Abstract
Objective
This study aimed to determine the combined effects of age and HIV infection on the risk of incident neurocognitive disorders.
Method
A total of 146 neurocognitively normal participants were enrolled at baseline into one of four groups based on age (≤ 40 years and ≥ 50 years) and HIV serostatus resulting in 24 younger HIV−, 27 younger HIV+, 39 older HIV−, and 56 older HIV+ individuals. All participants were administered a standardized clinical neuropsychological battery at baseline and 14.3 ±0.2 months later.
Results
A logistic regression predicting incident neurocognitive disorders from HIV, age group, and their interaction was significant (χ2[4] = 13.56, p = .009), with a significant main effect of HIV serostatus (χ2[1] = 5.01, p = .025), but no main effect of age or age by HIV interaction (ps > .10). Specifically, 15.7 percent of the HIV+ individuals had an incident neurocognitive disorder as compared to 3.2 percent of the HIV− group (odds ratio = 4.8 [1.2, 32.6]). Among older HIV+ adults, lower baseline cognitive reserve, prospective memory, and verbal fluency each predicted incident neurocognitive disorders at follow-up.
Conclusions
Independent of age, HIV infection confers a nearly 5-fold risk for developing a neurocognitive disorder over approximately one year. Individuals with lower cognitive reserve and mild weaknesses in higher-order neurocognitive functions may be targeted for closer clinical monitoring and preventative measures.
Keywords: HIV, Incidence, Neuropsychology, Aging, Hepatitis C
The effectiveness and wide-spread availability of combination antiretroviral therapy (cART) has greatly improved life expectancies in persons infected with HIV. Accordingly, there has been an increase in the prevalence of adults aged 50 and older living with HIV infection (Brooks, Buchacz, Gebo, & Mermin, 2012). As individuals live increasingly longer with HIV disease, their risk of incident age-related morbidities across several different organ systems, including the brain, may also rise. Cross-sectional research to date indicates that older age and HIV disease confer largely independent, additive effects on brain structure (e.g., Becker et al., 2012) and function (e.g., Thomas, Brier, Snyder, Vaida, & Ances, 2013). There is accumulating cross-sectional evidence showing that older HIV+ adults have a higher prevalence of HIV-associated neurocognitive disorders (HAND; Becker, Lopez, Dew, & Aizenstein, 2004; Valcour et al., 2004), which involves mild-to-moderate deficits across a wide array of ability areas, such as executive function, information processing speed, attention, and memory (e.g., Sacktor et al., 2007; Woods, Moore, Weber, & Grant, 2009). Older age and HIV infection also have a synergistic adverse effect on everyday functioning (Morgan, Iudicello, et al., 2012), which is greatly worsened by the presence of neurocognitive deficits (e.g., Thames et al., 2011). However, the vast majority of the research on the combined effects of HIV disease and aging is cross-sectional; therefore, we know little about the evolution and trajectory of HAND in older adults. Such longitudinal research on the incidence of HAND is important because it can more directly identify clinicodemographic factors (e.g., specific cognitive vulnerabilities) that place an individual at greatest risk for incipient pathological neurocognitive decline. Thus, it is imperative that we investigate the trajectory of HAND across the adult lifespan, including its origins in neurocognitively normal older HIV+ persons.
The annual incidence rate of HAND across the lifespan is currently estimated to be around 15–20% (Robertson et al., 2007), with cART era studies ranging from 1 to 25% (Brodt, Hamps, Gute, Knupp, Staszewski, & Helm, 1997; Mateen et al., 2012; Sacktor et al. 2001, 2002). Neurocognitive decline in HIV disease is associated with an array of immunovirological factors (Heaton et al., 2015; cf. Roberston et al., 2007), including AIDS status, viremia, and lower baseline CD4 counts (Cysique et al., 2010; Marcotte et al., 2003), as well as comorbidities such as mood and substance use disorders (Heaton et al., 2015). In parallel, baseline HIV-associated neurocognitive impairment (i.e., Asymptomatic Neurocognitive Impairment, or ANI) is associated with an approximately 2- to 6-fold risk of subsequent declines in everyday functioning (Grant et al., 2014), which are the hallmark of syndromic HAND diagnoses of Minor Neurocognitive Disorder (MND) and HIV-Associated Dementia (HAD).
To date, we are aware of only four longitudinal studies that have prospectively examined the role of aging in the trajectory of HIV-associated neurocognitive functioning. Seider et al. (2014) found that older HIV+ individuals showed an exaggerated decline in delayed verbal memory at a one-year follow-up as compared to HIV− individuals. Similarly, Sacktor et al. (2010) observed greater declines in complex psychomotor processing speed over one-year in older versus younger HIV+ individuals. Heaton et al. (2015) did not observe an association between baseline age and global neurocognitive change in largely middle-aged HIV+ adults. A recent longitudinal study by Molsberry et al. (2015) found that cognitive decline in aging HIV-infected individuals manifests as one of three distinct trajectories (normal aging, premature aging, and unhealthy aging), which were associated with HIV infection, AIDS status, Hepatitis C (HCV) infection, and other clinicodemographic variables such as depression and ethnicity/race. Given the emerging longitudinal evidence linking older age to more rapid domain-specific neurocognitive decline in the setting of HIV disease, the present study aims to extend the prior literature by: 1) determining whether the combined effects of age and HIV infection confer an increased risk for incident clinical neurocognitive disorders; and 2) identifying baseline predictors of incident neurocognitive disorders in older HIV+ individuals.
Method
Participants
Baseline Parent Study Cohort
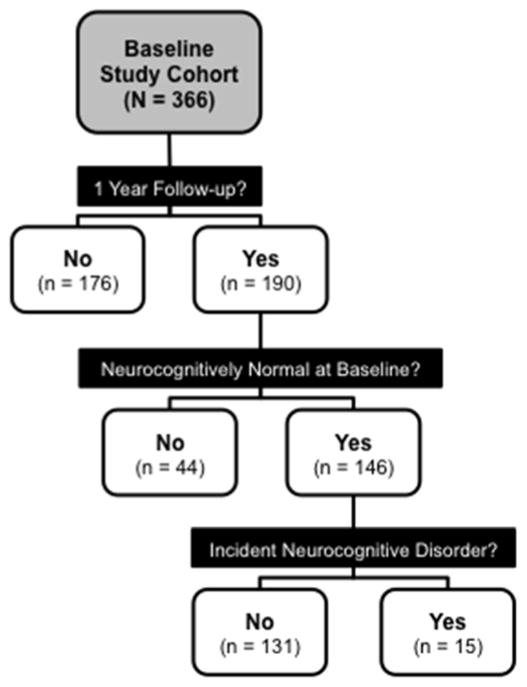
The study sample was derived from an NIH-funded study on aging and memory in HIV disease that originally enrolled 366 baseline eligible participants from the community and urban HIV clinics between 2008 and 2013 (see Figure 1). Participants were enrolled at baseline into one of four groups based on age (younger group ≤ 40 years, older group ≥ 50 years) and HIV serostatus, which was confirmed using standard Western blot and/or a point-of-care test (MedMira Inc., Nova Scotia, Canada). Baseline exclusion criteria for the parent study included diagnosis of severe psychiatric disorder (e.g., schizophrenia) or neuromedical condition including an active central nervous system opportunistic infection, a seizure disorder, head injury with loss of consciousness for more than 30 minutes, stroke with neurological sequelae, presence of a non–HIV-associated dementia or an estimated verbal IQ score less than 70 on the Wechsler Test of Adult Reading (WTAR; Psychological Corporation, 2001). Individuals were also excluded if they had current substance dependence or tested positive on a breathalyzer or urine toxicology screen for illicit drugs (except marijuana) on any day of testing.
Figure 1.
Flow diagram of study enrollment at baseline, retention at 14-month follow-up, exclusions based on baseline neurocognitive impairment, and the final cohort of participants with and without incident neurocognitive disorders.
