Abstract
Differentiating bipolar disorders (BD) from unipolar depression (UD) remains a major clinical challenge. The identification of neurobiological markers may help to differentiate these disorders, particularly during depressive episodes. This cross-sectional study, including 33 patients with UD, 33 patients with BD, and 34 healthy controls, is one of the first to directly compare UD and BD with respect to reward processing. A card-guessing paradigm was employed and brain activity associated with reward processing was investigated by means of fMRI. A 3 (group) × 2 (condition: reward>control, loss>control) ANOVA was conducted using the nucleus accumbens (NAcc) as ROI. Furthermore, a whole-brain approach was applied. A functional connectivity analysis was performed to characterize diagnosis-related alterations in the functional coupling between the NAcc and other brain areas. The ANOVA revealed higher activity for healthy controls (HCs) than for BD and UD in the NAcc during reward processing. Moreover, UD showed a higher functional connectivity between the NAcc and the VTA than HC. The patients groups could be differentiated in that BD showed a decreased activation, in the reward condition, of the NAcc, caudate nucleus, thalamus, putamen, insula, and prefrontal areas compared with UD. These results may help to refine the understanding of neural correlates of reward processing in both disorders, and to understand the neural underpinnings of anhedonia, a core symptom of depressive episodes.
INTRODUCTION
Among patients suffering from bipolar disorder (BD), misdiagnosis rates up to 70% have been reported, leading to inappropriate medication treatment and poor prognosis (Hirschfeld et al, 2003; Phillips and Kupfer, 2013). The main reason for the failure to accurately identify BD is that diagnostic criteria for a depressive episode are the same in both disorders (Almeida and Phillips, 2013). Therefore, the identification of neurobiological markers may help to differentiate these disorders, particularly during depressive episodes, and may also identify shared neuronal alterations.
Previous neuroimaging studies have already addressed the differentiation of BD and unipolar depression (UD) employing structural (Redlich et al, 2014a; Versace et al, 2010) and functional MRI (Almeida and Phillips, 2013; Benson et al, 2014; Grotegerd et al, 2014). These studies yielded differences in regions that contribute to the dysregulation of emotional and cognitive functions. However, only few studies focused on neural systems associated with reward processing in BD and, to our knowledge, only one study directly compared BD and UD (Chase et al, 2013).
A basic function of reward is to induce a subjective feeling of pleasure and positive emotion. Altered responsiveness to reward, and to reinforcing stimuli, could therefore contribute to the generation and maintenance of depressive symptoms (Pizzagalli et al, 2009). Diminished responsiveness to commonly rewarding stimuli has already been observed in both disorders, and appears to be mainly driven by the mesolimbic dopamine system including the ventral tegmental area (VTA), a central structure in the reward processing circuitry (Keller et al, 2013).
There are different stages of reward processing, and it is widely discussed whether the nucleus accumbens (NAcc) is predominantly involved in reward anticipation (see, eg, Knutson et al, 2001) or in reward outcome (see, eg, Elliott et al, 2000; Ernst et al, 2005). Given the heterogeneity of previous studies, with different paradigms focusing on different aspects of reward processing, it is difficult to gain a clear picture. In a meta-analysis, Liu et al (2011) showed that reward outcome often activated the NAcc and medial orbitofrontal cortex, whereas reward anticipation activated the anterior cingulate cortex (ACC), insula, and areas within the brainstem.
Several studies examining alterations in the mesolimbic system in UD found reduced activity during reward feedback, relative to healthy controls (HCs), in the ventral striatum (VS) including the NAcc (Knutson et al, 2008; Pizzagalli et al, 2009). Studies in BD reported more heterogeneous results, probably because of a higher variation of mood states. A recent review by Nusslock et al (2014) suggested increased NAcc responsiveness across all mood states in BD. However, not all available data support this notion. Compared with HCs, individuals with BD showed elevated VS activity during hypomania (O'Sullivan et al, 2011), no differences or elevated activity in euthymic states (Caseras et al, 2013; Nusslock et al, 2012), but activity was decreased in euthymic to mildly depressed patients (Trost et al, 2014).
Chase et al (2013) is the first study that examined reward feedback in BD during depressive episodes and directly compared BD and UD. With a paradigm that included both anticipation and feedback phases, BD and UD showed less activity in the ACC than HCs during reward anticipation, but there were no differences during reward outcome. Given the characteristics of the outcome phase used, it is likely that the activation during reward feedback not only reflects the reaction to the outcome, but is also influenced by the signed prediction error and reward. Therefore, the present study focuses in contrast to Chase et al (2013) on reward outcome, examining neuronal correlates of reward processing, and directly compares UD and BD suffering from depressive episodes.
Based on the previous research in UD (see, eg, Pizzagalli et al, 2009), we hypothesized BD and UD to show reduced activity in the NAcc during reward feedback as compared with HCs. Because of the lack of studies exclusively focusing on this phase of reward processing, no strong a priori hypotheses regarding group differences were made.
When investigating reward processing, alterations in one structure are likely associated with connectivity abnormalities within a larger system. Thus, it seems important to further investigate the functional interplay between the NAcc and other brain areas. Functional connectivity (Friston, 1994) allows to identify networks of brain regions showing patterns of coactivation throughout the time course of a task. With respect to the functional coupling between the NAcc and other reward-relevant brain areas, we predicted altered functional connectivity to prefrontal and striatal areas in both BD and UD based on previous research (Diekhof and Gruber, 2010; Diekhof et al, 2008).
