Abstract
Several mouse models of SLE, including FcγRIIB-KO and TLR7tg mice, develop an expansion of an atypical NK cell subset with functional similarity to cells referred as IKDCs or pre-mNKs in other systems. Here we show that atypical NKs purified from spleens of SLE-prone mice, and identified as NK1.1+CD11c+CD122+MHC-II+, induce persistent autoimmune disease in an IFN-I and CD40L-dependent manner when transferred to WT mice. A single transfer of 4x106 NK1.1+ cells from TLR7tg into WT induces a 2-week-long wave of inflammatory cytokines in the serum, a sustained increase in T cell activation and follicular helper cells for the following months, and a progressive expansion of dendritic cells, monocytes and granulocytes. Furthermore IL15 deficiency, which impedes development of NK cells, ameliorates the autoimmune pathology of TLR7tg mice. These results suggest that cells of the NK lineage can develop into cytokine producing/antigen-presenting cells that affect the priming and progression of systemic autoimmune disease.
Keywords: SLE, autoimmunity, NK cells
Introduction
Systemic lupus disease is characterized by a break of immune tolerance to common self-antigens, an interferon signature gene expression and elevated granulocyte numbers (1, 2). Cytokine-producing cells and antigen-presenting cells (APCs) such as dendritic cells (DCs) play a central role in the development of autoimmunity due to their ability to activate naïve T cells and promote antibody production (3). In addition to DCs, autoimmunity-induced chronic inflammation promotes the appearance of normally rare immune populations with activated phenotype and multiple functionalities that might affect the outcome of disease. For example, we have detected the expansion of cells belonging to the NK cell lineage with an atypical profile in various mouse models of lupus (Voynova et al. JI in press). These cells resemble those previously described as IKDCs (4, 5) or pre-mNKs (6) because they express the surface markers NK1.1 and CD11c and they are efficient cytokine producers and APCs, with high MHC-II expression and cross-presentation ability. NK1.1+CD11c+ cells from the lupus-prone FcγRIIB Yaa and TLR7tg mice have an immature but activated profile; they are also highly proliferative and survive for months in adoptive transfer experiments (Voynova et al JI in press). Up to date IKDCs cells have mostly been linked to tumor environments (7) or TLR7-induced activation (8). In the context of autoimmunity, IKDCs have also been linked to encephalomyelitis (9). We now seek to find whether the type of NK cells that expand in lupus models have in vivo relevance to disease. In this manuscript we present evidence that NK1.1+CD11c+ cells from lupus mice can break lymphocyte tolerance and promote long-term myeloid cell expansion when adoptively transferred to WT mice.
Material and Methods
Mice
The generation of TLR7tg and B6.FcγRIIB−/− mice as well as IL15−/−, IL18−/−, IFNRαβ1−/− has been described earlier (10–14). Mice were used at 8-12 weeks of age, except in survival studies. Housing at the NIH facility met the instructional animal care and use committee (IACUC) and NIH guidelines.
Adoptive transfer
Transferred NK1.1+CD11c+ cells were sorted by FACSAria (BD Bioscience) or purified by a combination of CD11c and NK1.1-positive bead selection (RoboSep, Stemcell Technologies) with comparable results. 4x106 cells were injected i.v. per mouse.
Pathology
Elisa was used to measure serum RNA antibodies (Immco Diagnostics) and total IgG (Southern Biotech). Hematological scores were determined by a Hemavet (Drew Sci.). Serum cytokines were quantified by CBA (BD Bioscience). For histology, 10% formalin-fixed organs were stained with hematoxylin and eosin and inflammatory scores were as described (15).
Statistical analysis
Statistical significance was determined by the student t-test, one-way ANOVA and by Kaplan-Meier for the survival curve.
