Abstract
Fbxw7 has been identified as an oncosuppressor protein in many types of cancer. We have recently shown that loss of Fbxw7 in bone marrow-derived stromal cells (BMSCs) promotes cancer metastasis by increasing production of the chemokine CCL2, which attracts monocytic myeloid-derived suppressor cells (Mo-MDSCs) and macrophages to the metastatic niche.
Keywords: chemokine, F-box protein, metastasis, niche, ubiquitin ligase
Fbxw7 (also known as Fbw7, Sel-10, hCdc4, or hAgo) is an evolutionarily conserved F-box protein that serves as a substrate receptor for the Skp1-Cul1-F-box protein–Rbx1 (SCF) ubiquitin ligase complex.1 Most Fbxw7 substrates are encoded by proto-oncogenes for diverse human cancer types and include c-Myc, Notch, cyclin E, c-Jun, KLF5, Mcl-1, and mTOR. Recent studies have revealed that loss-of-function mutations in Fbxw7 give rise to both hematopoietic and solid tumors in mice. Moreover, mutations in Fbxw7 have been identified in multiple types of human cancer, including cholangiocarcinoma and T cell acute lymphoblastic leukemia. These various observations suggest that Fbxw7 functions as a tumor suppressor in a cell-autonomous manner, whereas little is known about the non-cell-autonomous roles of this protein.
We recently discovered a new aspect of the role of Fbxw7 in tumor suppression – namely, its function in the host environment to suppress cancer metastasis (Fig. 1). We generated mice that are deficient in Fbxw7 specifically in bone marrow-derived cells (Fbxw7Δ/Δ mice). Intravenous injection of melanoma (B16/F10 or B16/F1) cells or of lung cancer (Lewis lung carcinoma) cells into these mutant mice revealed that lung metastasis was enhanced and that the animals died sooner compared with injected wild-type controls.2 Orthotopic transplantation of E0771 murine breast cancer cells also showed that metastasis to the lungs was markedly enhanced in Fbxw7Δ/Δ mice, whereas the size of the primary tumors did not differ significantly between the mutant and wild-type animals. Both the number of metastatic tumor nodules and the average area per nodule in the lungs were greater for Fbxw7Δ/Δ mice than for controls.
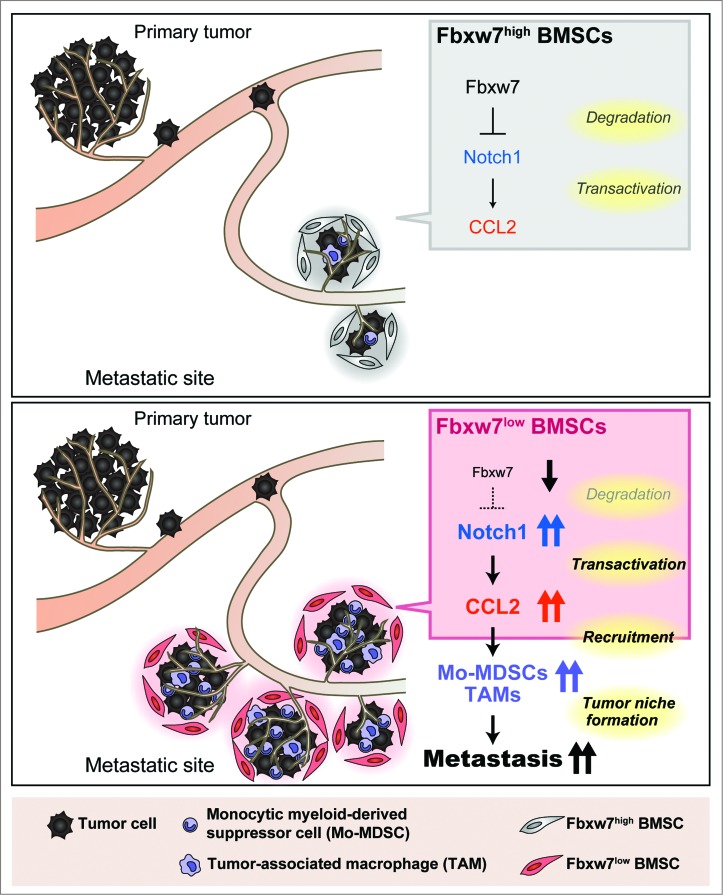
Figure 1.
