Leishmania.
Therefore, the goal of this study was to determine the frequency of distinct CD8+ T-lymphocyte subsets [late-differentiated effector (LDE), central memory (CM) and effector memory (EM)] obtained from patients with active CL disease and clinically cured patients (PCC) in order to increase our understanding of the immune responses associated with the progression of human CL and its healing process. We also measured correlations between the frequency of these subpopulations expressing diverse Vβ chains and clinical indicators of human CL.
SUBJECTS, MATERIALS AND METHODS
Ethics statement - This study was in accordance with the ethical standards of the National Ethical Clearance Committee of Brazil as well as with the Ethical Committee for Human Research of the Oswaldo Cruz Foundation (CEP-FIOCRUZ) and the Evandro Chagas Clinical Research Institute (CEP-IPEC/FIOCRUZ) and with the Helsinki Declaration of 1975, as revised in 1983. Written informed consent was obtained from all volunteers prior to blood collection.
Patients and controls - The study included healthy subjects (HS) (n = 18) and two cohorts of CL patients: patients with active disease (PAD) (n = 14), showing ulcerated skin lesions, and PCC (n = 11), evaluated eighty day after the beginning of treatment, at which time clinical cure was defined as full epithelialisation of ulcerated lesions, regression of crust, desquamation and infiltration. All adults included were between 18-60 years of age with a mean age of 35.1 ± 13.2 years.
All HS enrolled in this study were from nonendemic areas in RJ, showing neither a previous history of leishmaniasis nor any other comorbidity. All CL patients resided in RJ (de Oliveira-Neto et al. 2000) and were recruited at the Leishmaniasis Surveillance Laboratory, IPEC/FIOCRUZ.
The diagnosis of leishmaniasis was based on clinical and epidemiological evidence; positive Montenegro skin test (MST) and parasitological exams. All patients were submitted to meglumine antimoniate treatment independent of their enrolment in our study, according to the guidelines of the Brazilian Ministry of Health (SVS/MS 2010). We investigated clinical and epidemiologic information, such as age, sex, number and diameter of lesion at active phase and evolution period. Patients who presented with comorbidity, reactivation of CL, reinfection or nonresponse to antimonial therapy were excluded from the study.
Analysis of the Vβ repertoire and cell phenotyping - Venous blood was collected from CL patients and HS in heparinised tubes and peripheral blood mononuclear cells (PBMC) were obtained by Ficoll-Hypaque density gradient centrifugation (Sigma Aldrich, USA). Vβ repertoire and cell phenotype staining was performed as previously described (Barral-Netto et al. 1995). In brief, we performed six-colour cell surface staining using the IOTest Beta Mark TCR-Vβ Repertoire kit (Beckman Coulter Inc, USA), which contains eight tubes with three antibodies in each against the following TCR-Vβ chains: Vβ1, Vβ2, Vβ3, Vβ4, Vβ5.1, Vβ5.2, Vβ5.3, Vβ7.1, Vβ7.2, Vβ8, Vβ9, Vβ11, Vβ12, Vβ13.1, Vβ13.2, Vβ13.6, Vβ14, Vβ16, Vβ17, Vβ18, Vβ20, Vβ21.3, Vβ22, Vβ23 belonging to 19 of 26 Vβ human families known. These 24 Vβ chains cover 70% of the T-lymphocyte TCR-Vβ repertoire in a normal individual. The staining protocol included one aliquot of the Vβ repertoire kit, which combines TCR antibodies labelled with fluorescein isothiocyanate (FITC), phycoerythrin (PE) or both PE and FITC, with one aliquot of each monoclonal antibody anti-CD8 allophycocyanine (APC), anti-CD45RA PE-Texas Red, anti-CD27 PE-cyanine 7 (Beckman Coulter) and anti-CD3 APC-cyanine 7 (BD Biosciences, USA). This antibody combination allowed us to simultaneously identify the Vβ repertoire of total CD8+ T-lymphocytes and the Vβ repertoire of naïve, LDE, CM and EM subsets. The staining was performed in phosphate-buffered saline containing 0.1% sodium azide (Sigma Aldrich) and 2% foetal calf serum (Sigma Aldrich) and incubated for 30 min at 4ºC, protected from light and finally washed for subsequent acquisition in a flow cytometer.
Flow cytometry - Fifty-thousand events were acquired in by Beckman Coulter Cyan ADP (Beckman Coulter) or BD FACSAria II (Becton & Dickinson, USA) flow cytometers. All flow cytometry data were analysed using Kaluza 1.2 software (Beckman Coulter) where the distribution of the CD8+ T-lymphocyte TCR-Vβ repertoire was determined. In this manner, the total CD8+ T-lymphocytes was determined in a CD3 vs. CD8 dot-plot created from a region encompassing lymphocyte population in a side vs. forward dot-plot. To evaluate naïve, LDE, CM and EM CD8+ T-lymphocyte subsets, a CD27 vs. CD45RA dot-plot was created based on CD3+CD8+ region. Simultaneously, TCR-Vβ repertoire distributions were determined by analysing the frequency of 24 Vβ families, three for each staining tube based on FITC vs. PE dot-plot, gated on five different populations: total, naïve, LDE, CM and EM CD8+ T-cell subsets (Fig. 1). To ensure confidence in cytofluorimetric Vβ analyses from different dates, the limits for the regions created in Vβ dot-plots, quadrant markers and histograms were set, each time, based on nonstaining cells and negative isotypic controls. Additionally, fluorescence compensation adjustments were performed based on single-labelled samples.
Fig. 1: representative flow cytometry protocol to determine Vβ repertoire of total CD8+ T-lymphocyte and subpopulations. Peripheral blood mononuclear cells from cutaneous leishmaniasis patients were stained ex vivo with CD3-allophycocyanine-cyanine (APC) 7, CD8-APC, CD45RA-PE-Texas Red, CD27-PE-cyanine 7 and 24 different anti-Vβ monoclonal antibodies conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE) and FITC-PE. The lymphocytes were gated on forward (FSC) vs. side (SSC) scatter dot-plot (A) and CD8+ T-lymphocytes were defined by double positive staining to CD3 and CD8 (CD3+CD8+) (B). Based on lymphocyte gate we analysed the expression of three different Vβ families (in each of the 8 tubes) (C) and CD8+ T lymphocyte subsets were defined by CD45RA and CD27 staining as late-differentiated effector (LDE) (CD45RA+CD27-), early-differentiated (naïve) (CD27+CD45RA+), late-differentiated effector memory (EM) (CD45RA-CD27-) and central memory (CM) (CD45RA-CD27+) (D). We also performed the Vβ analysis of naïve (E), LDE (F), EM (G) and CM (H) CD8+ T-lymphocytes.

