Abstract
Natural killer (NK) cells are innate lymphocytes postulated to mediate resistance against primary haematopoietic but not solid tumor malignancies. Cancer stem cells (CSCs) are a small subset of malignant cells with stem-like properties which are resistant to chemo- and radiotherapies and are able to repopulate a tumor after cytoreductive treatments. We observed increased frequencies of stem-like tumor cells after irradiation, with increased expression of stress ligands on surviving stem-like cells. Ex vivo NK cells activated by low dose IL2 in vitro and IL15 in vivo displayed an increased ability to target solid tumor stem-like cells both in vitro and in vivo after irradiation. Mechanistically, both upregulation of stress-related ligands on the stem-like cells as well as debulking of non-stem populations contributed to these effects as determined by data from cell lines, primary tumor samples, and most relevant patient derived specimens. In addition, pretreatment of tumor-bearing mice with local radiation prior to NK transfer resulted in significantly longer survival indicating that radiation therapy in conjunction with NK cell adoptive immunotherapy targeting stem-like cancer cells may offer a promising novel radio-immunotherapy approach in the clinic.
Keywords: natural killer cells, adoptive immunotherapy, cancer stem cell, radiotherapy
Introduction
CSCs represent a small proportion of heterogeneous cancer cells within a tumor. They have the ability to maintain long-term growth potential, and due to a reduced rate of proliferation, are resistant to conventional cytoreductive cancer treatments in comparison to non-CSCs.1,2 The presence of CSC subpopulations has been identified in many human malignancies including pancreatic cancer,3,4 sarcoma,5-7 and breast cancer.8-10 Mounting studies examining CSC engraftment in immune-compromised mice and CSC repopulation in long-term in vitro outgrowth assays have validated the CSC phenotype.11,12
CSCs remain dormant within the tumor niche. Although the mechanism is incompletely understood, CSCs are capable of repopulating the tumor mass after cytoreductive treatments, leading to eventual relapse. CSC resistance to treatment-induced DNA damage is also related to increased levels of the DNA checkpoint kinases Chk1 and Chk2.13 CSCs can also utilize ATP-binding cassette (ABC) transporters to actively transport chemotherapeutic agents out of the cell, conferring resistance to cytotoxic chemotherapeutics in a variety of tumor types.14
Radiotherapy (RT) is a standard treatment modality for many cancers. It exerts its antitumor effects primarily through the induction of single or double stranded DNA breaks and the formation of damaging reactive oxygen species within cancer cells.15 The known immune modulating effects of RT include the release of TLR ligands and other tumor antigens from dying cancer cells.16 The influence of RT on cancer immunotherapy is highlighted by the synergistic responses seen when combined with checkpoint blockade.17 While CSC resistance to RT has been well characterized, it is unclear how RT may impact immune-mediated recognition of CSCs. Furthermore, little is known regarding the effect of RT on NK cell recognition or cytolysis of CSCs from solid human tumors. Given that in radiobiological models tumor repopulation by CSCs is a key factor limiting the probability of cure from therapy, understanding ways to limit tumor repopulation could greatly improve the efficacy of RT.
Previous reports have indicated that NK cells may be uniquely capable of exhibiting their cytotoxic functions toward cells with a stem cell phenotype.18,19 However, this effect has not been examined in combination with a standard cytotoxic therapy such as RT. NK cells recognize target cells through the interactions of activating and inhibitory receptors with their cognate ligands expressed on malignant or virally infected cells.20 NKG2D is a key NK cell activating receptor which binds MHC-1b molecules upregulated by malignant cells.21 The predominant inhibitory receptors for NK cells are the killer cell immunoglobulin like receptors (KIRs), which bind to distinct HLA molecules expressed on the surface of nearly all cells.22 Some reports indicate that HLA is expressed by CSCs,23 while others indicate that CSCs may downregulate their expression of HLA molecules, making them an attractive target for NK cell attack.24,25
Here, we report that RT uniquely sensitizes CSCs from multiple solid tumor types including pancreatic cancers, breast cancers, and sarcomas to the cytolytic effector functions of NK cells. We demonstrate that RT upregulates the expression of NK cell recognition ligands by CSCs in vitro, and sensitizes CSCs to NK cell cytolysis in vitro and in vivo. Lastly, we demonstrate that our combination radio-immunotherapy approach effectively targets CSCs and leads to improved antitumor efficacy in vivo in pre-clinical models of advanced metastatic cancer.
Results
Irradiation enriches heterogeneous tumors for cells with a CSC phenotype
We first sought to evaluate the effects of RT on CSC populations in our model systems. In order to evaluate stem-like properties of tumor cells, we relied on a combination of phenotypic markers which we and others have validated previously to confer the CSC phenotype.12 Numerous pre-clinical and clinical studies have linked expression of aldehyde dehydrogenase (ALDH) with a stem-like phenotype and worse oncologic outcome in a broad range of tumor types including pancreatic cancer,3,4 sarcoma,5-7 and breast cancer.8-10 We also evaluated the expression of additional stemness-associated markers, including CD24 and CD44, as the differential expression of these surface proteins identify CSC populations in breast (CD24−/CD44+) and pancreatic cancer (CD24+/CD44+).26-28 We also validated these alternative markers using cell sorting. As shown in Fig. S1, the CD24+/CD44+/ALDHbright population in clinical pancreatic cancer specimens showed characteristics of CSCs when implanted into NSG mice (Fig. S1A) or grown in liquid culture (Fig. S1B).