Model for the promotion of cancer metastasis by loss of Fbxw7 in the host environment. Excessive signaling by Notch1 due to the impairment of its degradation caused by Fbxw7 ablation gives rise to increased production of CCL2 by bone marrow-derived stromal cells (BMSCs). The consequent recruitment of Mo-MDSCs and macrophages facilitates metastatic tumor growth.
Consistent with the results obtained with these mouse models, low levels of FBXW7 mRNA in peripheral blood of breast cancer patients were found to be associated with poor prognosis.2 In particular, the prognosis of patients with triple-negative breast cancer differed profoundly between women with high or low levels of FBXW7 expression in peripheral blood cells. FBXW7 expression in peripheral blood was highly correlated with the abundance of Fbxw7 protein in stromal cells that surround tumor cells. Collectively, our data suggest that a reduced abundance of Fbxw7 in the host environment results in poor prognosis in both humans and mice with breast cancer.
Interactions between tumor cells and the associated microenvironment play a key role in cancer progression. Increases in the number of inflammatory cells in the tumor niche as well as in the concentration of soluble inflammatory mediators, such as cytokines and chemokines, are associated with poor prognosis.3,4 We found that the numbers of Ly6C+ Mo-MDSCs and F4/80+ macrophages, both of which promote tumor growth as well as tumor cell survival and proliferation, were increased at sites of metastasis and in peripheral blood of Fbxw7Δ/Δ mice compared with control animals.2 Furthermore, the serum level of chemokine C-C motif ligand 2 (CCL2), an inflammatory cytokine that promotes the accumulation of myeloid-derived cells at metastatic sites, was found to be greater in Fbxw7Δ/Δ mice than in wild-type mice. Treatment with propagermanium, an antagonist of the CCL2 receptor CCR2,5 significantly reduced the size, but not the number, of metastatic nodules in the lungs of Fbxw7Δ/Δ mice transplanted orthotopically with breast cancer cells. The number of Mo-MDSCs in the lungs was also markedly reduced by this treatment. These results suggest that increased production of CCL2 in Fbxw7Δ/Δ mice promotes the growth of established metastatic tumors.
Neither the serum level of CCL2 nor tumor metastasis to the lungs differed between control mice and Fbxw7−/Δ mice that lack Fbxw7 specifically in the myeloid lineage, suggesting that loss of Fbxw7 in Mo-MDSCs or tumor-associated macrophages does not contribute to the observed increase in cancer metastasis in the original Fbxw7Δ/Δ mice.2 Rather, Fbxw7 deficiency in BMSCs appears to promote transcription of the CCL2 gene and secretion of the chemokine. BMSCs which are thought to be the precursors of tumor-associated fibroblasts, are a major component of the tumor microenvironment and source of CCL2 production. Cancer-associated BMSCs have been shown to promote tumor growth as a result of their high-levels of expression of CCR2 ligands such as CCL2.6 We found that BMSCs colocalized with metastatic tumor cells and Mo-MDSCs in the lungs of Fbxw7Δ/Δ mice. Co-transplantation of BMSCs with melanoma cells revealed that increased production of CCL2 by Fbxw7Δ/Δ BMSCs contributes to promotion of metastasis.
