Abstract
Glioblastoma multiforme (GBM) is the most aggressive form of primary brain tumor and is associated with poor survival. Virotherapy is a promising candidate for the development of effective, novel treatments for GBM. Recent studies have underscored the potential of virotherapy in enhancing antitumor immunity despite the fact that its mechanisms remain largely unknown. Here, using a syngeneic GBM mouse model, we report that intratumoral virotherapy significantly modulates the tumor microenvironment. We found that intratumoral administration of an oncolytic adenovirus, AdCMVdelta24, decreased tumor-infiltrating CD4+ Foxp3+ regulatory T cells (Tregs) and increased IFNγ-producing CD8+ T cells in treated tumors, even in late stage disease in which a highly immunosuppressive tumor microenvironment is considered to be a significant barrier to immunotherapy. Importantly, intratumoral AdCMVdelta24 treatment augmented systemically transferred tumor-antigen-specific T cell therapy. Furthermore, mechanistic studies showed (1) downregulation of Foxp3 in Tregs that were incubated with media conditioned by virus-infected tumor cells, (2) downregulation of indoleamine 2,3 dioxygenase 1 (IDO) in glioma cells upon infection by AdCMVdelta24, and (3) reprograming of Tregs from an immunosuppressive to a stimulatory state. Taken together, our findings demonstrate the potency of intratumoral oncolytic adenoviral treatment in enhancing antitumor immunity through the regulation of multiple aspects of immune suppression in the context of glioma, supporting further clinical development of oncolytic adenovirus-based immune therapies for malignant brain cancer.
Keywords: glioblastoma, immunosuppression, immunotherapy, oncolytic adenovirus, regulatory T cells, Tregs, tumor microenvironment, virotherapy
Introduction
GBM is among the most invasive and aggressive of cancers, with a median survival of less than 15 months post-diagnosis. This poor survival rate indicates that conventional treatments including surgery, radiotherapy, and/or chemotherapy are not effective, and novel therapies that can efficiently control disease progression are required. Both virotherapy and immune therapy have recently emerged as promising candidates for the treatment of multiple types of cancer, including glioma.1-6
Remarkable progress has been achieved in oncolytic virotherapy over the past decade with regard to developing novel vectors and understanding the mechanisms by which viruses specifically replicate in tumor cells.7-12 It was also reported that certain oncolytic viruses can target cancer stem cells that are resistant to conventional cancer therapy, leading to efficient cell death.13,14 Several oncolytic viruses have entered into clinical studies as anticancer agents for the treatment of multiple types of cancer patients. However, the therapeutic efficacy in most of these studies has been limited.3,15 Recently, accumulated evidence has suggested that virotherapy may have the potential to induce immune responses against cancer, which will open opportunities to integrate virotherapy into immunotherapeutic approaches to develop novel oncolytic virus-based treatments for cancer.4,6
Immunotherapy has been proposed for decades as cancer treatment. Very recent studies on the blockade of immune checkpoints CTLA-4 or PD-1/PD-L1 for the treatment of melanoma patients,16,17 and on the use of adoptively transferred chimeric CD19 specific T cell therapy for leukemia,18 have shown promise. However, it is well known that immune suppression within the tumor microenvironment is a significant barrier to cancer immunotherapy, particularly within solid tumors.19-22 In glioma, a highly immunosuppressive microenvironment has been characterized by the presence of Tregs.23,24 Treg cells are the major source of TGF-β and IL-10, which are documented as mediators of immune suppression within glioma.25,26 Moreover, our most recent work identifies the role of indoleamine 2,3 dioxygenase 1 (IDO) as one of the key regulators of intracranial Tregs and demonstrates that combining an IDO inhibitor with the immune checkpoints antibodies against CTLA-4 and PD-L1 results in a decrease of Treg cells in glioma and a durable survival advantage.27,28 Having shown the presence of immune regulatory factors associated with glioma and the significant therapeutic efficacy achieved through modulation of these factors, we hypothesized that the use of an oncolytic virus, due to its potentiation of antitumor immunity, may be a promising strategy to improve the antitumor effects of immunotherapies for glioma. Further understanding of the immunological mechanisms by which the oncolytic virus itself, or combined with other immunotherapeutic approaches, generates potent antitumor immune responses will advance clinical development of novel therapies for glioma.
Here, we extend our previous studies on using an oncolytic adenovirus in the treatment of glioma10,29-32 to investigate whether intratumoral adenoviral treatment would modulate the immune suppressive microenvironment associated with glioma, and whether these responses would facilitate immunotherapy for glioma. To study this, we chose the recombinant replication-competent adenovirus, AdCMVdelta24, that we have previously shown to induce oncolysis and viral replication in several tested glioma cell lines including murine glioma cells (GL261) and primary human glioma cells.10 In the present study, we observed that intratumoral AdCMVdelta24 treatment modulates the tumor microenvironment toward immune activation, which was evidenced by a significant decrease of Tregs, an increase of IFNγ-producing CD4+ and CD8+ T cells, and an increased ratio of CD4+ effector T cells compared to Tregs within GL261 glioma. Mechanistically, we observed viral treatment-mediated downregulation of Foxp3 in Tregs, downregulation of IDO expression in glioma cells, and reprogramming of Tregs from an immunosuppressive to a stimulatory state. Moreover, viral treatment enhanced systemically transferred adoptive tumor-antigen-specific T cell therapy for glioma. Together, these results indicate that combinatorial therapy of intratumoral oncolytic adenoviral treatment and immunotherapeutic approaches may be promising for the treatment of glioma.
Results
Intratumoral viral treatment increases infiltration of leukocytes
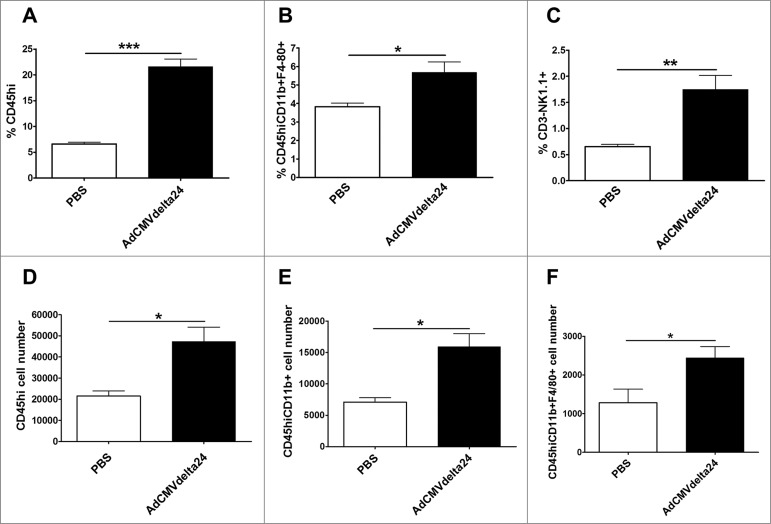
C57BL/6 mice bearing 5 d-established intracranial GL261 tumors were injected intratumorally (i.t.) with AdCMVdelta24 or control PBS. Seventy-two hours after viral injection, an increased percentage of CD45high leukocytes in mice bearing GL261 glioma brain was observed as compared with PBS-injected control mice (Fig. 1A). In further analysis of the subsets of innate immune cells among these CD45high leukocytes, we observed an increased percentage of CD11b+F4/80+ macrophages (Fig. 1B) as well as CD3− NK1.1+ NK cells (Fig. 1C). Besides the frequency increase, the total numbers of infiltrating-leukocytes, monocytes, and macrophages into brains were also increased (Fig. 1D–F, respectively) confirming that inflammatory responses are induced shortly after i.t. injection of AdCMVdelta24. Indeed, in line with our study, it was reported that the early inflammatory responses mediated by i.t. injection of oncolytic adenovirus are prerequisite for effective immunotherapy for cancer.33 Thus, our results suggest that intratumoral oncolytic adenoviral treatment may modify the tumor immunological microenvironment at early time points, which may facilitate the generation of immune responses against glioma.