MATERIALS AND METHODS
Participants and Questionnaires
This study comprised 33 individuals with BD (mean age: 38.1, SD=12.6 years), 33 individuals with UD (mean age: 38.5, SD=12.1 years), and 34 HCs (mean age: 38.6, SD=12.3 years). The groups did not differ in age (P=0.88), sex (P=0.72), and years of education (P=0.18). Furthermore, both patient groups were comparable regarding several clinical variables including number of depressive episodes, time since onset of depression, total duration in depressive state, total duration of acute episode, and medication load (all Ps>0.17). However, more time since first inpatient treatment had elapsed for BD patients, and their cumulative life-time duration of inpatient treatment was also longer (Ps<0.02; see Table 1). Patients were recruited from the inpatient service of the Department of Psychiatry, University of Muenster. HCs were recruited by public notices and newspaper announcements. Diagnoses were verified with the structured clinical interview for DSM-IV (SCID-IV; Wittchen et al, 1997). All patients suffered from a current major depressive episode and fulfilled the criteria of either MDD or bipolar-I disorder. For HCs, any life-time psychiatric disorder was an exclusion criterion. For patients, additional comorbid life-time diagnoses of any organic mental disorders, dementia, substance-related disorders, and schizophrenia/schizoaffective disorders were exclusion criteria. There were no significant differences in comorbidity frequencies between both patient groups (all Ps>0.15, see Table 2). All participants were free from any history of neurological abnormalities or brain injury, had normal or corrected-to-normal vision, and had adequate knowledge of German and cognitive abilities (verbal IQ >80; multiple-choice vocabulary intelligence test MWT-B; Lehrl, 2005). All participants received a financial compensation. The study was approved by the local IRB, and all participants provided written informed consent before study participation.
Table 1. Sociodemographic and Clinical Characteristics.
|
BD sample (n=33) |
UD sample (n=33) |
t-test or χ2 testa |
HC sample (n=34) |
||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | P-value | Mean | SD | |
| Sociodemographic characteristics | |||||||
| Age | 38.12 | 12.55 | 38.48 | 12.08 | 0.91 | 38.59 | 12.28 |
| Sex (m/f) | 17/16 | 16/17 | 0.81 | 18/16 | |||
| Total education time | 14.76 | 2.06 | 14.18 | 1.83 | 0.23 | 14.85 | 2.20 |
| Verbal IQ | 111.58 | 16.10 | 111.55 | 11.76 | 0.99 | 118.71 | 11.53 |
| Questionaires | |||||||
| BDI | 24.85 | 8.60 | 27.88 | 9.29 | 0.17 | 1.88 | 2.51 |
| HAMD | 22.88 | 4.55 | 24.56 | 5.92 | 0.20 | 1.24 | 1.37 |
| YMRS | 2.45 | 2.43 | 1.56 | 1.66 | 0.09 | 0.29 | 0.68 |
| SHAPS | 4.35 | 4.03 | 6.26 | 4.05 | 0.07 | 0.52 | 1.23 |
| STAI | 57.56 | 10.75 | 60.94 | 9.10 | 0.18 | 30.47 | 6.20 |
| Clinical characteristics | |||||||
| Duration of current episode (weeks) | 14.09 | 13.20 | 23.76 | 29.31 | 0.09 | NA | |
| Number of depressive episodes | 6.73 | 5.47 | 4.33 | 4.02 | 0.47 | NA | |
| Life-time cumulative duration of depressive states (months) | 26.18 | 21.57 | 29.68 | 28.23 | 0.57 | NA | |
| Number of manic episodes | 3.52 | 3.73 | NA | NA | |||
| Life-time cumulative duration of manic states (months) | 5.72 | 6.30 | NA | NA | |||
| Time since onset of first depressive episode (months) | 138.45 | 123.07 | 100.15 | 97.02 | 0.17 | NA | |
| Time since first inpatient treatment (months) | 83.41 | 18.54 | 25.47 | 9.86 | 0.01 | NA | |
| Life-time cumulative duration of inpatient treatment (weeks) | 26.12 | 41.40 | 8.21 | 10.64 | 0.02 | NA | |
| Medical characteristics | |||||||
| Medication Load Index | 3.18 | 2.13 | 2.58 | 1.54 | 0.19 | NA | |
| Antidepressants | |||||||
| SSNRI | 8 | 26 | <0.01 | ||||
| SSRI | 5 | 6 | 0.74 | ||||
| SNRI | 1 | 0 | 0.31 | ||||
| Tricyclic antidepressants | 2 | 3 | 0.64 | ||||
| MAO inhibitor | 2 | 0 | 0.15 | ||||
| Agomelantine | 1 | 7 | 0.02 | ||||
| Mood stabilizer | 17 | 3 | <0.01 | ||||
| Antipsychotics | 24 | 14 | <0.01 | ||||
| No medication | 1 | 1 | 1 | ||||
| Monotherapy | 8 | 15 | 0.07 | ||||
Abbreviations: BD, bipolar-I disorder; BDI, Beck Depression Inventory; HAMD, Hamilton Depression Rating Scale; MAO, monoamine oxidase inhibitor; STAI, State Trait Anxiety Inventory; SSNRI, Selective Serotonin Noradrenaline Reuptake Inhibitor; SSRI, Selective Serotonin Reuptake Inhibitor, SNRI, Selective Noradrenaline Reuptake Inhibitor; UD, unipolar depressive disorder; YMRS, Young Mania Rating Scale.
The t-tests and χ2 tests refer to the comparison between UD and BD.
Table 2. Life-Time Comorbidities.
| BD sample (n=33) | UD sample (n=33) | X2 P-value | |
|---|---|---|---|
| Panic disorder/agoraphobia | 5 | 8 | 0.35 |
| Social phobia | 4 | 3 | 0.69 |
| Specific phobia | 3 | 3 | 1 |
| Obsessive compulsive disorder | 3 | 1 | 0.31 |
| Post-traumatic stress disorder | 2 | 1 | 0.56 |
| Somatoform disorder | 0 | 2 | 0.15 |
| Eating disorder | 1 | 1 | 1 |
Abbreviations: BD, bipolar depression; UD; unipolar depression.
