Abstract
Dendritic cells (DCs) play a pivotal role in the connection of innate and adaptive immunity of hosts to mycobacterial infection. Studies on the interaction of monocyte-derived DCs (MO-DCs) using Mycobacterium leprae in leprosy patients are rare. The present study demonstrated that the differentiation of MOs to DCs was similar in all forms of leprosy compared to normal healthy individuals. In vitro stimulation of immature MO-DCs with sonicated M. leprae induced variable degrees of DC maturation as determined by the increased expression of HLA-DR, CD40, CD80 and CD86, but not CD83, in all studied groups. The production of different cytokines by the MO-DCs appeared similar in all of the studied groups under similar conditions. However, the production of interleukin (IL)-12p70 by MO-DCs from lepromatous (LL) leprosy patients after in vitro stimulation with M. leprae was lower than tuberculoid leprosy patients and healthy individuals, even after CD40 ligation with CD40 ligand-transfected cells. The present cumulative findings suggest that the MO-DCs of LL patients are generally a weak producer of IL-12p70 despite the moderate activating properties ofM. leprae. These results may explain the poor M. leprae-specific cell-mediated immunity in the LL type of leprosy.
Keywords: leprosy, dendritic cells, IL-12p70
Mycobacterium leprae causes leprosy and it affects peripheral nerves and skin (Scollard et al. 2006). The clinical manifestations of leprosy are dependent on the type of host immune response. Tuberculoid (TT) patients are characterised by effective M. leprae-specific T-helper (Th)1 responses that generate interleukin (IL)-2 and interferon (IFN)-gamma and show few lesions with a low bacillary load. Lepromatous leprosy (LL) presents with specific Th2 responses pattern accompanied by IL-10 and IL-4 production and numerous bacilli-loaded lesions. Intermediate forms of leprosy, called borderline leprosy, exist between these two polar leprosy forms and these forms display variable levels ofM. leprae-specific cellular and humoral immune responses (Scollard et al. 2006, Worobec 2009, Modlin 2010).
Dendritic cells (DCs) are specialised in antigen presentation to naïve T-cells and strategically distributed in different tissues to capture foreign antigens (Palucka & Banchereau 1999, Steinman & Hemmi 2006). Immature DCs (iDCs) capture pathogens, which activate DCs to become mature and efficient antigen-presenting cells (APCs) that activate T-cells for the generation of antigen-specific effector and memory T-cells. Antigen presentation occurs via major histocompatibility complexes (MHCs) on DCs and the expression of costimulatory molecules and the pattern of cytokine production by DCs are also important features for the effective activation of T-cells and subsequent differentiation to antigen-specific Th1 or Th2 populations (Steinman & Hemmi 2006). Therefore, an understanding of the interaction between the organism and DCs is an essential pre-requisite for the elucidation of the pathology of microbial immunity, including the immunopathology of leprosy.
There are surprisingly few studies of the interaction between M. lepraeand DCs and these studies reported controversial results (Hashimoto et al. 2002, Maeda et al. 2005, Murray et al. 2007). M. leprae usually presents with low variability (Monot et al. 2009) and most individuals are naturally resistant to the demonstrable infection (Lázaro et al. 2010). Therefore, the genetic background of the host is relevant to disease outcome. However, most of the reported studies of DC-M. leprae interactions were performed using cells obtained from healthy donors, which are most likely resistant to the demonstrable infection by M. leprae. Studies using DCs from disease-free donors are of limited value in understanding the activation of DCs byM. leprae that results or contributes to leprosy as a disease entity.
Several studies suggest that M. leprae is not efficiently presented by DCs to trigger an organism-specific immune response (Hashimoto et al. 2002), such as is in the case of LL leprosy. Notably, one study demonstrated that M. le-prae inhibits or acts as a neutral factor in the activation and maturation of DCs and relevant APCs (Murray et al. 2007). Another study demonstrated that the major membrane protein (MMP)-II, which is a component of the M. lepraemembrane, stimulated and effectively matured DCs, accompanied by the production of tumour necrosis factor (TNF) and the bioactive form of IL-12p70 (Maeda et al. 2005). The latter cytokine is essential for the generation of an effective antigen-specific effector Th1 response.
Therefore, this study principally focused on comparative studies of the maturation of monocyte-derived (MO) DCs of leprosy patients and healthy controls (HCs) after in vitro stimulation with sonicated M. leprae (ML DCs) compared to DCs that were induced to maturation in the presence of a standard maturation cocktail (mDCs). The cumulative findings of the present study will further our understanding of anergy in cell-mediated immunity in the LL type of leprosy compared to TT patients and HCs.