Figure 1.
AdCMVdelta24 increases infiltration of leukocytes into tumor-bearing brains. Flow cytometry analysis of CD45 high leukocytes, CD45 high CD11b+ monocytes, CD45high CD11b+ F4/80+ macrophages, and CD3− NK1.1+ NK cells isolated from the brain was performed. Changes of the frequency (top panel, Leukocytes (A); Macrophages (B); NK cells (C)) and the total number (bottom panel, Leukocytes (D); Monocytes (E); Macrophages (F)) of the cells in mice brains are presented. C57BL/6 mice bearing 5 d-established intracranial GL261 tumor were injected intratumorally with AdCMVdelta24 or control PBS. Three days later, the flow cytometry analysis was performed. Data are presented as mean ± SEM in three or four mice for each treatment group. *p < 0.05, **p < 0.01 and ***p < 0.001 (unpaired, two-tailed Student's t-test). CD45Hi, CD45high.
Intratumoral viral treatment decreases regulatory T cells and increases the ratio of effector T cells to Treg cells in intracranial tumors but not in cervical lymph nodes and spleens
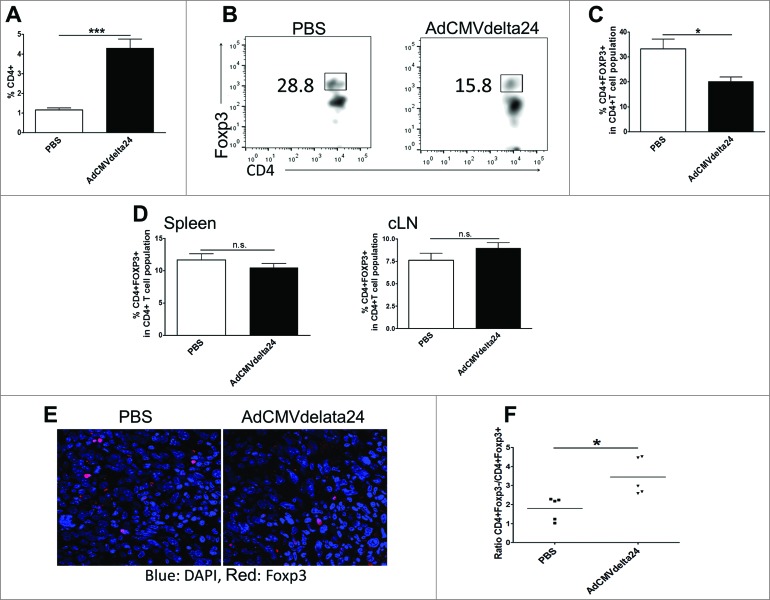
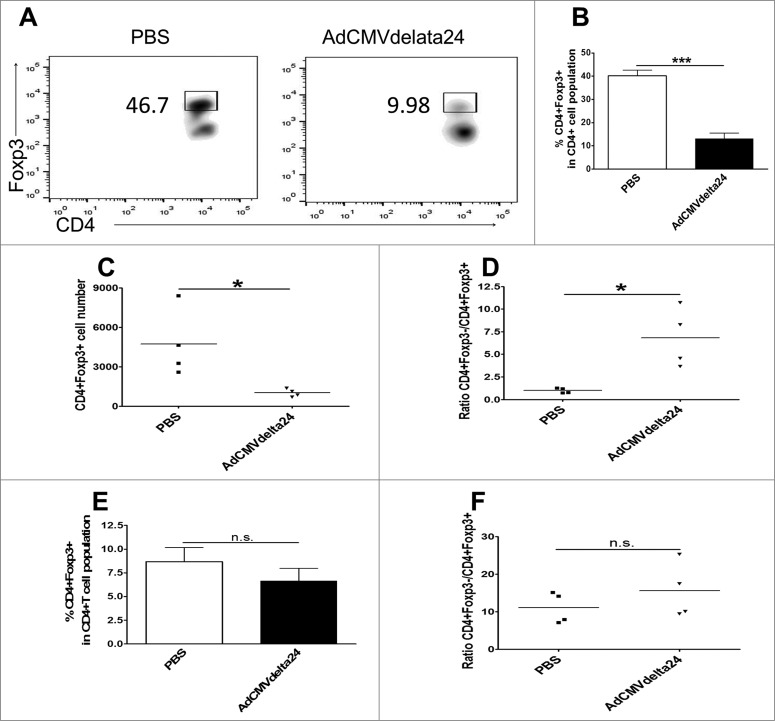
Tregs are well characterized immune suppressive cells which have been identified as a major component responsible for tumor tolerance to therapies.20 In a murine glioma model, it has been shown that the depletion of Tregs by anti-CD25 antibody results in enhanced antitumor immunity and prolonged mice survival.34,35 More importantly, in human patients, it was reported that the preferential accumulation of Tregs is associated with higher grades of glioma.23 Thus, we investigated whether intratumoral viral treatment could regulate Treg cells in the context of glioma. The results show that the frequency of CD4+ T cells increased (Fig. 2A), but the frequency of Foxp3+ Tregs in glioma bearing brains was significantly decreased at one week after viral treatment (Fig. 2B and C). A decreased infiltration of Tregs into the tumor was confirmed via microscopy (Fig 2E). Moreover, an increased ratio of effector T cells to Treg cells was observed in virus-treated mice brains as compared to control PBS-treated mice brains (Fig. 2F). Two weeks after viral treatment, when a more immunosuppressive microenvironment is induced by late stage tumors, the decreased frequency and total cell number of Tregs, as well as the increased ratio of effector T cells to Tregs within glioma, was continually observed (Fig. 3A–D). Alternatively, neither the frequency of Tregs nor the ratio of effector T cells to Treg cells was altered in spleens or in cervical lymph nodes (Figs. 2D and 3E–F). These results suggest that intratumoral AdCMVdelta24 treatment may modulate the immune suppressive tumor microenvironment toward immune stimulation through the deregulation of Tregs in glioma.
Figure 2.