Longitudinal Study Cohort
One hundred and ninety participants from the baseline parent cohort also completed a one-year follow-up evaluation. With the exception of current substance dependence, the above-referenced exclusion criteria also were applied at follow-up testing. Of note, there were no new occurrences of these major exclusions and as such no participants were excluded at follow-up for this reason. We examined possible differences on each of the variables listed in Table 1 between the group of 176 individuals who were lost at follow-up and the 190 individuals who were retained. Thus, the independent variable was a 2-level categorical variable of lost at follow-up (n = 176) and retained (n = 190). The dependent variables for this series of analyses were each variable in Table 1. We used analysis of variance (ANOVA; or non-parametric equivalent) tests for continuous dependent variables, and we used chi-square tests for categorical dependent variables. The 190 participants who completed the one-year follow-up assessment were older, χ2(3) = 23.50, p < .001, had a higher prevalence of lifetime Major Depressive Disorder, χ2(1) = 6.24, p = .016, and had longer estimated durations of HIV infection, F(1,209) = 6.87, p = .009. No other baseline variables presented in Table 1 differed significantly by retention status (all ps > .05).
Table 1.
Mean (standard error) baseline demographic, psychiatric, medical, and HIV disease characteristics of the study groups.
| Younger HIV− (n = 24) | Younger HIV+ (n = 27) | Older HIV− (n = 39) | Older HIV+ (n = 56) | p | Group Differences | |
|---|---|---|---|---|---|---|
| Age (years) | 30.1(1.3) | 32.8(1.0) | 56.0(0.8) | 56.7(0.8) | < .001 | Y−, Y+ < O−, O+ |
| Education (years) | 13.5 (0.4) | 12.8(0.4) | 14.7(0.3) | 14.6(0.3) | < .001 | Y+ < O−, O+ |
| Ethnicity (%) | -- | -- | ||||
| African-American | 25.0 | 25.9 | 12.8 | 21.4 | ||
| Asian | 4.2 | 7.4 | 0.0 | 0.0 | ||
| Hispanic | 29.2 | 11.1 | 18.0 | 10.7 | ||
| White | 41.7 | 55.6 | 69.2 | 67.9 | ||
| Gender (% men) | 62.5 | 85.2 | 66.7 | 83.9 | -- | -- |
| Estimated Verbal IQ (WTAR) | 102.8(2.0) | 100.7(2.0) | 106.0(1.5) | 103.3(1.3) | -- | -- |
| POMS total (of 200) | 45.1(5.1) | 55.0(9.0) | 45.1(4.9) | 54.1(4.9) | -- | -- |
| Major Depression a (%) | 50.0 | 66.7 | 46.2 | 58.9 | -- | -- |
| Generalized Anxiety a (%) | 4.2 | 11.1 | 5.1 | 25.0 | .013 | Y−, O− < O+ |
| Substance Dependence b (%) | 50.0 | 63.0 | 53.8 | 50.0 | -- | -- |
| Hepatitis C infection (%) | 4.2 | 3.7 | 15.4 | 32.1 | < .001 | Y−, Y+ < O+ |
| Estimated Duration of Infection (months) | - | 80.4(12.2) | - | 207.7(11.4) | < .001 | Y+ < O+ |
| AIDS (%) | - | 40.7 | - | 66.1 | .035 | Y+ < O+ |
| CD4 count (cells/μL) | - | 550.8(45.9) | - | 560.8(36.3) | -- | -- |
| Nadir CD4 (cells/μL) | - | 242.9(27.8) | - | 180.2(21.3) | .028c | Y+ > O+ |
| cART status (%) | - | 85.2 | - | 89.3 | -- | -- |
| Plasma RNA detectable (%) | - | 32.0 | - | 20.0 | -- | -- |
| Among subjects on cART (%) | - | 19.05 | - | 14.29 | -- | -- |
Note. Younger ≤ 40 years; Older ≥ 50 years; WTAR = Wechsler Test of Adult Reading; POMS = Profile of Mood States; AIDS = Acquired Immune Deficiency Syndrome; CD4 = Cluster of Differentiation 4; cART = combination antiretroviral therapy.
Includes both current (e.g., the last 30 days) and lifetime diagnosis.
Any lifetime diagnosis of dependence on alcohol or illicit substances.
Determined with Wilcoxon rank-sum test.
Baseline Neurocognitive Status of the Longitudinal Cohort
Our focus on the incidence of neurocognitive disorders necessitated that we also excluded participants with a neurocognitive disorder at baseline. This criterion was operationalized in a manner consistent with Frascati research guidelines for determining neurocognitive impairment when diagnosing HAND (Antinori et al., 2007). Participants with impairment in 2 or more neurocognitive domains at baseline as measured by a global clinical rating score of 5 or greater were excluded (see Woods et al., 2004). Thus, all study participants were neurocognitively normal at baseline. These neurocognitive disorder exclusion criteria eliminated 44 of the 190 individuals who were retained in the longitudinal arm of the study and thus provided a total of 146 eligible participants for the study sample (see Figure 1). We examined possible differences on each of the variables listed in Table 1 between the group of individuals who were excluded due to baseline neurocognitive impairment and individuals who were retained. Each variable in Table 1 was treated as a dependent variable and the independent variable for each test was a 2-level categorical variable comprised of individuals who were excluded due to a baseline neurocognitive disorder (n = 44) versus individuals who were neurocognitively normal at baseline and therefore retained in this study (n = 146). We conducted a series of individual one-way ANOVA tests (or Wilcoxon rank-sum tests for non-parametric data) for continuous dependent variables, and we used chi-square tests for categorical dependent variables. Individuals who were excluded at baseline due to having a neurocognitive disorder had significantly fewer years of education, F(1,188) = 10.50, p = 0.001, a lower estimated verbal IQ WTAR score, F(1,188) = 8.78, p = 0.003, a higher total mood disturbance scores on the Profile of Mood States (POMS; McNair, Lorr, & Droppleman, 1981), F(1,177) = 6.64, p = 0.011, as well as a higher prevalence of lifetime substance dependence (Fischer’s exact test p = 0.039) and HIV infection (Fischer’s exact test p = .008).
Final Study Sample
The final sample therefore included 146 participants who were neurocognitively normal at baseline and completed follow-up evaluations. This group included 24 younger HIV-seronegative (HIV−), 27 younger HIV-seropositive (HIV+), 39 older HIV−, and 56 older HIV+ individuals. The demographic and clinical characteristics of these groups are displayed in Table 1. The mean (standard error) number of months between baseline and follow-up testing for all 146 retained participants included in analyses was 14.27 (0.23) months.
Materials and Procedure
All participants provided written, informed consent prior to completing a comprehensive medical, psychiatric, and neuropsychological research evaluation for which they received nominal financial compensation. The university’s human subjects committee approved the parent study from which these participants were drawn. Participants completed the following procedure at baseline and at one-year follow-up.
Medical Evaluation
Participants were administered a brief medical evaluation conducted by a research nurse, which included a review of systems, medications, history, urine toxicology, and a blood draw.
Psychiatric Evaluation
Current mood symptoms were assessed using the POMS, which is a 65-item self-report measure of affective distress in the week prior to evaluation. Current (i.e., within the last 30 days) and lifetime Major Depressive Disorder, Generalized Anxiety Disorder, and Substance Use Disorder were determined using the Composite International Diagnostic Interview (version 2.128).
Neuropsychological Assessment
Comprehensive Neuropsychological Test Battery
All participants were administered a comprehensive neuropsychological test battery by certified research assistants. The domains included for a determination of neurocognitive disorders included (1) learning, (2) delayed memory, (3) attention, (4) speed of information processing, (5) executive functions, and (6) motor skills. The measures for each domain included (1) the Logical Memory I subtest from the Wechsler Memory Scale, 3rd edition (WMS-III; Wechsler, 1997) and Total Trials 1–5 of the California Verbal Learning Test, 2nd edition (CVLT-II; Delis, Kramer, Kaplin, & Ober, 2000) for learning; (2) the Logical Memory II subtest from the WMS-III and the long Delay Free Recall trial of the CVLT-II for delayed memory; (3) the Digit Span subtest from the WMS-III and Trial 1 from the CVLT-II for attention; (4) Trailmaking Test, Part A time (Army Individual Test Battery, 1944) and the Total Execution Time from the Tower of London Test (Drexel Version; Culbertson & Zillmer, 1999) for speed of processing; (5) Trailmaking Test, Part B time and the Total Moves score from the Tower of London Test for executive functions; and (6) Grooved Pegboard (Kløve, 1963) dominant and non-dominant hand completion times for motor skills. Raw scores from these measures were converted to demographically-adjusted normative T-scores, which were then used to derive global clinical rating scores (Woods et al., 2004).