SUBJECTS, MATERIALS AND METHODS
Subjects - Leprosy patients were selected from the outpatient unit at the Lauro de Souza Lima Institute (ILSL), Bauru, state of São Paulo, Brazil, retrospectively with specified diagnoses for the different types of leprosy. Diagnoses were confirmed using clinical and histopathological criteria. Patients were classified in accordance with Ridley and Jopling (1966) criteria. Tuberculoid-tuberculoid (TT) and borderline-tuberculoid (BT) patients were grouped as TT patients, and lepromatous (LL) patients included lepromatous-lepromatous (LL) and borderline-lepromatous (BL) forms. No intermediate or true borderline patients were included in this study. Age and gender-matched normal HCs were selected from the ILSL staff and these individuals served as the control group.
Ethics - The Ethical Committee of Human Experimentation at ILSL approved this study (protocol 171/09), which was performed in accordance with the Helsinki Declaration of 1975, as revised in 1983. Written informed consent was obtained from all individuals included in the study.
Generation of MO-DCs and their maturation - Fourteen TT patients (7 females/7 males, consisting of 6 TT and 8 BT) and 12 LL patients (1 female/11 male consisting of 8 LL and 4 BL) and 16 HC individuals (7 females, 9 males) were included in the evaluations of differentiation and maturation of MO-DCs.
Peripheral mononuclear blood cells were obtained using density gradient centrifugation with Histopaque-1077 (Sigma, USA) followed by MO purification using a Percoll gradient (GE Healthcare, Sweden). The purity of MOs was 95-99% as determined using a flow cytometer with CD14 positivity as the only criteria. Further purification was not possible because of the unavailability of specialised software and sorting apparatus and an adequate supply of other relevant reagents for the gating and re-analysis for the presence of contamination with CD14-negative cells. The same purification procedure was adopted for all of the studied samples, with the expectation that if the purified CD14+ MOs were contaminated with other CD14-negative cells, such as CD16+CD14 cells, these cells were uniformly present in all samples.
Purified CD14+ MOs were cultured for six days in Iscove’s modified Dulbecco’s medium (Gibco, USA) supplemented with 10% foetal bovine serum (FBS) (Life Technologies, USA) and antibiotics (penicillin/streptomycin) in 24-well culture plates at a concentration of 5 x 105 cells per well in the presence of rGM-CSF (500 U/mL) and rIL-4 (250 U/mL) (PeproTech, USA) as stimuli for the differentiation of MOs to DCs (Sallusto & Lanzavecchia 1994).
The reagents for differentiation were used as supplied by the manufacturer and the bioactivities of these reagents were not further checked prior to use.
iDCs were cultured for another two days after differentiation in the presence of stimulants in either (i) a standard maturation cocktail (MC) composed of IL-1β (25 ng/mL), IL-6 (1,000 U/mL), TNF (50 ng/mL) and prostaglandin E2 (PGE2) (10-6 M) as previously described (Mailliard et al. 2004) to induce full mDCs or (ii) sonicated M. leprae (10 μg/mL) (kindly provided by Dr Patrick Brennan, Colorado State University) (ML-DCs). Nonstimulated cultures were maintained as iDCs. MO-DCs were examined using flow cytometry [fluorescence-activated cell sorting (FACS)] after eight days in culture for differentiation and maturation.
FACS analysis of MO-DCs surface markers - The phenotypic characteristics of MO-DCs were evaluated using the following markers: CD11c, CD1a, DC-SIGN, HLA-DR, ICAM-1, CD40, CD80, CD86 and CD83 (BD Biosciences, USA) according to the manufacturer’s instructions. Data collection was performed using a FACSCalibur flow cytometer (BD Biosciences) and included a minimum of 10,000 events.
Cytokine evaluation - The levels of TNF, IL-10, IL-15, transforming growth factor (TGF)-β1, IL-12p40 and IL-12p70 cytokines in MO-DCs supernatants were measured two days after the stimulation of iDCs (i.e., 8th day of culture) using commercial ELISA kits (R&D Systems, USA). Assays of IL-12p70 were performed on two separate occasions using two different ELISA kits (R&D Systems on the 1st occasion and BD Biosystems on the 2nd occasion). The detection limits for all kits were checked prior to use despite the manufacturers’ specifications by producing the standard curves in our laboratory set up. All of the kits demonstrated similar detection limits as stated in the manufacturers’ work sheet, except IL-12p70. Our standard curves for this cytokine revealed that the lowest detection limit was 3 pg/mL (not 31.5 pg/mL as specified by the manufacturer for R&D Systems) and 4 pg/mL for the BD Biosystems.
