Abstract
Introduction:
Melioidosis is a rapidly fatal infectious disease caused by Burkholderia pseudomallei, an agent of potential biothreat, endemic in several parts of India. Most melioidosis-induced infected aneurysms are located in the abdominal or thoracic aorta.
Case Presentation:
We reported two unusual cases of melioidosis resulting in pseudoaneurysm of the descending thoracic aorta. In both cases, blood cultures yielded B. pseudomallei. The first patient was managed with resection of aneurysm and reconstruction with Dacron graft followed by medical treatment and was discharged uneventfully. The second patient died within one week of admission before the infecting etiological agent was identified and aneurysmal repair was planned.
Conclusions:
A high clinical index of suspicion, especially in areas of endemicity is essential for timely management of intracavitary infected pseudoaneurysms caused by B. pseudomallei and use of rapid microbiological techniques, such as bact/alert 3D system, which enables rapid and early recovery of the etiological agent.
Keywords: Infected Aneurysm, Sepsis, Bacteremia, Virulence
1. Introduction
Pseudoaneurysms are usually saccular and most commonly the result of trauma, infection, instrumentation or penetrating atherosclerotic ulcers (1). Infected aneurysms represent only 1-2.6% of all aortic aneurysms and is associated with significant morbidity and mortality (2). Most melioidosis-induced infected aneurysms of the aorta are located in the abdominal or thoracic aorta and rarely involve other major arteries (3). We reported two cases of pseudoaneurysm of the descending thoracic aorta caused by B. pseudomallei.
2. Case Presentation
2.1. Case 1
A 57-year-old male patient evaluated in a private hospital for complaints of chest pain and sudden onset of breathlessness was diagnosed to have pseudoaneurysm of the aortic arch and was referred to our hospital for further management. Patient gave a history of hypertension and diabetes for six months. The coronary angiogram showed stenosis of the left anterior descending coronary artery (LAD) with mild diffuse disease noted in proximal and mid segments. A 50 - 60% stenotic lesion was noted in the distal segment of LAD. Carotid Doppler showed atheromatous changes in the form of a fibrofatty plaque in left carotid bifurcation. Computed tomography (CT) of aortogram showed diffuse ectasia of ascending and descending thoracic aorta with associated multiple thin mixed and calcified intimal plaques. There was a 2.8 × 3.9 cm pseudoaneurysm arising from the posterior wall of the descending thoracic aorta at D9-level with a large surrounding hypodensity suggestive of hematoma. Coronary artery bypass grafting (CABG) and aneurysmal repair were performed. Intraoperative findings showed cardiomegaly, dilated descending thoracic aorta with saccular aneurysm just above the diaphragm adherent to the left lung. The pseudo-sac was blood stained with thick purulent discharge. After the repair of aneurysm, the thick purulent pus and two sets of blood cultures were collected in Standard and Fan plus bottles (Biomerieux, Marcy’l Etoile, France) and sent to microbiology laboratory for further analysis. Intravenous (IV) Sulbactum + cefoperazone (Magnex) 1 gram and Zanocin 200 mg IV twice daily were administered for the patient. Intraoperative cultures of pus from the pseudoaneurysm grew B. pseudomallei after 48 hours of incubation.
Based on the microbiology report and antibiogram of the isolate, injection Ceftazidime 2 grams IV every six hours and Injection Co-trimoxazole 1600: 320 IV twice daily, for 14 days were administered for patient. Blood cultures were sterile even after five days of incubation. Post-operative ECHO showed good biventricular function, without vegetation. Patient had an uneventful recovery and discharged with an advice to continue oral Bactrim-DS twice daily for six months.
2.2. Case 2
A 48-year-old male patient with complaints of fever, hoarseness of voice, sleeplessness and irritability, for one month was admitted to our hospital. He is not a known diabetic; however had high blood sugar after admission along with 3 plus urine sugar at the time of admission.
Two sets of blood cultures were collected in Standard and Fan BacT/alert plus bottles (Biomerieux, Marcy’l Etoile, France) and submitted to microbiology laboratory. IV meropenem, one gram every 12 hours was started for him. The patient had no response and his febrile episodes were persisting. Total leucocyte count was 14700/cu.mm with predominance of neutrophils. Patient developed chest pain and on evaluation with CT coronary aortogram, a large saccular pseudoaneurysm arising from the posterior wall of descending thoracic aorta was detected. Aneurysm repair was planned on an emergency basis. However, before intubation could be initiated, the patient developed aspiration pneumonitis. Though he was immediately ventilated, he had cardiorespiratory arrest and died. The blood cultures were positive after 72 hours of incubation and showed growth of Gram negative bacilli.