Incident Neurocognitive Disorder Determinations
Follow-up characterizations of incident neurocognitive disorder were determined via Frascati research guidelines for assessing HAND using the methods and a marginally different battery (e.g., different form versions) detailed in Woods et al. (2013). Specifically, a global clinical rating score was generated from neurocognitive test scores across the six cognitive domains, which ranged from 1 (above average, T score ≥ 55) to 9 (severely impaired, T score < 20). Since HAND by definition requires cognitive and functional impairment to be likely due to HIV infection, we elected to use disease neutral terminology of classifying participants across HIV− and HIV+ groups with neurocognitive disorders as either subsyndromic (i.e., ANI) or syndromic disorder (i.e., MND, HAD). Subsyndromic neurocognitive disorder was operationalized by a global clinical rating score greater than or equal to 5 without impairment in everyday functioning (see Woods et al., 2004). Syndromic neurocognitive disorder was operationalized by a global clinical rating score greater than or equal to 5 in addition to 2 or more areas of “dependence” among the following indicators: (1) unemployment (not due to elective retirement), (2) self-reported impairment in 2 or more instrumental activities of daily living (ADLs) on a modified form (Heaton et al., 2004) of the Lawton and Brody (Lawton & Brody, 1969) ADL Scale (i.e., finance management, purchasing groceries, cooking, using transportation, shopping, medication management, and social activity planning), (3) self-reported impairment in 2 or more basic ADL domains on the modified ADL Scale (i.e., housekeeping/cleaning, laundry, home repairs, bathing, and dressing), (4) impairment on the Confusion/Bewilderment subscale of the POMS defined by an age and gender-corrected z score greater than or equal to 1, or (5) a score < 90 on the clinician-rated Karnofsky Scale of Performance Status (Karnofsky & Burchenal, 1949), which has been previously used in HIV (e.g., Gandhi et al., 2011; Morgan et al., 2012).
Confirming Incident Neurocognitive Disorder Determinations
Across the entire study cohort of 146 neurocognitively normal participants at baseline, 15 individuals met diagnostic criteria for an incident neurocognitive disorder (see Figure 1). In order to determine: 1) the presence of neurocognitive declines in individuals with incident neurocognitive disorders; and 2) stability in those who remained in the normal range, we conducted paired samples t-tests of the baseline and follow-up global clinical rating scores within these two subgroups. Among individuals with an incident neurocognitive disorder at follow-up (n = 15), there was a significant decline in global clinical rating score from baseline, t(14) = −6.96, p < .001, g = −2.35. However, in the group of individuals who did not have a neurocognitive disorder at follow-up (n = 131), there was no significant decline in global clinical rating score from baseline t(130) = −0.15, p = 0.883, g = −0.01.
Supplementary Cognitive Predictors of Incident Neurocognitive Disorders
In addition to the abovementioned neuropsychological domains, we also assessed possible cognitive predictors of incident neurocognitive disorders that were not part of the neurocognitive diagnoses, thereby reducing potential concerns regarding criterion contamination. These predictor domains included: (1) cognitive reserve, (2) verbal fluency, (3) semantic memory, and (4) prospective memory. These supplementary domains were selected due to their: 1) Availability in the parent study; 2) relevance to cognitive aging and neuroAIDS; and 3) relevance as potential predictors of neurocognitive decline. The measures for each domain included: (1) years of education, estimated verbal IQ as measured by the WTAR, and Hollingshead score of highest occupation level (Hollingshead, 1975) for cognitive reserve; (2) total number of correct switches in the animal-musical instrument category switching subtest from the Delis Kaplan Executive Function System (D-KEFS; Delis, Kaplan, & Kramer, 2001) and total words generated on a verbal (action) fluency test (see Piatt, Fields, Paolo, & Tröster, 1999 and Woods et al., 2006 for further details) for verbal fluency; (3) total correct on the Famous Faces subtest of the Kaufman Adolescent and Adult Intelligence Test (KAIT; Kaufman & Kaufman, 1993) and total score from the Boston Naming Test (BNT: Goodglass, Kaplan, & Barresi, 2001) for semantic memory; and (4) the modified version (Woods et al., 2008) of the Memory for Intentions Screening Test (MIST; Raskin, Buckheit, & Sherrod, 2010) for prospective memory. With the exception of semantic memory measures, for which raw scores were converted to demographically adjusted normative T-scores, raw scores from these measures were converted to unadjusted population-based T-scores in order to derive average composite scores. Unadjusted population-based T-scores were computed for each individual by comparing their raw score to the average performance within the older HIV+ sample, which allows measures without normative data (e.g., the MIST) to be on the same descriptive scale as the demographically adjusted normative T-scores.
Results
Baseline Characteristics Across Study Groups
The baseline demographic and clinical characteristics of the four study groups are shown in Table 1. To determine if there were any between-group differences on these baseline characteristics, a series of individual one-way ANOVA or chi-square tests were conducted using each individual dependent variable in Table 1 and the four-level age/HIV grouping independent variable (i.e., younger HIV−, younger HIV+, older HIV−, and older HIV+); any significant omnibus tests were further examined using post hoc independent t-test (or its non-parametric equivalent) or chi-square test pair-wise comparisons. The younger HIV+ group had significantly fewer years of education than the older HIV+ and older HIV− groups, F(3,142) = 5.95, p < .001. Older HIV+ individuals were more likely to have a lifetime diagnosis of Generalized Anxiety Disorder compared to the older and younger HIV− groups, χ2(3) = 10.73, p = .013, and had higher rates of HCV compared to the younger HIV− and younger HIV+ groups, χ2(3) = 16.12, p = .001. Within the HIV+ cohort, the older group had significantly longer estimated durations of infection, F(1,81) = 47.68, p < .001, a higher prevalence of AIDS, χ2(1) = 4.78, p = .035, and a lower nadir CD4 count (Z = 2.19, p = 0.028), compared to the younger group.
Selection of Model Covariates
As detailed below, our primary study hypotheses were tested with a logistic regression predicting incident neurocognitive disorders from HIV, age group, and their interaction. In order to determine the optimal covariates for this model, we individually tested each potential covariate in Table 1 that showed significant between-group differences (as detailed in the paragraph above) for its association with the outcome (i.e., a 2-level categorical variable of incident neurocognitive disorder versus neurocognitive normality). Among the 6 variables listed above that differed across the study groups, only HCV also showed a significant association with incident neurocognitive disorder (Fischer’s exact test p = .006). As such, HCV was included as a covariate in the primary logistic regression model detailed below.
Age and HIV Predicting Incident Neurocognitive Disorders
Next we conducted a logistic regression to determine the impact of two between-subjects predictor variables HIV serostatus (HIV+, HIV−) and age group (younger, older) and their interaction on the dichotomous outcome variable of incident neurocognitive disorders (no incident neurocognitive disorder group, incident neurocognitive disorder group). The overall logistic regression model was significant, χ2(4) = 13.56, p = .009, and showed a significant main effect of HIV serostatus in predicting the likelihood of incident neurocognitive disorder, χ2(1) = 5.01, p = .025. HIV+ individuals were 4.84 (95% CI [1.20, 32.58]) times more likely to have a neurocognitive disorder at follow-up than were HIV− individuals (see Figure 2). There was no main effect of age group, χ2(1) = 0.69, p = .410, and no interaction between HIV serostatus and age group, χ2(1) = 0.03, p = .860.
Figure 2.
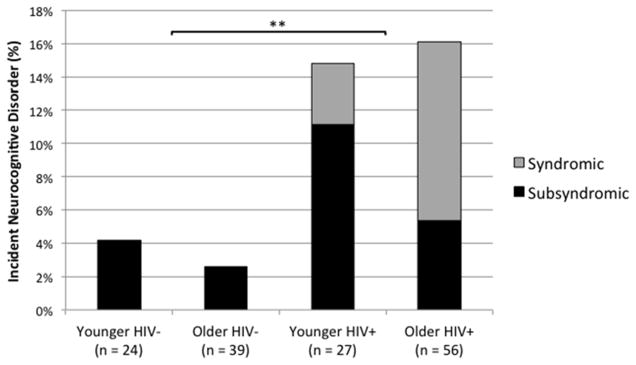
Incidence of syndromic and subsyndromic neurocognitive disorders at 14-month follow-up for younger and older individuals with and without HIV disease.