AdCMVdelta24 treatment decreases regulatory T cells and increases the ratio of Teff /Treg cells in tumor bearing brains. C57BL/6 mice bearing 5 d-established intracranial GL261 tumor were injected intratumorally with AdCMVdelta24 or PBS. One week later, the flow cytometry analysis of CD4+ T cells, CD4+ Foxp3+ regulatory T cells (Treg), CD4+ Foxp3− effector T cells (Teff) isolated from brains, spleens and cervical lymph nodes (cLNs) were performed (n = 4 or 5 mice in each group). (A) Frequency of CD4+T cells in brain. (B–D) Frequency of Tregs: Representative flow cytometry of Tregs in brain (B) (Gated on CD4+T cells) and quantitative data (mean±SEM) in brains (C) and in spleens and cLNs (D). (E) Tumor sections from brain stained for Foxp3 and imaged under confocal microscope. (F) Ratio of Teff :Treg in brains. *p < 0.05, ***p < 0.001 and n.s., not significant (unpaired, two-tailed Student's t-test).
Figure 3.
Viral treatment-induced decrease of tumor-infiltrating Tregs and the increase of ratio of Teff /Treg cells were retained at late stage of disease. The experiment in Fig. 2 was repeated, except that the mice were sacrificed at two weeks, instead of one week, after viral treatment. Data are shown in (A–B) Frequency of Tregs in brain, Representative flow cytometry results (A) and quantitative data (B) (mean ± SEM), (C) Total number of Tregs in brain, (D) Ratio of Teff:Treg in brain, and (E–F) Frequency of Tregs (E) and ratio of Teff :Treg (F) in cLNs. *p < 0.05, ***p < 0.001 and n.s., not significant (unpaired, two-tailed Student's t-test).
Intratumoral viral treatment modulates the tumor microenvironment toward immune activation in late stage disease
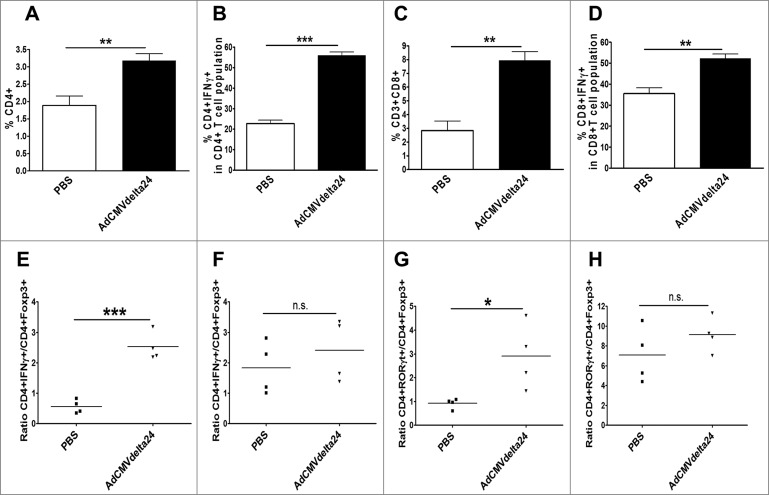
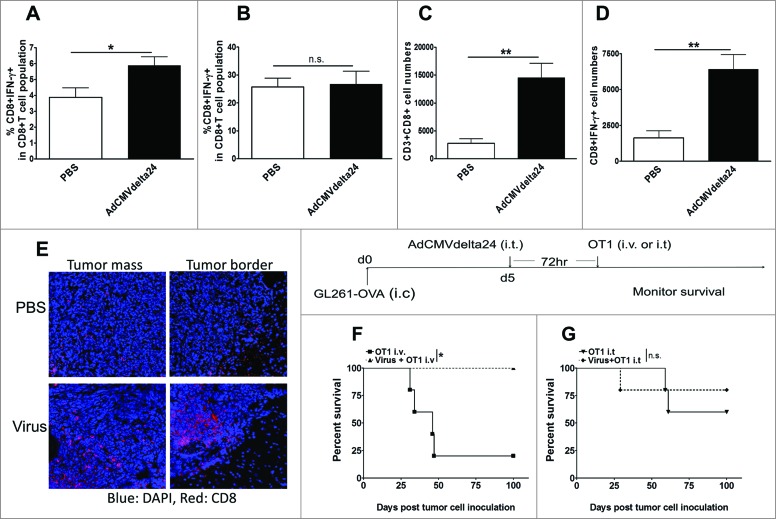
The immune suppressive tumor microenvironment is a significant barrier to effective therapies,19-22 and immunosuppression becomes more pronounced with further recruitment of immunosuppressive cells as the tumor progresses. Having shown the decrease of Tregs within glioma, we further investigated whether intratumoral administration of AdCMVdelta24 could modulate and eventually lead to a shift of the tumor microenvironment from an immune suppressive to an immune stimulatory state at a late stage of disease. Two weeks after viral treatment, flow cytometry analysis of brain-infiltrating lymphocytes demonstrated that not only did the frequency of either CD4+ or CD8+ T cells increase (Figs. 4A and C), but the frequency of IFNγ-producing CD4+ or CD8+ T cells, among total CD4+ or CD8+ cells respectively, was also increased (Figs. 4B and D). Again, despite the late stage of tumors with a character of high levels of immune suppression at 2 weeks after viral treatment, increased ratios of CD4+IFNγ+ to CD4+Foxp3+ (Fig. 4E) and CD4+RORγt+ to CD4+Foxp3+ (Fig. 4G) cells are observed in treated tumors. In contrast to the intracranial gliomas, we did not find the differences in these ratios in cLNs between the virotherapy and control PBS groups (Fig. 4F and H). These results suggest that intratumoral viral treatment may convert the immune suppressive tumor microenvironment into an immune stimulatory state that benefits immunotherapy for glioma.
Figure 4.
Intratumoral injection of AdCMVdelta24 modulates tumor microenvironment toward immune stimulatory state. C57BL/6 mice bearing 5 d intracranial GL261 glioma were injected intratumorally with AdCMVdelta24 virus or control PBS. Two weeks after viral treatment, flow cytometry analysis was performed on brains and cLNs leukocytes. (A–D) An increased percentage of CD4+ (A), CD4+IFNγ+ (B), CD8+ (C), and CD8+IFNγ+ (D) T cells in brains was observed. (E–G) An increased ratio of CD4+IFNγ+ to CD4+Foxp3+T cells (E), and CD4+RORγt+ to CD4+Foxp3+ (G) T cells was observed in brains, but not in cLNs (F, H). Data are presented as mean ±SEM in four to five mice for each treatment group. *p < 0.05, **p < 0.01, ***p < 0.001, and n.s., not significant (unpaired, two-tailed Student's t-test).