To measure total medication load, we used a strategy as described earlier (Redlich et al, 2014a). Each psychotropic medication was coded as absent=0, low=1 (equal or lower average dose), or high=2 (greater than average dose), relative to the midpoint of the daily dose range recommended by Physician's-Desk-Reference. We calculated a composite measure of total medication load for each individual, reflecting dose and variety of different medications taken, by summing all individual medication.
The Beck Depression Inventory (BDI; Beck and Steer, 1987; Hautzinger et al, 1994) was used to assess the presence of depressive symptoms. In addition, the Hamilton Rating Scale of Depression (HAMD; Hamilton, 1960) was applied by a clinical interviewer as an objective depression measure. The Young Mania Rating Scale (YMRS, Young et al, 1978) was used to assess manic symptoms. The German version of the Snaith-Hamilton Pleasure Scale (SHAPS-D, Franz et al, 1998; Snaith et al. 1995) was used to assess self-reported anhedonia. In order to control for effects of trait anxiety, the State-Trait Anxiety Inventory (STAI-trait version; Spielberger et al, 1970) was administered.
Materials and Procedure
We employed a card-guessing paradigm (Forbes et al, 2009; Opel et al, 2015) to detect brain activity associated with reward processing, more precisely reward feedback. Participants were told that reaction times were irrelevant for the task outcome and the final amount of their monetary reward would depend on their guessing performance on the card game, and were unaware that the outcome was actually fixed (10€). The pseudorandom block design paradigm comprised 9 blocks: 3 ‘win' blocks (blocks 1, 4, and 7), 3 ‘lose' blocks (blocks 2, 5, and 8), and 3 control blocks (blocks 3, 6, and 9), with each block consisting of 5 trials. For details see Supplementary Material.
fMRI Data Acquisition and Analysis
T2* functional data were acquired with a 3-Tesla scanner (Gyroscan Intera 3T, Philips Medical Systems, Best, The Netherlands), using a single-shot echoplanar sequence, with parameters selected to minimize distortion in the region of central interest, while retaining adequate signal-to-noise ratio (S/N) and T2* sensitivity. Volumes consisting of 34 slices were acquired (matrix 64 × 64, resolution 3.6 mm × 3.6 mm × 3.6 mm; TR=2.1 s, TE=30 ms, FA=90°). The slices were tilted 25° from the AC/PC line in order to minimize dropout artifacts in the mediotemporal and orbitofrontal regions.
All stimuli were projected to the rear end of the scanner (Sharp XG-PC10XE with additional HF shielding). During the experiment, subjects lay supine in the MRI scanner with the response box in their right hand. The head position was stabilized with a vacuum head cushion.
Data were analyzed using statistical parametric mapping software (SPM8, Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm). Functional data were preprocessed, including realignment, unwarping, and spatial normalization of each participant's functional images to the Montreal Neurological Institute International Consortium (MNI) for Brain Mapping template. Images were smoothed with a Gaussian kernel of 6 mm full width at half maximum (FWHM).
The onsets and durations of the experimental conditions (win, loss, control) were modeled using a canonical hemodynamic response function in the context of a GLM, and the model was corrected for serial correlations. A high-pass filter of 128 s was used to remove low-frequency noise.
For each subject, two contrast images were generated in each individual's first-level analysis (win>control, loss>control). One bipolar depressive patient and two unipolar depressive patients had to be excluded because of excessive head movement (exclusion criterion 3 mm/3°).
Second-level analyses
We calculated a 3 (group= UD vs BD vs HC) × 2 (condition= reward>control vs loss>control) ANOVA, using a full-factorial model, with group as between-subjects factor and reward condition as within-subjects factor. To explore the nature of the interaction, post hoc analyses were conducted.
To address our hypotheses on differential NAcc responsiveness to reward feedback, ROI analyses of the bilateral NAcc were performed. A whole-brain analysis was also conducted.
The mask for bilateral NAcc was created with the aid of the WFU PickAtlas (Maldjian et al, 2003), dilating the defined mask by 1 mm according to the IBASPM atlas (http://www.fil.ion.ucl.ac.uk/spm/ext/#IBASPM; Aleman-Gomez et al, 2006). To control for multiple statistical testing, cluster-level false-positive detection rate was kept at P<0.05, using a voxel-level threshold of P<0.01 with a cluster extent (k) empirically determined by Monte Carlo simulations (n=1000 iterations). This was performed by means of the AlphaSim (Forman et al, 1995) procedure, implemented in the REST toolbox (http://restfmri.net/forum/index.php) as reported in previous publications (Dannlowski et al, 2014). The empirically determined cluster threshold was k=14 voxel for the bilateral NAcc mask. A more conservative voxel-level threshold of P<0.0005 was used for the whole-brain analysis. The ascertained cluster threshold was k=79 voxel. The anatomical labeling was performed by means of the AAL-Toolbox (Tzourio-Mazoyer et al, 2002), and the Brodmann areas (BAs) were identified with the Talairach Daemon atlas (http://www.talairach.org).
Functional Connectivity Analysis
An exploratory functional connectivity analysis was conducted to characterize alterations associated with diagnostic status in the functional coupling between the NAcc and other brain areas. The methods for functional connectivity analyses have been described previously (Dannlowski et al, 2009; Redlich et al, 2014b). Briefly, for each subject the signal time course of the entire left NAcc (‘seed' region) was extracted and entered into a new first-level model of the same subject predicting brain activity by the NAcc time series. The experimental conditions were modeled as nuisance regressors to avoid coactivation by the task. Based on the resulting contrast images, we performed a second-level one-way ANOVA with experimental group as factor, using the same statistical threshold as above (P<0.0005, k=79).
