Abstract
A phase II clinical trial for head and neck squamous cell cancer (HNSCC) patients using multiple peptides vaccine derived from tumor-associated antigens (TAAs) was performed. The therapy was well tolerated and overall survival was statistically significantly longer in vaccinated patients, and the patients exhibiting cytotoxic T lymphocytes (CTL) responses to multiple peptides exhibited better prognosis.
Keywords: cancer vaccine therapy, cytotoxic T lymphocytes, head and neck squamous cell cancer, multiple peptides vaccine, phase II clinical trial, tumor-associated antigens
Introduction
In recent years, chemoradiotherapy for the locoregional advanced HNSCC has become the standard care. The treatment of HNSCC has shifted from primary surgery to organ preservation using combination chemoradiotherapy. The current approach attempts to achieve both organ preservation and function.1 Despite the use of aggressive treatment modalities, such as surgical tumor resection with radical neck dissection and chemoradiotherapy, the maintenance of long-term disease control of advanced HNSCC remains difficult. Some chemoradiotherapy regimens have a higher treatment effect; however, the 5-year survival rates have not been extended. Although, various drugs are used in combination with chemotherapy and intensity modulated radiation therapy, no molecular targeting agents against HNSCC have been developed, except for cetuximab. Therefore, the development of novel treatment modalities is eagerly awaited and immunotherapy is one of attractive candidates of treatments for HNSCC.
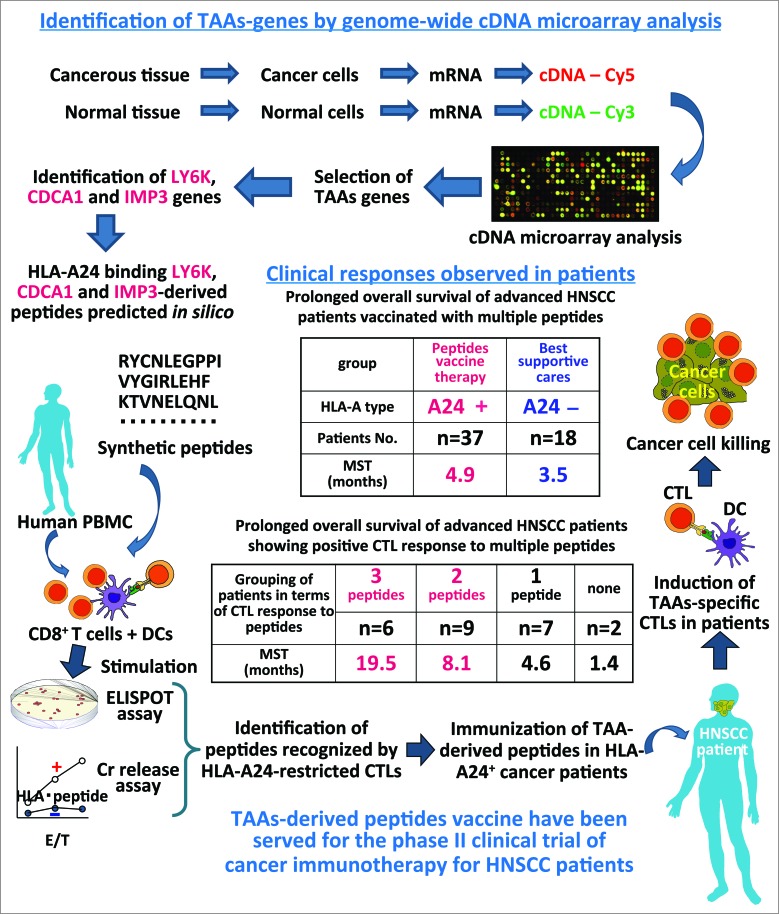
Nakamura et al. analyzed the gene expression profiles of various cancers and normal tissues using a genome-wide cDNA microarray analysis covering more than 27,000 genes. By using this technology, we have identified new TAAs which were not expressed in normal tissues except for testis and fetal organs. These studies have provided a number of ideal oncogenic TAAs and the expressions of TAAs were correlated with poor prognosis. As shown in Fig. 1, we have identified many HLA-A24 (A*24:02)-restricted CTLs epitopes that induced tumor reactive CTLs but not autoimmunity. LY6K, CDCA1 and IMP3 were TAAs frequently overexpressed in HNSCC cells but not in normal cells, so we thought these TAAs would be applicable to peptides-based vaccines for advanced HNSCC patients. We used LY6K 177–186 (RYCNLEGPPI), CDCA1 56–64 (VYGIRLEHF) and IMP3 508–516 (KTVNELQNL) peptides that can induce tumor-reactive and HLA-A24 (A*24:02)-restricted CTLs without stimulating autoimmunity. A phase II clinical cancer vaccine therapy trial using a combination of three peptides in HLA-A*24:02(+) patients with advanced esophageal squamous cell carcinoma was previously reported,2-6 and the positive results encouraged us to apply this therapy for HNSCC patients.