Viral treatment augments systemically transferred antigen-specific T cell therapy for glioma
We have already shown that viral treatment modulates the tumor microenvironment toward an immunostimulatory state. Meanwhile, at 72 h post-viral treatment, we also found an increased frequency of IFNγ-producing CD8+ cells among total CD8+ T cells in the cLNs (Fig. 5A), rather than in the glioma-bearing brains (Fig. 5B), of virus-treated mice as compared to the control group. Moreover, one week later, in the tumor-bearing brains, an increased absolute number of CD8+ cells, as well as of IFNγ-producing CD8+ T cells, was observed (Fig. 5C and D). An increase of tumor-infiltrating CD8+T cells into both periphery and center of tumors was also observed via microscopy (Fig. 5E). As such, we further explored whether these virotherapy induced immune responses could provide a platform in favor of immunotherapy against glioma. Several tumor vaccine strategies have been tested and have shown promise in the treatment of glioma patients, whereas adoptive T cell therapy administrated through either intravenous (i.v) or intracranial route has not been successful.36 We decided to determine whether intratumoral AdCMVdelta24 treatment would improve adoptive T cell therapy. As a murine model of adoptive T cell therapy, we used OT1 CD8+T cells that express a transgenic T cell receptor specific for the SIINFEKL epitope of the ovalbumin (OVA) protein presented in the context of the H-2Kb MHC class I molecule expressed by GL261-OVA tumor cells. The results showed that, following virotherapy, adoptively transferred OT1 cell therapy through the i.v route was significantly better than T cell therapy alone against GL261-OVA tumors growing in syngeneic C57BL/6 mice (Fig. 5F). In contrast to this, when the same number of OT1 cells used for i.v injection were given i.t., additional virotherapy did not augment T cell therapy (Fig. 5G). Taken together, these results indicate that intratumoral AdCMVdelta24 treatment can augment systemically transferred antigen-specific T cell therapy against tumors, and that enhanced immunostimulatory responses in cLNs at the time (Fig. 5A) when OT1 cells are given may play a critical role in facilitating systemically transferred OT1 cell therapy against glioma.
Figure 5.
Intratumoral AdCMVdelta24 treatment augments intravenously transferred OT1 cell therapy. (A–D) C57BL/6 mice bearing 5 d intracranial GL261glioma were injected intratumorally with AdCMVdelta24 virus or control PBS. Flow cytometry analysis of CD8+ T cells, IFNγ+ CD8+T cells isolated from brains and cervical lymph nodes (cLNs) were performed at 72 h and 1 week after viral treatment. (A–B) Frequency of CD8+IFNγ+ T cells in cLNs (A) or in brains (B) at 72 h post treatment. (C–D) Total number of CD8+T cells (C) and IFNγ+CD8+ T cells (D) in brains at one week after viral treatment. (E) Tumor sections from brain at one week after viral treatment, stained for CD8+ T cells and imaged under confocal microscope (Top panel, PBS treatment; Bottom panel, AdCMVdelta24 treatment). (F–G) C57BL/6 mice (n = 5) were intracranially injected with GL261-OVA cells at 1 × 105 in 5 uL of PBS (d0). Five days later (d5), AdCMVdelta24 was intratumorally injected, followed by 1 × 106 OT1 cells infusion through either tail vein (i.v) (F) or intratumoral (i.t.) injection (G) at 72 h post viral treatment. Mice were then monitored for survival. *p < 0.05, and n.s., not significant (log-rank test).
Oncolytic adenoviral treatment downregulates multiple aspects of immune suppression in the context of glioma
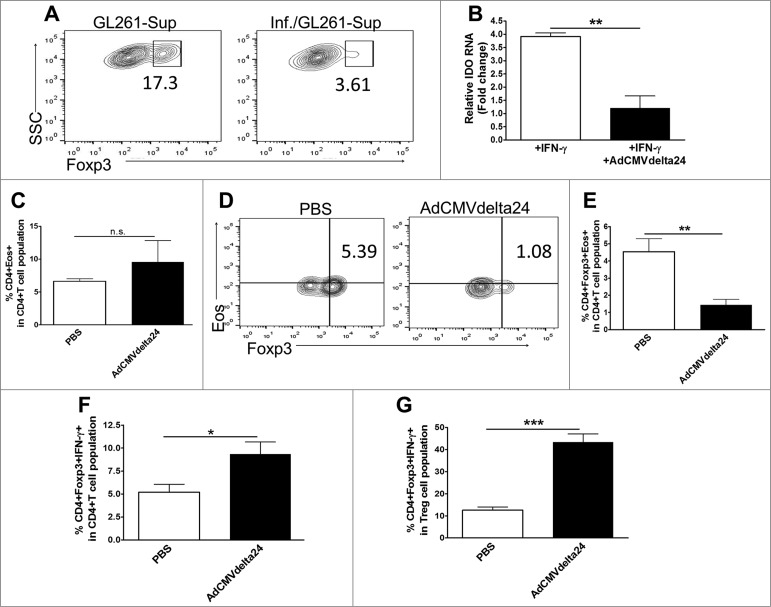
We showed that viral treatment decreases Tregs within glioma in mice (Fig. 2). Thus, we further tested whether viral treatment of tumor cells has effects directly on Tregs. CD4+ CD25 + Treg cells were isolated from C57BL/6 mice and were incubated in the presence of supernatants harvested from virus infected tumor cells. We cultured GL261 tumor cells under normoxia or hypoxia conditions, and harvested the supernatants for use in Treg cell culture experiments. Interestingly, we found that a significantly higher Foxp3 expression was observed in Tregs cultured with the supernatants harvested from hypoxic GL261 cells as compared to that from normoxic cells (17% vs. 4%, respectively, data not shown). This is in accordance with the hypoxic features within malignant glioma,37,38 as such we only focused on the effects on Tregs by viral infection of GL261 tumor cells under hypoxia conditions in current study. Our results showed that Foxp3 expression was decreased in Treg cells that were incubated with supernatants from viral-infected GL261 cells as compared to those incubated with supernatants from non-infected GL261 cells (Fig. 6A), suggesting that soluble mediators from viral infected glioma cells deregulate Foxp3 expression in Treg cells. Moreover, to extend our previous study that Indoleamine 2,3 dioxygenase 1 (IDO) plays a critical role in immune suppression in glioma,27,28 we tested whether infection of GL261 glioma cells by AdCMVdelta24 could regulate IDO expression in tumor cells. The results showed a significant decrease of IFNγ-induced IDO expression at the mRNA level in virus infected GL261 cells as compared to that in non-infected cells (Fig. 6B). Next, we explored the possibility that Tregs could be reprogrammed by viral treatment. For this, we analyzed the tumor-infiltration of Eos+ CD4+ Foxp3+ Tregs. Eos plays a critical role in CD4+ Foxp3+ Treg cells-mediated immune suppression. Loss or knockdown of Eos in Treg cells can reprogram these cells,39,40 resulting in the re-expression of immune stimulatory genes that are silenced in a Foxp3-dependent manner, such as IFNγ gene in Tregs,39 and can even result in the conversion of Treg cells into Foxp3+ T helper cells which was shown to enhance antitumor T cell responses.40 In the current study, we found that intratumoral AdCMVdelta24 treatment did not change the frequency of Eos-positive CD4+ T cells in brains (Fig. 6C). Intriguingly, however, such treatment not only decreased the percentage of Eos-positive Tregs in total CD4+ T cell population (Fig. 6D and E), but also increased the frequency of IFNγ-producing Treg cells (Foxp3+ helper T cells) among total CD4+ T cells (Fig. 6F), as well as among total CD4+Foxp3+ Treg cells (Fig. 6G), suggesting the reprograming of Treg cells. Collectively, these results indicate that intratumoral AdCMVdelat24 treatment may alter the tumor microenvironment toward immune activation by targeting multiple immune suppressive mechanisms.
Figure 6.
