Abstract
Melanoma has a propensity for lymphatogenous metastasis. Improved understanding of the sentinel lymph node (SLN) immunological environment may improve outcomes. The immune phenotype of fresh melanoma SLNs (n = 13) were compared to fresh control lymph nodes (n = 13) using flow cytometry. RNA was isolated from CD4+ T cells of the SLN and control lymph node and assessed for Th1/Th2 gene expression pathways using qRT-PCR. In addition, VEGF expression was compared between primary melanoma (n = 6) and benign nevi (n = 6) using immunohistochemistry. Melanoma SLNs had fewer CD8+ T cells compared to controls (9.2% vs. 19.5%, p = 0.0005). The CD8+ T cells within the SLN appeared to have an exhausted phenotype demonstrated by increased PD-1 mRNA expression (2.2% vs. 0.8%, p = 0.004) and a five-fold increase in CTLA-4 mRNA expression. The SLN also contained an increased number of CD14 (22.7% vs. 7.7%, p = 0.009) and CD68 (9.3% vs. 2.7%, p = 0.001) macrophages, and CD20 B cells (31.1% vs. 20.7%, p = 0.008), suggesting chronic inflammation. RT-PCR demonstrated a significant Th2 bias within the SLN. In vitro studies demonstrated a similar Th2 polarization with VEGF treatment of control lymph nodes. The primary melanoma demonstrated strong VEGF expression and an increase in VEGFR1 within the SLN. Melanoma is associated with Th2-mediated “chronic inflammation,” fewer cytotoxic T cells, and an exhausted T cell phenotype within the SLN combined with VEGF overproduction by the primary melanoma. These immunologic changes precede nodal metastasis and suggests consideration of VEGF inhibitors in future immunotherapy studies.
Keywords: CD4 T cells, CD8 T cells, immunotherapy, inflammation, lymphocytes, melanoma, sentinel lymph node, Th2 polarization, VEGF
Introduction
Metastasis to the regional lymph nodes is one of the most important prognostic factors in melanoma and is associated with a high risk of subsequent recurrence and death.1 Lymphatic mapping and SLN biopsy are techniques currently utilized to identify the first tumor-draining lymph nodes which represent the first site of contact between tumor-associated antigens, the adaptive immune system and paradoxically, the first expected site of metastasis. In order for melanoma tumor cells to metastasize they must escape immune surveillance and mobilize to the regional lymph nodes while avoiding immune detection within the SLN. Previous studies have demonstrated that the regional lymph node basin is immunosuppressed in a gradient fashion with less mitogenic and alloantigen-induced lymphocyte proliferation in the lymph nodes nearest the primary melanoma compared to those farthest from the effects of the tumor.2 Studies demonstrate fewer and less responsive CD8+ T cells and an increase of suppressive CD4+ T cells within the lymph nodes nearest the tumor.3,4 The CD8+ T cells that are present have a significant reduction in the expression of the co-stimulatory molecule CD28 indicating that the CD8+ T cells may not be adequately activated and primed.5 This suggests that primary tumor-derived factors are suppressing the regional immune response at an early stage. This immune subversion may be critical to facilitate regional lymph node metastasis. A better understanding of the regional immunological changes and what potential soluble mediators are being released by the primary tumor may lead to novel targeted therapeutic agents to prevent metastasis of melanoma.
Patients with more advanced disease have profound systemic immunological changes; including a significant decrease in activated dendritic cells, increase in regulatory T cells and exhausted PD-1 expressing T cells, poor T cell function and a Th2 cytokine profile.6 Th2 dominance and chronic inflammation may be permissive to tumor progression and metastases as patients with elevated inflammatory markers have a poor survival.7 We hypothesize that these immunological changes may occur much earlier, prior to hematogenous dissemination and may affect the regional lymphatic system. Specifically, the Th2 dominance that is present in metastatic melanoma may be present in the regional lymph nodes and could be contributing to lymphatic metastasis of melanoma. Th2 polarization may be a therapeutic target in early stage melanoma preventing or treating lymphatogenous metastasis.
VEGF is an important immune modulator that suppresses dendritic cell maturation and modulates lymphocyte endothelial trafficking. VEGF is found in increased quantities within peripheral blood of melanoma patients and is predictive of response to immunotherapy with anti-CTLA-4 antibodies.8 VEGF has been implicated in Th2 polarization of systemic immunity in metastatic melanoma, and treatment with anti-VEGF antibodies has resulted in a significant increase in CD8+ cell infiltration and a significant decrease in the Th2 cytokine IL-6.9,10 We hypothesize that VEGF production by the primary melanoma induces Th2 polarization of the SLN and impairment of the T cell response to tumor antigens. This immune dysfunction may occur within the SLN at a very early stage, prior to recognizable metastasis to the SLN, and may be necessary to prepare the SLN for seeding of metastatic disease. Herein, we provide prospective data in humans demonstrating the immunological dysfunction of the SLN that precedes nodal metastasis.