Statistical analysis - For a comparison of the means between groups, the Mann-Whitney U nonparametric test was applied. The differences were considered statistically significant when p < 0.05. Correlation analyses were performed using Spearman’s correlation coefficient and were reported with its associated r and p-value. All statistical calculations and graphical representations of data were obtained using the GraphPad Prism v.5.0 software (GraphPad Software Inc, USA).
RESULTS
Clinical characteristics of CL patients - All patients were from endemic areas in RJ and were included in this study after informed consent and donation of peripheral blood. All CL patients received Glucantime®therapy, as suggested by the Brazilian Ministry of Health (SVS/MS 2010) and, at the end of therapy, all of them showed epithelialised lesions with an absence of erythema and were considered clinically cured. All patients presented with a positive MST. The duration of lesion ranged from one-six months and the largest measured diameter of the ulcers varied from 15-60 mm (mean 42.18 ± 12.51 mm). Basic demographic and clinical information of the studied groups are summarised in Table.
TABLE. Clinical information of patients included in this study.
| HS | PAD | PCC | |
|---|---|---|---|
| Volunteers (n) Sex (M/F) | 18 11/7 | 14 9/5 | 11 8/3 |
| Age (years) | 29 ± 9.7 | 35.7 ± 13.4 | 41 ± 15.1 |
| Lesions (n) | NA | 1 | 1 |
| Diameter of lesion [(mm) mean ± SD] | NA | 42 ± 12.51 | 41.4 ± 13.98 |
| MST (mm) Duration of disease [(months) median (range)] | NA NA | 11.71 ± 3.6 2 (1-6) | 12.3 ± 3.68 2 (1-5) |
HS: healthy subjects; M/F: male/female; MST: Montenegro skin test; NA: not applicable; PAD: patients with active disease; PCC: patients clinical cured; SD: standard deviation.
Definition of TCR-Vβ repertoire of total CD8 + T-lymphocytes - Due to the diversity of the CD8+ T-lymphocyte TCR repertoire, the antigenic specificity of these cells and the role they play in a CL-associated immune response is of fundamental importance to assess the preferential expression of certain clones, associating them with active disease and the healing process.
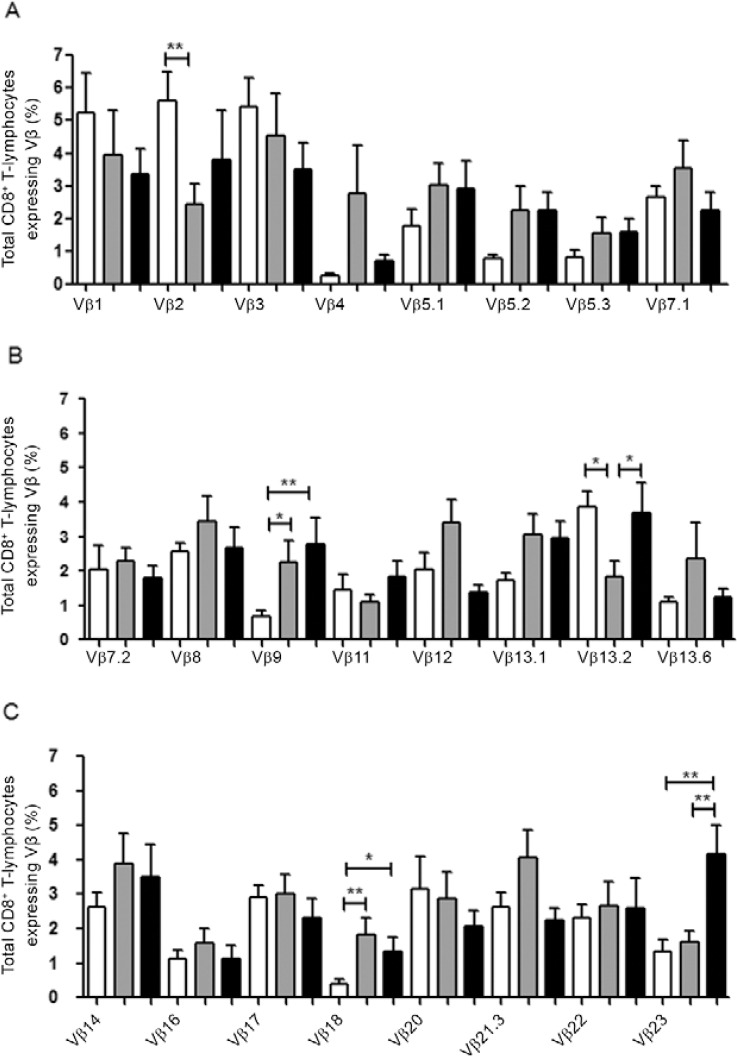
To this end, we determined the frequency of total CD8+ T-lymphocytes expressing 24 TCR Vβ chains in CL PAD and PCC as well as in HS. In these three groups, we observed a polyclonal distribution of these Vβ families. In a comparative analysis between groups, we observed a significant difference in frequencies of total CD8+ T-lymphocytes expressing Vβ2, Vβ9, Vβ13.2, Vβ18 and Vβ23 chains in PAD compared to HS and/or to PCC (Fig. 2). PAD showed lower frequencies of CD8+ T-cells expressing Vβ2 (mean 2.5% ± 1.9%) and Vβ13.2 regions (mean 1.8% ± 1.5%) compared to HS (Vβ2: mean 5.6% ± 3.1%; Vβ13.2: mean 3.8% ± 1.6%; p < 0.01 and p < 0.05, respectively). Conversely, PAD showed higher frequencies of CD8+ T-lymphocytes expressing Vβ9 (mean 2.9% ± 0.6%) and Vβ18 (mean 1.8% ± 0.4%) when compared to HS (Vβ9: mean 0.6% ± 0.1%; Vβ18: 0.3% ± 0.1%; p < 0.05 and p < 0.01, respectively). These results suggest that during the active phase of disease there is a down-modulation in the frequencies of CD8+ T-lymphocytes expressing Vβ2 and Vβ13.2 and an up-modulation of CD8+ T-lymphocytes expressing Vβ9 and Vβ18. In PCC, the normal distribution of Vβ18 appears to be restored, whereas frequencies of CD8+ T-lymphocytes expressing Vβ9 remain high. In contrast, PCC showed higher frequencies of total CD8+ T-lymphocytes expressing Vβ18 (mean: 1.3% ± 0.4%) and Vβ23 (mean 4.1% ± 1.7%) when compared to HS (Vβ18: mean 0.3% ± 0.1%; Vβ23: mean 1.3% ± 1%; p < 0.05 and p < 0.01, respectively), whereas the frequency of CD8+ T-lymphocytes expressing Vβ13.2 was higher (3.6% ± 0.85%) when compared to PAD (mean 1.8% ± 1.5%; p < 0.05) (Fig. 2).