Viral treatment downregulates the expression of Foxp3 in Treg cells and IDO in glioma cells and reprograms regulatory T cells. (A) Downregulation of Foxp3 expression in Treg cells. CD4+CD25+Treg cells sorted from the spleen and cLNs from C57/BL mice were stimulated with anti-CD3, anti-CD28 and IL-2, and incubated with the medium harvested from tumor cells. GL261 glioma cells were infected by AdCMVdelta24 at a MOI of 100 (Inf./GL261), or left uninfected (GL261), 4 h later, the cells were washed to remove viruses and incubated in the hypoxia chamber (1% O2). After 24 h, the supernatants were harvested and added into Treg cell culture. Treg cells were grown in the presence of conditioned-medium for 24 h and were analyzed by flow cytometry for intracellular expression of Foxp3. The cells were gated on CD4+ T cells. (B) Downregulation of IFNγ-induced IDO expression by GL261 glioma cells. GL261 cells were infected by AdCMVdelta24 at a MOI of 100 in the presence of 20 ng/mL mIFNγ (+IFNr+Adv). 24 h later, the cells were collected for q-PCR analysis of the expression of IDO. mIFNγ was used to induce IDO expression and served as a control (+IFNr). (C–H) Mice bearing 5 d GL261 tumor were injected intratumorally by AdCMVdelta24 or PBS (n = 4 or 5 mice in each group). Two weeks later, mice were killed and flow cytometric analysis was performed on leukocytes isolated from brains. (C) The percentage of Eos-positive CD4+ among the total CD4+ T cell population was not different between two groups. (D–E) A decreased percentage of Eos-positive Tregs in the CD4+ T cell population was observed in viral treated mice; (D) Representative flow cytometry results (gated on CD4+ T cells) and (E) The quantitative data (mean±SEM). (F–G) An increased percentage of IFNγ-producing Foxp3+ helper Treg cells among total CD4+ T cell population (F) and among total CD4+Foxp3+ Treg cell populations (G). *p < 0.05, **p < 0.01, ***p < 0.001, and n.s., not significant (unpaired, two-tailed Student's t-test).
Discussion
Virotherapy has shown promising results in the treatment of a variety of types of cancer in animal studies. However, the efficacy of virotherapy has been limited in the clinic. Recent evidence that virotherapy can potentiate antitumor immunity has shed light on this approach.6 Further understanding of the immunological mechanisms by which the oncolytic virus itself or combined with other immunotherapeutic approaches to generate effective antitumor immunity would advance oncolytic virus-based therapy for cancer patients. Using the GL261 syngeneic murine glioma model, our study provides the first evidence that intratumoral oncolytic AdCMVdelta24 treatment modulates the tumor microenvironment toward immune activation in glioma through deregulating immune suppression and reprograming Tregs. Additionally, we showed that intratumoral virotherapy significantly augments the adoptively transferred tumor-antigen-specific T cell therapy against glioma.
It has been reported that intratumoral adenoviral treatment induces early inflammatory immune responses within tumor, which plays a key role in generating the effectiveness of DC vaccine-based immunotherapy with subcutaneous tumor models.33 In line with this study, we observed an increased intratumoral infiltration of total leukocytes, as well as monocytes, macrophages and NK cells in GL261 glioma model at 3 d following intratumoral AdCMVdelta24 treatment, suggesting the development of early inflammatory responses. Furthermore, we observed that when antigen-specific T cells against tumor were given intravenously at 3 d post-intratumoral AdCMVdelta24 treatment, a prolonged mice survival benefit was achieved as compared to T cell therapy alone (Fig. 5F). Conversely, in the studies of Herpes Simplex Virus (HSV)-based virotherapy, it has been reported that macrophages and NK cells mediate antiviral immune responses in human glioma patients and in animal models, suggesting that the reduction of these cells during HSV-based virotherapy would improve viral infection of tumor cells and increase oncolytic activity.41-44 During adenoviral treatment for glioma, whether these tumor-infiltrating cells have the similar antiviral effects is unclear. Further experiments are ongoing to dissect the mechanisms of these cells mediated-antiviral and -antitumoral immune responses, which will benefit to the development of novel treatment strategies specifically preventing antiviral immune responses but retaining antitumor immunity in adenoviral therapy for glioma.
In the current study, an increased frequency of intracranial CD4+ T cells concurrent with a decreased frequency and the number of CD4+Foxp3+ Treg cells suggests that intratumoral adenoviral treatment may preferably targets Treg cells in glioma. Recent studies have shown that both murine and human CD4+Foxp3+ Tregs display phenotypic and functional plasticity under certain stimulatory circumstances,45,46 such as losing the Foxp3 expression to convert into other types of inflammatory cells47 or reprograming Treg cells into immune stimulatory “Helper” Treg cells with retaining Foxp3 expression.39,40 Here, we observed that viral infection of GL261 cells directly downregulates Foxp3 expression in Tregs. Moreover, intratumoral AdCMVdelta24 treatment decreased suppressive Eos positive CD4+ Foxp3+ Tregs, and subsequently increased the frequency of IFNγ-producing Tregs. Eos, as a co-repressor of Foxp3, plays dominant roles ensuring Foxp3-mediated immune suppression of Treg cells. Loss or downregulation of Eos expression in Foxp3+ Treg cells results in reprograming Tregs from an immune suppressive into an immune stimulatory state, which is advantageous for eliciting efficient antitumor immunity.39,40 Additionally, in line with our previous study that IDO is one of key regulators of glioma-infiltrating Tregs,27 we also observed the downregulation of IDO expression in GL261 glioma cells upon infection by AdCMVdelta24. Besides IDO, as an immunosuppressive molecule, having impacts on suppressing T cell immunity through various mechanisms,48 collectively, our results indicate that intratumoral AdCMVdelta24 treatment modulates the glioma microenvironment toward immune activation through multiple mechanisms, at least including the downregulation of Foxp3 in Tregs and IDO expression in glioma cells, as well as the reprograming of tumor-infiltrating Tregs. We are currently undertaking to determine how AdCMVdelta24 treatment transcriptionally or epigenetically affects expression, as well as the stability of Foxp3 in Treg cells, IDO in tumor cells, and Eos levels in Treg cells within glioma.
Further, we hypothesized that intratumoral viral treatment-induced host immune stimulatory responses may facilitate immunotherapy for glioma. In support of this hypothesis, we observed the augmentation of adoptively transferred tumor-antigen-specific T cell therapy for glioma in viral-treated mice. Interestingly, when the effector CD8+OT1 cells were injected i.t., viral treatment did not further enhance T cell therapy. We believe one of the reasons for this discrepancy is the fact that, at the time of T cells administration (3 d post-viral treatment, Fig. 5A and B), an increase of IFNγ-producing CD8+ T cells was observed in cLNs but not in brains, suggesting that intravenously infused T cells may be further boosted in peripheral cLNs. Nevertheless, when effector T cells infused through either route arrive into the tumor sites, the viral-modulated tumor microenvironment does not dampen T cell therapy and may even enhance T cell therapy in the case of intravenously transferring CD8+OT1 cells. Given that the immunosuppressive microenvironment in cancer patients correlates to poor prognosis and limits the success of cancer immunotherapy, developing strategies to modulate the tumor microenvironment toward immune activation is important for achieving therapeutic effectiveness. Since our data showed the modulation of the tumor microenvironment toward immune activation even in late-stage tumors (Fig. 4), we will investigate whether intratumoral viral treatment can improve either systemic or intratumoral T cell therapy in treating more clinically-relevant, advanced glioma. With the role of targeting immune suppression within glioma, intratumoral oncolytic adenoviral treatment is also expected to be tested in combination with other immunotherapeutic approaches, such as tumor vaccine strategies or inhibitors of immune checkpoints, to achieve improved immunotherapies.