Results and Discussion
Adoptive transfer of NK1.1+ CD11c+ cells induces long-term autoreactivity and inflammation
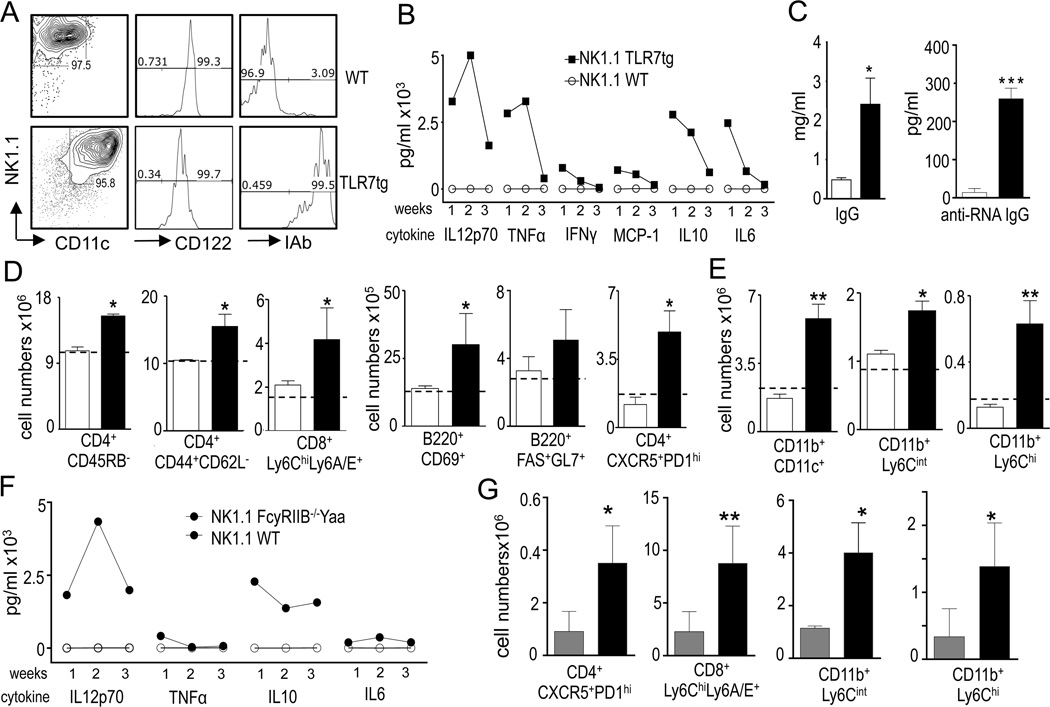
We have previously reported the expansion of a NK subset identified as NK1.1+CD11c+CD3−CD122+MHC-II+E4BP4+Tbet+ in autoimmune prone mice (Voynova et al., JI in press). To investigate the role of these cells in vivo, we purified NK1.1+ splenocytes from WT and TLR7tg spleens and i.v. injected 4x106 cells into untouched WT mice. This represented the transfer of about all the NK1.1+ cells from a single TLR7tg spleen into 3 recipient mice. TLR7tg-derived NK1.1+ cells were determined to be MHC-II+CD122+CD11c+ in all experiments (Fig. 1A). We also confirmed on at least one experiment that this population was devoid of T cells, mature NK cells or pDCs, as they were CD3−CD4−DX5−PDCA−. As control, NK1.1+ from WT mice represented conventional NK cells and were all DX5+CD11c−MHC-II+ (Fig. 1A). Donor populations were purified either by cell sorting TLR7tg NK1.1+CD11c+ and WT NK1.1+CD11c− populations, or by NK1.1 positive bead purification. We obtained the same results regardless of the type of purification. Within a week after adoptive transfer of TLR7tg NK1.1+ cells into WT mice, we detected increased expression of inflammatory cytokines that returned to normal or near normal levels by week 4 (Fig. 1B). At four weeks, we detected augmented titers of total serum IgG and RNA-specific antibodies (Fig. 1C), increased number of activated CD4+ cells (CD4+CD45RBlo), effector/memory CD4 cells (CD4+CD44+CD62L−), TFH cells (CD4+CXCR5+PD1+), germinal center (GC) B cells (B220+GL7+FAS+), activated B cells (B220+CD69+) and CD8+ cells expressing the Ly6 antigens in the spleen of recipient mice that received NK1.1+ cells from TLR7tg spleen (Fig. 1D). Thus, this transfer induced autoreactivity that we measured by the presence of autoantibodies and GC-associated lymphocytes. High Ly6A/C expression on CD8s was consistent with increased interferon expression. We also detected a progressive myeloid expansion apparent by 4 weeks and maximal 4 months after the transfer of NK1.1+TLR7tg cells. This expansion included conventional DCs (CD11c+CD11b+), inflammatory monocytes (CD11b+Ly6Chi) and granulocytes (CD11bhiLy6Cint) (Fig. 1E). In comparison, transfer of the same number of WT NK1.1+ did not induce a phenotype in recipient mice (Fig. 1B-E).
Figure 1. Adoptive transfer of NK1.1+ cells isolated from TLR7tg mice.