Fig. 2: 24 Vβ chain repertoire profile of total CD8+ T-lymphocytes. A: Vβ chains 1-7.1; B: Vβ chains 7.2-13.6; C Vβ chains 14-23. Statistical analyses were performed by Mann-Whitney U test. Results were considered significant when p < 0.05. HS: healthy individuals (white bars; n = 18); PAD: patients with active disease (grey bars; n = 14); PCC: patients clinically cured (black bars; n = 11); *: p < 0.05; **: p <0.01.
Evaluation of TCR-Vβ repertoire of memory, LDE and naïve CD8 + T-lymphocyte subsets - To evaluate a link between the type of CD8+ T subsets and the diversity of the Vβ repertoire of these cells, we also performed a detailed analysis of the distribution and alterations of Vβ repertoire in naïve and LDE, CM and EM CD8+ T-lymphocytes in CL patients and HS. These frequencies were obtained by a gating strategy according to the expression of CD45RA and/or CD27 (see Subjects, Materials and Methods): CD27+CD45RA+ (naïve), CD45RA+CD27-(LDE), CD45RA-CD27- (EM) and CD45RA-CD27+ (CM) (Fig. 1). The subset analysis showed a polyclonal distribution of TCR Vβ repertoires; hence, all 24 Vβ chains were expressed in the TCR of these four subpopulations (data not shown).
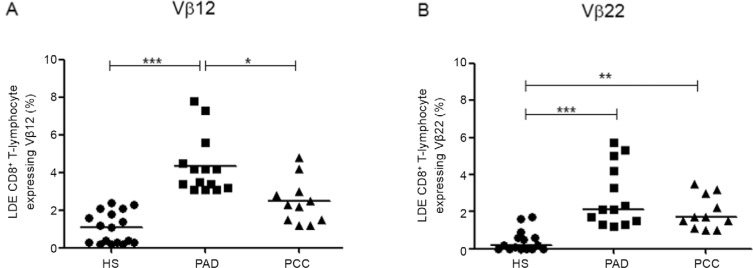
Frequencies of LDE CD8 + T-lymphocytes expressing Vβ12 and Vβ22 in CL patients - An analysis of the Vβ repertoire of LDE CD8+ T-lymphocytes revealed that two Vβ families appeared to expand in the LDE CD8+ T-lymphocyte subset from CL patients. PAD showed higher frequencies of LDE CD8+ T-lymphocytes expressing Vβ12 (mean 3.8% ± 1.5%), when compared to both HS (mean 1.1% ± 0.8%; p < 0.001) and PCC (mean 2.3% ± 1.1%; p < 0.05) (Fig. 3A). Similarly, the frequency of LDE CD8+ T-lymphocytes expressing Vβ22 was higher in PAD (mean 2.1% ± 1.5%) when compared to HS (mean 0.2% ± 0.5%; p < 0.001) and to PCC (mean 1.7% ± 0.9%; p < 0.01) (Fig. 3B).
Fig. 3: percentage of late-differentiated effector (LDE) CD8+ T-lymphocyte expressing Vβ12 (A) and Vβ22 (B). Statistical analyses were performed by Mann-Whitney U test. Results were considered significant when p < 0.05. Each point represents one subject. HS: healthy subjects (n = 18); PAD: patients with active disease (n = 14); PCC: patients clinically cured (n = 11); *: p< 0.05; **: p < 0.01; ***: p< 0.001.
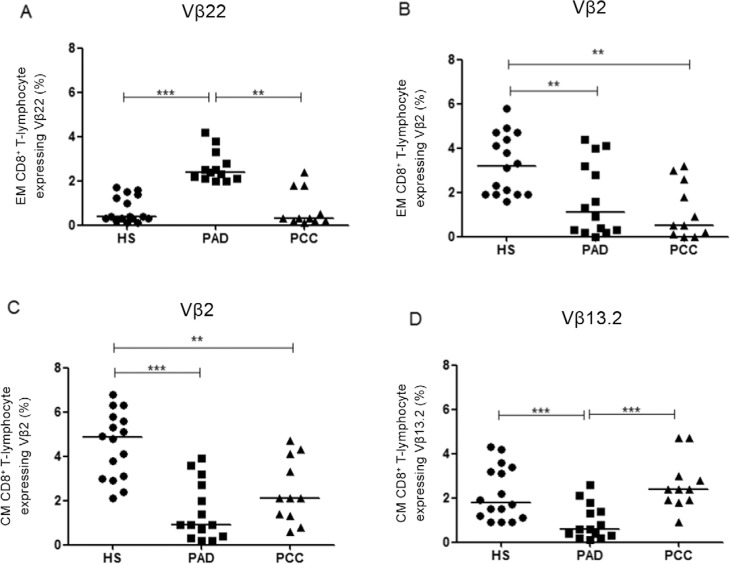
CL patients exhibited higher frequencies of EM CD8 + T-cell subsets expressing Vβ22 and lower frequencies of both EM and CM CD8 + T-cell subsets expressing Vβ2 - Evaluating EM CD8+ T-lymphocytes expressing Vβ22, we observed a pronounced clonal expansion in the active CL, as PAD showed higher frequencies (mean 2.6% ± 0.1%) when compared to HS (mean 0.7% ± 0.1%; p < 0.001). Notably, PCC (mean 0.9% ± 0.3%) showed similar frequencies of this subset in expressing Vβ22 when compared to HS and lower frequencies compared to PAD (p < 0.01) (Fig. 4A). In contrast, EM CD8+ T-cells expressing Vβ2 have lower frequencies in PAD (mean 1.6% ± 0.4%; p < 0.01) and also in PCC (mean 0.9% ± 0.2; p < 0.01) when compared to HS (3.3% ± 0.3%) (Fig. 4B). This contraction of the Vβ2 family was also observed in CM CD8+ T-lymphocytes from PCC (mean 2.4% ± 0.4%) and in PAD (mean 1.4% ± 0.3%), showing lower frequencies of these cells when compared to HS (mean 4.5% ± 0.3%; p < 0.01 and p < 0.001, respectively) (Fig. 4C). Another contraction could also be observed in CM CD8+ T-lymphocyte clones expressing Vβ 13.2, as PAD presented lower frequencies of these cells (mean 0.9% ± 0.2%) when compared to both HS (mean 2.2% ± 0.3%; p < 0.01) and PCC (mean 2.6% ± 0.3%; p < 0.001) (Fig. 4D).