Stimulation with CD40-CD40 ligand (CD40L) to induction of IL-12p70- We evaluated the cytokine production in MO-DC preparations from 13 HC, 12 TT/BT and 12 LL/BL patients without CD40 ligation to examine the ability of freshly prepared MO-DC to produce IL-12p70 without a second signal. The production of this cytokine is augmented by the cognate interaction of CD40L; therefore, DCs were harvested, washed, plated in 96-well plates at 2 x 104 cells/well and cocultured with either a CD40L-expressing cell line (CD40L+ transfected J588 myeloma cell line) at 5 x 104 cells/well or a CD40L-ve cell line (a kind gift from Dr Peter Lane, University of Birmingham, UK) to mimic this interaction. This experiment was performed in a limited number of representative samples in each group because of the constraint of the availability of all of the reagents necessary to repeat experiments using a larger number of samples. A preliminary experiment also demonstrated that the level of IL-12p70 in the presence and absence of mock-transfected J588 cell line (also a gift from Dr Peter Lane) was similar (data not shown).
Briefly, MO-DCs from three TT (2 males, 1 female), three LL patients (3 males) and three HCs (2 males, 1 female) were used and iDCs were cultured in parallel with either mDCs or the sonicated antigen of M. leprae and the absence of any stimulant for 48 h, as described above. Twenty-four-hour supernatants were analysed for IL-12p70 using ELISA (BD Biosystems). The fold increase in IL-12p70 production due to CD40 ligation for the individuals in each group were calculated and compared between groups.
Statistics - Data comparisons between different groups (LL patients, TT patients and HCs) were performed using Kruskal-Wallis one-way ANOVA followed by Dunn’s post-test for independent samples. Wilcoxon matched-pairs test was used to compare two dependent samples (i.e., different stimuli in the same group) and Friedman nonparametric analysis of variance followed by Dunn’s post-test was used for comparison of three different stimuli. A significance level of 5% was adopted.
RESULTS
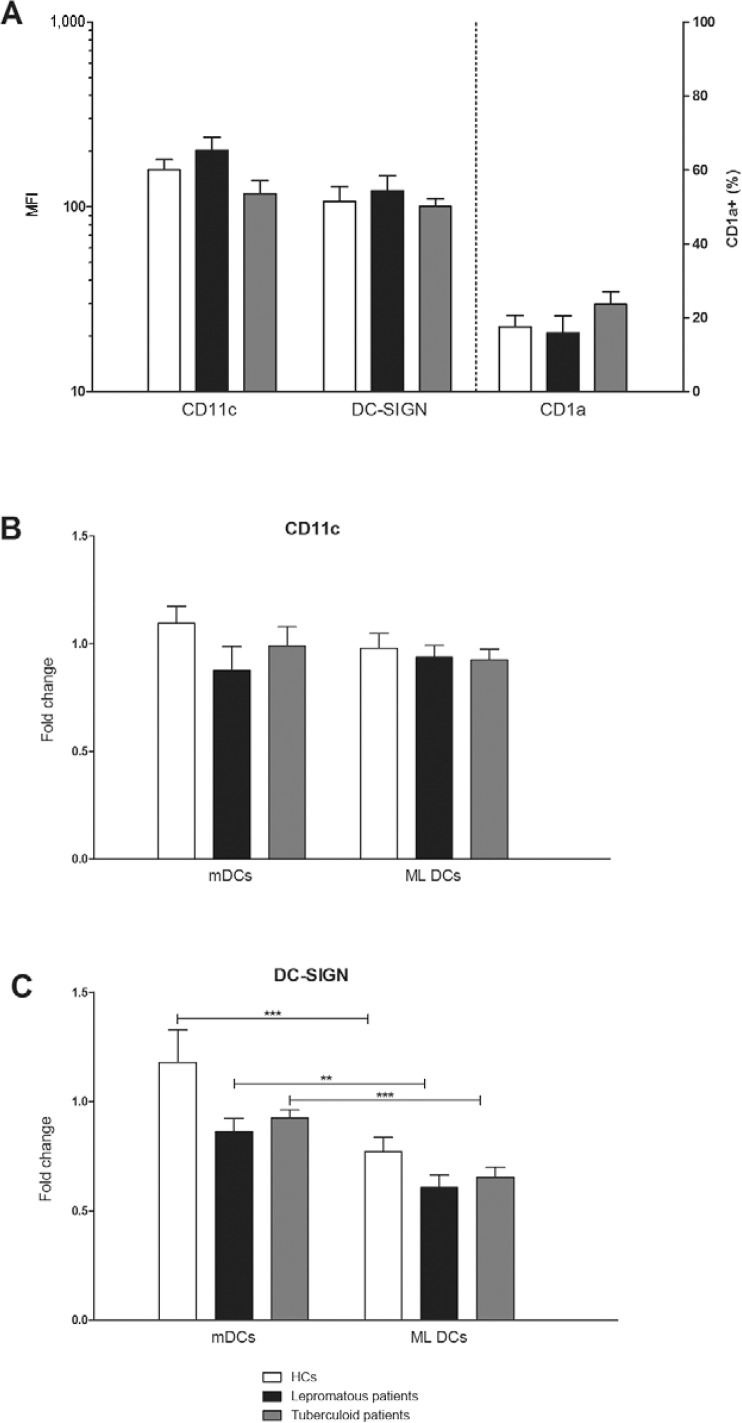