Figure 1.
Identification of TAAs-derived CTL epitopes and application to the clinical trials of cancer immunotherapy. The ideal TAAs genes were identified by using genome wide cDNA microarray analyses of cancer and normal tissues. Peripheral blood mononuclear cells (PBMCs) derived from healthy donors and cancer patients were used to investigate the immunogenicity of candidate TAAs-derived peptides. Then we started the phase II clinical trial using three TAAs-derived peptides vaccine for HNSCC patients. As a result, we observed prolonged OS of advanced HNSCC patients treated with peptides vaccine, and a better prolonged OS of vaccinated patients showing positive CTL response to multiple peptides.
We performed a phase II, open-label, non-randomized clinical cancer vaccine therapy.7 Vaccination with a mixture of three peptides derived from LY6K, CDCA1 and IMP3, and incomplete Freund's adjuvant (Montanide ISA51, SEPPIC) was performed in HNSCC patients (n = 37) with locally advanced, recurrent and/or metastatic tumors resistant to standard therapy. The background characteristics of the patients were not statistically different between the HLA-A*24:02-positive group (n = 37) treated with peptides vaccination and the HLA-A*24:02-negative group (n = 18) received best supportive cares. Among the 37 patients, one case was judged to have achieved a complete response for 37 months and nine cases were found to have stable disease for 3 months, according to the RECIST criteria. When the patients were classified into A24(+) and A24(−) groups, the overall survival (OS) of the A24(+) group was statistically significantly longer than that of the A24(−) group (4.9 vs. 3.5 months at mean survival time (MST), respectively, p <0.05, Fig. 1). The progression free survival (PFS) of the A24(+) group was not significantly better than that of the A24(−) group (1.9 vs. 1.0 months at MST, respectively, p = 0.13).
In the A24(+) group, in vitro cultured T cells were subjected to ELISPOT and HLA-A24-peptides multimer assays, and positive CTL responses specific to the LY6K-, CDCA1- and IMP3-derived peptides after vaccination were observed in 85.7%, 64.3% and 42.9% of the patients, respectively. When the OS was compared between the A24(+) patients in the CTL response-positive and -negative groups, the patients showing a CTL response specific to the LY6K peptide exhibited a significantly longer OS than those without a LY6K-specific CTL response. Similarly, the patients demonstrating a positive response specific to the CDCA1 peptide exhibited a significantly longer OS than those without a CTL response. The PFS of the patients with LY6K-, CDCA1- and IMP3-specific CTL responses tended to be longer than that of the patients without CTL responses. Interestingly, when the patients were divided into four groups according to the number of antigenic peptides to which they showed a positive CTL response, the OS was longer in the groups in which the patients demonstrated a positive CTL response to a larger number of peptides; the MST of the patients exhibiting CTL responses to three peptides was longer (19.5 months) than that observed in the other patient groups (Fig. 1). These observations indicate that the CTL responses induced by the peptides vaccination contributed to improvement of the prognosis of these patients.
In this phase II clinical trial, we suggested that cancer vaccine therapy using multiple TAAs-derived peptides inducing HLA-A24-restricted CTLs may provide a survival benefit in patients with advanced HNSCC. Furthermore, as demonstrated in the vaccinated patients group, specific CTL responses to multiple peptides may improve the OS in comparison to the patients who showed CTL response to no or only one peptide. Although treatment with TAAs vaccination has been shown to result in increased levels of circulating TAAs-specific T cells,8 we herein provided another evidence of a positive correlation between the larger extent of the peptides-specific CTL responses and a longer OS.
In conclusion, our clinical study supports the possibility that multiple TAAs-derived peptides vaccination-induced immune responses contribute to improve the prognosis of patients with advanced HNSCC. Recently, these TAAs-derived long peptides activating both tumor-specific T helper type 1 cells and CTLs were identified,9,10 we hope that these peptides vaccine therapy would provide an effective treatment for HNSCC patients.
Disclosure of Potential Conflicts of Interest
Y Nakamura is a stockholder and scientific advisor for OncoTherapy Science, Inc.