Hepatitis C Predicting Incident Neurocognitive Disorders
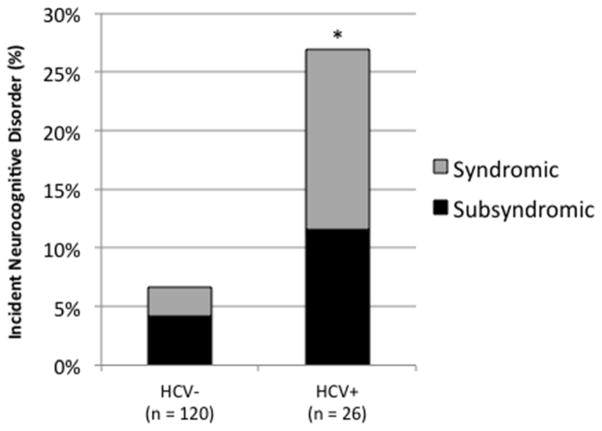
As shown in Figure 3, there also was a significant main effect of the HCV covariate, such that individuals with HCV were 5.45 (95% CI [1.49, 21.48]) times more likely to have a neurocognitive disorder at follow-up than were HCV− individuals, χ2(1) = 6.54, p = .011. In an effort to determine if the effects of HCV may be particularly pronounced among HIV+ persons, we undertook post hoc chi-square analyses examining the association of HCV serostatus with incident neurocognitive disorders in the HIV− and HIV+ groups separately. In the HIV− group, there was a significant effect of HCV on incident neurocognitive disorders, such that 28.6% of HIV-HCV+ individuals had an incident neurocognitive disorder as compared to 0.0% of HIV-HCV− individuals (Fischer’s exact test p = .011; Odd ratio = 51.36, 95% CI [2.18, 1210.8]). However, there was no significant effect of HCV serostatus on incident neurocognitive disorders in the HIV+ sample. Specifically, 26.3% of HIV+HCV+ individuals had an incident neurocognitive disorder as compared to 12.5% of HIV+HCV− individuals (Fischer’s exact test p = 0.162; Odds ratio = 0.4, 95% CI [0.11, 1.41]).
Figure 3.
Incidence of syndromic and subsyndromic neurocognitive disorder at 14-month follow-up for individuals with and without with Hepatitis C virus infection.
Incidence of Syndromic Versus Subsyndromic HAND by Age Group
A chi-square test was used to examine the possible differences in incidence rates of neurocognitive disorders between the older and younger HIV+ groups. There was no significant difference in the rate of incident neurocognitive disorders between older HIV+ (16.07%) and younger HIV+ (14.81%) individuals (p > .10). We did not have adequate statistical power to compare the rates of incident syndromic versus subsyndromic HAND by age group (i.e., for a chi-square test using a critical alpha level of .05 our 1−β to detect a large effect size was 0.44). However, at the descriptive level, 6 of the 7 (85.7%) incident syndromic neurocognitive disorder cases were in the older HIV+ group. The single case of syndromic neurocognitive disorder in the younger HIV+ group met criteria for MND, while 55.6% (n = 5) and 11.11% (n = 1) of the older HIV+ group with a neurocognitive disorder met criteria for MND and HAD, respectively. No participants in the HIV− groups were designated as having a syndromic neurocognitive disorder.
Baseline Clinical Predictors of Incident HAND in Older HIV+ Adults
We were also interested in determining whether there were baseline predictors of incident neurocognitive disorders in older HIV+ participants (NB. as there were only 4 participants in the younger HIV+ group with incident HAND, we focused our attention on the older HIV+ sample). To determine if any baseline characteristics shown in Table 1 differed between individuals with an incident neurocognitive disorder versus neurocognitively normal individuals, a series of individual one-way ANOVA or chi-square tests were conducted using each individual dependent variable in Table 1 and the 2-level categorical variable of incident neurocognitive disorder versus neurocognitive normality as the independent variable; any significant omnibus tests were further examined using post hoc independent t-test (or its non-parametric equivalent) or chi-square test pair-wise comparisons. No baseline characteristic variables presented in Table 1 (e.g., nadir CD4 count) were associated with incident neurocognitive disorders in the older HIV+ group (all ps > .05).
Baseline Clinical Neurocognitive Predictors of Incident HAND
As presented in Table 2, separate one-way ANOVA tests revealed that lower baseline composite neuropsychological domain predictor variable scores for learning, memory, executive functions, and speed of information processing were each associated with incident neurocognitive disorder in older HIV+ individuals (all ps < .05). Hedge’s g was utilized as a measure of effect size given our continuous baseline neuropsychological domain scores and a dichotomous incident neurocognitive disorder outcome variable. Hedge’s g effect sizes for these analyses were large and ranged from 0.80 (learning) to 1.18 (executive functions). Classification accuracy using baseline neuropsychological scores that maximized both sensitivity and specificity as determined by Youden’s index in predicting incident neurocognitive disorders was analyzed using receiver operating characteristic (ROC) curves, and significant predictor area under the curve (AUC) values ranged from 0.75 (learning, odds ratio = 12.89, 95% CI [2.11, 249.44]) to 0.81 (executive functions, odds ratio = 18.86, 95% CI [3.05, 367.08]).
Table 2.
Mean (standard error) baseline neurocognitive characteristics of older HIV+ individuals grouped by incident HIV-associated neurocognitive disorder.
| Neurocognitive Domain T-scores | Normal (n = 47) | Incident HAND (n = 9) | p | g | AUC | Sens | Spec | PPV | NPV | Odds Ratio [95% CI] |
|---|---|---|---|---|---|---|---|---|---|---|
| Diagnostic Battery Measures | ||||||||||
| Learning | 57.0(1.2) | 50.6(2.5) | 0.029 | 0.80 | 0.75 | 0.89 | 0.62 | 0.31 | 0.97 | 12.89 [2.11, 249.44] |
| Memory | 56.2(1.1) | 49.4(1.8) | 0.013 | 1.04 | 0.77 | 0.78 | 0.75 | 0.37 | 0.95 | 10.21 [2.13, 75.20] |
| Attention | 51.3(1.3) | 45.6(2.5) | 0.075 | 0.65 | 0.70 | 0.56 | 0.85 | 0.42 | 0.91 | 7.14 [1.55, 35.98] |
| Executive Functions | 52.2(1.1) | 43.5(2.5) | 0.001 | 1.18 | 0.81 | 0.89 | 0.70 | 0.36 | 0.97 | 18.86 [3.05, 367.08] |
| Speed of Information Processing | 50.5(0.9) | 44.5(1.4) | 0.005 | 1.05 | 0.80 | 0.89 | 0.62 | 0.31 | 0.97 | 12.89 [2.11, 249.44] |
| Motor Skills | 46.7(1.1) | 43.4(2.3) | 0.243 | 0.42 | 0.61 | 0.44 | 0.83 | 0.33 | 0.89 | 3.9 [0.82, 18.25] |
| Supplementary Measures | ||||||||||
| Cognitive Reservea | 51.5(1.0) | 42.0(2.3) | 0.001 | 1.33 | 0.84 | 0.56 | 1.00 | 1.00 | 0.92 | 116.11 [5.49, 2457.25] |
| Verbal Fluencya | 51.5(1.2) | 42.6(2.0) | 0.003 | 1.13 | 0.83 | 1.00 | 0.66 | 0.36 | 1.00 | 36.27 [1.98, 662.92] |
| Semantic Memory | 54.2(1.3) | 48.4(2.1) | 0.065 | 0.68 | 0.71 | 0.89 | 0.51 | 0.26 | 0.96 | 8.34 [1.37, 161.15] |
| Prospective Memoryab | 51.5(1.4) | 42.3(2.6) | 0.010 | 0.96 | 0.78 | 0.78 | 0.79 | 0.41 | 0.95 | 12.95 [2.66, 96.69] |
Note. HAND = HIV-associated Neurocognitive Disorder; g = Hedge’s g; AUC = Area under the curve; Sens = Sensitivity; Spec = Specificity; PPV = Positive predictive value; NPV = Negative predictive value. Values reflect normatively-adjusted T-scores unless otherwise indicated; Cutoff T-scores (Youden’s index): Learning = 54.83; Memory = 50.00; Attention = 41.00; Speed of Information Processing = 49.00; Executive Functions = 48.83; Motor Skills = 39.50; Cognitive Reserve = 37.90; Verbal Fluency = 48.63; Semantic Memory = 52.67; Prospective Memory = 43.85.