Fig. 4: percentage of late-differentiated effector memory (EM) CD8+ T-lymphocyte expressing Vβ2 (A) and Vβ22 (B) and of central memory (CM) CD8+ T-lymphocyte expressing Vβ2 (C) and Vβ13.2 (D). Statistical analyses were performed by Mann-Whitney U test. Results were considered significant when p < 0.05. Each point represents one subject. HS: healthy subjects (n = 18); PAD: patients with active disease (n = 14); PCC: patients clinically cured (n = 11); **: p < 0.01; ***: p <0.001.
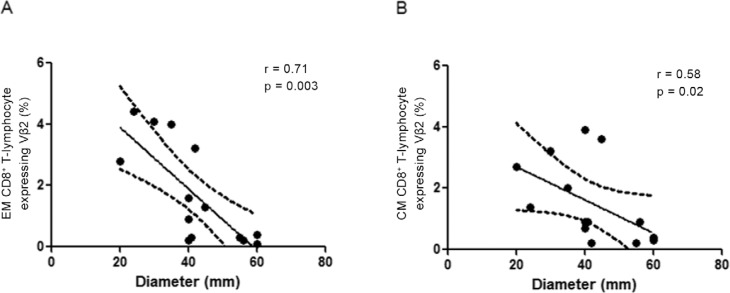
Lower frequencies of CD8 + T-cells expressing Vβ2 correlate with larger lesion size - Due to relationship between clinical features and immune response in CL, we verified whether the frequency of CD8+ T-cell expressing Vβ2, Vβ12, Vβ13.2 and Vβ22 was associated with the size of the CL lesion. An analysis of the PAD data revealed an inverse correlation between frequencies of EM and CM CD8+T-cells expressing Vβ2 and lesion size; the greater the lesion size, the lower the frequency of EM and CM CD8+ T-lymphocytes expressing Vβ2 (Fig. 5). No statistically significant correlation was observed in PCC and in other evaluated Vβ families.
Fig. 5: correlation analysis between lesion size and frequency of effector memory (EM) (A) and central memory (CM) (B) CD8+ T-lymphocyte expressing Vβ2. The lesion size is correlated negatively with frequency of EM CM CD8+ T -lymphocytes from patients with active disease (n = 14). The central line represents medians values and the graphs show the best fitted lines with 95% confidence interval. The lesion size was measured through the largest diameter (mm). Statistical analyses were performed by Spearman test. Results were considered significant when p< 0.05. Each point represents one subject. r: correlation coefficient.
DISCUSSION
The analysis of the T-cell profiles involved in local or systemic immune responses has been shown to be very important in clarifying many immunoclinical phenomena, including autoimmunity, response to viral or bacterial superantigens, alloimmunity and tumour immunity (Gorski et al. 1994). The establishment of an effective immune response against the protozoanLeishmania spp is essential to limit or prevent tissue damage and is responsible for CL outcomes. An important characteristic of CL is that most patients, especially in RJ, have a satisfactory response to antimonial therapy (de Oliveira-Neto et al. 2000). Therefore, the treatment may be associated with the development of an efficient immune response that is able to control the infection.
CL immune response leads to lesion resolution mainly through CD4+ T-helper 1 lymphocytes with activation of CD8+ T-cells and macrophages/dendritic cells via cytokine production (Da-Cruz et al. 1994, 2002, Barral et al. 1995, Coutinho et al. 1996, Brodskyn et al. 1997). This response is characterised by an antigenic presentation with the involvement of particular Vβ families to guide to antigen-specific immunological events, including T-cell activation and the triggering of effector and regulatory mechanisms. Some authors suggest that CD8+ T-lymphocytes have a central role in the process of healing CL resolution (Bertho et al. 2000, Toledo et al. 2001, Da-Cruz et al. 2002,Colmenares et al. 2003, Bousoffara et al. 2004, Rostami et al. 2010a, Ferraz et al. 2015). Another study suggests that the high frequencies of CD8+ T-lymphocytes in the CL lesion milieu support the hypothesis that these cells may be involved in the healing process of lesions (Da-Cruz et al. 2005) and the IFN-γ production of CD8+ T-lymphocytes correlates with the control of infection (Rostami et al. 2010b). In contrast, some reports have suggested that cytotoxicity may be involved in CL pathology (Novais et al. 2013, Cardoso et al. 2015) and others have shown that the high expression of IFN-γ increases cytotoxic activity, which may be responsible for the lack of control of the inflammatory response in mucosal leishmaniasis (Faria et al. 2005). Thus, faced with the paradoxical role of CD8+ T-cells linked to immune responses in CL (Novais & Scott 2015), we may assume that a particular expansion or contraction of specific Vβ in CD8+ T-lymphocyte TCR may be associated with disease control or its progression.
The analysis of individual T-cell subsets based on their TCR expression is a powerful tool for studying T-cell responses, allowing for the evaluation of the diversity and distribution of different clones in peripheral blood from CL patients. Our analysis of the total CD8+ T-lymphocyte Vβ repertoire revealed a polyclonal distribution that includes the expression of 24 different Vβ chains by all studied individuals. Although all Vβ families included in our study were expressed by CD8+ T-cells, we observed a heterogeneity in the frequency of expression of different families that comprise the repertoire, as some chains, such as Vβ3 and Vβ8, had a tendency to be more frequently expressed in the three studied cohorts, whereas others, such as Vβ16 and Vβ18, were less frequently expressed. These data corroborate those reported by others, showing that CD4+ and CD8+ T-lymphocytes from healthy individuals present a nonrandom usage profile of Vβ families (Giacoia-Gripp et al. 2005).
Considering that the Vβ repertoire of healthy individuals may serve as a parameter for comparing the Vβ repertoire in CL patients, we may observe a modulation of the repertoire of CD8+ T-lymphocytes both in PAD and PCC, suggesting an oligoclonal induction of these lymphocytes. According to our analysis of the total CD8+ T-cell compartment, we observed a disturbance in the expression of some clones/families of TCR/Vβ repertoire, such as that of the Vβ2, Vβ9, Vβ13.2, Vβ18 and Vβ23 families, which showed lower or higher frequencies in CL patients when compared to HS. This may indicate that these clones are involved in the response against L. braziliensis. It is important to highlight that few Vβ chains have been associated with immunopathogenesis of CL to date. In the lesions ofL. braziliensis-infected patients, the expansion of Vβ6 has already been observed (Uyemura et al. 1993) and, in another report, it was suggested that T-lymphocytes expressing Vβ12 are involved in the CL immune response (Clarêncio et al. 2006). Moreover, exposure to Leishmania guyanensispreferentially induces CD8+ T-lymphocytes expressing Vβ14 (Kariminia et al. 2007). In contrast, in Chagas disease patients, the preferential expression of Vβ3.1 (Menezes et al. 2004) and Vβ5 (Costa et al. 2000) may be associated with the pathology of this disease.