We did not observe the efficacy of AdCMVdelta24 virotherapy alone,10 (and data not shown), suggesting that the generation of more potent oncolytic adenovirus by engineering viral vector to include interest genes such as cytotoxic genes and/or immunotherapeutic genes is required. It is unclear whether the mutant E1delta24,49 rather than wild-type E1 viral protein, plays a key role in modulating the immunological tumor microenvironment. Moreover, unlike most commonly used and tested adenoviruses in both pre-clinical and clinical studies, AdCMVdelta24 retains the entire E3 viral region,7 which may lead to ADP (adenovirus death protein, located in E3)-mediated enhanced tumor cell death; contributing to immune responses again tumor cells. Therefore, further identification of the role of key viral proteins in the context of viruses-based immunotherapy will help to rationally design better oncolytic adenoviral vectors to improve virotherapy alone or combined with immunotherapies. In light of data presented here, further exploration of this modulation of the glioma microenvironment by intratumoral oncolytic adenoviral treatment is a highly promising strategy for the development of virus-based immunotherapies for glioma.
Materials and Methods
Cells and viruses
GL261 cells are murine glioma cells described previously.27 GL261-quad OVA cells (kindly provided by Dr John R. Ohlfest, department of Pediatrics, University of Minnesota) were derived from GL261 cells and encode chicken OVA 257–264 peptide (presented by H-2Kb).50 Cells were cultured with Dulbecco's modified Eagle's minimal essential medium (Gibco Invitrogen) and supplemented with 10% fetal calf serum and 100 U/mL of penicillin/streptomycin. GL261-OVA were also maintained in the presence of 800 μg/mL G418 and 0.1 mg/mL of Normocin (Invivogen).50 The replication-competent adenovirus, AdCMVdelta24, was described previously (Ref.7), and the virus was rescued by transfecting the pDNA of AdCMVdelta24 into HEK293 cells. The virus was propagated in HEK293 cells and purified by two rounds of CsCl gradients based on equilibrium centrifugation.10 Viral titer was determined using the Adeno-X™ Rapid Titer Kit (Clontech Cat. No. 632250) and presented as infection units (ifu)/mL according to the manufacture's instruction. The purified virus was stored at –80°C prior to use.
Mice
C57BL/6 mice (6–8 weeks of age) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). The OT-1 mouse strain was on a C57BL background (H-2Kb) (kindly provide by Dr Yang-xin Fu, department of pathology and committee on immunology, University of Chicago) and expressed a transgenic T-cell receptor, Vα2, specific for the SIINFEKL peptide of OVA in the context of MHC class I, H2-Kb.51
Abs and flow cytometry analysis (FACS)
Anti-CD3 (Pacific Blue), Anti-CD8 (PE) and Anti-CD45 (PE) antibodies were purchased from BioLegend. Anti-CD11b (APC) was purchased from BD PharMingen. All other antibodies were purchased from eBioscience. For intracellular Foxp3, RORγt, and Eos detection, cells were first stained by specific antibodies against mouse CD3, CD4, and CD8. Cells were then fixed and permeabilized with Perm/Fix solution (eBioscience) and then stained with anti-Foxp3 (Clone FJK-16s), anti-RORγt (Clone B2D), and anti-Eos (Clone ESB7C2). For intracellular cytokine IFNγ detection, the cells were treated with Cell Stimulation Cocktail (plus protein transport inhibitors) (eBiosciece) for 5 h, followed by antibodies against CD3, CD4, and CD8 cell surface markers first and then IFNγ staining as described above. Flow cytometry data was collected on LSR Fortessa (BD Biosciences) and analyzed with FlowJo software (TreeStar).
Preparation of activated OT-I cells
Preparation of activated OT-1 cells was performed as previously described.52 Naive OT-1 cells were isolated from the spleen and lymph nodes of OT-1 mice. Red blood cells were lysed by ACK Lysing Buffer (LONZA), and the dissociated single cell suspension was grown in Iscove's modified Dulbecco's medium plus 5% fetal bovine serum in the presence of 1μg/mL of SIINFEKL peptide (InvivoGen), 50 IU/mL of rIL-2 (R&D System), and 50 μM of β-mercaptoethanol. Three days after activation, the cells were harvested and purified via centrifugation in a lympholyte-M density gradient (Cedarlane) before use for in vivo injection.
Quantitative PCR
Total RNA was isolated with the RNeasy Plus Mini Kit (QIAGEN). RNA was reverse transcribed into cDNA using the iScript cDNA kit (Biorad), and the cDNA was used for Quantitative PCR (SYBR green qPCR kit, Biorad). Each qPCR reaction was run in triplicate and was normalized to the GAPDH gene transcript. Gene expression was quantified using the 2−ΔΔCT method (mean fold change of gene expression = 2−ΔΔCT). The primers used for real-time PCR analysis of IDO were as follows: sense: ACT GTG TCC TGG CAA ACT GGA AG, antisense: AAG CTG CGA TTT CCA CCA ATA GAG.
Immunostaining and image acquisition
For immunostaining, mouse brains were removed, immersed into Tissue Tek O.C.T. Compound (Sakura Finetek USA, Inc.), and frozen by dry ice. Frozen brains were sectioned on a Microm cryostat with vacutome system (Themo Scientific) at 5 μm intervals. For Foxp3 immunostaining, a modified protocol Biolegend's website was used. Briefly, slides were fixed for 5 min in Acetone at –20°C, washed 3X with TBS-T, and then blocked with 5% BSA (in TBS-T) for 1 h. Slides were then washed 3X TBS-T, and then probed with Foxp3-Biotin or CD8-Biotin (Ebioscience) diluted 1:100 in antibody dilution buffer (1% BSA / 0.5% Triton X-100/ in PBS) overnight at 4°C. The following day, slides were washed 3X with TBS-T, followed by incubation with 1:400 streptavidin-Alexa-488 (Jackson Immuno) in TBS-T for 45 min. Slides were then washed with 3X TBS-T and wet mounted with Fluoroshield with DAPI (Sigma). Slides were imaged using a Leica TCS-SP5 inverted confocal microscope. Images were acquired with a 63X oil immersion lens, and analyzed using ImageJ software (NIH).
In vivo studies
All procedures were approved by the Institutional Animal Use Committee of the University of Chicago. To establish intracranial syngeneic mouse glioma, C57BL/6 mice were used, and 5 × 104 of GL261 cells in 5 μL of PBS were stereotactically injected through an entry site centered 2 mm posterior to the coronal suture and 2 mm lateral to the sagittal suture, and 3 mm below the surface of skull of anesthetized mice by using a stereotactic frame. Five days later, mice were randomly assigned, and AdCMVdelta24 at 1 × 108 ifu in 5 μL of PBS was administrated slowly into the tumors. Mice were examined daily for survival studies.