Results
Clinical parameters
Fresh SLNs were prospectively collected from 13 melanoma patients and compared to fresh intramammary or low lying axillary lymph nodes from 13 patients undergoing prophylactic mastectomy. The clinical and pathologic characteristics of the melanoma SLN cohort are illustrated in Table 1. Briefly, the median age of the melanoma patients was 61 (range 45–65) years, eight (62%) were males, and the most common location was the axilla (11, 85%). The median Breslow depth was 1.2 (0.9–2.7) mm and only 2 (15%) were ulcerated. Tumor infiltrating lymphocytes were non-brisk or absent in 12 (92%) and brisk in only 1 (8%) tumor. Four (31%) patients had a positive SLN, while the rest were negative on H&E and IHC. The median age of the patients from whom the control lymph nodes were taken was 43 (range 38–59) years which was not different than the melanoma patients (p = 0.2); all the controls were female. There was no association between the clinical parameters of age, sex, Breslow depth, ulceration, lymph node metastasis, and SLN expression of immune cell type.
Table 1.
Clinical and pathological characteristics of the thirteen patients who underwent sentinel lymph node biopsy for melanoma and the thirteen control patients undergoing prophylactic mastectomy (median and IQR or number and frequency are reported)
| Characteristics | Melanoma (n = 13) | Breast (n = 13) | p value |
|---|---|---|---|
| Age | 61 (45–65) | 43 (38–59) | 0.2 |
| Sex | |||
| Male | 8 (62%) | 0 (0%) | <0.001 |
| Female | 5 (38%) | 13 (100%) | |
| Location | |||
| Axilla | 11 (85%) | 16 (100%) | 0.33 |
| Inguinal | 2 (15%) | 0 (0%) | |
| Breslow depth | 1.2 (0.9–2.7) | NA | |
| Ulceraton | 2 (15%) | ||
| TIL response | NA | ||
| Brisk | 1 (8%) | ||
| Non-brisk | 11 (85%) | ||
| Absent | 1 (8%) | ||
| SLN status | NA | ||
| Positive | 4 (31%) | ||
| Negative | 9 (69%) |
CD8 T cell depletion of the SLN
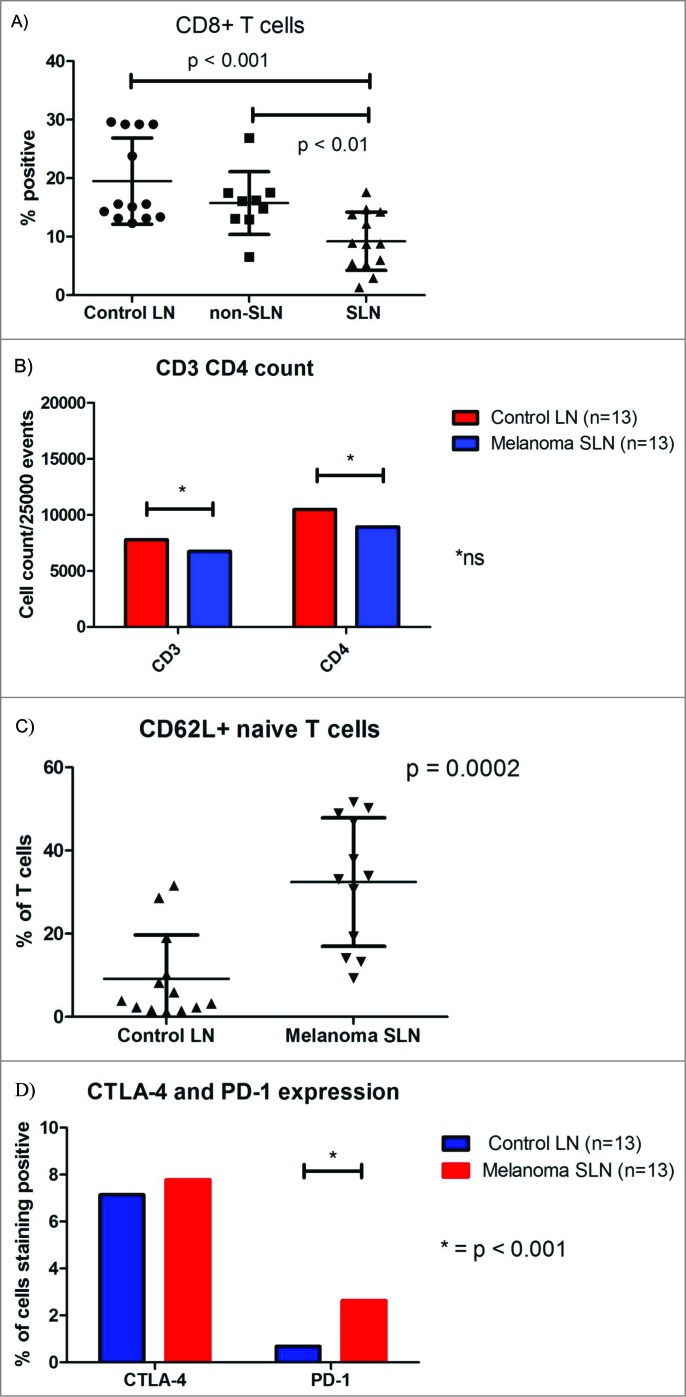
We previously demonstrated depletion of CD8+ T cells within the melanoma draining SLN compared to healthy control lymph nodes using immunohistochemistry. This finding was evident regardless whether the SLN harbored metastasis.5 Whether this depletion of CD8+ T cells was due to apoptosis, failure of T cell influx into the SLN, increased efflux of CD8+ T cells out of the SLN, or decreased expression of the CD8+ receptor is unknown. Using flow cytometry, we again found decreased expression of CD3/CD8+ cytotoxic T cells within the melanoma SLN compared to the control lymph nodes (SLN 9.2% ± 4.9% vs. Control 19.5% ± 7.4%, p = 0.0005) (Figs. 1A and B). The decrease