The differentiation of MOs into DCs is not impaired regardless of the clinical form of leprosy - Fig. 1A-C shows the comparative expression of DC phenotypic markers. Fig. 1A shows that the in vitro differentiation of MOs into DCs, as represented by CD11c, DC-SIGN and CD1a expression, was similar in leprosy patients and HCs. The generation of DCs examined by different markers revealed that CD11c- and DC-SIGN expressing DC populations were considerable, as expected, but CD1a-expressing DCs were low in all of the samples (data not shown). Neither mDCs nor ML DCs exhibited changes in CD11c expression compared to iDCs (Fig. 1B). However, a decrease in DC-SIGN levels was observed in ML DCs compared to mDCs in leprosy patients and HCs (Fig. 1C). The phenotypic expression of DC markers exhibited variable degrees of expression, despite the presence of the same number of CD14+ MOs in each case.
Fig. 1: analysis of differentiation of monocytes into dendritic cells (DCs) in healthy controls (HCs) and leprosy patients. A: expression of CD11c and DC-SIGN in mean intensity of fluorescence (MFI) and percentage of positive cells for CD1a in nonstimulated immature DCs (iDCs); B, C: fold change in the expression of CD11c and DC-SIGN (MFI) in mature DCs (mDCs) stimulated for 48 h with a specific maturation cocktail [interleukin (IL)-1β (25 ng/mL), IL-6 (1,000 U/mL), tumour necrosis factor (50 ng/mL) and prostaglandin E2 (10-6M)] or in Mycobacterium leprae stimulated DCs (ML DCs) pulsed with sonicated antigen of M. leprae (10 μg/mL) compared to nonstimulated iDCs. DCs were obtained from HCs (n = 16), tuberculoid (n = 14) or lepromatous leprosy patients (n = 12); **: p < 0.01; ***: p < 0.001, Wilcoxon matched-pairs test.
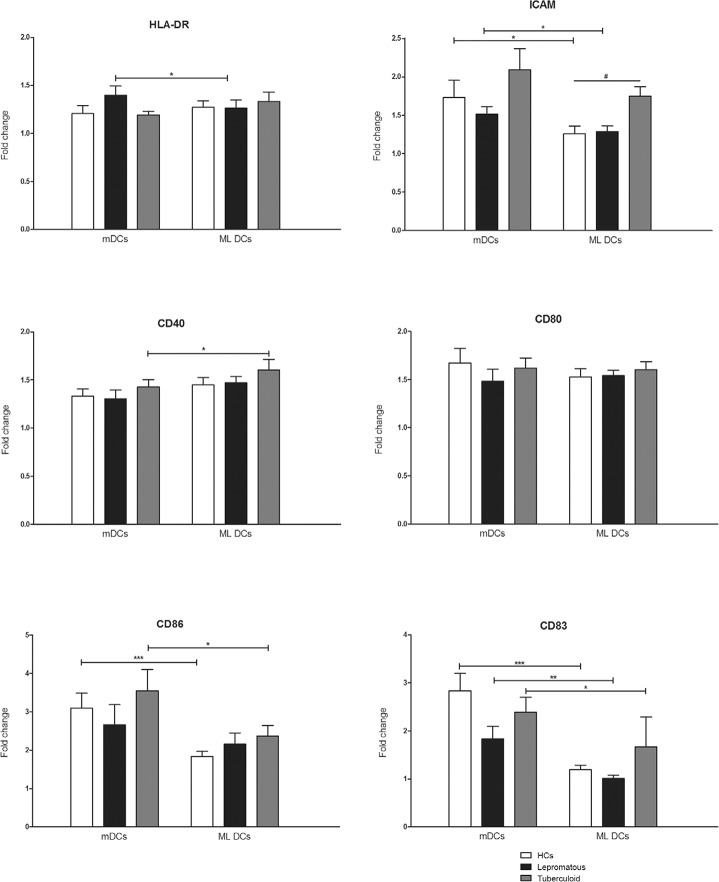
M. leprae induces variable maturation of MO-DCs and increases expression levels of HLA-DR and costimulatory molecules, but not CD83 - We evaluated the maturation of MO-DCs using the expression of cell surface markers.Fig. 2 indicates increased expression of HLA-DR, CD40, CD80 and CD86 in ML DCs compared to iDCs, expressed as a fold-change in leprosy patients and HCs. However, CD83, which is the most important representative marker of DC maturation, did not exhibit increased expression in ML DCs from leprosy patients or HCs. ICAM-1 expression (Fig. 2) was increased in ML DCs from TT patients compared to HCs. No differences in the gene expression of cell surface markers were observed between leprosy patients and HCs (Supplementary data). We also used LPS as a routine positive control for MO-DCs maturation, but we did not observe any higher expression of maturation markers (data not shown).