T-scores based on unadjusted sample-based mean and standard deviation of the older HIV+ sample.
Memory for Intentions Screening Test (MIST) Summary Score.
Baseline Supplementary Neurocognitive Predictors of Incident HAND
Separate one-way ANOVAs of the supplementary neurocognitive measures showed that lower baseline cognitive reserve, verbal fluency, and prospective memory performance were each associated with incident neurocognitive disorder in the older HIV+ group (all ps < .05). There was no association between semantic memory and incident neurocognitive disorders in the older HIV+ group (p > .10). Hedge’s g effect sizes for significant baseline supplementary measure associations with incident neurocognitive disorders were large and ranged from 0.96 (prospective memory) to 1.33 (cognitive reserve). Classification accuracy of baseline supplementary measure predictor scores were analyzed in the same method as performed for baseline neuropsychological scores, and significant baseline supplementary measure AUC values ranged from 0.78 (prospective memory, odds ratio = 12.95, 95% CI [2.66, 96.69]) to 0.84 (cognitive reserve, odds ratio = 116.11, 95% CI [5.49, 2457.25]). To determine which specific baseline cognitive reserve components may have been driving the association with incident neurocognitive disorders, we conducted separate one-way ANOVA tests with years of education, Hollingshead score, and WTAR estimated verbal IQ as the predictor variables and the 2-level categorical variable of incident neurocognitive disorder versus neurocognitive normality as the outcome variable. The results showed that neither years of education, F(1,54) = 1.81, p = 0.169, Hedge’s g = 0.48), nor WTAR estimated verbal IQ were significantly associated with incident neurocognitive disorders, F(1,54) = 3.89, p = .054, Hedge’s g = 0.70, although both showed broadly medium effect sizes. In contrast, lower Hollingshead scores were significantly associated with incident neurocognitive disorders, F(1,53) = 13.81, p < .001, Hedge’s g = 1.33.
Incident Clinical Factors Associations with Incident Neurocognitive Disorders
Finally, in order to rule out the possibility that other incident clinical factors may have played a role in the elevated rates of incident neurocognitive disorders in HIV disease, we coded the incidence of mood (i.e., major depression disorder, generalized anxiety disorder) and substance use disorders (i.e., any substance dependence), as well as clinical changes in health status (i.e., detectable plasma RNA at follow-up, new-onset AIDS, and incident HCV) at one-year follow-up. The overall rate of incident changes in these variables was quite low, ranging from 0% (any substance dependence) to 3.4% (generalized anxiety disorder). We then conducted separate chi-square tests examining the associations between these variables and: 1) the four-level HIV/age groups; and 2) presence of an incident neurocognitive disorder. None of the incident mood, drug, or health variables were significantly associated with study group or incident neurocognitive disorders (all ps > .10).
Discussion
The results of the present study indicate that among adults who were neurocognitively normal at baseline, HIV infection conferred a nearly five-fold increased odds of incident neurocognitive disorders over a 14-month period. The approximately 16% incidence rate of neurocognitive disorders evident in the current HIV+ sample is broadly within the range of previous incidence studies in the cART era (e.g., Robertson et al., 2007). Further, the elevated rate of incident neurocognitive disorders in HIV+ individuals was not better explained by baseline demographic (e.g., age, ethnicity) or non-HIV-associated clinical factors (e.g., mood, substance use, or co-infection with HCV), nor by any incident changes in mood, substance, or health-related variables (i.e., AIDS status, plasma RNA detectable, HCV).
Contrary to expectations, the risk of incident neurocognitive disorders was not significantly higher among older (16.1%) versus younger HIV+ adults (15.8%). One possible reason for the discrepancy between prior studies and our findings is that all participants in the current study were neurocognitively normal at baseline, whereas prior studies have included samples with varying prevalence of neurocognitive disorders at baseline that may have increased the older adults’ susceptibility to further decline (Grant et al., 2014). Another difference is that previous longitudinal studies have primarily utilized continuous raw scores for neurocognitive variables (e.g., Sacktor et al., 2010; Seider et al., 2014). By way of contrast, we used rigorous Frascati criteria to determine clinically-meaningful levels of impairment and decline. However, it is also possible that the robustness of the effect of HIV infection on incident neurocognitive disorders dampens the importance of age-related changes for individuals in their mid-50s. In support of this hypothesis, cART era studies have found that HIV infection predicts incidence and prevalence rates of HAND over and above clinicodemographic variables—including age (Heaton et al. 2010, 2011; Robertson et al., 2007). The extent to which the incidence of HAND is modulated by age among persons in their 60s and 70s remains to be determined, because so few individuals infected with HIV have reached this age range in which age-related cognitive decline becomes more prevalent and may therefore affect the trajectory of HAND.
Another possibility is that the everyday expression of incident HAND may differ between older and younger adults. Although the low incidence rates of HAND in this study preclude us from drawing reliable statistical inferences due to limited power, the descriptive data are nevertheless compelling. Specifically, 6 out of the 7 cases of incident syndromic HAND—and the only case of HIV-associated dementia—were observed the older HIV+ group. This observation is consistent with prior cross-sectional studies suggesting that the adverse real-world impact of neurocognitive impairment is exacerbated in older adults (e.g., Jacks et al., in press). For example, Jacks et al. (in press) observed that older HIV+ persons with neurocognitive impairment were at disproportionate risk of being lost to follow-up care in an HIV clinic. The vulnerability of HIV+ adults with neurocognitive impairment to such functional declines may reflect decreased ability to deploy and utilize compensatory strategies in everyday life.
Despite the small sample size of older HIV+ adults with incident HAND, the present study identified multiple baseline neurocognitive indicators of incipient decline. Among the battery of clinical neurocognitive measures used to classify HAND, baseline assessments of learning, memory, executive functions, and speed of information processing were all associated with large effect sizes in predicting incident HAND. Individuals with lower (albeit still normal) average T-scores in these domains were 10 to 19 times more likely to have incident HAND. Overall, T-scores that fell above the cutoff scores as determined by Youden’s index (see Table 2) were strong indicators of not evidencing incident HAND at follow-up (negative predictive values ranged between .95 and .97), while scores below the cutoff score were less powerful predictors (positive predictive value range = .31 to .37). In other words, individuals with baseline performance in the high average and above range were highly unlikely to experience incident HAND, whereas there was some greater variability in whether persons with low average scores converted to HAND at one-year follow-up. The extent to which such individuals with low average scores are at greater risk for incident HAND at longer follow-up intervals remains to be determined.
Complementing the predictive value of the clinical measures used to diagnose HAND, our data showed that baseline performance on other neuropsychological tasks that place demands on memory and executive functions (viz., verbal fluency and prospective memory) were also predictive of incident HAND. Both verbal fluency and prospective memory are affected by age and HIV (Iudicello, Woods, Deutsch, Grant, & the HNRP Group, 2012; Weber et al., 2011; Woods, Dawson, Weber, Grant, & the HNRC Group, 2010), and deficits in these domains are associated with impairments in everyday functioning outcomes (Woods et al., 2006, 2008). Deficits in these domains also are predictive of incident impairment in other populations in which frontal systems are affected, including Parkinson’s disease (Jacobs et al., 1995). Collectively, the neuropsychological associations observed herein suggest that suboptimal memory and executive functioning in HIV may be important risk factors for progression to HAND.