To investigate whether clinical variables and current mood state influenced our findings, the peak contrast values of the 3 (group) × 2 (condition) interaction analysis of the bilateral NAcc and significant cluster from the functional connectivity analysis were extracted for each patient and further analyzed with PASW Statistics 22 (IBM, Armonk, NY). An additional analysis of covariance (ANCOVA) was conducted on NAcc responsiveness, with the factor group (UD, BD) as well as BDI, HAMD scores, SHAPS-D scores, medication load index, number of depressive episodes, and time since onset of first depressive episode as covariates. To cover for multicollinearity effects, we additionally conducted separate ANCOVAs for each individual covariate. Furthermore, each of these clinical variables was separately correlated with NAcc responsiveness to reward stimuli for patients with UD and patients with BD.
RESULTS
Behavioral Data
The results of the reaction times are provided in the Supplementary Material.
fMRI Analyses
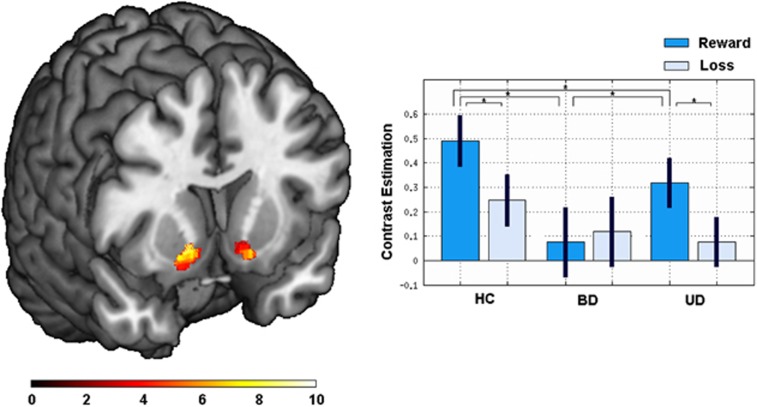
The ROI analysis with the 3 (group) × 2 (condition) ANOVA revealed a significant condition × group interaction within the bilateral NAcc (right: x=14, y=10, z=−6; F(2, 188)=9.05; P<0.001; k=79 voxels, left: x=−14, y=14, z=−12; F(2, 188)=7.21; P=0.001; k=38 voxels). The post hoc analyses revealed significantly lower activation of the NAcc in BD compared with UD (right: x=18, y=6, z=−10; T(61)=3.64; P<0.001; k=71 voxels, left: x=−8, y=6, z=−8; T(61)=3.02; P<0.001; k=47 voxels) and to HC (right: x=18, y=10, z=−10; T(63)=4.62; P<0.001; k=90 voxels, left: x=−16, y=8, z=−12; T(63)=3.93; P<0.001; k=54 voxels). These differences only emerged in the reward>control condition, but not in the loss>control condition (see Figure 1 for details). Next, UD also showed a significantly lower activation of the NAcc than HC (right: x=14, y=14, z=−8; T(62)=3.08; P=0.001; k=40 voxels), again exclusively in the reward>control condition. There was also a significant main effect of condition in the bilateral NAcc (right: x=10, y=8, z=−10; F(1, 188)=53.85; P<0.001; k=88 voxels, left: x=−8, y=8, z=10; F(1, 188)=55.45; P<0.001; k=54 voxels), resulting from overall higher activity for reward>control than for loss>control. Furthermore, a main effect of group emerged (right: x=20, y=10, z=−12; F(2, 188)=7.84; P=0.001; k=60 voxels, left: x=−14, y=10, z=−10; F(2, 188)=5.41; P=0.005; k=30 voxels). The post hoc T-tests revealed overall higher activity for HC than for BD (right: x=20, y=10, z=−12; T(63)=3.68; P<0.001, 86 voxels; left: x=−14, y=10, z=−10, T(63)=3.18; P=0.001, k=47 voxels) as well as for UD (right: x=14, y=14, z=−8, T(62)=3.08; P=0.001, k=40 voxels; see Figure 1).
Figure 1.
Left: Coronal slice (MNI coordinates at y=−4) depicting the results of the 2 × 3 ANOVA interaction within the NAcc. Color bar: F-value. Right: The bars depicting the estimated contrast values for healthy controls (HCs), bipolar disorder (BD), and unipolar depression (UD) for the reward>control (dark blue) and loss>control (light blue) condition. Asterisks indicate significant differences corrected using AlphaSim (voxel threshold, P<0.05; minimum cluster volume threshold k=14 voxels). MNI, Montreal Neurological Institute.
The whole-brain analysis of the 3 (group) × 2 (condition) ANOVA yielded 6 clusters showing an condition × group interaction, comprising the caudate nucleus including the NAcc, thalamus, putamen, insula, and prefrontal areas including the orbitofrontal cortex. The interaction was because of decreased activation in the reward condition in BD compared with HC and UD (see Table 3 for details). There were no significant group differences for the loss>control condition.
Table 3. Results of the 3 (Group) × 2 (Condition) ANOVAa.