Fig. 2: analysis of dendritic cells (DCs) maturation. Fold change in the expression of surface markers in mature DCs (mDCs) stimulated for 48 h with a standard maturation cocktail [interleukin (IL)-1β (25 ng/mL), IL-6 (1,000 U/mL), tumour necrosis factor (50 ng/mL) and prostaglandin E2 (10-6M)] or in Mycobacterium leprae stimulated DCs (ML DCs) pulsed with sonicated antigen of M. leprae (10 μg/mL) compared to nonstimulated immature DCs. DCs were obtained from healthy controls (HCs) (n = 16), tuberculoid (n = 14) or lepromatous leprosy patients (n = 12). *: p < 0.05; **: p < 0.01; ***: p < 0.001, Wilcoxon matched-pairs test; #: p < 0.05, Kruskal-Wallis test (nonparametric ANOVA) with Dunn’s Multiple Comparisons test.
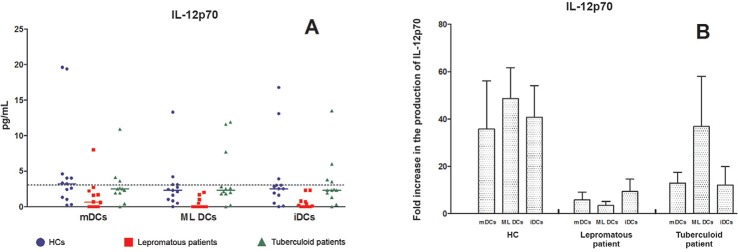
MO-DCs from LL patients produce less IL-12p70 - Fig. 3A shows the profile of IL-12p70 production by MO-DCs from all of the studied groups. Notably, the production of IL-12p70 was almost zero in most of LL patients in absence of ligation with CD40L. Only 1/36 (2.7%) of LL/BL, 10/36 (28.6%) TT patients and 16/39 (41%) HCs produced this cytokine above the cut-off value for the detection limit. The scale of these differences was too narrow to justify any conclusions, despite the noticeable difference in the production of this cytokine. We repeated the experiments in a few representative samples using a transfected cell line expressing CD40L (J588) and mock-transfected J588 cell line to confirm the above-stated findings. This restriction in the use of the number of samples must be accepted because of the constraint in the supply of commercial kits and reagents compounded with the limited availability of CD40L-transfected and mock-transfected cell lines from the UK. Fig. 3B shows the results of these experiments as a fold-increase of IL-12p70. These results clearly demonstrated that the production of IL-12p70 by ML DCs increased considerably in HCs upon CD40 ligation (22.5-61.9-fold) and TT patients (3.2-76.1-fold). This augmentation was considerably lower in LL patients (1.1-6.8-fold), which confirms that the MO-DCs of the LL/BL group are weak producers of IL-12p70.
Fig. 3: production of interleukin (IL)-12p70 by monocytes derived-dendritic cells (MO-DCs). A: production of IL-12p70 by healthy controls (HCs) (n = 13), tuberculoid (n = 12) and lepromatous leprosy patients (n = 12). The dashed line represents the detection limit of IL-12p70 (3 pg/mL); B: fold increase in the production of IL-12p70 represented by the levels found in DCs cocultured with CD40-CD40 ligand (CD40L) expressing J588 cell line normalised by the production in MO-DCs cocultured with not transfected J588 cell line [HCs (n = 3), tuberculoid patients (n = 3) and lepromatous patients (n = 3)]; mDCs: mature DCs stimulated for 48 h with a specific maturation cocktail [IL-1β (25 ng/mL), IL-6 (1,000 U/mL), tumour necrosis factor (50 ng/mL) and prostaglandin E2 (10-6 M)]; ML DCs: Mycobacterium leprae stimulated DCs pulsed with sonicated antigen of M. leprae (10 μg/mL); iDCs: nonstimulated immature DCs.