Baseline cognitive reserve was among the strongest predictors of incident neurocognitive disorders in older HIV+ adults, with positive and negative predictive values greater than 90. Cognitive reserve is a process whereby potential performance deficits due to insult to neural systems are assuaged by a built-up ability to more efficiently utilize brain networks (Stern, 2002). Higher levels of cognitive reserve may serve multiple protective purposes in HIV, including 1) having a lowered risk of cross-sectional neurocognitive deficits (Vázquez-Justo, Blanco, Vergara-Moragues, Gestoso, & Pérez-García, 2014; for review, see Vance, McDougall, Wilson, Debiasi, & Cody, 2014) and 2) a decreased risk of concurrent everyday functioning problems (Morgan, Woods, et al., 2012). Closer examination of the variables of which cognitive reserve was comprised showed that Hollingshead occupation scores were significantly associated with incident neurocognitive disorders in the older HIV+ adults. Such findings converge with prior studies showing the importance of lower occupational status as a risk factor for dementia (e.g., Stern et al., 1994). The extent to which this association reflects the benefits of higher levels of cognitive activity, availability, and utilization of compensatory strategies and/or actual brain reserve remains to be determined. The present data suggest that higher levels of cognitive reserve in older HIV+ individuals may indeed assuage longitudinal decline in neurocognitive status as measured by Frascati HAND criteria. However, determining whether this protective role also applies to subsequent incident everyday functioning decline should be a point of emphasis in future studies.
Traditional clinical markers of HIV disease severity were not associated with incident HAND in the older HIV+ group. This included null findings for baseline predictors (e.g., nadir CD4 count) and incident immunovirological changes (e.g., detectable HIV RNA in plasma), which prior cohort studies suggest may be related to HAND (e.g., Heaton et al., 2015; Ellis et al., 2011). The absence of findings in the current study could relate to the small samples of incident HAND, which were not sufficiently powered to detect the quite modest associations observed in the prior large-scale neuroepidemiological studies that have shown such effects. Nevertheless, our findings are consistent with studies arguing that clinical factors other than HIV disease severity (e.g., vascular disease) may be more relevant to the development and evolution of HAND in the era of cART (e.g., Becker et al., 2009). Future work in this regard may factor in more detailed biomarkers of neuroAIDS (e.g., MCP-1) and other aspects of neural injury (e.g., tau) as predictors of incident HAND among older HIV+ persons.
HCV infection was also associated with increased incidence of neurocognitive disorders, regardless of HIV serostatus. Specifically, HCV infection conferred nearly a 5-fold increased risk for developing a neurocognitive disorder at follow-up. This finding was not better explained by any of the clinicodemographic variables in Table 1. Imaging studies have implicated abnormalities in both gray and white matter of frontosriatal regions of individuals with HCV (Bladowska et al., 2013; Forton et al., 2008; Weissenborn et al., 2004). In parallel, neuropsychological findings implicate attention and psychomotor speed deficits in persons with HCV infection (Hilsabeck, Hassanein, Carlson, Ziegler, & Perry, 2003; Hinkin, Castellon, Levine, Barclay, & Singer, 2008; Senzolo et al., 2011), as well as an increased risk for incident dementia (Chiu et al., 2014). One limitation of this finding is that HCV status was determined by self-report; however, post hoc analyses revealed that higher levels of liver biomarkers (AST and ALT) were significantly associated with HCV status (data not shown), which lends some credence to the self-report HCV variable. The HCV-associated risk of incident neurocognitive disorders was independent of HIV serostatus. Although the literature on the effects of co-infection on the central nervous system are mixed, our post hoc analyses present data that do not support an additive effect of HIV and HCV in the context of incident neurocognitive disorders. Rather, these data more simply suggest that the presence of either neurotropic infection increases one’s risk of an incident neurocognitive disorder. Therefore, it is suggested that HCV and HIV infection be considered concurrently when assessing risk for developing a neurocognitive disorder.
The present study was limited to a relatively short 14-month period of time between baseline and follow-up compared to other incident impairment studies in HIV (e.g., Heaton et al., 2015), although previous studies have also used a one year interval and found similar declines (e.g., Seider et al., 2014). Of interest clinically, a study by Salthouse (2014) found that seronegative adults who were tested multiple times within an interval in which one might expect to see cognitive decline (e.g., a 3 year interval) were less likely to have cognitive change over the entire interval. Thus, the lack of a significant risk for incident neurocognitive disorder associated with age in the present study may offer support for clinicians who opt to utilize longer intervals between assessments in order to detect broad cognitive decline as the population of HIV+ individuals continues to age. In regard to the generalizability of the present findings, these data should be interpreted cautiously in light of the findings that individuals with lower cognitive reserve and neuropsychiatric disorders (e.g., depression, substance use) in the present sample were less likely to have been retained at follow-up. Further, the present study sample consisted of predominantly white, educated men from an urban setting, and so external validity in broader national and international settings remains to be determined. One limitation inherent in longitudinal studies is the possibility of practice effects as possible confounding variables in the outcome of incident neurocognitive disorders. However, it is unlikely that any between group findings were a product of multiple test administrations as there is no reason to expect that these effects would be limited to one age or HIV status group. We also utilized parallel forms of tests when possible. Finally, even though small sample sizes should increase the likelihood of committing a type II error, we nevertheless observed large effect sizes across significant neuropsychological and supplementary cognitive measures.
In summary, the results of the present study revealed an increased risk of incident neurocognitive disorders in HIV, the effect of which may be more likely to result in the syndromic subtype for older adults. These findings necessitate addressing the question of whether interventions might be implemented to attenuate the deleterious effects of cognitive and functional decline in the growing older HIV+ population. In healthy older adult samples, there is considerable evidence suggesting that aerobic exercise improves cognitive outcomes (Colcombe & Kramer, 2003), which may be facilitated by improved neural structural integrity (for review, see Hayes, Alosco, & Forman, 2014). In HIV, poor aerobic fitness has been found to be associated with cognitive impairment (Mapstone et al., 2013). A recent study by Fazeli et al. (2014) found that increased numbers of self-reported active lifestyle factors (i.e., physical exercise, social activity, and current employment) were associated with improved neurocognitive performance in addition to a lower prevalence of HAND. Successful cognitive aging in HIV also has been associated with better health-related quality of life (Moore et al., 2014) and lower rates of Major Depressive Disorder and current affective distress (Malaspina et al., 2011). Finally, determining risk factors associated with incident syndromic neurocognitive disorders may help identify persons who may benefit from preemptive compensatory strategy training for everyday living tasks (e.g., medication management programs, automatic bill pay) which may improve functional outcomes for these at-risk individuals. Researchers and clinicians should aim to identify the potential avenues through which interventions and compensatory training might yield consistent optimal outcomes for older HIV+ individuals at risk for syndromic neurocognitive disorders.
Table 3.
Mean (standard error) demographic, psychiatric, medical, and HIV disease characteristics across HCV groups.
| HCV− (n = 120) | HCV+ (n = 26) | p | Group Difference | |
|---|---|---|---|---|
| Age (years) | 46.8(1.3) | 52.0(1.3) | .069 | -- |
| Education (years) | 14.3(0.2) | 13.0(0.5) | .006 | HCV− > HCV+ |
| Ethnicity (%) | -- | -- | ||
| African-American | 17.5 | 34.6 | ||
| Asian | 2.5 | 0.0 | ||
| Hispanic | 15.8 | 15.4 | ||
| White | 64.2 | 50.0 | ||
| Gender (% men) | 76.7 | 73.1 | -- | -- |
| Estimated Verbal IQ (WTAR) | 104.3(0.9) | 99.8(1.7) | .039 | HCV− > HCV+ |
| POMS total (of 200) | 49.2(3.2) | 55.4(7.4) | -- | -- |
| Major Depressiona (%) | 53.3 | 65.4 | -- | -- |
| Generalized Anxietya (%) | 10.0 | 30.77 | .010 | HCV− < HCV+ |
| Substance Dependenceb (%) | 47.5 | 80.77 | .002 | HCV− < HCV+ |
| HIV Infection c (%) | 53.33 | 73.1 | .082 | -- |
| Estimated Duration of Infection c (months) | 157.5(12.7) | 195.9(18.9) | -- | -- |
| AIDS c (%) | 54.7 | 68.4 | -- | -- |
| CD4 count c (cells/μL) | 559.6(33.9) | 550.5(51.4) | -- | -- |
| Nadir CD4 c (cells/μL) | 198.1(19.7) | 208.8(36.4) | -- | -- |
| cART status c (%) | 89.1 | 84.2 | -- | -- |
| Plasma RNA detectable c (%) | 24.2 | 22.2 | -- | -- |
| Among subjects on cART (%) | 14.6 | 20.0 | -- | -- |
Note. HCV = Hepatitis C virus; WTAR = Wechsler Test of Adult Reading; POMS = Profile of Mood States; AIDS = Acquired Immune Deficiency Syndrome; CD4 = Cluster of Differentiation 4; cART = combination antiretroviral therapy.