|
MNI (at peak) |
|||||||
|---|---|---|---|---|---|---|---|
| BA | Cluster size (k) | x | y | z | Side | F-value/T-value | |
| ANOVA | |||||||
| Caudate nucleus incl. NAcc/thalamus | — | 1108 | 16 | 10 | 16 | R | 18.34 |
| Superior frontal gyrus/middle frontal gyrus | 10 | 223 | −22 | 54 | 12 | L | 17.87 |
| Inferior frontal gyrus, orbital part/putamen | 47/13/34 | 663 | −30 | 24 | −22 | L | 14.87 |
| Superior frontal gyrus, medial part/anterior cingulate gyrus | 9/10 | 155 | 12 | 42 | 26 | R | 14.08 |
| Superior temporal gyrus/operculum | 42/40/41 | 110 | 64 | −24 | 14 | R | 12.24 |
| Putamen, caudate nucleus incl. NAcc | — | 131 | 6 | 12 | 2 | R | 11.96 |
| HC>BD (Reward>Control) | |||||||
| Insula/caudate nucleus incl. NAcc/putamen/thalamus/hippocampus/inferior frontal gyrus, orbital part/pallidum/amygdala | 47/13/45/11/22/44/34/28 | 4636 | −36 | 14 | 6 | L | 5.44 |
| Inferior parietal gyrus/superior parietal gyrus | 40/7 | 166 | 44 | −60 | 46 | R | 5.06 |
| Fusiform gyrus/cerebellum | 19 | 202 | 32 | −60 | −14 | R | 4.94 |
| Inferior parietal gyrus/superior parietal gyrus/angular gyrus | 9/10 | 333 | −28 | −66 | 44 | L | 4.78 |
| Middle Cingulate gyrus/anterior cingulate gyrus/superior frontal gyrus | 24/32/6 | 426 | 2 | 12 | 28 | R | 4.61 |
| Middle frontal gyrus/precentral gyrus | 9/8 | 526 | −46 | 24 | 40 | L | 4.59 |
| Cuneus/calcarine gyrus/superior occipital gyrus | 18/31/19 | 119 | 20 | −80 | 24 | R | 4.56 |
| Superior medial frontal gyrus | 9 | 186 | 2 | 46 | 30 | R | 4.50 |
| Precentral gyrus/postcentral gyrus | 4/6/3 | 173 | 46 | −22 | 60 | R | 4.36 |
| UD>BD (Reward>Control) | |||||||
| Precentral gyrus/postcentral gyrus | 3/6/4 | 208 | 44 | −24 | 60 | R | 4.85 |
| Insula/operculum/temporal pole | 13/22/6 | 319 | 50 | −4 | 2 | R | 4.69 |
| Insula/superior temporal gyrus/transverse temporal gyrus | 13/22 | 135 | −44 | −8 | 0 | L | 4.41 |
| Superior temporal gyrus | 41/40/42 | 150 | 62 | −24 | 16 | R | 4.36 |
| Putamen/caudate nucleus incl. NAcc | — | 122 | −10 | 0 | 6 | L | 4.06 |
| Insula/putamen | 47/13 | 115 | 26 | 16 | −18 | R | 3.95 |
Abbreviations: BA, Brodmann area; BD, bipolar disorder; HC, healthy controls; MNI, Montreal Neurologic Institute; NAcc, nucleus accumbens; UD, unipolar depression.
Analyses were conducted with a voxel threshold of P<0.0005 and a minimum cluster volume threshold k≥79 as determined by AlphaSim. Coordinates based on MNI atlas.
Functional Connectivity Analyses
The functional connectivity analysis of the NAcc yielded a significant main effect of group, mapping to the VTA (x=−2, y=−24, z=−6; F(94)=15.03; P<0.00001; k=81 voxels). The post hoc T-tests revealed a higher functional connectivity in UD compared with HC between the NAcc and the VTA (x=−2, y=−24, z=−8; T(62)=5.01; P<0.0001; k=158 voxels). No significant differences were found in BD compared with both UD and HC with this rigorous threshold. A higher connectivity in BD compared with HC emerged as a trend that did not survive the cluster-extent threshold of 79 voxels (x=0, y=−24, z=−4; T(63)=4.25; P<0.0001; k=60 voxels). To explore the nature of these results, we additionally conducted a psychophysiological interaction analysis (PPI, see Supplementary Material) that yielded no significant differences between the groups at the applied threshold (P<0.0005, k>79).
The conducted ANCOVAs regarding clinical parameter and current mood state revealed that adding clinical variables as covariates only slightly weakened the results regarding the condition × group interaction (NAcc right: Ps<0.004, NAcc left Ps<0.015). The bivariate correlation analyses yielded no significant associations between clinical variables and the reported findings, neither for the NAcc (all Ps>0.122) nor VTA functional connectivity (all Ps>0.100; for a detailed overview see Supplementary Table 2).
To explore the applicability of the present findings to discriminate UD and BD, a linear discriminant analysis was additionally performed (see Supplementary Material). The discriminant analysis yielded 66.6% accuracy rate (sensitivity, correct classified patients with UD=74.2% specificity, correct classified patients with BD=59.4% Eigenvalue=0.25; Wilks's λ=0.80; P=0.004).
DISCUSSION
This study investigated neural correlates of reward processing and directly compared patients with UD and BD, and also included functional connectivity analyses. Our results revealed an overall lower activity in the NAcc in both BD and UD, compared with HC, as well as differences between the patient groups, with reduced reward responsiveness in BD when compared with UD in the NAcc, thalamus, putamen, insula, and prefrontal areas. We also found alterations in functional connectivity between the NAcc and the VTA when comparing UD with HC, and a trend for such changes when comparing BD and HC. The changes involved a higher functional coupling between the NAcc and the VTA in the patients.
These results indicate changes in reward processing in UD and BD during depressive mood states. In contrast to Chase et al (2013), both groups showed reduced activity in the NAcc. Given the differences between our paradigm and that of Chase et al (2013), the findings are not necessarily conflicting. However, in light of recent findings that indicate state-independent elevated striatal activity (Nusslock et al, 2014), but other researchers reporting results in the opposite direction (Trost et al, 2014), it seems to be important to separate the investigation of reward anticipation and reward outcome processing to gain a clearer picture in future studies.
The NAcc is described as a region that integrates reward-related information and, in case of increased dopaminergic transmission, contributes to positive emotion (Schultz, 1998). More explicitly, patients who suffer from a depressive episode seem to have a reduced hedonic effect of rewarding stimuli than healthy subjects. The inability to experience pleasure from commonly pleasant and rewarding stimuli is one of the two core symptoms in depressive episodes. Reduced reactivity of the NAcc observed in depressed subjects could therefore represent the neurobiological basis of anhedonia, as already suggested in other studies (Keedwell et al, 2005; Keller et al, 2013). In theory, rewards are needed for the organization of voluntary goal-directed behavior (Schultz, 2000). With a lack of this hedonic effect, it seems more likely that patients could be seeking less frequently for rewards.