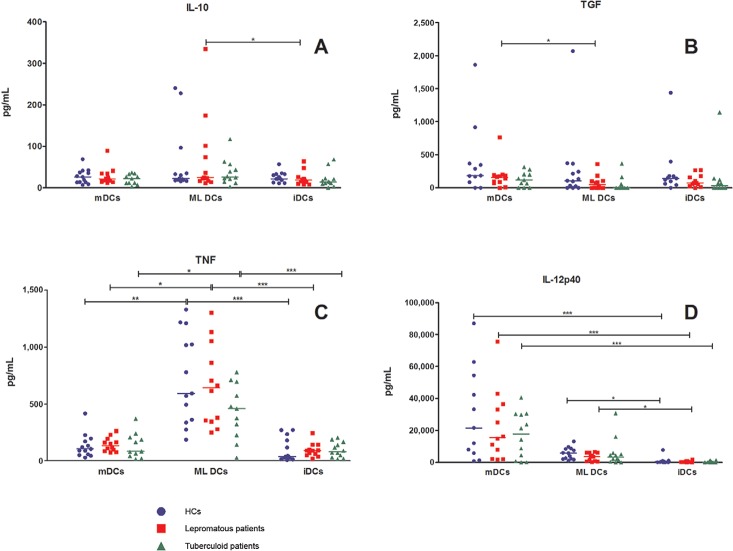
TNF and IL-12p40 levels were higher in ML DCs compared to iDCs in leprosy patients and HCs, but no significant difference was observed in the production of TGF-β1 (Fig. 4). IL-15 production by MO-DCs was not detected in our study.
Fig. 4: production of cytokines by monocytes derived-dendritic cells (DCs). Production of interleukin (IL)-10, transforming growth factor (TGF), tumour necrosis factor (TNF) and IL-12p40 [healthy controls (HCs) (n = 13), tuberculoid (n = 12) and lepromatous leprosy patients (n = 12)]. mDCs: mature DCs stimulated for 48 h with a specific maturation cocktail [IL-1β (25 ng/mL), IL-6 (1,000 U/mL), TNF (50 ng/mL) and prostaglandin E2 (10-6M)]; ML DCs: Mycobacterium leprae stimulated DCs pulsed with sonicated antigen of M. leprae (10 μg/mL); iDCs: nonstimulated immature DCs; *: p < 0.05; **: p < 0.01; ***: p < 0.001, Friedman test (nonparametric repeated measures ANOVA) with Dunn’s Multiple Comparisons test.
DISCUSSION
DCs are pivotal for the orchestration of immunity to intracellular organisms, such asMycobacterium tuberculosis and M. leprae by connecting the two arms of host immunity (Rossi & Young 2005). However, studies on the role of DCs in the triggering of the immune response against M. leprae are rare.
We studied the differentiation of MO-DCs from patients with polar forms of leprosy (TT and LL) and MO-DC activation induced by a standard MC or sonicated M. leprae. No differences in the differentiation of CD14+ MO to DCs were observed between leprosy patients and HCs. Sieling et al. (1999) observed similar results for the differentiation of MO-DCs from leprosy patients and HCs. Taken together, our results and the results of Sieling et al. (1999) suggest no defects in the de novo generation of DCs in leprosy patients. All studied groups also exhibited similar extents of maturation of MO-DCs, including CD83 expression, under standard MC stimulation (Fig. 2). However, it is possible that M. leprae acts directly on MOs in vivo and blocks their differentiation to DCs and further maturation towards the capability to activate lymphocytes. Sieling et al. (1999) suggested that the high bacterial load in lepromatous leprosy lesions may impair the migration/differentiation of MOs into mDCs, as shown by the lack of CD83 expression in the lesions of these patients. This study did not investigate the in situ expression of this marker in the lesions of our patients. Therefore, our present data cannot be compared withSieling et al. (1999) in toto.
MO-DCs from leprosy patients and HCs exhibited a decreased expression of the DC-SIGN receptor after stimulation with sonicated M. leprae. Considering that M. leprae binds DC-SIGN (Barreiro et al. 2006), the resultant complex after this ligation is likely internalised for further degradation and antigen presentation, resulting in lower expression of this receptor on the surface of ML DCs. However, this hypothesis requires further experimentation.