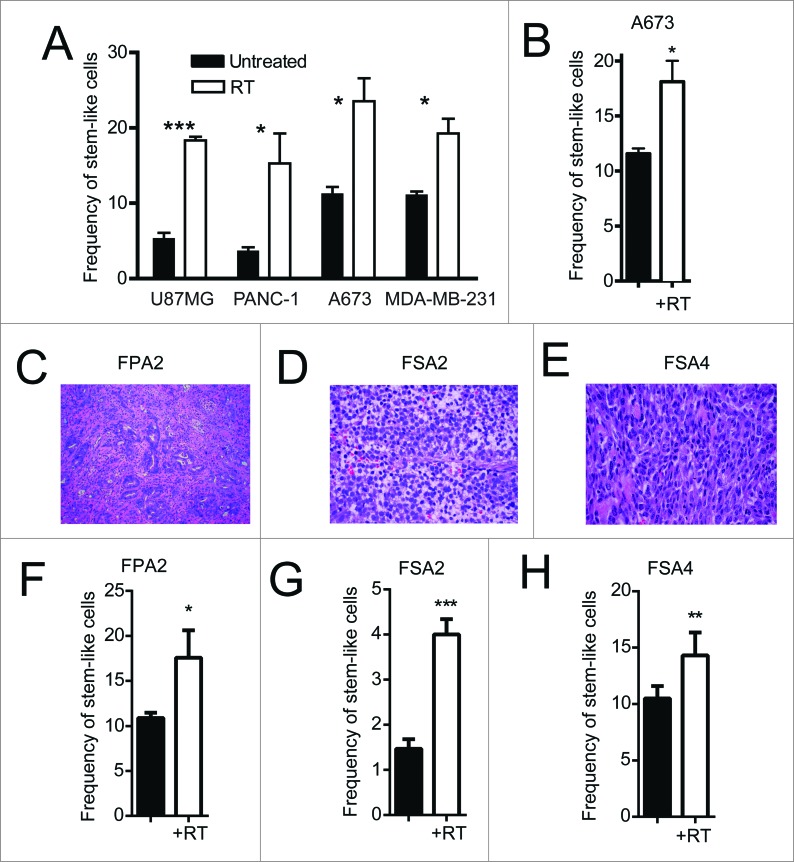
We observed enrichments in ALDHbright stem-like populations 24 h after irradiating in vitro cultures of multiple cell lines, including U87MG (glioblastoma), PANC-1 (pancreas), A673 (Ewing's sarcoma) and MDA-MB-231 (breast) (Fig. 1A). We next implanted the Ewing's sarcoma cell line, A673, into the flanks of NSG mice to determine the effect of conformal, multi-collimated local RT on CSC enrichment in vivo. Similar to our results in vitro, a single 8 Gy fraction of locally administered RT led to a significant (p < 0.05) increase in the proportion of cells with high ALDH activity 24 h after irradiation (Fig. 1B). Fresh tumor tissue was then obtained from surgical resections from patients with pancreatic ductal adenocarcinoma (FPA2; Fig. 1C), Ewing's sarcoma (FSA2; Fig. 1D) and leiomyosarcoma (FSA4; Fig. 1E). FPA2 was assessed for frequencies of CD24, CD44 and ALDH while the sarcoma specimens were assessed for ALDH alone. Flow cytometry revealed a small population of FPA2 tumor cells expressing these stem-like markers (Fig. S1C). Dissociated primary tumor cells from FPA2 were subjected to a single dose of RT and analyzed 24 h later to assess for changes in stem-like tumor cell frequency (Fig. 1F–H). These freshly isolated tumor specimens showed a similar significant increase in the percentage of stem-like cells after RT. Taken together, these data indicate that RT enriches for CSCs in diverse pre-clinical models and in human primary tissues.
Figure 1.
Irradiation enriches for cells with a CSC phenotype from primary solid tumors. (A) Indicated human tumor cell lines, were exposed to 4–10 Gy γ-irradiation, and then assessed for the percentage of remaining cells expressing a stem-like phenotype by ALDH expression 18–24 h later by flow cytometry. (B) ALDH expression of dissociated A673 xenograft tumors 48 h after 8 Gy local radiotherapy in vivo. (C–E) H&E staining of primary human pancreatic adenocarcinoma (FPA2), Ewing's sarcoma (SA2) and leiomyosarcoma (SA4). (F–H) Primary tumor specimens in C–E were given 8 Gy γ-irradiation, then assessed for stem-like phenotypic markers by flow cytometry 18–24 h later. Stem-like cells in FPA2 were quantified as CD24+/CD44+/ALDHbright cells while stem-like cells from soft-tissue sarcoma samples FSA2 and FSA4 were quantified as ALDHbright cells. Statistical significance was determined by Student's t-test. * p < 0.05; ** p < 0.01, ***p < 0.001.
Irradiation enhances the expression of NK activation ligands by stem-like tumor cells
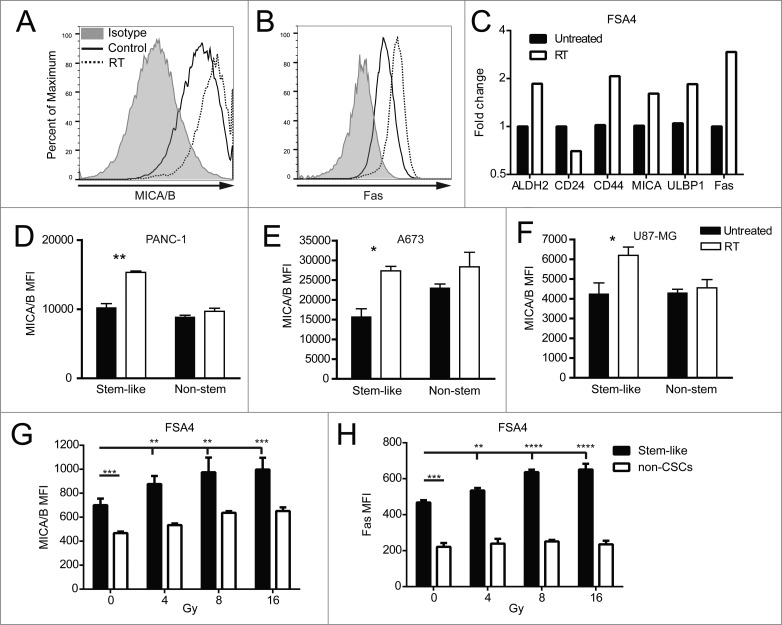
Given that stem-like cells resisted RT and were enriched after treatment, we wanted to assess whether RT would induce changes in the expression of cell surface markers on CSCs which may confer a heightened sensitivity to immunological targeting. Single-cell suspensions of the fresh primary sarcoma sample FSA4 were irradiated shortly after surgical removal from the patient. Twenty-four hours following RT, we observed an upregulation of the stress markers MICA/B and Fas on these unsorted primary tumor cells in vitro by flow cytometry median fluorescent intensity (MFI) (Fig. 2A,B). We also assessed stem-like phenotypic markers and death markers on FSA4 by qRT-PCR and observed changes in the expression of ALDH, CD24 and CD44 transcripts consistent with an increased number of stem-like cells after irradiation (Fig. 2C). Additionally, transcripts of the NKG2D ligands MICA and ULBP1 were elevated along with the death receptor Fas.
Figure 2.
Irradiation increases the expression of NK cell ligands on stem-like tumor cells. (A, B) Fresh sarcoma specimen FSA4 was dissociated and exposed to 8 Gy γ-irradiation. Twenty-four hours later, tumors were assessed for fluorescent intensity of MICA/B and Fas by flow cytometry. (C) FSA4 cells were assessed for stem-like phenotypic markers and death markers by qRT-PCR. Data are presented as fold change compared to unirradiated samples. (D–F) Expression of MICA on irradiated tumor cells was assessed for ALDHbright (stem-like) and ALDHdim (non-stem like) populations from PANC-1 (D), A673 (E), and U87-MG (F). (G, H) FSA4 cells were exposed to 8 Gy γ-irradiation, then assessed for the expression of the NKG2D ligands MICA/B (E) or the death receptor Fas (F) by flow cytometry on stem and non-stem tumor populations. Stem-like populations in FSA4 were defined as ALDHbright whereas non-stem were defined as ALDHdim. Statistical significance was determined using a one-way ANOVA with multiple comparison test or Tukey post-test. *p < 0.05; **p < 0.01, ***p < 0.001.