Along with the lower activity in the NAcc in BD and UD, compared with HC, we observed a higher functional connectivity between the NAcc and the VTA in UD than in HC, and a threshold trend in the same direction between BD and HC. There were no reliable differences between the patient groups. Reward processing is based on a neuronal circuitry including regions of the mesolimbic dopamine system, consisting of dopamine-producing midbrain nuclei (particularly VTA) and their subcortical (eg, NAcc) and cortical (eg, OFC and MPFC) target regions (Diekhof et al, 2008; Liu et al, 2011). The VTA–NAcc pathway seems to play a crucial role in reward processing, and its manipulation via dopaminergic transmission can regulate depression-like behavior (Keller et al, 2013). Thus, it is not surprising that altered connectivity is observed in UD as well as in BD—as a trend that failed statistical criteria. It is interesting that the VTA was the only region showing a significant functional connectivity with the NAcc. Note that connectivity was higher in patients than in controls, which seems counterintuitive. A higher functional coupling between these regions should—regarding their noninhibitory connection—lead to more innervation of the NAcc by the VTA, but our data demonstrate the opposite. A possible explanation for the higher functional connectivity between NAcc and VTA might be compensatory mechanisms such as upregulation of postsynaptic dopamine receptors due to a reduction in dopamine release. In the light of the PPI results, higher functional connectivity rather seems to reflect a general alteration in the mesolimbic system that seems to be independent from reward conditions. However, studies of dopamine receptor binding in major depressive disorder have been inconsistent (for an overview see Dunlop and Nemeroff, 2011). It is possible that there is a potentially stronger neural projection from the VTA and blunted NAcc activity results from an excessive prefrontal regulation. The presence of prefrontal cortex modulation upon the NAcc has been demonstrated by several studies (Richard and Berridge, 2013) and could already take place during anticipation processing, and this was not explored in our experiment. Further research is needed to clarify the influence of prefrontal regulation processes during different stages of reward processing in affective disorders.
The comparison of the patient groups further revealed a significantly reduced reward responsiveness of the NAcc in BD compared with UD, and this might be because of a greater impairment in the structures of the mesolimbic system in BD. The difference between BD and UD may be related to the course of disease: The mesolimbic system of subjects with BD has to deal with manic and hypomanic mood states, phases of elevated mood, during which patients excessively seek for rewarding activities and stimuli (American Psychiatric Association, 2000). Studies with hypomanic patients show elevated VS activity in response to rewarding stimuli (O'Sullivan et al, 2011). A downregulation of NAcc sensitivity in consequence of these mood states could explain the observed lower reactivity of the NAcc—despite a tendency of higher functional connectivity between NAcc and VTA. The idea of blunted neural responses toward reward has also been proposed for addictive disorders (Martinez et al, 2005; Volkow et al, 2010). However, this interpretation should be taken with care, because our cross-sectional study design does not allow for more specific conclusions. An alternative interpretation of these results could be greater abnormal regulation processes in BD. Different studies reported changes in prefrontal regulation in affective disorders that differentiates UD and BD, particular connectivity patterns between the orbital frontal cortex and the amygdala (Almeida et al, 2009; Robinson et al, 2008). Similar processes could be in play in the context of reward processing, meaning that the reported result of a most blunted NAcc activity is not necessarily related to reward outcome, but with regulation abnormalities that take part before the outcome processing. However, our paradigm was not designed to measure these early regulation processes. Therefore, it would be interesting to investigate this hypothesis in future studies.
Besides differences in the NAcc, the whole-brain analysis revealed reduced activity during reward processing in other reward-related structures, such as the putamen, the caudate nucleus, and the insula, only in BD compared with UD and HC. These specific differences were not found in UD, indicating that the alterations of the mesolimbic system in BD involve the VTA–NAcc pathway as well as larger parts of the reward circuitry. These results correspond well with findings from meta analyses investigating structural alterations in BD, repeatedly reporting altered insula and basal ganglia structure in BD compared with HC (Bora et al, 2012). Similarly, functional MRI studies highlighted the role of the insula for reward processing in BD (Phillips et al, 2008a).
Together, the results indicate a decisive alteration of brain function associated with the dopamine system particularly in BD. The reported neurobiological alterations might reflect a more severe course of disease, and a prevalently poorer outcome in BD than in UD.
CONCLUSION
Our results may help to refine the neural correlates of reward processing in both affective disorders, and to understand the neural underpinnings of anhedonia as a core symptom of depressive episodes. Although the differentiation of BD and UD disorders remains difficult in clinical practice, we showed that they are associated with different patterns of neural activation during reward processing. This seems to concern primarily parts of the VS and the insula. The reward system has an important role in neurobiology and in the treatment of affective disorders, especially in BD. Future studies should aim to replicate and refine these results.
Limitations
First, all but two patients were medicated and thus differed from healthy controls. Furthermore, the patient groups differed regarding the distribution of medication. However, the total medication load did not differ between patient groups. Furthermore, studies on the effect of psychotropic medication found only a limited impact on fMRI results, revealing normalizing effects, if any (Hafeman et al, 2012; Phillips et al, 2008b). Nevertheless, we cannot completely rule out specific medication effects. In light of studies that demonstrate a reduction of reward-related activation by antipsychotics in regions such as the ventral striatum (Abler et al, 2007), it is possible that our findings are still confounded. Therefore, these findings need replications in unmedicated patients, or studies with a longitudinal design controlling for medication as proposed before (Hafeman et al, 2012).