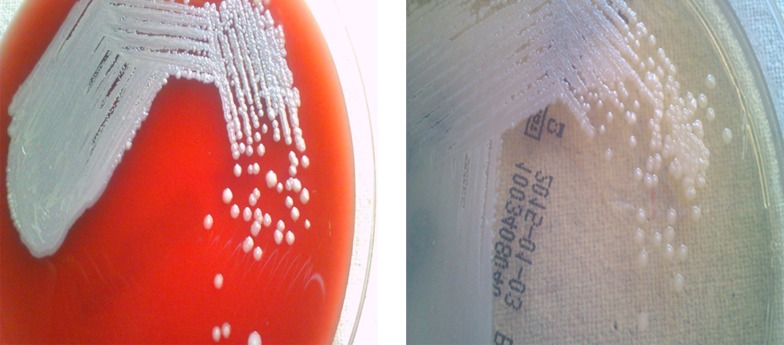
Microbiology: The purulent specimen and positive blood broths were inoculated on 5% sheep blood agar (COS) and chrome agar (CPS) (Biomerieux) and incubated aerobically at 37°C for 24 and 48 hours. Characteristic shiny carrom coin type of colonies were observed (Figure 1) on all the agar plates from the pus and blood cultures after 48 hours. They were hemolytic on the blood agar. The isolate was identified as B.pseudomallei by the Vitek-2 system using ID GN panel. Antibiotic susceptibility was performed with ATB PSE5 panel of the API system (Biomerieux). The isolate was susceptible to cotrimoxazole, ceftazidime, imipenem and meropenem.
Figure 1. Colony Morphology of B. pseudomallei on 5% Sheep Blood Agar and Chrome Agar With 1-2 mm Shiny Carrom Coin Type of Colonies.
3. Discussion
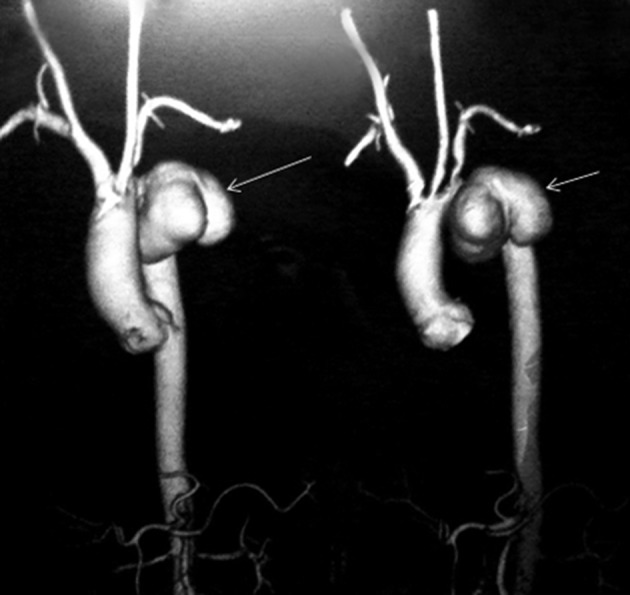
An aneurysm is an abnormal focal arterial dilation due to destruction of vessel wall by an infection. It can be classified as true or false aneurysm and developed when a new aneurysm is produced by infection of the normal arterial valve or when a pre-existing aneurysm becomes secondarily infected. The source of infection may be intrinsic, usually a complication of infective endocarditis with embolization and an arrest of septic embolus at some point within a vessel. The usual etiological agents in such conditions are Staphylococcus aurous and Streptococcus viridans and non-typhoidal Salmonella species. However, aneurysms can get infected by extrinsic sources as a result of bacteremia caused by hematogenous seeding in patients with preexisting atherosclerotic diseases (4). In our first case, the patient who survived, Carotid Doppler showed atheromatous changes in the form of a fibrofatty plaque in the left carotid bifurcation. The aorta is the most common site of involvement due to higher incidence of underlying atherosclerotic plaques and aneurysms with a larger vasa vasorum where infected emboli may dislodge (1). In both cases, we reported pseudoaneurysm originated from the descending thoracic aorta (Figure 2). Both patients were paddy field workers and probably got the infection from the soil leading to bacteremia with a subsequent seeding of the atheromatous aorta.
Figure 2. 3D Reconstruction of CT Aortogram Showing Pseudoaneurysm (Arrow Mark) Arising From the Descending Thoracic Aorta Distal to Origin of the Left Subclavian Artery.
The most frequent etiology of extrinsically infected aortic aneurysm is determined by regional conditions and varies according to a region’s endemic diseases. After an extensive literature search, we found several reported cases of melioidosis involving the thoracoabdominal aorta (5-7), abdominal aorta (4, 8), renal artery (6), intrathoracic subclavian artery (9) and iliac artery (10). Intracavitary infected aneurysms present a significant challenge with a reported mortality of 16 - 44% unless surgical repair of the aneurysm is performed within the appropriate time (11). Management of melioidosis with pseudoaneurysm consists of antibiotic therapy combined with aggressive surgical debridement of the involved tissue and vascular reconstruction, as needed (4).