Knowing that naïve and effector LDE, CM and EM subsets are inserted in the pool of total CD8+ T-lymphocytes, some expansions and contractions of Vβ regions in these subsets may be missed in general population analysis. In this context, a particular evaluation of these CD8+ T-lymphocyte subset frequencies and their profiles is of great interest to investigate the involvement of specific clones of CD8+ T-lymphocyte subsets expressing a particular TCR/Vβ region in the immune response context. Therefore, the disturbance of the total CD8+ T-lymphocyte repertoire was also observed in at least one of the four analysed CD8+ T-lymphocyte subsets, except Vβ18. Hence, we determined that these repertoire perturbations may be subset-specific and should be studied individually in each subpopulation, as this expression is not observed in total CD8+ T-lymphocyte populations. Proposing to more clearly understand the differentiation status of specific subsets of CD8+ T-lymphocytes based on Vβ expression, we performed Vβ repertoire analysis of LDE, naïve, EM and CM CD8+ T-lymphocytes. The distribution of Vβ-clone CD8+T-lymphocyte subsets in the CL patients had not yet been described and an evaluation of differences between the frequencies of these subsets represent one possible method for assessing the role of these cell populations in the CL immune response.
T-lymphocytes directly involved in antigen recognition and specific responses may be led to clonal expansion in response to antigenic presentation or to contraction due to chronic re-stimulation and subsequent T-cell death (Menezes et al. 2004, Keesen et al. 2011). In the present report, we observed high frequencies of LDE CD8+ T-lymphocytes expressing Vβ12 and Vβ22 in PAD, which led us to hypothesise that this cell clone selection may be related to this phase of the disease and to the LDE roles of these cells. These findings were corroborated by a previous report (Clarêncio et al. 2006) that showed that the expansion of CD4+ and CD8+ T-lymphocytes expressing Vβ12 in CL patients was associated with an in vitro proliferation in response to the L. amazonensis antigen. Moreover, in vaccinated individuals, the increase in IFN-γ production by CD4+ and CD8+T-lymphocytes was associated with higher frequencies of both T-cells expressing Vβ12. In a more recent study, increased frequencies of CD4+ T-lymphocytes expressing Vβ12 after stimulation of PBMC in infected CL patients with solubleL. braziliensis antigen was observed. These findings suggest that Vβ12 could be expanded by different species of Leishmania and could be related to the LDE status in CL immune responses (Keesen et al. 2011). Taking all these data together, we contend that Vβ12 is actually involved in the anti-Leishmania T-cell response and that CD8+ T-lymphocytes expressing Vβ12 have a central role in the Leishmania-specific immune response displaying a LDE profile.
Because PCC showed lower frequencies of LDE CD8+ T-lymphocytes expressing Vβ12, we hypothesise that these clones are down-modulated after clinical cure. Moreover, we were unable to confirm that CD8+ T-lymphocytes expressing Vβ12 were associated with protective or deleterious effects, but the LDE CD8+ T-cells expressing Vβ12 appear to be selected for during active disease, suggesting an involvement of these clones in the CL immune response.
A similar phenomenon was observed in the LDE CD8+ T-lymphocyte subpopulation expressing Vβ22 in PAD, suggesting that these clones, as well as Vβ12 CD8+ T-cells, may be associated with a LDE role during the active phase of CL. Moreover, these Vβ22-expressing clones may be activated and/or differentiated in EM CD8+ T-lymphocytes, as we observed an expansion of these effector-memory cells expressing Vβ22 during active disease. Thus, notably, these data indicate that CD8+ T-cells expressing Vβ22 may be a candidate clone involved in the effector response as well as in the protective immune response in CL.
In contrast, we observed a contraction of EM CD8+ T-lymphocytes expressing Vβ2 in PAD. Similar results were observed by Clarêncio et al. (2006), who showed that total CD8+T-lymphocytes expressing Vβ2, when cultured in the presence of antigens ofL. amazonensis, had a decrease in their frequencies compared to cells cultured without antigen stimulation. These authors attributed such decrease to the apoptosis of these clones after their antigenic stimulation. Remarkably, we also observed this decrease in the frequencies of CM CD8+ T-lymphocytes expressing Vβ2 from PAD and the frequency of these clones appears to be restored in PCC. Conversely, PCC showed a lower frequency of EM expressing Vβ2 when compared to PAD and HS, suggesting a down-modulation of these clones in different subsets, depending on the stage of disease. Notably, Menezes et al. (2004) identified a decrease in the frequency of activated-CD28+-CD8+ T-lymphocytes expressing Vβ2 in patients with Chagas disease. Although Leishmania andTrypanosoma are different parasites and drive different pathologies, it is relevant that activated and/or LDE CD8+T-lymphocyte expressing Vβ2 may be modulated and have a key role in the immune response occurring in these two distinct pathologies. An analysis of the CM CD8+ T-lymphocyte repertoire revealed observed small frequencies Vβ2 CD8+ T-cells from PAD, suggesting a selected down-modulation of these clones in both memory subsets of CD8+ T-lymphocytes.
Additionally, the largest lesions found in CL patients were correlated to the low frequency found in EM and in CM CD8+ T-lymphocytes expressing Vβ2. Because lesion size characterises the severity of disease and is considered to be the most significant clinical feature of CL (Oliveira et al. 2011), the low frequency of these memory subsets expressing Vβ2 may be associated with the development or maintenance of the lesions. We still observed low frequencies of both memory subsets expressing Vβ2 in PAD, suggesting that these memory CD8+ T subsets are down-modulated during active disease. We therefore hypothesise that CD8+ T-lymphocytes expressing Vβ2 are an important clone in the protective immune response.
In conclusion, we have demonstrated that distinct CD8+ T-lymphocyte subsets expressing specific Vβ families are involved in the immune response of CL patients infected with L. braziliensis. Particular Vβ expansions suggest that determined TCR/Vβ clones of CD8+ T-lymphocytes are selected during the CL immune response during disease and/or after antimonial therapy, supporting the hypothesis that CD8+ T-lymphocytes expressing Vβ12 and Vβ22 are involved in a LDE anti-Leishmania immune response. In contrast, the TCR/Vβ2 contractions in memory CD8+ T subsets appear to be a result of the down-modulation of these clones during active disease, which was associated with tissue damage. The identification of antigens inLeishmania that activate specific Vβ clones is still lacking and we hope that our results contribute to the definition of preferentially used immunodominant antigens. We therefore argue that expansions or contractions of CD8+ T-cells expressing Vβ12, Vβ22 and Vβ2 play an important role in the anti-Leishmania immune response. Moreover, the information gleaned by our study clearly points to specific clonally related CD8+ T subpopulations recruited by the immune system, with the aim of killingLeishmania parasites, leading to the resolution of human CL.