Second, we did not assess smoking. In light of increased rates of smoking in mood disorders (Lasser et al, 2000; Lawrence et al, 2009) and emerging findings of the effects of nicotine on striatal functions (Exley et al, 2013), our results could be influenced by smoking status.
Third, the results of the functional connectivity analysis are a correlative approach only and should therefore not be interpreted as proving the presence of structural or causal connections. In view of neurochemical studies (see, eg, Wickham et al, 2013) the assumption that correlations between these areas are primarily based on neuronal connections from VTA to NAcc seems likely. However, as functional connectivity follows a more exploratory approach, confirmatory, hypothesis-driven approaches like dynamic causal modeling (Friston et al, 2003) could provide additional information regarding the specific effective connectivity between the NAcc and VTA as well as prefrontal areas, and thus should be applied in future studies.
Finally, given the pseudorandom block design with no real influence on the outcome and the instruction regarding required speed, our paradigm was not appropriate to investigate questions based on behavioral data. Future studies could modify the paradigm in order to investigate, for example, associations between cognitive impairment and reward processing.
FUNDING AND DISCLOSURE
V Arolt is a member of the advisory board of, or has given presentations on behalf of, the following companies: Astra-Zeneca, Janssen-Organon, Lilly, Lundbeck, Servier, Pfizer, Otsuka, and Trommsdorff. These affiliations are of no relevance to the work described in the manuscript. H Kugel has received consultation fees from MR:comp GmbH, Testing Services for MR Safety. This cooperation has no relevance to the work that is covered in the manuscript. The other authors declare no conflict of interest.
Acknowledgments
We thank Ahmad Hariri for providing the fMRI paradigm. We further thank Nina Nagelmann for her skillful technical support during the fMRI sessions. The study was supported by grants of the German Research Foundation (DFG; grant FOR 2107; DA1151/5-1 to UD), Innovative Medizinische Forschung (DA120903 to UD, DA111107 to UD, and DA211012 to UD), and Rolf-Dierichs-Stiftung (ZUW80037 to UD).
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Abler B, Erk S, Walter H. Human reward system activation is modulated by a single dose of olanzapine in healthy subjects in an event-related, double-blind, placebo-controlled fMRI study. Psychopharmacology. 2007;191:823–833. doi: 10.1007/s00213-006-0690-y. [DOI] [PubMed] [Google Scholar]
- Aleman-Gomez Y, Melie-Garcia L, Valdes-Hernandez P.2006Toolbox for automatic parcellation of brain structures12th Annual OHBM, Florence, Italy.
- Almeida JRC, Phillips ML. Distinguishing between unipolar depression and bipolar depression: current and future clinical and neuroimaging perspectives. Biol Psychiatry. 2013;73:111–118. doi: 10.1016/j.biopsych.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida JRC, Versace A, Mechelli A, Hassel S, Quevedo K, Kupfer DJ, et al. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry. 2009;66:451–459. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association 2000. Diagnostical and Statistical Manual of Mental Disorders: DSM-IV-TR: 4th Edition Text Revision. APA Press: Washington, DC, USA, 2000.
- Beck AT, Steer RA.1987. Beck Depression Inventory: Manual. The Psychological Corporation, Harcourt Brace Jovanovich: San Antonio, 1987.
- Benson BE, Willis MW, Ketter T, Speer A, Kimbrell T, Herscovitch P, et al. Differential abnormalities of functional connectivity of the amygdala and hippocampus in unipolar and bipolar affective disorders. J Affect Disord. 2014;168:243–253. doi: 10.1016/j.jad.2014.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Fornito A, Pantelis C, Yücel M. Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 2012;138:9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Caseras X, Lawrence NS, Murphy K, Wise RG, Phillips ML. Ventral striatum activity in response to reward: differences between bipolar I and II disorders. Am J Psychiatry. 2013;170:533–541. doi: 10.1176/appi.ajp.2012.12020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HWH, Nusslock R, Almeida JRC, Forbes EE, LaBarbara EJ, Phillips ML. Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar Disord. 2013;15:839–854. doi: 10.1111/bdi.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Grabe HJ, Wittfeld K, Klaus J, Konrad C, Grotegerd D, et al. Multimodal imaging of a tescalcin (TESC)-regulating polymorphism (rs7294919)-specific effects on hippocampal gray matter structure. Mol Psychiatry. 2014;20:398–404. doi: 10.1038/mp.2014.39. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Konrad C, Domschke K, Bauer J, Kugel H, et al. Reduced amygdala-prefrontal coupling in major depression: association with MAOA genotype and illness severity. Int J Neuropsychopharmacol. 2009;12:11–22. doi: 10.1017/S1461145708008973. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Falkai P, Gruber O. Functional neuroimaging of reward processing and decision-making: a review of aberrant motivational and affective processing in addiction and mood disorders. Brain Res Rev. 2008;59:164–184. doi: 10.1016/j.brainresrev.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Gruber O. When desire collides with reason: functional interactions between anteroventral prefrontal cortex and nucleus accumbens underlie the human ability to resist impulsive desires. J Neurosci. 2010;30:1488–1493. doi: 10.1523/JNEUROSCI.4690-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2011;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. J Neurosci. 2000;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Exley R, Clements M, Hartung H, McIntosh JM, Franklin M, Bermudez I, et al. Striatal dopamine transmission is reduced after chronic nicotine with a decrease in α6-nicotinic receptor control in nucleus accumbens. Eur J Neurosci. 2013;38:3036–3043. doi: 10.1111/ejn.12298. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Brown SMM, Kimak M, Ferrell REE, Manuck SBB, Hariri AR, et al. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun Ma, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Franz M, Lemke MR, Meyer T, Ulferts P, Puhl P, Snaith RP. Deutsche Version der Snaith-Hamilton-Pleasure-Scale (SHAPS-D) Fortschr Neurol Psychiat. 1998;66:407–413. doi: 10.1055/s-2007-995279. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp. 1994;2:56–78. [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Grotegerd D, Stuhrmann A, Kugel H, Schmidt S, Redlich R, Zwanzger P, et al. Amygdala excitability to subliminally presented emotional faces distinguishes unipolar and bipolar depression – an fMRI and pattern classification study. Hum Brain Mapp. 2014;35:2995–3007. doi: 10.1002/hbm.22380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman DM, Chang KD, Garrett AS, Sanders EM, Phillips ML. Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disord. 2012;14:375–410. doi: 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg. 1960;23:56–63. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautzinger M, Bailer M, Worall H, Keller F.1994. Beck Depressions-Inventar (BDI). Testhandbuch. Hans Huber: Bern, 1994.