We next assessed if the expression of these stress markers was upregulated on both stem-like and non-stem populations of tumor cells. The cell lines U87MG, PANC-1 and A673 were irradiated then incubated 24 h before performing flow cytometry. Analysis of total populations of these cell lines after RT revealed significant (p < 0.05) upregulation of the NK cell activating ligands MICA/B and the death receptor DR5 (Fig. S2). Importantly, the greatest increase in the expression of these death markers was found on the stem-like population rather than the non-stem population (Fig. 2D–F). Irradiated cell suspension of the freshly resected FSA4 sarcoma specimen also showed a significant increase in MFI of MICA/B (Fig. 2G) and Fas (Fig. 2H) with increasing doses of RT. Again, the stem-like tumor population showed the greatest upregulation of these markers. These data indicate that while stem-like tumor populations are less susceptible to irradiation-induced death, these cells display higher levels of stress markers after irradiation which may make them more susceptible to NK cell immunotherapy.
Human cancer patients show an increase in CSCs and NKG2D ligands following radiation therapy
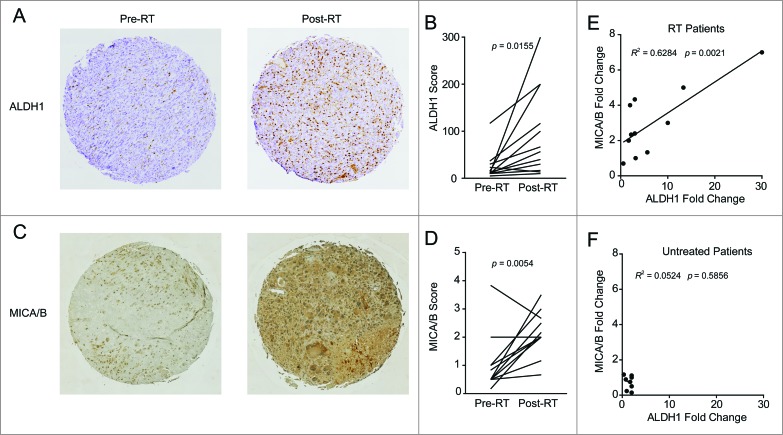
Given the enrichment for stem-like cells and upregulation of NKG2D ligands observed after irradiation in our diverse pre-clinical models, we assessed these parameters in a cohort of soft tissue sarcoma patients receiving neoadjuvant RT. Analysis of a tissue microarray (TMA) from 12 patients with matched pre- and post-RT samples revealed a statistically significant increase in immunohistochemical expression of the ALDH1 stem-like marker (Fig. 3A, B; p = 0.0155). Similarly, MICA/B staining intensity also increased following RT in these patients (Fig. 3C, D; p = 0.0054). Furthermore, there was a strong correlation between increased expression of ALDH1 following RT and increased expression of MICA/B after RT (Fig. 3E; R2 = 0.6284, p = 0.0021). In contrast, a control TMA was prepared from a cohort of sarcoma patients who did not receive neoadjuvant treatment (Fig. 3F). These patients showed no significant differences in MICA/B or ALDH1 expression between matched diagnostic biopsy and tumor tissue collected at the time of surgical resection. These clinical data provide compelling evidence that, similar to our pre-clinical studies including patient-derived specimens, patients treated with RT demonstrated a local enrichment for stem-like cells with simultaneous upregulation of NKG2D ligands.
Figure 3.
Patients treated with radiation therapy show enrichment for CSCs and NKG2D ligands. A cohort of 12 soft tissue sarcoma patients was biopsied before the start of neoadjuvant radiation therapy (RT) and compared with samples taken at the time of surgical resection. Alternatively, a separate cohort of eight patients who did not receive neoadjuvant treatment was assessed as a negative control. Tissue microarrays (TMA) were prepared from all patients and stained by IHC for ALDH1 and MICA/B. (A) Representative TMA punches pre- and post-RT showing changes in ALDH1 staining. (B) Changes in ALDH1 expression score as determined by a blinded clinical pathologist. ALDH1 staining intensity was scored from 0 to 4 and multiplied by the percentage of cells staining positive. (C) Representative TMA punches pre- and post-RT showing changes in MICA/B staining. (D) Changes in MICA/B expression score as determined by a blinded clinical pathologist scores (M.C.). MICA/B was scored based on staining intensity alone (from 0 to 4). (E, F) Fold change in ALDH1 scores and MICA/B scores was correlated among the RT treated cohort (E) or the untreated cohort (F). Statistics in B and D were performed by two-tailed Student's paired t-test.
Radiation enhances NK cell cytotoxicity toward CSCs
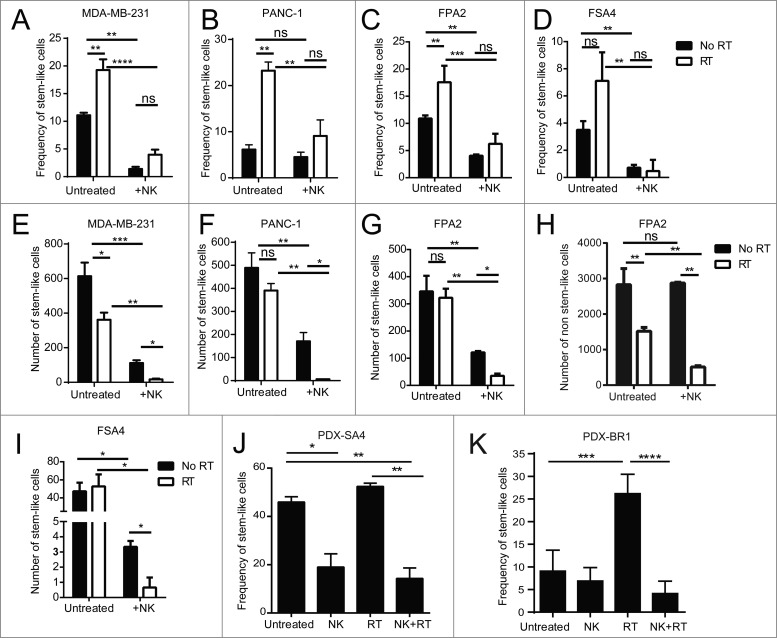
Based on the enrichment of cells with a CSC phenotype and the preferential induction of NK cell activation ligands on these cells following RT, we hypothesized that NK cells might be especially effective at targeting CSCs following RT. Tumor samples were irradiated with 8 Gy γ-irradiation then cultured for 24 h before being mixed with activated allogeneic NK cells for an additional 24 h before being analyzed. As shown in Fig. 4A–I, in agreement with our previous data, irradiated tumor cell lines (MDA-MB-231, A; and PANC-1, B) and fresh primary tumor specimens (FPA2, C; and FSA4, D) showed an increase in the percentage of stem-like cells. This dose of 8 Gy generally had a small effect on reducing the overall numbers of stem-like tumor cells (Fig. 4E–I), consistent with our previous observations. For the cell lines MDA-MB-231 and PANC-1, co-culture with NK cells (1:1 ratio) for 24 h without RT reduced both the percentage (Fig. 4A, B) and numbers (Fig. 4E, F) of remaining stem-like tumor cells. Similarly, NK co-culture (0.125:1 NK:tumor ratio) of cell suspensions from primary tumors, FPA2 and FSA4, significantly reduced (p < 0.01) the percentage (Fig. 4C, D) and numbers (Fig. 4G, I) of stem-like cells. When analyzing the absolute numbers of non-CSCs following the indicated treatments (Fig. 4H), we observed that the non-CSCs decreased after RT consistent with the known debulking effects of this cytotoxic treatment modality. However, NK cells by themselves (without RT) had minimal effects on the absolute numbers of non-CSCs, and the relative decrease in non-CSCs following combined RT and NK co-culture was less than the decreases observed in the CSC populations with the RT/NK combination, consistent with preferential NK killing of CSCs, especially after RT. In addition, the combination of NK cells and RT offset the enrichment for stem-like cell frequencies (Fig. 4A–D) seen with RT alone and allowed for superior targeting of stem-like tumor cells. This combination led to a near eradication of all stem-like tumor cells (Fig. 4F–I). Taken together, these results suggest that RT not only suppresses the non-CSC population, but also sensitizes CSCs to NK attack.