ACKNOWLEDGEMENTS
To Alessandro Marins, for Flow Cytometry Multiparametric Analysis Core Facility (IOC-FIOCRUZ), to Iris Maria Peixoto Alvim, from Platform of Flow Cytometry (PDTIS-FIOCRUZ), for helping the flow cytometry acquisitions, and to Michele Moreira, for patient’s assistant care in IPEC/FIOCRUZ.
Funding Statement
Financial support: IOC-FIOCRUZ, PROEP-CNPq-IOC (402557/211-5), FAPERJ APQ-1 (E-26/110.332/2014)
Footnotes
Financial support: IOC-FIOCRUZ, PROEP-CNPq-IOC (402557/211-5), FAPERJ APQ-1 (E-26/110.332/2014)
REFERENCES
- Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7: doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baars PA, do Couto LMR, Leusen JH, Hooibrink B, Kuijpers TW, Lens SM, van Lier RA. Cytolytic mechanisms and expression of activation-regulating receptors on effector-type CD8+CD45RA+CD27- human T cells. J Immunol. 2000;165:1910–1917. doi: 10.4049/jimmunol.165.4.1910. [DOI] [PubMed] [Google Scholar]
- Barral A, Guerreiro J, Bomfim G, Correia D, Barral-Netto M, Carvalho EM. Lymphadenopathy as the first sign of human cutaneous infection by Leishmania braziliensis. Am J Trop Med Hyg. 1995;53:256–259. doi: 10.4269/ajtmh.1995.53.256. [DOI] [PubMed] [Google Scholar]
- Barral-Netto M, Barral A, Brodskyn C, Carvalho EM, Reed SG. Cytotoxicity in human mucosal and cutaneous leishmaniasis. Parasite Immunol. 1995;17:21–28. doi: 10.1111/j.1365-3024.1995.tb00962.x. [DOI] [PubMed] [Google Scholar]
- Bertho AL, Santiago MA, Da-Cruz AM, Coutinho SG. Detection of early apoptosis and cell death in T CD4+ and CD8+ cells from lesions of patients with localized cutaneous leishmaniasis. Braz J Med Biol Res. 2000;33:317–325. doi: 10.1590/s0100-879x2000000300010. [DOI] [PubMed] [Google Scholar]
- Bousoffara T, Louzir H, Salah AB, Dellagi K. Analysis of granzyme B activity as a surrogate marker of Leishmania-specific cell-mediated cytotoxicity in zoonotic cutaneous leishmaniasis. J Infect Dis. 2004;189:1265–1273. doi: 10.1086/382031. [DOI] [PubMed] [Google Scholar]
- Bretschneider I, Clemente MJ, Meisel C, Guerreiro M, Streitz M, Hopfenmüller W, Maciejewski JP, Wlodarski MW, Volk H-D. Discrimination of T-cell subsets and T-cell receptor repertoire distribution. Immunol Res. 2014;58:20–27. doi: 10.1007/s12026-013-8473-0. [DOI] [PubMed] [Google Scholar]
- Brodskyn CI, Barral A, Boaventura V, Carvalho E, Barral-Netto M. Parasite-driven in vitro human lymphocyte cytotoxicity against autologous infected macrophages from mucosal leishmaniasis. J Immunol. 1997;159:4467–4473. [PubMed] [Google Scholar]
- Campbell JP, Riddell NE, Burns VE, Turner M, van Zanten JJCSV, Drayson MT, Bosch JA. Acute exercise mobilises CD8+T-lymphocytes exhibiting an effector-memory phenotype. Brain Behav Immun. 2009;23:767–775. doi: 10.1016/j.bbi.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Cardoso TM, Machado A, Costa DL, Carvalho LP, Queiroz A, Machado P, Scott P, Carvalho EM, Bacellar O. Protective and pathological functions of CD8+ T cells in Leishmania brazi- liensis infection. Infect Immun. 2015;83:898–906. doi: 10.1128/IAI.02404-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarêncio J, Oliveira CI, Bomfim G, Pompeu MM, Teixeira MJ, Barbosa TC, Souza S, Neto, Carvalho EM, Brodskyn C, Barral A, Barral-Netto M. Characterization of the T-cell receptor Vbeta repertoire in the human immune response against Leishmania parasites. Infect Immun. 2006;74:4757–4765. doi: 10.1128/IAI.00265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmenares M, Kima PE, Samoff E, Soong L, McMahon-Pratt D. Perforin and gamma interferon are critical CD8+ T-cell-mediated responses in vaccine-induced immunity against Leishmania amazonensis infection. Infect Immun. 2003;71:3172–3182. doi: 10.1128/IAI.71.6.3172-3182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa RP, Gollob KJ, Fonseca LL, Rocha MO, Chaves AC, Medrano-Mercado N, Araújo-Jorge TC, Antas PR, Colley DG, Correa-Oliveira R, Gazzinelli G, Carvalho-Parra J, Dutra WO. T-cell repertoire analysis in acute and chronic human Chagas disease: differential frequencies of Vbeta5 expressing T-cells. Scand J Immunol. 2000;51:511–519. doi: 10.1046/j.1365-3083.2000.00706.x. [DOI] [PubMed] [Google Scholar]
- Coutinho SG, Da-Cruz AM, Bertho AL, Santiago MA, De-Luca P. Immunologic patterns associated with cure in human American cutaneous leishmaniasis. Braz J Med Biol Res. 1998;31:139–142. doi: 10.1590/s0100-879x1998000100019. [DOI] [PubMed] [Google Scholar]
- Coutinho SG, Oliveira MP, Da-Cruz AM, Luca PM, Mendonça SC, Bertho AL, Soong L, McMahon-Pratt D. T-cell responsiveness of American cutaneous leishmaniasis patients to purified Leishmania pifanoi amastigote antigens and Leishmania braziliensis promastigote antigens: immunologic patterns associated with cure. Exp Parasitol. 1996;84:144–155. doi: 10.1006/expr.1996.0100. [DOI] [PubMed] [Google Scholar]
- Coutinho SG, Pirmez C, Da-Cruz AM. Parasitological and immunological follow-up of American tegumentary leishmaniasis patients. Trans R Soc Trop Med Hyg. 2002;96(Suppl. 1):173–178. doi: 10.1016/s0035-9203(02)90072-6. [DOI] [PubMed] [Google Scholar]
- Da-Cruz AM, Bertho AL, Oliveira MP, Neto, Coutinho SG. Flow cytometric analysis of cellular infiltrate from American tegumentary leishmaniasis lesions. Br J Dermatol. 2005;153:537–543. doi: 10.1111/j.1365-2133.2005.06647.x. [DOI] [PubMed] [Google Scholar]