Includes both current (e.g., the last 30 days) and lifetime diagnosis.
Any lifetime diagnosis of dependence on alcohol or illicit substances.
Sample size for HIV disease characteristic variables: HCV− n=64, HCV+ n=19.
Acknowledgments
This work was supported by the National Institutes of Health under Grants R01-MH073419, T32-DA31098, L30-DA0321202, P30-MH62512 and K24-AG026431.
The authors thank Marizela Cameron and P. Katie Riggs for their help with study management and Donald Franklin and Stephanie Corkran for their help with data processing.
Footnotes
The authors have no financial conflicts of interest related to this work. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
References
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Army Individual Test Battery. Manual of directions and scoring. Washington, DC: War Department, Adjutant General’s Office; 1944. [Google Scholar]
- Becker JT, Kingsley L, Mullen J, Cohen B, Martin E, Miller EN Multicenter AIDS Cohort Study. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology. 2009;73(16):1292–1299. doi: 10.1212/WNL.0b013e3181bd10e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Maruca V, Kingsley LA, Sanders JM, Alger JR, Barker PB Multicenter AIDS Cohort Study. Factors affecting brain structure in men with HIV disease in the post-HAART era. Neuroradiology. 2012;54(2):113–121. doi: 10.1007/s00234-011-0854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladowska J, Zimny A, Knysz B, Małyszczak K, Kołtowska A, Szewczyk P, Sąsiadek MJ. Evaluation of early cerebral metabolic, perfusion and microstructural changes in HCV-positive patients: a pilot study. Journal of Hepatology. 2013;59(4):651–657. doi: 10.1016/j.jhep.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Brodt HR, Kamps BS, Gute P, Knupp B, Staszewski S, Helm EB. Changing incidence of AIDS-defining illnesses in the era of antiretroviral combination therapy. AIDS (London, England) 1997;11(14):1731–1738. doi: 10.1097/00002030-199714000-00010. [DOI] [PubMed] [Google Scholar]
- Brooks JT, Buchacz K, Gebo KA, Mermin J. HIV infection and older Americans: the public health perspective. American Journal of Public Health. 2012;102(8):1516–1526. doi: 10.2105/AJPH.2012.300844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu WC, Tsan YT, Tsai SL, Chang CJ, Wang JD, Chen PC Health Data Analysis in Taiwan (hDATa) Research Group. Hepatitis C viral infection and the risk of dementia. European Journal of Neurology: The Official Journal of the European Federation of Neurological Societies. 2014;21(8):1068–e59. doi: 10.1111/ene.12317. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychological Science. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Culbertson WC, Zillmer EA. The Tower of London, Drexel University, research version: examiner’s manual. North Tonawanda: Multi-Health Systems; 1999. [Google Scholar]
- Cysique LA, Letendre SL, Ake C, Jin H, Franklin DR, Gupta S HIV Neurobehavioral Research Center group. Incidence and nature of cognitive decline over 1 year among HIV-infected former plasma donors in China. AIDS (London, England) 2010;24(7):983–990. doi: 10.1097/QAD.0b013e32833336c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. The California verbal learning test. 2. San Antonio: The Psychological Corporation; 2000. [Google Scholar]
- Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D CHARTER Group. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS (London, England) 2011;25(14):1747–1751. doi: 10.1097/QAD.0b013e32834a40cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli PL, Woods SP, Heaton RK, Umlauf A, Gouaux B, Rosario D HNRP Group. An active lifestyle is associated with better neurocognitive functioning in adults living with HIV infection. Journal of Neurovirology. 2014;20(3):233–242. doi: 10.1007/s13365-014-0240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forton DM, Hamilton G, Allsop JM, Grover VP, Wesnes K, O’Sullivan C, Taylor-Robinson SD. Cerebral immune activation in chronic hepatitis C infection: a magnetic resonance spectroscopy study. Journal of Hepatology. 2008;49(3):316–322. doi: 10.1016/j.jhep.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Gandhi NS, Skolasky RL, Peters KB, Moxley RT, Creighton J, Roosa HV, Sacktor N. A comparison of performance-based measures of function in HIV-associated neurocognitive disorders. Journal of Neurovirology. 2011;17(2):159–165. doi: 10.1007/s13365-011-0023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, Barresi B. Boston diagnostic aphasia examination. 3. Philadelphia: Lippincott WilliamsWilkins; 2001. [Google Scholar]
- Grant I, Franklin DR, Deutsch R, Woods SP, Vaida F, Ellis RJ CHARTER Group. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. 2014;82(23):2055–2062. doi: 10.1212/WNL.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Alosco ML, Forman DE. The Effects of Aerobic Exercise on Cognitive and Neural Decline in Aging and Cardiovascular Disease. Current Geriatrics Reports. 2014;3(4):282–290. doi: 10.1007/s13670-014-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F CHARTER Group. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Deutsch R, Letendre S, Ellis RJ, Casaletto K CHARTER Group. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2015;60(3):473–480. doi: 10.1093/cid/ciu862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S HNRC Group. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of Neurovirology. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H HNRC Group. The impact of HIV-associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychological Society: JINS. 2004;10(3):317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Hilsabeck RC, Hassanein TI, Carlson MD, Ziegler EA, Perry W. Cognitive functioning and psychiatric symptomatology in patients with chronic hepatitis C. Journal of the International Neuropsychological Society: JINS. 2003;9(6):847–854. doi: 10.1017/S1355617703960048. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Levine AJ, Barclay TR, Singer EJ. Neurocognition in individuals co-infected with HIV and hepatitis C. Journal of Addictive Diseases. 2008;27(2):11–17. doi: 10.1300/J069v27n02_02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale University Press; 1975. [Google Scholar]
- Iudicello JE, Woods SP, Deutsch R, Grant I HIV Neurobehavioral Research Program Hnrp Group. Combined effects of aging and HIV infection on semantic verbal fluency: a view of the cortical hypothesis through the lens of clustering and switching. Journal of Clinical and Experimental Neuropsychology. 2012;34(5):476–488. doi: 10.1080/13803395.2011.651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks A, Wainwright D, Salazar L, Grimes R, York M, Strutt AM, Shahani L, Woods SP, Hasbun R. Neurocognitive deficits increase risk of poor retention in care among older adults with newly diagnosed HIV infection. AIDS. doi: 10.1097/QAD.0000000000000700. in press. [DOI] [PubMed] [Google Scholar]
- Jacobs DM, Marder K, Côté LJ, Sano M, Stern Y, Mayeux R. Neuropsychological characteristics of preclinical dementia in Parkinson’s disease. Neurology. 1995;45(9):1691–1696. doi: 10.1212/wnl.45.9.1691. [DOI] [PubMed] [Google Scholar]
- Karnofsky DA, Burchenal JH. The clinical evaluation of chemo-therapeutic agents in cancer. In: Maclead CM, editor. Evaluation of Chemotherapeutic Agents. New York, NY: Columbia University Press; 1949. pp. 191–205. [Google Scholar]
- Kaufman AS, Kaufman NL. Manual for the Kaufman Adolescent and Adult Intelligence Test (KAIT) Circle Pines, MN: American Guidance Service; 1993. [Google Scholar]
- Kløve H. Grooved pegboard. Indiana: Lafayette Instruments; 1963. [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- Malaspina L, Woods SP, Moore DJ, Depp C, Letendre SL, Jeste D HIV Neurobehavioral Research Programs (HNRP) Group. Successful cognitive aging in persons living with HIV infection. Journal of Neurovirology. 2011;17(1):110–119. doi: 10.1007/s13365-010-0008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapstone M, Hilton TN, Yang H, Guido JJ, Luque AE, Hall WJ, Shah K. Poor Aerobic Fitness May Contribute to Cognitive Decline in HIV-infected Older Adults. Aging and Disease. 2013;4(6):311–319. doi: 10.14336/AD.2013.0400311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte TD, Deutsch R, McCutchan JA, Moore DJ, Letendre S, Ellis RJ San Diego HIV Neurobehavioral Research Center (HNRC) Group. Prediction of incident neurocognitive impairment by plasma HIV RNA and CD4 levels early after HIV seroconversion. Archives of Neurology. 2003;60(10):1406–1412. doi: 10.1001/archneur.60.10.1406. [DOI] [PubMed] [Google Scholar]