Figure 4.
Irradiation sensitizes CSCs to NK cell attack in vitro and in vivo. (A, B) CSC frequency of indicated tumor cell lines, as assessed by ALDH activity, following 24 h incubation under the indicated treatment conditions. (C, D) CSC frequency of indicated primary tumor sample as assessed by flow cytometric analysis of CD24+/CD44+/ALDH bright (C) or ALDH bright (D) remaining following 24 h incubation under the indicated treatment conditions. (E–I) Numbers of CSCs remaining from experiments described in C and D. (H) Numbers of non-CSCs in primary pancreatic tumor sample, FPA2, after RT and with/without NK co-culture. (J) Frequency of CSCs (ALDH bright) remaining from patient-derived xenografts of the leiomyosarcoma, PDX-SA4, as assessed using flow cytometry, 5 d after the indicated treatments in vivo. (K) expression of CSCs as assessed by flow cytometric analysis of ALDH expression or CD24−CD44+ expression on patient-derived xenografts of the mammary carcinoma, PDX-BR1, 5 d after the indicated treatments in vivo. Statistical significance was determined by one-way ANOVA with a Bonferroni post-test where *p ≤ 0.05; **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001.
We then assessed if activated NK cells would similarly be able to target human CSCs in vivo in our patient-derived xenograft (PDX) models. Mice with 2nd or 3rd generation PDX tumors from leiomyosarcoma (PDX-SA4) or breast cancer (PDX-BR1) were treated with a single fraction of 8 Gy local RT and then injected with NK cells directly into the tumor site. Mice were sacrificed 6 d after NK cell transfer, and dissociated tumors were assessed for stem-like cell frequencies (Fig. 4J, K). In support of the concept that NK cells could target stem-like cells in vivo following local RT, we observed that the frequency of stem-like cells was significantly reduced in vivo after combination RT and NK therapy in the sarcoma PDX (p < 0.01, Fig. 4J) and breast PDX (p < 0.0001, Fig. 4K) when compared with mice receiving RT alone. These data suggest that RT enriches for CSCs in vivo and sensitizes CSCs to the cytotoxic effects of exogenously transferred NK cells.
NK cell transfer improves the antitumor effects of radiation therapy
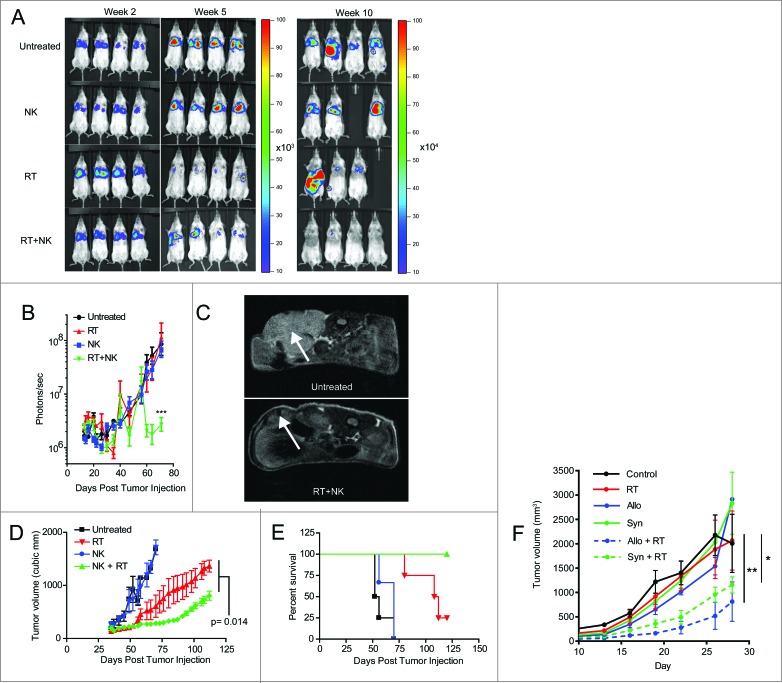
Given the synergistic effects of combination RT and NK therapy on CSC sensitization and elimination, our next objective was to assess the therapeutic effects of this combination in xenograft models of transplanted human tumors. First, we injected PANC-1 cells, transfected with luciferase, intravenously into NSG mice, establishing xenograft models of metastatic pancreatic cancer. Mice then received local irradiation (8 Gy) to the lungs after lung metastases were established. After intravenous injection of hydrodynamic IL-15 to maintain in vivo activation, 2 × 107 NK cells were injected intravenously. Tumor burden was then assessed by bioluminescent imaging (Fig. 5A) 2–3 times per week. As depicted in Fig. 5A–B, in this model and treatment schema, administration of NK cells alone failed to show any significant antitumor effects. Although mice treated with RT monotherapy showed an initial decrease in luciferase intensity by day 35 (Fig. 5A, B), RT-treated tumors subsequently relapsed, leading to bioluminescent intensity comparable to controls at later time points. In contrast, mice treated with the combination of activated NK cells and RT showed tumor intensities more than 50-fold lower than mice treated with RT or NK alone (Fig. 5B, p < 0.001).