(A-G) 4x106 sorted NK1.1+CD11c+ TLR7tg splenoctyes (black bars), or the same number of NK1.1+ CD11c− WT splenocytes (white bars) were injected i.v. into a WT recipient. (A) Flow cytometry analysis of NK1.1+ sorted cells from WT or TLR7tg. (B) Serum cytokines measured on the first (1w), second (2w) and fourth (4w) week after injection NK1.1 cells; (C) Total serum IgG and anti-RNA antibodies 4 weeks after cell transfer. (D, E) Cells counts of the specified immune populations in the spleen of recipient mice one (D) or four (E) months after injections of NK1.1+ cells. Levels of immune populations in WT C57Bl/6 mice are shown as dotted lines in each graph; (F-G) NK1.1+ sorted cells from WT (grey bar) or B6.FcγRIIB−/−Yaa (black bar) spleens were injected i.v. into WT mice and inflammatory cytokines were measured in serum (F) while FACS analysis was performed in the spleen 4 weeks after injection (G). *p<0.05; **p<0.01; ***p<0.001 in Student T-test. Experiments were performed in at least 3 mice per group and repeated 5 times.
The ability of NK1.1+CD11c+ cells to break tolerance upon cell transfer is not unique to cells purified from TLR7tg mice. NK1.1+ cells isolated from B6.FcγRIIB−/−Yaa mice also increased serum levels of inflammatory cytokines (Fig. 1F), and induced lymphocyte activation and myeloid expansion in recipient WT mice (Fig. 1G). This is a rare case of long-term induction of autoimmunity and inflammatory pathology from a single injection of a lupus-associated cell population into a non-lupus-prone recipient. Up to date, adoptive transfer of B or T lymphocytes or even serum from lupus mouse models has failed to initiate pathology in WT mice recipients. Only the transfer of DCs has been reported to lead to autoimmunity, but even then it does not induce long-term pathology (16, 17). A notable difference that could explain why our adoptive transfer experiments lead to persistent disease and not in those described for DC transfers, is the long survival times and high proliferative capacity of NK1.1+ cells purified from lupus-prone mice (Voynova et al, JI in press).
IL15-deficiency ameliorates and delays pathology in TLR7tg mice
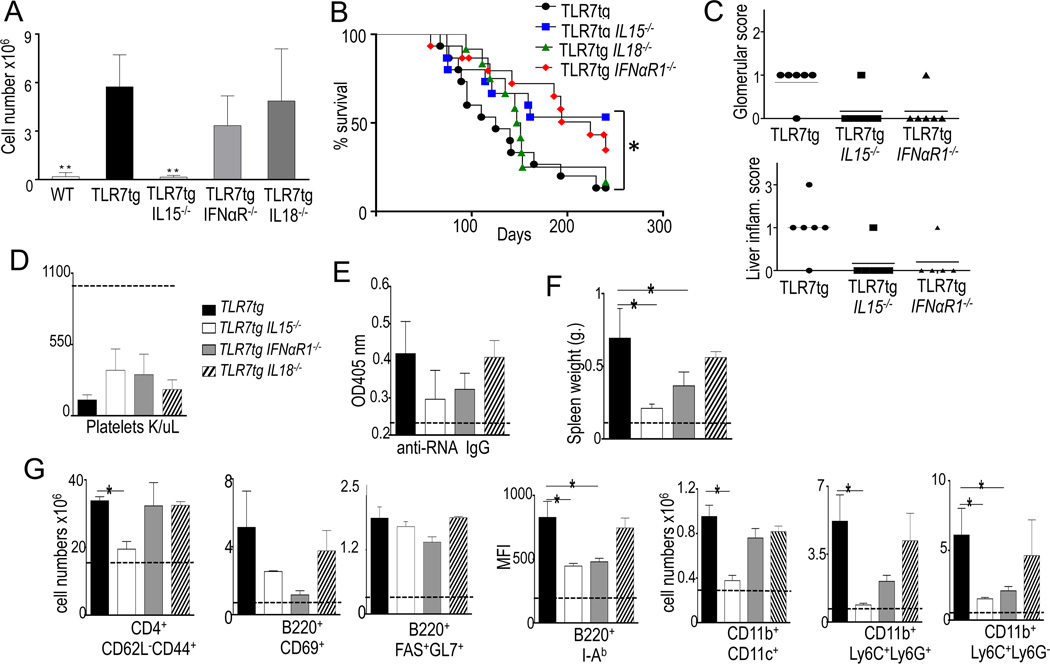
We tested cytokine requirements for the development of atypical NK cells by breeding TLR7tg mice to IL15-, IL18- and IFNαβR1- deficient mice. These cells were completely dependent on IL15 but their expansion was unaltered by IL18 or IFNαβR1 deficiencies (Fig. 2A). IL15 dependency suggests these cells are of the NK lineage (18), and unlike pDCs, they do not require IFN-I for development. IL-18 has been shown to be important for full activation of mature NK cells (19), therefore the presence of atypical NK cells in IL18-deficient TLR7tg implies that these cells do not represent activated mature NKs.