in CD8+ T cells within the SLN occurred regardless of whether the SLN harbored metastasis or not. The relative decrease in CD8+ T cells was not due to a relative increase in CD4+ helper T cells or total number of T cells, because these populations were similar within the SLN and control LN. Also, the decrease in CD8+ T cells was constant when the absolute cell count was analyzed. In addition, there was no change in the ratio of CD8+ and CD4+ positive CD3 T cells and no difference in the incidence of T cell death (as quantified by propidium iodine). Therefore, we questioned whether the trafficking of CD8+ T cells was altered within the SLN. Given the importance of CD62L and CD69 expression for both T cell entry into the SLN and efflux out of the SLN, respectively, we compared the expression of CD3+ CD62L+ and CD3+ CD69+ T cells between the different LNs. The SLN had greater expression of CD62L+ CD3+ T cells than the control LN, (32.4% ± 15.4% vs. 9.2% ± 10.5%, p = 0.0002, respectively, Fig. 1C suggesting an increased proportion of naive T cells.
Figure 1.
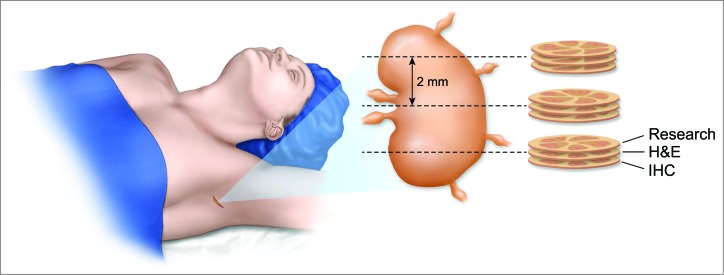
Artist depiction of technique for obtaining fresh nodal tissue from the sentinel lymph node for immunological analysis without compromising pathological analysis. The surrounding fat is removed from the sentinel lymph node and the node is sectioned into 2 mm gross sections. Each section is placed on a cryotome and a single shaving is removed from the top prior to freezing. This tissue is collected for immunological profiling. The remainder of the 2 mm section is then utilized for pathological analysis. On average there were four 2 mm sections processed resulting in four shavings from throughout the sentinel lymph node.
Further evidence of dysfunctional T cell trafficking in melanoma comes from RNA expression arrays from the CD4+ and CD8 T+ cells within the SLN and controls. The most striking differences we noted were an increase in the expression of CCR2 (six-fold) and CCR4 (six-fold) mRNA within the CD4+ T cells of the SLN (Table 2). These chemokines are associated with trafficking of Th2 and regulatory T cells which suggests that these cells are being recruited to the SLN prior to metastasis from the melanoma.11 Meanwhile, expression of CCL5 (RANTES), an important chemokine associated with delaying tumor growth by enhancing CD8+ T cell infiltration and effector function was decreased (five-fold) within CD8+ T cells residing in the melanoma SLN resulting in a 12-fold downregulation of CD40L expression and suggest impaired T cell priming and function (CD8+ data not shown).12
Table 2.