Figure 5.
NK cells provide superior antitumor effects when combined with radiotherapy. (A) Representative bioluminescence images showing tumor burden at weeks 2, 7, and 10 after injection of luciferase transfected PANC-1 tumors. (B) Quantification of captured tumor burden as assessed in A. (C) Representative axial MRI images of mice bearing established orthotopic PANC-1 tumors assessed 14 d after NK cell infusion. Tumors are indicated with white arrows. (D) Tumor growth of subcutaneous PANC-1 xenograft tumors from indicated treatment groups as assessed by external caliper. (E) Kaplan–Meier curve displaying survival fraction of mice from experiments described in C. (F) Immune-competent BALB/c mice were injected subcutaneously with RENCA mouse renal carcinoma cells, administered local RT, then treated with 2 × 107 activated C57BL/6 (allogeneic) or BALB/c (syngeneic) NK cells. Mice were monitored for tumor growth. All survival experiments were performed at least twice with four mice per group. Statistics were determined by two-way ANOVA with Bonferroni post-test or by Log-rank test where appropriate. *p < 0.05; **p < 0.01, ***p ≤ 0.001.
We then evaluated an orthotopic model of pancreatic cancer using mice surgically implanted with PANC-1 tumors in the distal pancreas. After MRI confirmation of xenograft formation, we treated the tumor-bearing mice with combination radio-immunotherapy. Mice received 8 Gy local RT, followed by 2×107 NK cells (i.v.). Twenty days after treatment, we imaged the mice using a small animal MRI to assess tumor burden. As depicted in Fig. 5C, mice treated with the combination of RT and NK immunotherapy showed significant antitumor responses in this orthotopic model.
We next examined RT and NK combination therapy in a subcutaneous PANC-1 xenograft model (Fig. 5D, E). Following a single intra-lesional injection of 2 × 107 NK cells, subcutaneous PANC-1 tumors again showed no significant tumor regression from NK cell treatment alone. In contrast, RT monotherapy again provided a modest decrease in tumor growth (Fig. 5D), while combination radio-immunotherapy was statistically superior to all other treatments, including RT alone (p = 0.014). Furthermore, all mice treated with combined RT and NK transfer survived > 120 d (Fig. 5D). Similarly, studies of radio-immunotherapy in immune-competent tumor-bearing Balb/c mice treated with syngeneic or allogeneic murine NK cells showed significant antitumor effects when NK cells were combined with RT (Fig. 5F), suggesting that the adoptive transfer of NK cells post RT remains beneficial in the setting of an immune competent host with a normal contingent of NK cells. Taken together, these data provide strong pre-clinical evidence that combination therapy, using local RT followed by infusions of ex vivo activated NK cells, imparts antitumor effects in both local and metastatic models of solid cancers.
Discussion
Despite profound advances in current cancer therapy, the inability to control and eradicate disseminated disease remains a central challenge, and overall, only modest progress has been observed with the use of empiric chemotherapy and other cytotoxic therapies for solid tumors. The CSC hypothesis postulates that a sub-population of quiescent cells exist within tumors that are resistant to conventional cytotoxic/anti-proliferative therapies. It is these CSCs that then seed tumor relapse, even in cases of apparent complete response to systemic therapy. Therefore, therapies that add a specific anti-CSC strategy to standard cytoreductive therapies may provide a greater and more durable therapeutic effect. The American Society of Clinical Oncology recognizes targeting of CSCs as an unmet need in the future of clinical oncology.29
Our results suggest that the ability of NK cells to target CSCs in multiple experimental models of solid cancers is significantly enhanced by pretreatment with RT. While NK cells have previously been shown to have the capacity to kill cancer cells with a stem like phenotype,30 our studies, which utilize relatively un-manipulated primary cell lines and primary tumor samples, may provide a better model for the translation of preclinical responses into clinically relevant therapeutic outcomes. In fact, a clear strength of this work is the extensive use of primary patient specimens to demonstrate differences in stem-like markers as well as expression of NK activation ligands after RT. This highlights the translational relevance of these studies, particularly with respect to the immune targeting of CSCs.
Although adoptive NK cell immunotherapy has demonstrated success in the treatment of hematologic malignancies, it has been less effective in the treatment of advanced solid tumors as monotherapy.31,32 In this study, NK cells were effective at targeting CSCs from a variety of solid tumor types, especially when combined with RT. The combination of NK cells with RT enhanced NK recognition of CSCs through two mechanisms. First, RT showed potent and direct killing of tumors, with non-CSCs being more sensitive to these effects. As a result, RT led to direct enrichment of CSC populations. Second, irradiation preferentially increased the levels of NK activating receptor ligands and death receptors which could lead to the death of CSCs through both granule and death ligand-mediated (Fas and TRAIL) cytolysis. However, in contrast to the in vitro results, our in vivo models showed little efficacy when NK cells were applied as a monotherapy. A hurdle NK cells must overcome in therapeutic models is migration to the tumor site. Irradiation-induced damage may be important in recruiting NK cells to the tumor site and providing local activation signals.
While this preclinical data provides promising insights into a combined radio-immunotherapy approach targeting CSCs and non-CSCs, several factors require consideration for the advancement of this therapeutic strategy. A key priority for future studies utilizing this approach will be to determine the optimal treatment window after RT in which NK cell transfer would be most efficacious. The enrichment for CSCs and upregulation of stress ligands after RT may be transient, suggesting that NK cells should be infused shortly thereafter, and a one week interval between completion of RT and NK transfer may not be optimal. Further studies are needed to clarify this question. Similarly, it is unclear whether CSCs develop resistance to NK cells, especially if repeated NK transfer is used to maximize therapeutic effects. Recent reports suggest that primary breast CSCs, exhibiting high levels of ALDH activity, were capable of evading NK cell-mediated cytotoxicity through epigenetic downregulation of NKG2D ligand expression.33 However, this study by Wang et al., does not evaluate the impact of local RT which we show significantly increases the expression of these ligands in vitro, ex vivo, and in patient matched clinical samples and may reduce or prevent NKG2D downregulation as a mechanism of NK evasion. Therefore, further studies are also needed to address how repeated administrations of either NK cells, or RT, may result in shedding or downregulation of NKG2D ligands. It is also unclear if multiple infusions of NK cells would still show synergy with RT given the potential normalization of death ligands over time.
Despite these considerations, we are able to show with extensive use of primary clinical specimens that the use of allogenic NK cell infusion in a radio-immunotherapy approach can be used in a combination strategy against multiple solid tumors to better target residual CSCs after initial debulking of non-CSCs. If, as we hypothesize, the CSC population can be enriched and sensitized prior to the application of NK therapy, then a multi-modality approach using NK cells, RT and possibly other cytotoxic treatments may simultaneously eradicate CSCs and non-CSCs leading to complete and durable tumor eradication. This combination approach may translate to long-term meaningful clinical benefits in various solid cancers.