Statistical analysis
All statistical analyses were performed using Graphpad Prism 4 (GraphPad Software Inc.). Sample size for each group was ≥3 and numerical data was reported as Mean±SEM. Comparisons between two groups were conducted using Student's t test or Mann–Whitney test as appropriate. Kaplan–Meier survival curves were generated and log rank test was applied to compare survival distributions. All reported p values were two-sided and considered to be statistically significant at * p < 0.05, **p < 0.01, ***p < 0.001.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by the NIH grant R01CA122930 (MS Lesniak).
References
- 1.Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ, Nieva J, Hwang TH, Moon A, Patt R et al.. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature 2011; 477:99-102; PMID:21886163; http://dx.doi.org/ 10.1038/nature10358 [DOI] [PubMed] [Google Scholar]
- 2.Heimberger AB, Sampson JH. Immunotherapy coming of age: what will it take to make it standard of care for glioblastoma? Neuro Oncol 2011; 13:3-13; PMID:21149252; http://dx.doi.org/ 10.1093/neuonc/noq169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol 2012; 30:658-70; PMID:22781695; http://dx.doi.org/ 10.1038/nbt.2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Workenhe ST, Mossman KL. Rewiring cancer cell death to enhance oncolytic viro-immunotherapy. Oncoimmunology 2013; 2:e27138; PMID:24498567; http://dx.doi.org/ 10.4161/onci.27138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaufmann JK, Chiocca EA. Glioma virus therapies between bench and bedside. Neuro Oncol 2014; 16:334-51; PMID:24470549; http://dx.doi.org/ 10.1093/neuonc/not310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going viral with cancer immunotherapy. Nat Rev Cancer 2014; 14:559-67; PMID:24990523; http://dx.doi.org/ 10.1038/nrc3770 [DOI] [PubMed] [Google Scholar]
- 7.Nettelbeck DM, Rivera AA, Balague C, Alemany R, Curiel DT. Novel oncolytic adenoviruses targeted to melanoma: specific viral replication and cytolysis by expression of E1A mutants from the tyrosinase enhancer/promoter. Cancer Res 2002; 62:4663-70; PMID:12183423 [PubMed] [Google Scholar]
- 8.Kamizono J, Nagano S, Murofushi Y, Komiya S, Fujiwara H, Matsuishi T, Kosai K. Survivin-responsive conditionally replicating adenovirus exhibits cancer-specific and efficient viral replication. Cancer Res 2005; 65:5284-91; PMID:15958575; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-2657 [DOI] [PubMed] [Google Scholar]
- 9.Kirn DH, Wang Y, Le Boeuf F, Bell J, Thorne SH. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med 2007; 4:e353; PMID:18162040; http://dx.doi.org/ 10.1371/journal.pmed.0040353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulasov IV, Rivera AA, Nettelbeck DM, Rivera LB, Mathis JM, Sonabend AM, Tyler M, Wang M, Douglas JT, Lesniak MS. An oncolytic adenoviral vector carrying the tyrosinase promoter for glioma gene therapy. Int J Oncol 2007; 31:1177-85; PMID:17912445 [PubMed] [Google Scholar]
- 11.Tumilasci VF, Oliere S, Nguyen TL, Shamy A, Bell J, Hiscott J. Targeting the apoptotic pathway with BCL-2 inhibitors sensitizes primary chronic lymphocytic leukemia cells to vesicular stomatitis virus-induced oncolysis. J Virol 2008; 82:8487-99; PMID:18579592; http://dx.doi.org/ 10.1128/JVI.00851-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorer DE, Nettelbeck DM. Targeting cancer by transcriptional control in cancer gene therapy and viral oncolysis. Adv Drug Deliv Rev 2009; 61:554-71; PMID:19394376; http://dx.doi.org/ 10.1016/j.addr.2009.03.013 [DOI] [PubMed] [Google Scholar]
- 13.Cripe TP, Wang PY, Marcato P, Mahller YY, Lee PW. Targeting cancer-initiating cells with oncolytic viruses. Mol Ther 2009; 17:1677-82; PMID:19672244; http://dx.doi.org/ 10.1038/mt.2009.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiocca EA, Blair D, Mufson RA. Oncolytic viruses targeting tumor stem cells. Cancer Res 2014; 74:3396-8; PMID:24753541; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-0290 [DOI] [PubMed] [Google Scholar]
- 15.Pol J, Bloy N, Obrist F, Eggermont A, Galon J, Cremer I, Erbs P, Limacher JM, Preville X, Zitvogel L et al.. Trial Watch: oncolytic viruses for cancer therapy. Oncoimmunology 2014; 3:e28694; PMID:25097804; http://dx.doi.org/ 10.4161/onci.28694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al.. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/ 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K et al.. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122-33; PMID:23724867; http://dx.doi.org/ 10.1056/NEJMoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF et al.. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013; 368:1509-18; PMID:23527958; http://dx.doi.org/ 10.1056/NEJMoa1215134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature 2001; 411:375-9; PMID:11357145; http://dx.doi.org/ 10.1038/35077241 [DOI] [PubMed] [Google Scholar]
- 20.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 2006; 6:295-307; PMID:16557261; http://dx.doi.org/ 10.1038/nri1806 [DOI] [PubMed] [Google Scholar]
- 21.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 2009; 30:636-45; PMID:19464986; http://dx.doi.org/ 10.1016/j.immuni.2009.04.010 [DOI] [PubMed] [Google Scholar]
- 22.Devaud C, John LB, Westwood JA, Darcy PK, Kershaw MH. Immune modulation of the tumor microenvironment for enhancing cancer immunotherapy. Oncoimmunology 2013; 2:e25961; PMID:24083084; http://dx.doi.org/ 10.4161/onci.25961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heimberger AB, Abou-Ghazal M, Reina-Ortiz C, Yang DS, Sun W, Qiao W, Hiraoka N, Fuller GN. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res 2008; 14:5166-72; PMID:18698034; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-0320 [DOI] [PubMed] [Google Scholar]
- 24.Jacobs JF, Idema AJ, Bol KF, Grotenhuis JA, de Vries IJ, Wesseling P, Adema GJ. Prognostic significance and mechanism of Treg infiltration in human brain tumors. J Neuroimmunol 2010; 225:195-9; PMID:20537408; http://dx.doi.org/ 10.1016/j.jneuroim.2010.05.020 [DOI] [PubMed] [Google Scholar]
- 25.Crane CA, Ahn BJ, Han SJ, Parsa AT. Soluble factors secreted by glioblastoma cell lines facilitate recruitment, survival, and expansion of regulatory T cells: implications for immunotherapy. Neuro Oncol 2012; 14:584-95; PMID:22406925; http://dx.doi.org/ 10.1093/neuonc/nos014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ooi YC, Tran P, Ung N, Thill K, Trang A, Fong BM, Nagasawa DT, Lim M, Yang I. The role of regulatory T-cells in glioma immunology. Clin Neurol Neurosurg 2014; 119:125-32; PMID:24582432; http://dx.doi.org/ 10.1016/j.clineuro.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 27.Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon KS, Auffinger B, Tobias AL, Han Y, Lesniak MS. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res 2012; 18:6110-21; PMID:22932670; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wainwright DA, Chang AL, Dey M, Balyasnikova IV, Kim CK, Tobias A, Cheng Y, Kim JW, Qiao J, Zhang L et al.. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res 2014; 20:5290-301; PMID:24691018; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-0514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonabend AM, Ulasov IV, Han Y, Lesniak MS. Oncolytic adenoviral therapy for glioblastoma multiforme. Neurosurg Focus 2006; 20:E19; PMID:16709024; http://dx.doi.org/ 10.3171/foc.2006.20.4.12 [DOI] [PubMed] [Google Scholar]