Members of the Notch family of proteins are membrane-sequestered transcription factors, and the intracellular domain of each Notch protein is cleaved by the γ-secretase proteolytic complex and then translocates to the nucleus. Depletion of Fbxw7 in BMSCs resulted in a marked increase in the amount of the nuclear form of Notch1, consistent with previous observations that Notch1 is a target for the SCFFbxw7 ubiquitin ligase.7,8 Inhibition of Notch signaling in Fbxw7Δ/Δ BMSCs by treatment with a γ-secretase inhibitor resulted in a reduction in the amount of CCL2 mRNA in these cells. We found that the excess amount of Notch1 in the nucleus of these cells results in direct activation of CCL2 gene transcription. Additional ablation of RBP-Jκ, an important cofactor for Notch-dependent transcription, resulted in a reduced serum concentration of CCL2 and attenuation of enhanced metastasis in Fbxw7Δ/Δ mice. Collectively, these observations suggest that Notch1 accumulation as a result of Fbxw7 deficiency in BMSCs promotes metastasis by increasing transactivation of the CCL2 gene.
Overall, our results provide mechanistic insight into the suppression of cancer metastasis by Fbxw7. They also suggest that the level of FBXW7 expression in peripheral blood is a potentially valuable prognostic marker for cancer patients, and that targeting of the CCL2-CCR2 system is a possible rational approach to inhibition of metastasis. The CCR2 antagonist propagermanium is currently in use clinically for the treatment of hepatitis B virus infection. Measurement of FBXW7 mRNA in peripheral blood may provide a “companion diagnostic” for propagermanium treatment in cancer patients. Our discovery that Fbxw7 is an upstream regulator of CCL2 expression also has implications for the development of new treatment strategies for cancer patients.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Davis RJ, Welcker M, Clurman BE. Tumor suppression by the Fbw7 ubiquitin ligase: mechanisms and opportunities. Cancer Cell 2014; 26:455-64; PMID:25314076; http://dx.doi.org/ 10.1016/j.ccell.2014.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yumimoto K, Akiyoshi S, Ueo H, Sagara Y, Onoyama I, Ueo H, Ohno S, Mori M, Mimori K, Nakayama KI. F-box protein FBXW7 inhibits cancer metastasis in a non-cell-autonomous manner. J Clin Invest 2015; 125:621-35; PMID:25555218; http://dx.doi.org/ 10.1172/JCI78782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borsig L, Wolf MJ, Roblek M, Lorentzen A, Heikenwalder M. Inflammatory chemokines and metastasis–tracing the accessory. Oncogene 2014; 33:3217-24; PMID:23851506; http://dx.doi.org/ 10.1038/onc.2013.272 [DOI] [PubMed] [Google Scholar]
- 4.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013; 19:1423-37; PMID:24202395; http://dx.doi.org/ 10.1038/nm.3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokochi S, Hashimoto H, Ishiwata Y, Shimokawa H, Haino M, Terashima Y, Matsushima K. An anti-inflammatory drug, propagermanium, may target GPI-anchored proteins associated with an MCP-1 receptor, CCR2. J Interferon Cytokine Res 2001; 21:389-98; PMID:11440636; http://dx.doi.org/ 10.1089/107999001750277862 [DOI] [PubMed] [Google Scholar]
- 6.Ren G, Zhao X, Wang Y, Zhang X, Chen X, Xu C, Yuan ZR, Roberts AI, Zhang L, Zheng B et al.. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFalpha. Cell Stem Cell 2012; 11:812-24; PMID:23168163; http://dx.doi.org/ 10.1016/j.stem.2012.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta-Rossi N, Le Bail O, Gonen H, Brou C, Logeat F, Six E, Ciechanover A, Israël A. Functional interaction between SEL-10, an F-box protein, and the nuclear form of activated Notch1 receptor. J Biol Chem 2001; 276:34371-8; PMID:11425854; http://dx.doi.org/ 10.1074/jbc.M101343200 [DOI] [PubMed] [Google Scholar]
- 8.Oberg C, Li J, Pauley A, Wolf E, Gurney M, Lendahl U. The Notch intracellular domain is ubiquitinated and negatively regulated by the mammalian Sel-10 homolog. J Biol Chem 2001; 276:35847-53; PMID:11461910; http://dx.doi.org/ 10.1074/jbc.M103992200 [DOI] [PubMed] [Google Scholar]