Materials and Methods
Tumor models
NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) mice were given tumors through intravenous, subcutaneous or intrapancreatic injections. For intravenous tumors, NSG mice received 2.5 × 105 luciferase transfected PANC-1 cells in 0.5 mL PBS. For subcutaneous models, mice received 106 PANC-1 in 100 uL PBS in the rear flank. For the intrapancreatic model, mice underwent an upper laparotomy and received 5 × 105 PANC-1 cells in 50 μL of 1:1 Matrigel and PBS. For PDX models, NSG mice were implanted with 2 mm tumor cubes subcutaneously using a trocar. For the RENCA tumor model, BALB/c mice received 1 × 106 RENCA cells subcutaneously in the rear flank and 14 d later mice were irradiated locally at 8 Gy. Three days after RT, mice received syngeneic or allogeneic rhIL-2 activated NK cells. Experiments were performed twice with four mice per group. All experimental protocols were approved by the UC Davis Institutional Animal Care and Use Committee.
Tumor cell lines and primary specimens
Human tumor cell lines MDA-MB-231, U87MG, A673, and PANC-1 and the mouse tumor cell line RENCA were purchased from ATCC (Manassas, VA) and propagated in the culture medium recommended by ATCC. Fresh primary tumor specimens (FSA2, FSA4, and FPA2) were obtained following either surgical resection or biopsy, from the UC Davis Comprehensive Cancer Center Biorepository. Tumor samples were minced and incubated for 60 min with 1 mg/mL collagenase-IV and 0.1 mg/mL DNAse I at 37°C. Following incubation, tissue was mechanically dissociated and filtered into cell culture media supplemented with 20% FBS. FPA2, was dissociated as stated above, and expanded in culture 4–6 times in order to generate appropriate cell yield (short passaged). Patient consent was obtained for all samples in accordance with the UC Davis Institutional Review Board. PDX-BR1 (BR-0744) fragments were purchased from the Jackson Laboratory and immediately thawed upon arrival for implantation into NSG mice.
NK cell isolation
Human NK cells were isolated from fresh peripheral blood obtained from healthy donors (Delta Blood Bank, Stockton, CA). Leukocyte reduction filters were back-flushed with sterile PBS and lymphocytes were subsequently isolated using Lymphocyte Separation Medium (Cellgro, Manassas, VA) as per manufacturer's instructions. Lymphocytes were then resuspended at 5 × 107 cells/mL with RBCs added back at a 1000:1 ratio per lymphocyte and coincubated with RosetteSep™ Human NK Cell Enrichment Cocktail (STEMCELL Technologies, Vancouver, BC, Canada) as per manufacturer's instructions. This protocol typically yielded >95% CD45+/CD56+/CD3− cells.
NK cell activation and expansion
NK cells were expanded via co-culture with irradiated (100 Gy) EBV-SMI-LCL cells at a ratio of 20 lymphoma cells per NK cell. The EBV-transformed lymphoma cell line EBV-SMI-LCL was provided by Dr Richard Childs (NCI). NK cells were grown in X-VIVO 20 (Lonza, Basel, Switzerland) with 10% human male AB serum (Valley Biomedical, Winchester, VA) containing 500 IU/mL recombinant human IL-2 (Biological Resources Branch, NCI, Frederick, MD) in upright T-75 flasks each. Mouse-activated NK cells were prepared from C57BL/6 or BALB/c donor mice as previously described.34
Twenty-four-hour killing assay
Indicated tumor cells were plated in triplicate with varying ratios of NK cells in 96-well plates for 24 h. All wells contained 50% human NK cell medium and 50% tumor cell specific medium. Following 24 h co-culture, plates were washed and stained for flow cytometry. For all in vitro RT experiments, cells were first treated with RT, then washed and allowed to rest for 24 h. After 24 h, cells were recounted and plated with NK cells at the indicated effector to target ratio. Tumor expression of remaining CSCs was established based on analysis of SSChi CD45− 7AAD− populations in order to exclude NK cells.
Flow cytometry
All samples were acquired on an LSR Fortessa™ with a high throughput sampler (BD Biosciences, San Jose, CA) and analyzed with FlowJo software (TreeStar, Ashland, OR). Pacific Blue anti-CD45 (clone HI30) and 7-AAD were purchased from BD Biosciences (San Jose, CA). Alexa Fluor® 700 CD44 (clone IM7), PE-Cy7 CD24 (clone ML5), biotin anti-MICA/B (clone 6D4) and biotin anti-Fas (clone DX2) were purchased from BioLegend (San Diego, CA). PE anti-DR5 (DJR2–4) was purchased from eBioscience (San Diego, CA). Aldehyde dehydrogenase expression was detected using the ALDEFLUOR™ assay system (STEMCELL Technologies, Vancouver, BC, Canada) according to manufacturer's instructions. For each assay a discrete DEAB control was established, as well as for each individual E:T ratio. ALDH was gated as five times the MFI of the paired DEAB containing sample in order to standardize for the leakage of ALDH signal from samples over time (Fig. S1).
qRT-PCR
RNeasy Mini kits (Qiagen) were utilized for the extraction of total RNA from primary cells and cell lines. Extraced RNA was reverse transcribed to cDNA using the RNA to cDNA kit (Applied Biosystems). Gene specific primers were obtained from Integrated DNA Technologies. Quatitative real-time PCR was performed using the RT2 SYBR Green Mastermix (Qiagen) using the StepOnePlus™ Real-Time PCR system (Applied Biosystems).
Hydrodynamic IL-15 plasmid
As a method to sustain infused NK cells in vivo, we injected an IL-15-producing plasmid as previously described. 35 IL-15 expression is driven via the CMV promoter and HEF-1 enhancer. Briefly, 10 μg of plasmid were injected into mice via intravenous hydrodynamic delivery, 24 h prior to NK cell infusions.
Local irradiation
Mice received 8 Gy of electron irradiation with an Elekta Synergy (Stockholm, Sweden) linear accelerator. Radiation doses were directed with a square 3 × 3 cm Cerrobend attenuator for the lungs or upper abdomen or a circular 2 cm diameter attenuator for subcutaneous flank tumors. Mice were anesthetized prior to RT with 1.2% tribromoethanol. The AP:PA diameter of the mice was determined using calipers. RT was prescribed to midplane and treatment dosing was calculated to cover the entire thoracic cavity with at least 90% of the prescription dose, 8 Gy. The prescription and entire RT treatment protocol was developed by a board certified medical physicist and a board certified radiation oncologist (A.M.M) using multi-collimation. Anesthetized mice were placed in the prone position on 5 cm solid water block at laser isocenter and the treatment area was covered with 0.5 cm bolus to ensure adequate dose build up.