Figure 2. IL15 deficiency delays the development of autoimmune disease in TLR7tg mice.
(A) Numbers of splenic NK1.1+CD11c+ cells in each genotype shown, at least three mice per group. **p<0.01(Student t-test); (B) Survival curve for TLR7tg, TLR7tg.IL15−/− and TLR7tg.IFNαβR1−/− mice, 15 mice per group. (C ) Glomerular damage in kidney and liver inflammation scores measured as described in methods; Mice of the genotype shown, were analyzed for number of platelets (D), total serum IgG and anti-RNA IgG antibodies (E), spleen weight (F), splenocyte count (G); Data collected from three experiments with three mice per group. Asterisk represents p≤ 0.05 in Anova statistical analysis and Kaplan-Meier for survival curve.
We next characterized disease progression in TLR7tg mice that lacked NK1.1+CD11c+ cells (i.e., bred to IL15-KO) and compared them to TLR7tg mice that bore mutations that wouldn’t affect NK1.1+CD11c+ cell numbers: i.e., bred to either IL18-KO or IFNαβR1-KO (Fig. 2A). We observed that IL15 deficiency prolonged the survival of TLR7tg mice (Fig. 3B), also delayed and ameliorated disease in TLR7tg, even at higher extent as deficiency in IFNαR1 (Fig. 3B-G). One possible mechanism by which atypical NKs might promote lymphocyte activation is through secretion of IFNγ, a process directed by IL-18 (19). However IL-18 deficiency did not alter the phenotype of TLR7tg mice, neither their mortality (Fig. 3B), platelet deficiency (3D), autoantibody titer (Fig 3E), lymphocyte activation nor myeloid expansion (Fig. 3G). The reduction in mortality of TLR7tg with deficiencies in IL15 or IFNαR1 could be explained by the lower level of kidney and liver inflammation (Fig 3C). However one phenotype that was not reduced in TLR7tg mice with IL15, IL18 or IFNαR1 deficiencies was their low level of platelets, causing what was likely lethal thrombocytopenia even in delayed pathologies (Fig. 3D). This result implies a platelet-intrinsic effect of the TLR7tg that is independent of factors such as IFN or IL15.
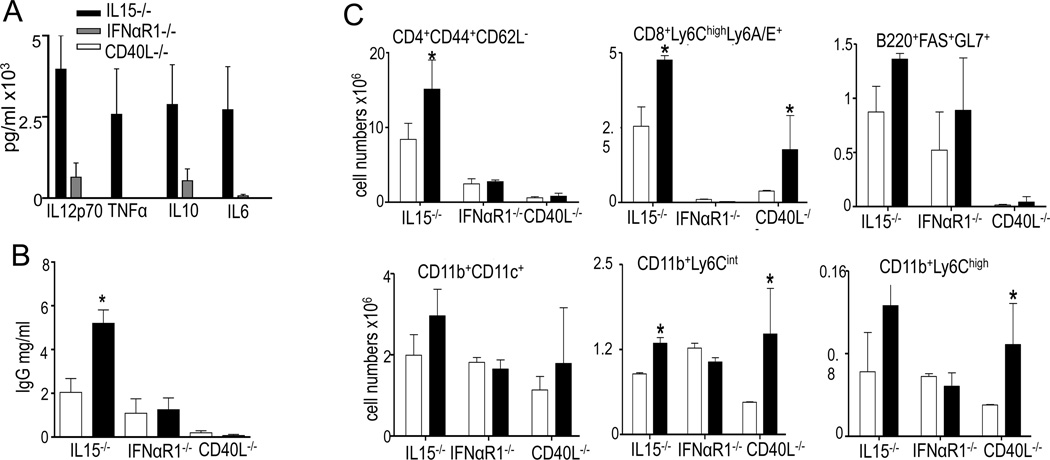
Figure 3. IFN and T cell help requirement in adoptive transfer of TLR7tg NK1.1+ cells.