Sonicated M. leprae induced the expression of costimulatory molecules (CD40, CD80 and CD86) and HLA-DR in leprosy patients and HCs in our experimental model. However, CD83 expression, which is an important marker of DC maturation, was not induced. This observation indicates a variable capability of maturation in MO-DCs after stimulation with M. leprae, which may prevent the activation of T-cells. Murray et al. (2007) evaluating the role of M. leprae in the activation of MO-DCs reported a null or suppressor action of dead irradiatedM. leprae on the differentiation of MO-DCs from HCs, which resulted in low expressions of CD40, CD80, CD86, CD83 and HLA-DR compared toM. tuberculosis and Mycobacterium bovis. Higher levels of Th2 cytokines were observed after stimulation with M. leprae, but the expression of genes encoding MHC class II costimulatory molecules, such as CD80 and the genes encoding IL-12 and TNF production were induced in MO-DCs stimulated with M. tuberculosis and M. bovis, but not M. leprae. These results demonstrate that M. leprae behaves differently from other mycobacteria by exerting an evasive action on DCs. Hashimoto et al. (2002) similarly investigated the effect of heat-killed M. leprae on MO-DCs from healthy individuals and found a decrease in HLA-ABC and HLA-DR expression and increased CD86 expression comparison to BCG, but CD83 expression and antigen presentation to T-cells, was only observed when a high dose of the bacillus was used. All of these previous studies and our own study indicate that M. leprae is not a robust inducer of DC maturation, despite some of the particularities observed, which are possibly related to the different antigens used. The best indicator of DCs maturation is the capability to activate lymphocytes; however, the observed low expression of CD83, which is a marker of DC maturation, suggests the poor stimulating nature of the bacillus.
Mihret et al. (2011) examined the interaction between DCs from HCs and M. tuberculosis in vitro and found that the bacillus induced DC maturation and activation with increased CD40, CD80, CD86, CD54 and HLA-DR expression and stimulatory capacity for T-cells. Hava et al. (2008) observed high expression of HLA-DR, CD80, CD86 and CD83, which indicates that M. tuberculosisinduces the maturation of DCs, but this maturation was rapid and compromised the antigen processing and presentation via MHC. These results demonstrate a possible mechanism of immune evasion by the bacillus. Hanekom et al. (2003) observed a limited maturation of DCs from HCs stimulated with M. tuberculosis in vitro, which impaired the APC function of DCs. The findings of this study are consistent with our finding using M. leprae. Together, these data on the interaction of M. tuberculosis and DCs present controversial results, as observed in the M. leprae interactions, which demonstrates the complexity of the relationship between host and parasites in the activation of the immune response in mycobacteriosis and indicates the need for further investigation.
The production of IL-12p70, which is the primary inducer of Th1-type immune responses by MO-DCs was low in mDCs and ML DCs from leprosy patients and HCs. However, the majority of LL patients produced decreased levels of IL-12p70 compared to TT patients and HCs. This lower production of the bioactive form of IL-12 may be characteristic of most LL patients and underlie the anergy of the Th1 response that is observed in these individuals. mDCs and ML DCs from HCs and leprosy patients were cocultured with a transfected cell line expressing CD40L to mimic the interaction with a CD40L-expressing T-cell and confirm these findings. A considerable increase in the production of IL-12p70 by ML DCs was observed under stimulation with CD40L in HCs and TT patients, but ML DCs of LL patients still produced considerably decreased levels of this cytokine under the same condition. These observations strongly indicate a weaker augmentation by CD40-CD40L during in vitro stimulation by sonicated M. leprae, which suggests that these patients are characteristically prone to a muted organism-specific cell-mediated immunity. It is tempting to conclude that these patients do not produce this important cytokine for Th1-type of immune responses, but no previous study evaluated the production of IL-12p70 by MO-DCs in LL patients. Maeda et al. (2005) evaluated the production of IL-12p70 by MO-DCs stimulated with MMP-II from M. leprae in healthy individuals and observed an augmented production of this cytokine upon stimulation with MMP-II plus soluble CD40L. Murray et al. (2007) did not observe IL-12 production in MO-DCs stimulated by irradiated-M. leprae. The combination of these results with the present study confirms the necessity of CD40L ligation in the stimulation of IL-12p70 production and the ability of HCs and TT patients to produce optimum levels of this cytokine to maintain effective Th1 immunity to M. leprae. However, this production is deficient on the LL side of the spectrum.
In summary, our results demonstrate that sonicated M. leprae antigen is a weak inducer of DC activation and MO-DCs from LL patients exhibited decreased bioactive IL-12 production, which may contribute to the anergy in the specific cell-mediated immune response in these individuals. Leprosy is important to public health because of the large number of cases that exist worldwide, especially in Brazil. Therefore, studies to improve our understanding of the immune response against the bacilli are justified to advance our knowledge of the disease pathomechanism.
ACKNOWLEDGEMENTS
To Dr DA Lammas, for re-reading the paper and correcting English.
Funding Statement
Financial support: FAPESP (2009/01436-1)
Footnotes
Financial support: FAPESP (2009/01436-1)
AFB and PG were supported by scholarships from CAPES. An award by AMC-UvA to PKD for the participation in this project is duly acknowledged.