In vivo imaging
Tumor burden of mice bearing metastatic, firefly luciferase transfected pancreatic tumors was assessed and recorded using an IVIS-Spectrum imaging system (Caliper Life Sciences, Hopkinton, MA) every 3–4 d Animals were anesthetized by inhaled isofluorene, and injected with 3 mg D-luciferin, prior to bioluminescent image capture. Bioluminescence data were analyzed using Living Image 3.0 (Caliper Life Sciences). Alternatively, mice were imaged by T2-weighted MRI on a Biospec 7T (Bruker, Billerica, MA) in collaboration with the UC Davis Center for Molecular and Genomic Imaging.
Evaluation of archived clinical sarcoma samples
TMA were constructed using formalin-fixed, paraffin-embedded sarcoma specimens obtained from the UC Davis Cancer Center Biorepository Core Facility. Twelve patients received RT for locally advanced extremity STS. Eight STS patients who were treated with primary surgical resection (without neoadjuvant RT) were used as controls. IRB approval for this retrospective analysis was obtained from the Institutional Review Board of UC Davis. TMA sections (4 μm) were stained and scored for ALDH by a blinded pathologist (M.C.) as described previously.12 Stained slides were scored in a comparable fashion for percentage and intensity of MICA/B -positive cells.
Statistical Considerations
Categorical variables were compared using a chi-squared test. Parametric continuous variables were compared using an independent samples t-test. Non-parametric continuous variables were compared using the Mann–Whitney U test. For comparison of more than two groups, statistical significance was determined using a one-way ANOVA followed by a Bonferroni multiple-group comparison test. ALDH and MICA/B scores before and after treatment were analyzed using the two-sided paired t-test. Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC) and Graph-Pad Prism 5. Significance was set at p < 0.05.
Acknowledgments
The authors would like to acknowledge Weihong Ma and Monja Metcalf for technical support and Douglas J Rowland from the UC Davis Center for Molecular and Genomic Imaging for assistance with bioluminescent and MRI imaging. We thank Julian R Perks and Shuaib Juma from the University of California, Davis, Comprehensive Cancer Center, Department of Radiation Oncology, for assistance in performing local RT dose calculations and in vivo dose delivery. We also thank Regina Gandour-Edwards, Irmgard Feldman, and Ramona Clarke from the Biorepository Core Facility at the University of California, Davis, Comprehensive Cancer Center, for support with the acquisition and evaluation of human specimens.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the National Institutes of Health (R01-HL089905, UC Davis Paul Calabresi K12 Career Development Award NIH 1K12CA138464-01A2, RJ Canter), the Sarcoma Foundation of America (RJ Canter), and the University of California Coordinating Committee for Cancer Control (RJ Canter).
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006; 444: 756-60; PMID:17051156; http://dx.doi.org/ 10.1038/nature05236 [DOI] [PubMed] [Google Scholar]
- 2.Bensimon J, Altmeyer-Morel S, Benjelloun H, Chevillard S, Lebeau J. CD24(−/low) stem-like breast cancer marker defines the radiation-resistant cells involved in memorization and transmission of radiation-induced genomic instability. Oncogene 2013; 32:251-8; PMID:22330142; http://dx.doi.org/ 10.1038/onc.2012.31 [DOI] [PubMed] [Google Scholar]
- 3.Kim MP, Fleming JB, Wang H, Abbruzzese JL, Choi W, Kopetz S, McConkey DJ, Evans DB, Gallick GE. ALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinoma. PloS One 2011; 6:e20636; PMID:21695188; http://dx.doi.org/ 10.1371/journal.pone.0020636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasheed Z, Wang Q, Matsui W. Isolation of stem cells from human pancreatic cancer xenografts. J Vis Exp 2010; 43:pii: 2169; PMID:20972397; http://dx.doi.org/ 10.3791/2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Awad O, Yustein JT, Shah P, Gul N, Katuri V, O'Neill A, Kong Y, Brown ML, Toretsky JA, Loeb DM. High ALDH activity identifies chemotherapy-resistant Ewing's sarcoma stem cells that retain sensitivity to EWS-FLI1 inhibition. PloS One 2010; 5:e13943; PMID:21085683; http://dx.doi.org/ 10.1371/journal.pone.0013943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohberger B, Rinner B, Stuendl N, Absenger M, Liegl-Atzwanger B, Walzer SM, Windhager R, Leithner A. Aldehyde dehydrogenase 1, a potential marker for cancer stem cells in human sarcoma. PloS One 2012; 7:e43664; PMID:22928012; http://dx.doi.org/ 10.1371/journal.pone.0043664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honoki K, Fujii H, Kubo A, Kido A, Mori T, Tanaka Y, Tsujiuchi T. Possible involvement of stem-like populations with elevated ALDH1 in sarcomas for chemotherapeutic drug resistance. Oncol Rep 2010; 24: 501-5; PMID:20596639; http://dx.doi.org/ 10.3892/or_00000885 [DOI] [PubMed] [Google Scholar]
- 8.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S et al.. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007; 1:555-67; PMID:18371393; http://dx.doi.org/ 10.1016/j.stem.2007.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcato P, Dean CA, Pan D, Araslanova R, Gillis M, Joshi M, Helyer L, Pan L, Leidal A, Gujar S et al.. Aldehyde dehydrogenase activity of breast cancer stem cells is primarily due to isoform ALDH1A3 and its expression is predictive of metastasis. Stem Cells 2011; 29:32-45; PMID:21280157; http://dx.doi.org/ 10.1002/stem.563 [DOI] [PubMed] [Google Scholar]
- 10.Croker AK, Goodale D, Chu J, Postenka C, Hedley BD, Hess DA, Allan AL. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J Cell Mol Med 2009; 13:2236-52; PMID:18681906; http://dx.doi.org/ 10.1111/j.1582-4934.2008.00455.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh SKHawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature 2004; 432:396-401; PMID:15549107; http://dx.doi.org/ 10.1038/nature03128 [DOI] [PubMed] [Google Scholar]
- 12.Canter RJ, Ames E, Mac S, Grossenbacher SK, Chen M, Li CS, Borys D, Smith RC, Tellez J, Sayers TJ et al.. Anti-proliferative but not anti-angiogenic tyrosine kinase inhibitors enrich for cancer stem cells in soft tissue sarcoma. BMC Cancer 2014; 14:756; PMID:25301268; http://dx.doi.org/ 10.1186/1471-2407-14-756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang WJ, Wu SP, Liu JB, Shi YS, Huang X, Zhang QB, Yao KT. MYC Regulation of CHK1 and CHK2 Promotes Radioresistance in a Stem Cell-like Population of Nasopharyngeal Carcinoma Cells. Cancer Res 2013; 73(3):1219-31; PMID:23269272; http://dx.doi.org/ 10.1158/0008-5472 [DOI] [PubMed] [Google Scholar]