Patients with melioidosis are managed with an initial intensive antibiotic regimen with intravenous (IV) ceftazidime 50 mg/kg every six hours for 14 days followed by eradication phase with oral co-trimoxazole 320/1600 mg/kg each 12 hours for three months. Switch to oral treatment should be made when there is clinical improvement and should be given until 20 weeks (4).
In the first case, the patient was managed with resection of aneurysm and reconstruction with Dacron graft followed by medical treatment and discharged uneventfully. The second patient died within one week of admission before the infecting etiological agent was identified and aneurysmal repair was planned.
Intracavitary infected pseudoaneurysms caused by B. pseudomallei are uncommon and present a significant challenge as a serious clinical condition associated with significant morbidity and mortality. A high clinical index of suspicion, especially in areas of endemicity is essential in timely management of these critical cases. Use of rapid microbiological techniques, such as bact/alert 3D system, which enables rapid and early recovery of organisms along with automated identification and antibiotic susceptibility testing systems facilitate rapid identification of the etiological agent. Early use of appropriate antibiotics and adequate surgical debridement would improve outcome of patients.
Footnotes
Authors’ Contributions:All authors contributed intellectually to the manuscript and the final manuscript was approved by all authors.
References
- 1.Rajiah P. CT and MRI in the Evaluation of Thoracic Aortic Diseases. Int J Vasc Med. 2013;2013:797189. doi: 10.1155/2013/797189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laohapensang K, Rutherford RB, Arworn S. Infected aneurysm. Ann Vasc Dis. 2010;3(1):16–23. doi: 10.3400/avd.AVDctiia09002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller BT, Wegener OR, Grabitz K, Pillny M, Thomas L, Sandmann W. Mycotic aneurysms of the thoracic and abdominal aorta and iliac arteries: experience with anatomic and extra-anatomic repair in 33 cases. J Vasc Surg. 2001;33(1):106–13. doi: 10.1067/mva.2001.110356. [DOI] [PubMed] [Google Scholar]
- 4.Rao J, Kaushal AS, Hoong CK. Abdominal aortic pseudoaneurysm secondary to melioidosis. Asian J Surg. 2009;32(1):64–9. doi: 10.1016/S1015-9584(09)60012-9. [DOI] [PubMed] [Google Scholar]
- 5.Patel MA, Schmoker JD, Moses PL, Anees R, D'Agostino R. Mycotic arch aneurysm and aortoesophageal fistula in a patient with melioidosis. Ann Thorac Surg. 2001;71(4):1363–5. doi: 10.1016/s0003-4975(00)02301-8. [DOI] [PubMed] [Google Scholar]
- 6.Noordin K, Abdullah MM, Natarajan C, Wahab YA, Abdullah K. Pseudoaneurysm of the renal artery associated with melioidosis. Br J Urol. 1995;75(5):680–1. doi: 10.1111/j.1464-410x.1995.tb07438.x. [DOI] [PubMed] [Google Scholar]
- 7.Steinmetz I, Stosiek P, Hergenrother D, Bar W. Melioidosis causing a mycotic aneurysm. Lancet. 1996;347(9014):1564–5. doi: 10.1016/s0140-6736(96)90722-9. [DOI] [PubMed] [Google Scholar]
- 8.Lee SS, Liu YC, Wang JH, Wann SR. Mycotic aneurysm due to Burkholderia pseudomallei. Clin Infect Dis. 1998;26(4):1013–4. doi: 10.1086/517640. [DOI] [PubMed] [Google Scholar]
- 9.Schindler N, Calligaro KD, Dougherty MJ, Diehl J, Modi KH, Braffman MN. Melioidosis presenting as an infected intrathoracic subclavian artery pseudoaneurysm treated with femoral vein interposition graft. J Vasc Surg. 2002;35(3):569–72. doi: 10.1067/mva.2002.118592. [DOI] [PubMed] [Google Scholar]
- 10.Luo CY, Ko WC, Lee HC, Yang YJ. Relapsing melioidosis as cause of iliac mycotic aneurysm: an indigenous case in Taiwan. J Vasc Surg. 2003;37(4):882–5. doi: 10.1067/mva.2003.164. [DOI] [PubMed] [Google Scholar]
- 11.Anunnatsiri S, Chetchotisakd P, Kularbkaew C. Mycotic aneurysm in Northeast Thailand: the importance of Burkholderia pseudomallei as a causative pathogen. Clin Infect Dis. 2008;47(11):1436–9. doi: 10.1086/592975. [DOI] [PubMed] [Google Scholar]