- Hirschfeld RM, Lewis L, Vornik LA. Perceptions and impact of bipolar disorder: how far have we really come? Results of the national depressive and manic-depressive association 2000 survey of individuals with bipolar disorder. J Clin Psychiatry. 2003;64:161–174. [PubMed] [Google Scholar]
- Keedwell P, Andrew C, Williams SCR, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Keller J, Young CB, Kelley E, Prater K, Levitin DJ, Menon V. Trait anhedonia is associated with reduced reactivity and connectivity of mesolimbic and paralimbic reward pathways. J Psychiatr Res. 2013;47:1319–1328. doi: 10.1016/j.jpsychires.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Biol Psychiatry. 2008;63:686–692. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, Mccormick D, Bor DH. Smoking and Mental Illness. JAMA. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Lawrence D, Mitrou F, Zubrick SR. Smoking and mental illness: results from population surveys in Australia and the United States. BMC Public Health. 2009;9:285. doi: 10.1186/1471-2458-9-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrl S.2005. Mehrfachwahl-Wortschatz-Intelligenztest MWT-B. Spitta Verlag: Balingen, 2005.
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang D, Huang Y, Perez A, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58:779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Almeida JRC, Forbes EE, Versace A, Frank E, LaBarbara EJ, et al. Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disord. 2012;14:249–260. doi: 10.1111/j.1399-5618.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Young CB, Damme KSF. Elevated reward-related neural activation as a unique biological marker of bipolar disorder: assessment and treatment implications. Behav Res Ther. 2014;62:74–87. doi: 10.1016/j.brat.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opel N, Redlich R, Grotegerd D, Dohm K, Haupenthal C, Heindel W, et al. 2015Enhanced neural responsiveness to reward associated with obesity in the absence of food-related stimuli Hum Brain Mappdoi: 10.1002/hbm.22773 [DOI] [PMC free article] [PubMed]
- O'Sullivan N, Szczepanowski R, El-Deredy W, Mason L, Bentall RP. fMRI evidence of a relationship between hypomania and both increased goal-sensitivity and positive outcome-expectancy bias. Neuropsychologia. 2011;49:2825–2835. doi: 10.1016/j.neuropsychologia.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Kupfer DJ. Bipolar disorder diagnosis: challenges and future directions. Lancet. 2013;381:1663–1671. doi: 10.1016/S0140-6736(13)60989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC.2008A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder Mol Psychiatry 13829833–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Travis MJ, Kupfer DJ, Fagiolini A. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlich R, Almeida JRC, Grotegerd D, Opel N, Kugel H, Heindel W, et al. Brain morphometric biomarkers distinguishing unipolar and bipolar depression: a voxel-based morphometry-pattern classification approach. JAMA Psychiatry. 2014;71:1222–1230. doi: 10.1001/jamapsychiatry.2014.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlich R, Grotegerd D, Opel N, Kaufmann C, Zwitserlood P, Kugel H, et al. Are you gonna leave me? Separation Anxiety is associated with increased amygdala responsiveness and volume. Social Cogn Affect Neurosci. 2014;10:278–284. doi: 10.1093/scan/nsu055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, Berridge KC. Prefrontal cortex modulates desire and dread generated by nucleus accumbens glutamate disruption. Biol Psychiatry. 2013;73:360–370. doi: 10.1016/j.biopsych.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Monkul ES, Tordesillas-Gutiérrez D, Franklin C, Bearden CE, Fox PT, et al. Fronto-limbic circuitry in euthymic bipolar disorder: evidence for prefrontal hyperactivation. Psychiatry Res. 2008;164:106–113. doi: 10.1016/j.pscychresns.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA.1970. State-Trait Anxiety Inventory, Manual for the State-Trait Anxiety Inventory. Consulting Psychologist Press: Palo Alto, 1970.
- Trost S, Diekhof EK, Zvonik K, Lewandowski M, Usher J, Keil M, et al. Disturbed Anterior Prefrontal Control of the Mesolimbic Reward System and Increased Impulsivity in Bipolar Disorder. Neuropsychopharmacology. 2014;39:1–37. doi: 10.1038/npp.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Versace A, Almeida JRC, Quevedo K, Thompson WK, Terwilliger RA, Hassel S, et al. Right orbitofrontal corticolimbic and left corticocortical white matter connectivity differentiate bipolar and unipolar depression. Biol Psychiatry. 2010;68:560–567. doi: 10.1016/j.biopsych.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain's control circuit. Bioessays. 2010;32:748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham R, Solecki W, Rathbun L, McIntosh JM, Addy N. Ventral tegmental area α6β2 nicotinic acetylcholine receptors modulate phasic dopamine release in the nucleus accumbens core. Psychopharmacology. 2013;229:73–82. doi: 10.1007/s00213-013-3082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen H-U, Wunderlich U, Gruschwitz S, Zaudig M.1997. Strukturiertes Klinisches Interview für DSM-IV. Hogrefe: Goettingen, 1997.
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.