(A-C); Splenic NK1.1+ cells isolated from wild type (white bar) or TLR7tg (black bar) were injected into IL15−/−IFNαβR1−/− or CD40L−/− mice. Mice from recipient injected with NK1.1+ from TLR7tg mice were tested one month after the transfer for serum cytokines (A); All experimental groups were tested for total serum IgG by ELISA (B). FACS analyses were performed on spenocytes of recipient mice 4 weeks after the injection (C). Experiments were performed in at least 3 mice per group and repeated three times. Asterisk represents p≤ 0.05.
Our analysis of immune functions showed that IL15 affected all autoimmunity and immune activation in TLR7tg mice: RNA autoantibodies (Fig. 3E), spleen size (Fig. 3F), lymphocyte activation and myeloid expansion (Fig 3G) were close to WT levels in TLR7tg mice with IL15 deficiency. IFNαR deficiency seemed to have only a partial protective effect while IL18 deficiency did not affect the disease of TLR7tg mice (Figs 3B-G).
Requirement of IFN-I and T cell help in the inflammatory effect of NK1.1+CD11c+ cells
To test functional requirements for the inflammatory function of TLR7tg NK1.1+ cells, we transferred them into IL15-, IFNαR1-, or CD40L- deficient recipient mice. We showed above that IL15 was essential for development and expansion of NK1.1+CD11c+ cells in TLR7tg mice (Fig. 2A). However IL15 in the recipient was dispensable in the development of inflammatory phenotype induced by the transfer of NK1.1+ cells from TLR7tg mice (with the caveat that donor cells could be using IL15 in autocrine fashion). Transferring TLR7tg NK1.1+ cells into IL15-deficient recipients resulted in elevated serum cytokines (Fig. 3A), IgG (Fig 3B), lymphocyte activation and myeloid cell numbers (Fig 3C). In contrast, IFNαR-deficiency had the opposite effect: IFNαR was dispensable for development of atypical NKs in TLR7tg mice (Fig. 2A) but this deficiency in recipient mice prevented all phenotypes normally induced by the transfer of TLR7tg NK1.1+ cells, i.e., the cytokine expansion (Fig. 3A), serum IgG (Fig. 3B), lymphocyte activation or myeloid expansion (Fig 3C). Finally, we determined the requirement for T cell-help by performing the adoptive transfer of TLR7tg NK1.1+ cells into CD40L-deficient recipients: this resulted in no autoimmunity, as measured by serum cytokines, serum IgG or activated CD4s (Fig. 3A-C). However CD40L-deficiency allowed the inflammatory monocyte (CD11b+Ly6Chi) and granulocyte (CD11b+ Ly6Cint) expansion detected upon transfer of TLR7tg NK1.1+ cells (Fig. 3C). Overall these results suggest that the phenotype induced by the transfer of atypical NK cells is fully IFN-I dependent and that T cell help is required for the increase in serum inflammatory cytokines but not for the myeloid expansion.
Overall our findings point toward a new role for immature NKs that expand and become activated in chronic inflammatory conditions. The appearance of these cells in autoimmune disease could exacerbate the phenotype and lead to a progressively more severe outcome. NK cells have been linked to human SLE in various studies although there is no information in reference to immature NK populations. NK KIR/HLA genotype has been correlated with autoimmune risk (20) and a lower number and impaired function of NK cells was reported in SLE patients compared to non-autoimmune individuals (21). An appealing theory is that the low level of mature NK cells in SLE patients represents a developmental blockade at the immature stage, and that might correlate with the appearance of the type of immature cells that we describe in these studies.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Author contributions
E.V. and S.B. designed the experiments, E.V., C.Q. and B.S. performed the experiments and analyzed the data, E.V. and S.B. interpreted the results and wrote the paper.
Conflict of interest
Authors have no conflicts of interest to declare.