REFERENCES
- Barreiro LB, Quach H, Krahenbuhl J, Khaliq S, Mohyuddin A, Mehdi SQ, Gicquel B, Neyrolles O, Quintana-Murci L. DC-SIGN interacts with Mycobacterium leprae, but sequence variation in this lectin is not associated with leprosy in the Pakistani population. Hum Immunol. 2006;67:102–107. doi: 10.1016/j.humimm.2006.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanekom WA, Mendillo M, Manca C, Haslett PA, Siddiqui MR, Barry C, Kaplan G. Mycobacterium tuberculosis inhibits maturation of human monocyte-derived dendritic cells in vitro. J Infect Dis. 2003;188:257–266. doi: 10.1086/376451. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Maeda Y, Kimura H, Suzuki K, Masuda A, Matsuoka M, Makino M. Mycobacterium leprae infection in monocyte-derived dendritic cells and its influence on antigen-presenting function. Infect Immun. 2002;70:5167–5176. doi: 10.1128/IAI.70.9.5167-5176.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hava DL, van der Wel N, Cohen N, Dascher CC, Houben D, León L, Agarwal S, Sugita M, van Zon M, Kent SC, Shams H, Peters PJ, Brenner MB. Evasion of peptide, but not lipid antigen presentation, through pathogen-induced dendritic cell maturation. Proc Natl Acad Sci USA. 2008;105:11281–11286. doi: 10.1073/pnas.0804681105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lázaro FP, Werneck RI, Mackert CC, Cobat A, Prevedello FC, Pimentel RP, Macedo GM, Eleutério MA, Vilar G, Abel L, Xavier MB, Alcaïs A, Mira MT. A major gene controls leprosy susceptibility in a hyperendemic isolated population from north of Brazil. J Infect Dis. 2010;201:1598–1605. doi: 10.1086/652007. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Mukai T, Spencer J, Makino M. Identification of an immunomodulating agent from Mycobacterium leprae. Infect Immun. 2005;73:2744–2750. doi: 10.1128/IAI.73.5.2744-2750.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, Kirkwood JM, Storkus WJ, Kalinski P. α-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- Mihret A, Mamo G, Tafesse M, Hailu A, Parida S. Dendritic cells activate and mature after infection with Mycobacterium tuberculosis. 247BMC Res Notes. 2011;4 doi: 10.1186/1756-0500-4-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlin RL. The innate immune response in leprosy. Curr Opin Immunol. 2010;22:48–54. doi: 10.1016/j.coi.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monot M, Honoré N, Garnier T, Zidane N, Sherafi D, Paniz-Mondolfi A, Matsuoka M, Taylor GM, Donoghue HD, Bouwman A, Mays S, Watson C, Lockwood D, Khamesipour A, Dowlati Y, Jianping S, Rea TH, Vera-Cabrera L, Stefani MM, Banu S, Macdonald M, Sapkota BR, Spencer JS, Thomas J, Harshman K, Singh P, Busso P, Gattiker A, Rougemont J, Brennan PJ, Cole ST. Comparative genomic and phylogeographic analysis of Mycobacterium leprae. Nat Genet. 2009;41:1282–1289. doi: 10.1038/ng.477. [DOI] [PubMed] [Google Scholar]
- Murray RA, Siddiqui MR, Mendillo M, Krahenbuhl J, Kaplan G. Mycobacterium leprae inhibits dendritic cell activation and maturation. J Immunol. 2007;178:338–344. doi: 10.4049/jimmunol.178.1.338. [DOI] [PubMed] [Google Scholar]
- Palucka K, Banchereau J. Dendritic cells: a link between innate and adaptive immunity. J Clin Immunol. 1999;19:12–25. doi: 10.1023/a:1020558317162. [DOI] [PubMed] [Google Scholar]
- Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255–273. [PubMed] [Google Scholar]
- Rossi M, Young JW. Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J Immunol. 2005;175:1373–1381. doi: 10.4049/jimmunol.175.3.1373. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338–381. doi: 10.1128/CMR.19.2.338-381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieling PA, Jullien D, Dahlem M, Tedder TF, Rea TH, Modlin RL, Porcelli SA. CD1 expression by dendritic cells in human leprosy lesions: correlation with effective host immunity. J Immunol. 1999;162:1851–1858. [PubMed] [Google Scholar]
- Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- Worobec SM. Treatment of leprosy/Hansen’s disease in the early 21st century. Dermatol Ther. 2009;22:518–537. doi: 10.1111/j.1529-8019.2009.01274.x. [DOI] [PubMed] [Google Scholar]