- 30.Nandi S, Ulasov IV, Tyler MA, Sugihara AQ, Molinero L, Han Y, Zhu ZB, Lesniak MS. Low-dose radiation enhances survivin-mediated virotherapy against malignant glioma stem cells. Cancer Res 2008; 68:5778-84; PMID:18632631; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-6441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ulasov IV, Sonabend AM, Nandi S, Khramtsov A, Han Y, Lesniak MS. Combination of adenoviral virotherapy and temozolomide chemotherapy eradicates malignant glioma through autophagic and apoptotic cell death in vivo. Br J Cancer 2009; 100:1154-64; PMID:19277041; http://dx.doi.org/ 10.1038/sj.bjc.6604969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim CK, Ahmed AU, Auffinger B, Ulasov IV, Tobias AL, Moon KS, Lesniak MS. N-acetylcysteine amide augments the therapeutic effect of neural stem cell-based antiglioma oncolytic virotherapy. Mol Ther 2013; 21:2063-73; PMID:23883863; http://dx.doi.org/ 10.1038/mt.2013.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woller N, Knocke S, Mundt B, Gurlevik E, Struver N, Kloos A, Boozari B, Schache P, Manns MP, Malek NP et al.. Virus-induced tumor inflammation facilitates effective DC cancer immunotherapy in a Treg-dependent manner in mice. J Clin Invest 2011; 121:2570-82; PMID:21646722; http://dx.doi.org/ 10.1172/JCI45585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Andaloussi A, Han Y, Lesniak MS. Prolongation of survival following depletion of CD4+CD25+ regulatory T cells in mice with experimental brain tumors. J Neurosurg 2006; 105:430-7; PMID:16961139; http://dx.doi.org/ 10.3171/jns.2006.105.3.430 [DOI] [PubMed] [Google Scholar]
- 35.Maes W, Rosas GG, Verbinnen B, Boon L, De Vleeschouwer S, Ceuppens JL, Van Gool SW. DC vaccination with anti-CD25 treatment leads to long-term immunity against experimental glioma. Neuro Oncol 2009; 11:529-42; PMID:19336528; http://dx.doi.org/ 10.1215/15228517-2009-004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reardon DA, Freeman G, Wu C, Chiocca EA, Wucherpfennig KW, Wen PY, Fritsch EF, Curry WT Jr, Sampson JH, Dranoff G. Immunotherapy advances for glioblastoma. Neuro Oncol 2014; 16:1441-58; PMID:25190673; http://dx.doi.org/ 10.1093/neuonc/nou212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meir EG. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro Oncol 2005; 7:134-53; PMID:15831232; http://dx.doi.org/ 10.1215/S1152851704001115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer U, Radermacher J, Mayer J, Mehraein Y, Meese E. Tumor hypoxia: Impact on gene amplification in glioblastoma. Int J Oncol 2008; 33:509-15; PMID:18695880; http://dx.doi.org/ 10.3892/ijo_00000034 [DOI] [PubMed] [Google Scholar]
- 39.Pan F, Yu H, Dang EV, Barbi J, Pan X, Grosso JF, Jinasena D, Sharma SM, McCadden EM, Getnet D et al.. Eos mediates Foxp3-dependent gene silencing in CD4+ regulatory T cells. Science 2009; 325:1142-6; PMID:19696312; http://dx.doi.org/ 10.1126/science.1176077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma MD, Huang L, Choi JH, Lee EJ, Wilson JM, Lemos H, Pan F, Blazar BR, Pardoll DM, Mellor AL et al.. An inherently bifunctional subset of Foxp3+ T helper cells is controlled by the transcription factor eos. Immunity 2013; 38:998-1012; PMID:23684987; http://dx.doi.org/ 10.1016/j.immuni.2013.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, Kaur B, Louis DN, Weissleder R, Caligiuri MA et al.. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci U S A 2006; 103:12873-8; PMID:16908838; http://dx.doi.org/ 10.1073/pnas.0605496103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fulci G, Dmitrieva N, Gianni D, Fontana EJ, Pan X, Lu Y, Kaufman CS, Kaur B, Lawler SE, Lee RJ et al.. Depletion of peripheral macrophages and brain microglia increases brain tumor titers of oncolytic viruses. Cancer Res 2007; 67:9398-406; PMID:17909049; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alvarez-Breckenridge CA, Yu J, Price R, Wojton J, Pradarelli J, Mao H, Wei M, Wang Y, He S, Hardcastle J et al.. NK cells impede glioblastoma virotherapy through NKp30 and NKp46 natural cytotoxicity receptors. Nat Med 2012; 18:1827-34; PMID:23178246; http://dx.doi.org/ 10.1038/nm.3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alvarez-Breckenridge CA, Yu J, Caligiuri MA, Chiocca EA. Uncovering a novel mechanism whereby NK cells interfere with glioblastoma virotherapy. Oncoimmunology 2013; 2:e23658; PMID:23734319; http://dx.doi.org/ 10.4161/onci.23658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc Natl Acad Sci U S A 2006; 103:7048-53; PMID:16632602; http://dx.doi.org/ 10.1073/pnas.0601554103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol 2013; 13:461-7; PMID:23681097; http://dx.doi.org/ 10.1038/nri3464 [DOI] [PubMed] [Google Scholar]
- 47.Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, Tanaka S, Bluestone JA, Takayanagi H. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med 2014; 20:62-8; PMID:24362934; http://dx.doi.org/ 10.1038/nm.3432 [DOI] [PubMed] [Google Scholar]
- 48.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest 2007; 117:1147-54; PMID:17476344; http://dx.doi.org/ 10.1172/JCI31178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, Shi YX, Levin VA, Yung WK, Kyritsis AP. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene 2000; 19:2-12; PMID:10644974; http://dx.doi.org/ 10.1038/sj.onc.1203251 [DOI] [PubMed] [Google Scholar]
- 50.Ohlfest JR, Andersen BM, Litterman AJ, Xia J, Pennell CA, Swier LE, Salazar AM, Olin MR. Vaccine injection site matters: qualitative and quantitative defects in CD8 T cells primed as a function of proximity to the tumor in a murine glioma model. J Immunol 2013; 190:613-20; PMID:23248259; http://dx.doi.org/ 10.4049/jimmunol.1201557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell 1994; 76:17-27; PMID:8287475; http://dx.doi.org/ 10.1016/0092-8674(94)90169-4 [DOI] [PubMed] [Google Scholar]
- 52.Qiao J, Wang H, Kottke T, Diaz RM, Willmon C, Hudacek A, Thompson J, Parato K, Bell J, Naik J et al.. Loading of oncolytic vesicular stomatitis virus onto antigen-specific T cells enhances the efficacy of adoptive T-cell therapy of tumors. Gene Ther 2008; 15:604-16; PMID:18305577; http://dx.doi.org/ 10.1038/sj.gt.3303098 [DOI] [PMC free article] [PubMed] [Google Scholar]