References
- 1.Tsokos GC. Systemic lupus erythematosus. The New England journal of medicine. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Lahita RG. Systemic Lupus Erythematosus. Elsevier Inc.; 2011. Copyright © 2011. [Google Scholar]
- 3.Ganguly D, Haak S, Sisirak V, Reizis B. The role of dendritic cells in autoimmunity. Nat Rev Immunol. 2013;13:566–577. doi: 10.1038/nri3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan CW, Crafton E, Fan HN, Flook J, Yoshimura K, Skarica M, Brockstedt D, Dubensky TW, Stins MF, Lanier LL, Pardoll DM, Housseau F. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med. 2006;12:207–213. doi: 10.1038/nm1352. [DOI] [PubMed] [Google Scholar]
- 5.Taieb J, Chaput N, Menard C, Apetoh L, Ullrich E, Bonmort M, Pequignot M, Casares N, Terme M, Flament C, Opolon P, Lecluse Y, Metivier D, Tomasello E, Vivier E, Ghiringhelli F, Martin F, Klatzmann D, Poynard T, Tursz T, Raposo G, Yagita H, Ryffel B, Kroemer G, Zitvogel L. A novel dendritic cell subset involved in tumor immunosurveillance. Nat Med. 2006;12:214–219. doi: 10.1038/nm1356. [DOI] [PubMed] [Google Scholar]
- 6.Guimont-Desrochers F, Boucher G, Dong Z, Dupuis M, Veillette A, Lesage S. Redefining interferon-producing killer dendritic cells as a novel intermediate in NK-cell differentiation. Blood. 2012;119:4349–4357. doi: 10.1182/blood-2011-11-395954. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhry UI, Plitas G, Burt BM, Kingham TP, Raab JR, DeMatteo RP. NK dendritic cells expanded in IL-15 exhibit antitumor responses in vivo. J Immunol. 2007;179:4654–4660. doi: 10.4049/jimmunol.179.7.4654. [DOI] [PubMed] [Google Scholar]
- 8.Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med. 2007;204:1441–1451. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huarte E, Rynda-Apple A, Riccardi C, Skyberg JA, Golden S, Rollins MF, Ramstead AG, Jackiw LO, Maddaloni M, Pascual DW. Tolerogen-induced interferon-producing killer dendritic cells (IKDCs) protect against EAE. J Autoimmun. 2011;37:328–341. doi: 10.1016/j.jaut.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM, Flavell RA, Bolland S. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolland S, Yim YS, Tus K, Wakeland EK, Ravetch JV. Genetic modifiers of systemic lupus erythematosus in FcgammaRIIB(−/−) mice. J Exp Med. 2002;195:1167–1174. doi: 10.1084/jem.20020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 14.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 15.Brunt EM. Grading and staging the histopathological lesions of chronic hepatitis: the Knodell histology activity index and beyond. Hepatology. 2000;31:241–246. doi: 10.1002/hep.510310136. [DOI] [PubMed] [Google Scholar]
- 16.Georgiev M, Agle LM, Chu JL, Elkon KB, Ashany D. Mature dendritic cells readily break tolerance in normal mice but do not lead to disease expression. Arthritis and rheumatism. 2005;52:225–238. doi: 10.1002/art.20759. [DOI] [PubMed] [Google Scholar]
- 17.Tzeng TC, Suen JL, Chiang BL. Dendritic cells pulsed with apoptotic cells activate self-reactive T-cells of lupus mice both in vitro and in vivo. Rheumatology (Oxford) 2006;45:1230–1237. doi: 10.1093/rheumatology/kel106. [DOI] [PubMed] [Google Scholar]
- 18.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 19.Shi FD, Takeda K, Akira S, Sarvetnick N, Ljunggren HG. IL-18 directs autoreactive T cells and promotes autodestruction in the central nervous system via induction of IFN-gamma by NK cells. Journal of immunology. 2000;165:3099–3104. doi: 10.4049/jimmunol.165.6.3099. [DOI] [PubMed] [Google Scholar]
- 20.Pellett F, Siannis F, Vukin I, Lee P, Urowitz MB, Gladman DD. KIRs and autoimmune disease: studies in systemic lupus erythematosus and scleroderma. Tissue antigens. 2007;(69 Suppl 1):106–108. doi: 10.1111/j.1399-0039.2006.762_6.x. [DOI] [PubMed] [Google Scholar]
- 21.Shibatomi K, Ida H, Yamasaki S, Nakashima T, Origuchi T, Kawakami A, Migita K, Kawabe Y, Tsujihata M, Anderson P, Eguchi K. A novel role for interleukin-18 in human natural killer cell death: high serum levels and low natural killer cell numbers in patients with systemic autoimmune diseases. Arthritis and rheumatism. 2001;44:884–892. doi: 10.1002/1529-0131(200104)44:4<884::AID-ANR145>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]