- Da-Cruz AM, Bittar R, Mattos M, Oliveira MP, Neto, Nogueira R, Pinho-Ribeiro V, Azeredo-Coutinho RB, Coutinho SG. T-cell-mediated immune responses in patients with cutaneous or mucosal leishmaniasis: long-term evaluation after therapy. Clin Diagn Lab Immunol. 2002;9:251–256. doi: 10.1128/CDLI.9.2.251-256.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da-Cruz AM, Conceição-Silva F, Bertho AL, Coutinho SG. Leishmania-reactive CD4+ and CD8+ T cells associated with cure of human cutaneous leishmaniasis. Infect Immun. 1994;62:2614–2618. doi: 10.1128/iai.62.6.2614-2618.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira CI, Brodskyn CI. The immunobiology of Leishmania braziliensis infection. Front Immunol. 2012;3(145) doi: 10.3389/fimmu.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira MP, Neto, Mattos MS, Perez MA, Da-Cruz AM, Fernandes O, Moreira J, Gonçalves-Costa SC, Brahin LR, Menezes CR, Pirmez C. American tegumentary leishmaniasis (ATL) in Rio de Janeiro state, Brazil: main clinical and epidemiologic characteristics. Int J Dermatol. 2000;39:506–514. doi: 10.1046/j.1365-4362.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- Faria DR, Gollob KJ, Barbosa J, Jr, Schriefer A, Machado PRL, Lessa H, Carvalho LP, Romano-Silva MA, Jesus AR, Carvalho EM, Dutra WO. Decreased in situ expression of interleukin-10 receptor is correlated with the exacerbated inflammatory and cytotoxic responses observed in mucosal leishmaniasis. Infect Immun. 2005;73:7853–7859. doi: 10.1128/IAI.73.12.7853-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraz R, Cunha CF, Gomes-Silva A, Schubach AO, Pimentel MI, Lyra MR, Mendonça SC, Valete-Rosalino CM, Da-Cruz AM, Bertho AL. Apoptosis and frequency of total and effector CD8+ T lymphocytes from cutaneous leishmaniasis patients during antimonial therapy. BMC Infect Dis. 2015;15(74) doi: 10.1186/s12879-015-0799-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacoia-Gripp CBW, Neves I, Jr, Galhardo MC, Morgado MG. Flow cytometry evaluation of the T-cell receptor Vbeta repertoire among HIV-1 infected individuals before and after antiretroviral therapy. J Clin Immunol. 2005;25:116–126. doi: 10.1007/s10875-005-2817-z. [DOI] [PubMed] [Google Scholar]
- Gorski J, Yassai M, Zhu X, Kissela B, Keever C, Flomenberg N. Circulating T-cell repertoire complexity in normal individuals and bone marrow recipients analyzed by CDR3 size spectratyping. Correlation with immune status. J Immunol. 1994;152:5109–5119. [PubMed] [Google Scholar]
- Hamann D, Baars PA, Rep MHG, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RAW. Phenotypic and functional separation of memory and effector human CD8+ T-cells. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Zang YC, Tejada-Simon MV, Kozovska M, Li S, Singh RA, Yang D, Rivera VM, Killian JK, Zhang JZ. A common TCR V-D-J sequence in Vbeta 13.1 T-cells recognizing an immunodominant peptide of myelin basic protein in multiple sclerosis. J Immunol. 1999;163:3530–3538. [PubMed] [Google Scholar]
- Kariminia A, Bourreau E, Ronet C, Couppie P, Sainte-Marie D, Tacchini-Cottier F, Launois P. Selective expression of the Vbeta 14 T-cell receptor on Leishmania guyanensis-specific CD8+ T-cells during human infection. J Infect Dis. 2007;195:739–747. doi: 10.1086/510912. [DOI] [PubMed] [Google Scholar]
- Keesen TSL, Antonelli LRV, Faria DR, Guimarães LH, Bacellar O, Carvalho EM, Dutra WO, Gollob KJ. CD4(+) T-cells defined by their Vβ T-cell receptor expression are associated with immunoregulatory profiles and lesion size in human leishmaniasis. Clin Exp Immunol. 2011;165:338–351. doi: 10.1111/j.1365-2249.2011.04430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura N, Toyonaga B, Yoshikai Y, Du RP, Mak TW. Sequences and repertoire of the human T-cell receptor alpha and beta chain variable region genes in thymocytes. Eur J Immunol. 1987;17:375–383. doi: 10.1002/eji.1830170312. [DOI] [PubMed] [Google Scholar]
- Lennon GP, Sillibourne JE, Furrie E, Doherty MJ, Kay RA. Antigen triggering selectively increases TCRBV gene transcription. J Immunol. 2000;165:2020–2027. doi: 10.4049/jimmunol.165.4.2020. [DOI] [PubMed] [Google Scholar]
- Libri V, Azevedo RI, Jackson SE, Di Mitri D, Lachmann R, Fuhrmann S, Vukmanovic-Stejic M, Yong K, Battistini L, Kern F, Soares MVD, Akbar AN. Cytomegalovirus infection induces the accumulation of short-lived, multifunctional CD4+CD45RA+CD27+ T-cells: the potential involvement of interleukin-7 in this process. Immunology. 2011;132:326–339. doi: 10.1111/j.1365-2567.2010.03386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonça SC, Luca PM, Mayrink W, Restom TG, Conceição-Silva F, Da-Cruz AM, Bertho AL, Costa CA, Genaro O, Toledo VP. Characterization of human T-lymphocyte-mediated immune responses induced by a vaccine against American tegumentary leishmaniasis. Am J Trop Med Hyg. 1995;53:195–201. doi: 10.4269/ajtmh.1995.53.195. [DOI] [PubMed] [Google Scholar]
- Menezes CAS, Rocha MOC, Souza PEA, Chaves ACL, Gollob KJ, Dutra WO. Phenotypic and functional characteristics of CD28+ and CD28- cells from chagasic patients: distinct repertoire and cytokine expression. Clin Exp Immunol. 2004;137:129–138. doi: 10.1111/j.1365-2249.2004.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer SC, Cooper DA, Hodgdon JC, Hussey RE, Fitzgerald KA, Schlossman SF, Reinherz EL. Identification of the receptor for antigen and major histocompatibility complex on human inducer T-lymphocytes. Science. 1983;222:1239–1242. doi: 10.1126/science.6606228. [DOI] [PubMed] [Google Scholar]