- 14.Burger H, van Tol H, Boersma AW, Brok M, Wiemer EA, Stoter G, Nooter K. Imatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pump. Blood 2004; 104:2940-42; PMID:15251980; http://dx.doi.org/ 10.1182/blood-2004-04-1398 [DOI] [PubMed] [Google Scholar]
- 15.Barcellos-Hoff MH, Park C., & Wright E.G.. Radiation and the microenvironment - tumorigenesis and therapy. Nat Rev. Cancer 2005; 5:867-75; PMID:16327765; http://dx.doi.org/ 10.1038/nrc1735 [DOI] [PubMed] [Google Scholar]
- 16.Kalbasi A, June CH, Haas N, Vapiwala N. Radiation and immunotherapy: a synergistic combination. J Clin Investig 2013; 123:2756-63; PMID:23863633; http://dx.doi.org/ 10.1172/JCI69219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E et al.. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012; 366:925-31; PMID:22397654; http://dx.doi.org/ 10.1056/NEJMoa1112824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hallett WH, Murphy WJ. Positive and negative regulation of Natural Killer cells: therapeutic implications. Semin Cancer Biol 2006; 16:367-82; PMID:16934486; http://dx.doi.org/ 10.1016/j.semcancer.2006.07.003 [DOI] [PubMed] [Google Scholar]
- 19.Perez-Cunningham J, Ames E, Smith RC, Peter AK, Naidu R, Nolta JA, Murphy WJ. Natural killer cell subsets differentially reject embryonic stem cells based on licensing. Transplantation 2014; 97:992-8; PMID:24704665; http://dx.doi.org/ 10.1097/TP.0000000000000063 [DOI] [PubMed] [Google Scholar]
- 20.Ames E, Murphy WJ. Advantages and clinical applications of natural killer cells in cancer immunotherapy. Cancer Immunol Immunother 2014; 63:21-8; PMID:23989217; http://dx.doi.org/ 10.1007/s00262-013-1469-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol 2008; 9:503-10; PMID:18425107; http://dx.doi.org/ 10.1038/ni1582 [DOI] [PubMed] [Google Scholar]
- 22.Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol 2011; 89:216-24; PMID:20567250; http://dx.doi.org/ 10.1038/icb.2010.78 [DOI] [PubMed] [Google Scholar]
- 23.Xu Q, Liu G, Yuan X, Xu M, Wang H, Ji J, Konda B, Black KL, Yu JS. Antigen-specific T-cell response from dendritic cell vaccination using cancer stem-like cell-associated antigens. Stem Cells 2009; 27:1734-40; PMID:19536809; http://dx.doi.org/ 10.1002/stem.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Tomaso T, Mazzoleni S, Wang E, Sovena G, Clavenna D, Franzin A, Mortini P, Ferrone S, Doglioni C, Marincola FM et al.. Immunobiological characterization of cancer stem cells isolated from glioblastoma patients. Clin Cancer Res 2010; 16:800-13; PMID:20103663; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao T, Kaufmann AM, Qian X, Sangvatanakul V, Chen C, Kube T, Zhang G, Albers AE. Susceptibility to cytotoxic T cell lysis of cancer stem cells derived from cervical and head and neck tumor cell lines. J Cancer Res Clin Oncol 2013; 139:159-170; PMID:23001491; http://dx.doi.org/ 10.1007/s00432-012-1311-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishizawa K, Rasheed ZA, Karisch R, Wang Q, Kowalski J, Susky E, Pereira K, Karamboulas C, Moghal N, Rajeshkumar NV et al.. Tumor-initiating cells are rare in many human tumors. Cell Stem Cell 2010; 7:279-82; PMID:20804964; http://dx.doi.org/ 10.1016/j.stem.2010.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang-Verslues WW, Kuo WH, Chang PH, Pan CC, Wang HH, Tsai ST, Jeng YM, Shew JY, Kung JT, Chen CH et al.. Multiple lineages of human breast cancer stem/progenitor cells identified by profiling with stem cell markers. PloS One 2009; 4:e8377; PMID:20027313; http://dx.doi.org/ 10.1371/journal.pone.0008377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003; 100:3983-88; PMID:12629218; http://dx.doi.org/ 10.1073/pnas.0530291100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masters GA, Krilov L, Bailey HH, Brose MS, Burstein H, Diller LR, Dizon DS, Fine HA, Kalemkerian GP, Moasser M et al.. Clinical Cancer Advances 2015: Annual Report on Progress Against Cancer From the American Society of Clinical Oncology. J Clin Oncol 2015; 33(7):786-809; PMID:25605863; http://dx.doi.org/ 10.1200/JCO.2014.59.9746 [DOI] [PubMed] [Google Scholar]
- 30.Tallerico R, Todaro M, Di Franco S, Maccalli C, Garofalo C, Sottile R, Palmieri C, Tirinato L, Pangigadde PN, La Rocca R et al.. Human NK cells selective targeting of colon cancer-initiating cells: a role for natural cytotoxicity receptors and MHC class I molecules. J Immunol 2013; 190:2381-90; PMID:23345327; http://dx.doi.org/ 10.4049/jimmunol.1201542 [DOI] [PubMed] [Google Scholar]
- 31.Farag SS, Fehniger TA, Ruggeri L, Velardi A, Caligiuri MA. Natural killer cell receptors: new biology and insights into the graft-versus-leukemia effect. Blood 2002; 100:1935-47; PMID:12200350; http://dx.doi.org/ 10.1182/blood-2002-02-0350 [DOI] [PubMed] [Google Scholar]
- 32.Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol 2013; 10:230-52; PMID:23604045; http://dx.doi.org/ 10.1038/cmi.2013.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang B, Wang Q, Wang Z, Jiang J, Yu SC, Ping YF, Yang J, Xu SL, Ye XZ, Xu C et al.. Metastatic consequences of immune escape from NK cell cytotoxicity by human breast cancer stem cells. Cancer Res 2014; 74:5746-57; PMID:25164008; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-2563 [DOI] [PubMed] [Google Scholar]
- 34.Sungur CM, Tang-Feldman YJ, Zamora AE, Alvarez M, Pomeroy C, Murphy WJ. Murine NK-cell licensing is reflective of donor MHC-I following allogeneic hematopoietic stem cell transplantation in murine cytomegalovirus responses. Blood 2013; 122:1518-21; PMID:23818546; http://dx.doi.org/ 10.1182/blood-2013-02-483503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barao I, Alvarez M, Redelman D, Weiss JM, Ortaldo JR, Wiltrout RH, Murphy WJ. Hydrodynamic delivery of human IL-15 cDNA increases murine natural killer cell recovery after syngeneic bone marrow transplantation. Biol Blood Marrow Transplant 2011; 17:1754-64; PMID:21906575; http://dx.doi.org/ 10.1016/j.bbmt.2011.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.