- Mateen FJ, Shinohara RT, Carone M, Miller EN, McArthur JC, Jacobson LP Multicenter AIDS Cohort Study (MACS) Investigators. Neurologic disorders incidence in HIV+ vs HIV- men: Multicenter AIDS Cohort Study, 1996–2011. Neurology. 2012;79(18):1873–1880. doi: 10.1212/WNL.0b013e318271f7b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1981. [Google Scholar]
- Molsberry SA, Lecci F, Kingsley L, Junker B, Reynolds S, Goodkin K, Becker JT. Mixed membership trajectory models of cognitive impairment in the multicenter AIDS cohort study. AIDS (London, England) 2015;29(6):713–721. doi: 10.1097/QAD.0000000000000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RC, Fazeli PL, Jeste DV, Moore DJ, Grant I, Woods SP HIV Neurobehavioral Research Program (HNRP) Group. Successful cognitive aging and health-related quality of life in younger and older adults infected with HIV. AIDS and behavior. 2014;18(6):1186–1197. doi: 10.1007/s10461-014-0743-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Iudicello JE, Weber E, Duarte NA, Riggs PK, Delano-Wood L HIV Neurobehavioral Research Program (HNRP) Group. Synergistic effects of HIV infection and older age on daily functioning. Journal of Acquired Immune Deficiency Syndromes (1999) 2012;61(3):341–348. doi: 10.1097/QAI.0b013e31826bfc53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Smith C, Weber E, Scott JC, Grant I HIV Neurobehavioral Research Program (HNRP) Group. Lower cognitive reserve among individuals with syndromic HIV-associated neurocognitive disorders (HAND) AIDS and behavior. 2012;16(8):2279–2285. doi: 10.1007/s10461-012-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatt AL, Fields JA, Paolo AM, Tröster AI. Action (verb naming) fluency as an executive function measure: convergent and divergent evidence of validity. Neuropsychologia. 1999;37:1499–1503. doi: 10.1016/s0028-3932(99)00066-4. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation. Manual for the Wechsler Test of Adult Reading (WTAR) San Antonio: Author; 2001. [Google Scholar]
- Raskin S, Buckheit C, Sherrod C. MIST Memory for Intentions Test professional manual. Lutz, FL: Psychological Assessment Resources, Inc; 2010. [Google Scholar]
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, Ellis RJ. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS (London, England) 2007;21(14):1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Lyles RH, Skolasky R, Kleeberger C, Selnes OA, Miller EN Multicenter AIDS Cohort Study. HIV-associated neurologic disease incidence changes:: Multicenter AIDS Cohort Study, 1990–1998. Neurology. 2001;56(2):257–260. doi: 10.1212/wnl.56.2.257. [DOI] [PubMed] [Google Scholar]
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. Journal of Neurovirology. 2002;8(2):136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Skolasky RL, Cox C, Selnes O, Becker JT, Cohen B Multicenter AIDS Cohort Study (MACS) Longitudinal psychomotor speed performance in human immunodeficiency virus-seropositive individuals: impact of age and serostatus. Journal of Neurovirology. 2010;16(5):335–341. doi: 10.3109/13550284.2010.504249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, Skolasky R, Selnes OA, Watters M, Poff P, Shiramizu B, Valcour V. Neuropsychological test profile differences between young and old human immunodeficiency virus-positive individuals. Journal of Neurovirology. 2007;13(3):203–209. doi: 10.1080/13550280701258423. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Frequent assessments may obscure cognitive decline. Psychological Assessment. 2014;26(4):1063–1069. doi: 10.1037/pas0000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seider TR, Luo X, Gongvatana A, Devlin KN, De la Monte SM, Chasman JD, Cohen RA. Verbal memory declines more rapidly with age in HIV infected versus uninfected adults. Journal of Clinical and Experimental Neuropsychology. 2014;36(4):356–367. doi: 10.1080/13803395.2014.892061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senzolo M, Schiff S, D’Aloiso CM, Crivellin C, Cholongitas E, Burra P, Montagnese S. Neuropsychological alterations in hepatitis C infection: the role of inflammation. World journal of gastroenterology: WJG. 2011;17(29):3369–3374. doi: 10.3748/wjg.v17.i29.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society: JINS. 2002;8(3):448–460. [PubMed] [Google Scholar]
- Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. Journal of the American Medical Association. 1994;271(13):1004–1010. [PubMed] [Google Scholar]
- Thames AD, Kim MS, Becker BW, Foley JM, Hines LJ, Singer EJ, Hinkin CH. Medication and finance management among HIV-infected adults: the impact of age and cognition. Journal of Clinical and Experimental Neuropsychology. 2011;33(2):200–209. doi: 10.1080/13803395.2010.499357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JB, Brier MR, Snyder AZ, Vaida FF, Ances BM. Pathways to neurodegeneration: effects of HIV and aging on resting-state functional connectivity. Neurology. 2013;80(13):1186–1193. doi: 10.1212/WNL.0b013e318288792b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, Sacktor N. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004;63(5):822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE, McDougall GJ, Wilson N, Debiasi MO, Cody SL. Cognitive Consequences of Aging with HIV: Implications for Neuroplasticity and Rehabilitation. Topics in Geriatric Rehabilitation. 2014;30(1):35–45. doi: 10.1097/TGR.0000000000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Justo E, Blanco AP, Vergara-Moragues E, Gestoso CG, Pérez-García M. Cognitive reserve during neuropsychological performance in HIV intravenous drug users. Applied Neuropsychology Adult. 2014;21(4):288–296. doi: 10.1080/23279095.2013.813852. [DOI] [PubMed] [Google Scholar]
- Weber E, Woods SP, Delano-Wood L, Bondi MW, Gilbert PE, Grant I HIV Neurobehavioral Research Program Group. An examination of the age-prospective memory paradox in HIV-infected adults. Journal of Clinical and Experimental Neuropsychology. 2011;33(10):1108–1118. doi: 10.1080/13803395.2011.604027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler memory scale-third edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Weissenborn K, Krause J, Bokemeyer M, Hecker H, Schüler A, Ennen JC, Böker KW. Hepatitis C virus infection affects the brain-evidence from psychometric studies and magnetic resonance spectroscopy. Journal of Hepatology. 2004;41(5):845–851. doi: 10.1016/j.jhep.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Woods SP, Dawson MS, Weber E, Grant I HIV Neurobehavioral Research Center (HNRC) Group. The semantic relatedness of cue-intention pairings influences event-based prospective memory failures in older adults with HIV infection. Journal of Clinical and Experimental Neuropsychology. 2010;32(4):398–407. doi: 10.1080/13803390903130737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Hoebel C, Pirogovsky E, Rooney A, Cameron MV, Grant I HIV Neurobehavioral Research Program Group. Visuospatial temporal order memory deficits in older adults with HIV infection. Cognitive and Behavioral Neurology: Official Journal of the Society for Behavioral and Cognitive Neurology. 2013;26(4):171–180. doi: 10.1097/WNN.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Iudicello JE, Moran LM, Carey CL, Dawson MS, Grant I HIV Neurobehavioral Research Center Group. HIV-associated prospective memory impairment increases risk of dependence in everyday functioning. Neuropsychology. 2008;22(1):110–117. doi: 10.1037/0894-4105.22.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychology Review. 2009;19(2):152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Morgan EE, Dawson M, Scott JC, Grant I HNRC Group. Action (verb) fluency predicts dependence in instrumental activities of daily living in persons infected with HIV-1. Journal of Cliniical and Experimental Neuropsychology. 2006;28:1030–1042. doi: 10.1080/13803390500350985. [DOI] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, Heaton RK. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. Journal of Clinical and Experimental Neuropsychology. 2004;26(6):759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]