- Murre C. Epigenetics of antigen-receptor gene assembly. Curr Opin Genet Dev. 2007;17:415–421. doi: 10.1016/j.gde.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novais FO, Carvalho LP, Graff JW, Beiting DP, Ruthel G, Roos DS, Betts MR, Goldschmidt MH, Wilson ME, Oliveira CI, Scott P. Cytotoxic T-cells mediate pathology and metastasis in cutaneous leishmaniasis. PLoS Pathog. 2013;9: doi: 10.1371/journal.ppat.1003504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novais FO, Scott P. CD8(+) T-cells in cutaneous leishmaniasis: the good, the bad and the ugly. Semin Immunopathol. 2015;37:251–259. doi: 10.1007/s00281-015-0475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira F, Bafica A, Rosato AB, Favali CBF, Costa JM, Cafe V, Barral-Netto M, Barral A. Lesion size correlates with Leishmania antigen-stimulated TNF-levels in human cutaneous leishmaniasis. Am J Trop Med Hyg. 2011;85:70–73. doi: 10.4269/ajtmh.2011.10-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G, Demarest JF, Soudeyns H, Graziosi C, Denis F, Adelsberger JW, Borrow P, Saag MS, Shaw GM, Sekaly RP. Major expansion of CD8+ T-cells with a predominant Vbeta usage during the primary immune response to HIV. Nature. 1994;370:463–467. doi: 10.1038/370463a0. [DOI] [PubMed] [Google Scholar]
- Pirmez C. Immunopathology of American cutaneous leishmaniasis. Mem Inst Oswaldo Cruz. 1992;87(Suppl. V):105–109. doi: 10.1590/s0074-02761992000900016. [DOI] [PubMed] [Google Scholar]
- Plasilova M, Risitano A, Maciejewski JP. Application of the molecular analysis of the T-cell receptor repertoire in the study of immune-mediated hematologic diseases. Hematology. 2003;8:173–181. doi: 10.1080/1024533031000107505. [DOI] [PubMed] [Google Scholar]
- Reithinger R, Dujardin J-C, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- Ria F, van den Elzen P, Madakamutil LT, Miller JE, Maverakis E, Sercarz EE. Molecular characterization of the T-cell repertoire using immunoscope analysis and its possible implementation in clinical practice. Curr Mol Med. 2001;1:297–304. doi: 10.2174/1566524013363690. [DOI] [PubMed] [Google Scholar]
- Rostami MN, Keshavarz H, Edalat R, Sarrafnejad A, Shahrestani T, Mahboudi F, Khamesipour A. CD8+ T-cells as a source of IFN-γ production in human cutaneous leishmaniasis. PLoS Negl Trop Dis. 2010a;4: doi: 10.1371/journal.pntd.0000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami MN, Valian HK, Eskandari SE, Mohammadi AM, Shahrestani ST, Sarraf-Nejad A, Khamesipour A. Differential in vitro CD4+/CD8+ T-cell response to live vs. killed Leishmania major. Parasite Immunol. 2010b;32:101–110. doi: 10.1111/j.1365-3024.2009.01164.x. [DOI] [PubMed] [Google Scholar]
- Salameire D, Solly F, Fabre B, Lefebvre C, Chauvet M, Gressin R, Corront B, Ciapa A, Pernollet M, Plumas J, Macintyre E, Callanan MB, Leroux D, Jacob M-C. Accurate detection of the tumor clone in peripheral T-cell lymphoma biopsies by flow cytometric analysis of TCR-Vβ repertoire. Mod Pathol. 2012;25:1246–1257. doi: 10.1038/modpathol.2012.74. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Langenkamp A, Geginat J, Lanzavecchia A. Functional subsets of memory T-cells identified by CCR7 expression. Curr Top Microbiol Immunol. 2000;251:167–171. doi: 10.1007/978-3-642-57276-0_21. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T-lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Santos CS, Boaventura V, Cardoso CR, Tavares N, Lordelo MJ, Noronha A, Costa J, Borges VM, Oliveira CI, Van Weyenbergh J, Barral A, Barral-Netto M, Brodskyn CI. CD8(+) granzyme B(+)-mediated tissue injury vs. CD4(+)IFNγ(+)-mediated parasite killing in human cutaneous leishmaniasis. J Invest Dermatol. 2013;133:1533–1540. doi: 10.1038/jid.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira FT, Lainson R, Gomes CMC, Laurenti MD, Corbett CEP. Immunopathogenic competences of Leishmania (V.) braziliensis and L. (L.) amazonensis in American cutaneous leishmaniasis. Parasite Immunol. 2009;31:423–431. doi: 10.1111/j.1365-3024.2009.01116.x. [DOI] [PubMed] [Google Scholar]
- SVS/MS - Secretaria de Vigilância em Saúde/Ministério da Saúde Brasil Manual de vigilância da leishmaniose tegumentar americana. 2010 bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_leishmaniose_tegumentar_americana.pdf.
- Toledo VPCP, Mayrink W, Gollob KJ, Oliveira MAP, Costa CA, Genaro O, Pinto JA, Afonso LCC. Immunochemotherapy in American cutaneous leishmaniasis: immunological aspects before and after treatment. Mem Inst Oswaldo Cruz. 2001;96:89–98. doi: 10.1590/s0074-02762001000100010. [DOI] [PubMed] [Google Scholar]
- Uyemura K, Pirmez C, Sieling PA, Kiene K, Paes-Oliveira M, Modlin RL. CD4+ type 1 and CD8+ type 2 T-cell subsets in human leishmaniasis have distinct T-cell receptor repertoires. J Immunol. 1993;151:7095–7104. [PubMed] [Google Scholar]
- van den Beemd R, Boor PP, van Lochem EG, Hop WC, Langerak AW, Wolvers-Tettero IL, Hooijkaas H, van Dongen JJ. Flow cytometric analysis of the Vbeta repertoire in healthy controls. Cytometry. 2000;40:336–345. doi: 10.1002/1097-0320(20000801)40:4<336::aid-cyto9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- van der Merwe PA, Davis SJ. Molecular interactions mediating T-cell antigen recognition. Annu Rev Immunol. 2003;21:659–684. doi: 10.1146/annurev.immunol.21.120601.141036. [DOI] [PubMed] [Google Scholar]
- Viola A, Contento RL, Molon B. Signaling amplification at the immunological synapse. Curr Top Microbiol Immunol. 2010;340:109–122. doi: 10.1007/978-3-642-03858-7_6. [DOI] [PubMed] [Google Scholar]
- WHO - Word Health Organization Control of leishmaniases. Report of a meeting of the WHO Expert Committee. 2010 whqlibdoc.who.int/trs/WHO_TRS_949_eng.pdf.