Funding
Y Nishimura is supported by a funding from OncoTherapy Science, Inc.
References
- 1.Brizel DM, Esclamado R. Concurrent chemoradiotherapy for locally advanced, nonmetastatic, squamous carcinoma of the head and neck: consensus, controversy, and conundrum. J Clin Oncol 2006; 24:2612-7; PMID:16763273; http://dx.doi.org/ 10.1200/JCO.2005.05.2829. [DOI] [PubMed] [Google Scholar]
- 2.Suda T, Tsunoda T, Daigo Y, Nakamura Y, Tahara H. Identification of human leukocyte antigen-A24-restricted epitope peptides derived from gene products upregulated in lung and esophageal cancers as novel targets for immunotherapy. Cancer Sci 2007; 98:1803-8; PMID:17784873; http://dx.doi.org/ 10.1111/j.1349-7006.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kono K, Mizukami Y, Daigo Y, Takano A, Masuda K, Yoshida K, Tsunoda T, Kawaguchi Y, Nakamura Y, Fujii H. Vaccination with multiple peptides derived from novel cancer-testis antigens can induce specific T-cell responses and clinical responses in advanced esophageal cancer. Cancer Sci 2009; 100:1502-9; PMID:19459850; http://dx.doi.org/ 10.1111/j.1349-7006.2009.01200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamabuki T, Daigo Y, Kato T, Hayama S, Tsunoda T, Miyamoto M, Ito T, Fujita M, Hosokawa M, Kondo S et al.. Genome-wide gene expression profile analysis of esophageal squamous cell carcinomas. Int J Oncol 2006; 28:1375-84; PMID:16685439; http://dx.doi.org/ 10.3892/ijo.28.6.1375 [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa N, Takano A, Yasui W, Inai K, Nishimura H, Ito H, Miyagi Y, Nakayama H, Fujita M, Hosokawa M et al.. Cancer-testis antigen lymphocyte antigen 6 complex locus K is a serologic biomarker and a therapeutic target for lung and esophageal carcinomas. Cancer Res 2007; 67:11601-11; PMID:18089789; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-3243. [DOI] [PubMed] [Google Scholar]
- 6.Kono K, Iinuma H, Akutsu Y, Tanaka H, Hayashi N, Uchikado Y, Noguchi T, Fujii H, Okinaka K, Fukushima R et al.. Multicenter, phase II clinical trial of cancer vaccination for advanced esophageal cancer with three peptides derived from novel cancer-testis antigens. J Transl Med 2012; 10:141-9; PMID:22776426; http://dx.doi.org/ 10.1186/1479-5876-10-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshitake Y, Fukuma D, Yuno A, Hirayama M, Nakayama H, Tanaka T, Nagata M, Takamune Y, Kawahara K, Nakagawa Y et al.. Phase II clinical trial of multiple peptide vaccination for advanced head and neck cancer patients revealed induction of immune responses and improved OS. Clin Cancer Res 2015; 21:312-21; PMID:25391695; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-0202. [DOI] [PubMed] [Google Scholar]
- 8.Keilholz U, Weber J, Finke JH, Gabrilovich DI, Kast WM, Disis ML, Kirkwood JM, Scheibenbogen C, Schlom J, Maino VC et al.. Immunologic monitoring of cancer vaccine therapy: results of a workshop sponsored by the Society for Biological Therapy. J Immunother 2002; 25:97-138; PMID:12074049; http://dx.doi.org/ 10.1097/00002371-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Tomita Y, Yuno A, Tukamoto H, Senju S, Kuroda Y, Hirayama M, Imamura Y, Yatsuda J, Sayem MA, Irie A et al.. Identification of immunogenic LY6K long peptide encompassing both CD4+ and CD8+ T-cell epitopes and eliciting CD4+ T-cell immunity in patients with malignant disease. OncoImmunol 2014; 3:e28100-1-15; PMID:25340007; http://dx.doi.org/ 10.4161/onci.28100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomita Y, Yuno A, Tukamoto H, Senju S, Yoshimura S, Osawa R, Kuroda Y, Hirayama M, Irie A, Hamada A et al.. Identification of CDCA1-derived long peptides bearing both CD4+ and CD8+ T-cell epitopes: CDCA1-specific CD4+ T-cell immunity in cancer patients. Int J Cancer 2014; 134:352-66; PMID:24734272; http://dx.doi.org/ 10.1002/ijc.28376. [DOI] [PubMed] [Google Scholar]