Melanoma SLN CD4+ T helper cell gene expression of particular interest to Th1 and Th2 function
| Gene name | Fold change | Function | ||
|---|---|---|---|---|
| Increased expression | ||||
| IL-13R | 20.28 | Receptor for IL-13 | ||
| TGFB3 | 15.33 | Inhibitory cytokine produced by regulatory T cells | ||
| STAT6 | 10.94 | Downstream mediator of IL-4 signaling | ||
| STAT4 | 9.43 | Th1 signaling | ||
| TNF | 7.48 | Chronic exposure impairs T cell activation | ||
| CCR4 | 6.36 | Preferentially expressed by Th2 cells | ||
| CCR2 | 6.17 | Chemokine for monocyte infiltration | ||
| IL-13 | 4.93 | Th2 cytokine | ||
| IL-6R | 4.66 | Th2 cytokine | ||
| CD40LG | 4.26 | Co-stimulatory and regulates B cell function | ||
| IL-17A | 3.83 | Chronic inflammatory cytokine | ||
| CREBBP | 3.79 | Th2 transcription factor | ||
| NFATC1 | 3.64 | Th2 transcription factor | ||
| CCR5 | 3.55 | Potentiates interaction between T cell and APC | ||
| NFATC2 | 3.43 | Th2 transcription factor | ||
| CSF2 | 3.32 | Stimulates granulocytes and monocytes | ||
| IL-4 | 3.05 | Th2 cytokine | ||
| Decreased expression | ||||
| CCL5 | 0.12 | Migration of Th1 T cells | ||
| SOCS2 | 0.13 | negative regulator of Th2 polarity | ||
| TLR6 | 0.18 | Drives Th1 response | ||
| IL-12B | 0.19 | Th1 cytokine | ||
| IRF4 | 0.21 | maintains Th1 polarity | ||
| IFNγ | 0.27 | Th1 cytokine | ||
| SOCS1 | 0.29 | maintains Th1 polarity | ||
| CD4 | 0.31 | T helper cell expression | ||
Exhausted T cells
The CD8+ T cells present within the SLN appear to have a functionally “exhausted” phenotype as demonstrated by a slightly greater expression of PD-1 (SLN 2.2% ± 1.1% vs. Control 0.8% ± 0.9%, p = 0.004) on flow cytometry (Fig. 1D). Although we did not find an increase in CTLA-4 expression by CD3 T cells using flow cytometry, we did find a five-fold increase in CTLA-4 RNA expression specifically by CD8+ T cells within melanoma SLN compared to controls (data not shown). Neither of these markers of exhaustion was associated with SLN metastasis. This analysis, however, is limited by our small (n = 4) sample size of positive SLNs. IFNγ gene expression by CD4+ T cells was decreased three-fold in the SLN (Table 2). Because IFNγ is a key cytokine in the antitumor immune response and CD4+ T cells are the major cellular source of IFNγ; this decrease in IFNγ gene expression by CD4+ T cells within the SLN may allow melanoma to disseminate and proliferate within the lymph node.13 The observed downregulation of the IFNγ signaling pathway in CD4+ T cells residing within the SLN appears to be an early step toward the systemic depression of IFNγ signaling found in metastatic melanoma.14
Th2 polarization of the SLN in melanoma
We have previously reported Th2 polarization of CD4+ T cells from patients with metastatic melanoma compared to healthy controls;6 however, we were unable to detect systemic Th2 polarization in non-metastatic melanoma. We hypothesized that Th2 polarization may occur within the regional lymph nodes in localized melanoma. In the current study, we utilized flow cytometry (n = 13) and found an influx of CD68+ macrophages (SLN 9.3% ± 6.8% vs. Control 2.7% ± 1.8%, p = 0.001), CD14 macrophages (SLN 22.7% ± 18.7% vs. 7.7% ± 5.6%, p = 0.009), as well as CD20 B cells (SLN 31.1% ± 5.4% vs. Control 20.7% ± 2.8%, p = 0.008) within the SLN, suggesting a chronic inflammatory state (Table 3). Using non-supervised hierarchical clustering of gene expression data, we found significant increases in Th2 proliferation, migration, and cell quantity with a concomitant decrease in Th1 proliferation, migration, and cell quantity within the melanoma SLN compared to controls (Fig. 2). Of the 86 Th1 and Th2 pathway genes evaluated, 57 were expressed at comparable levels in CD4+ T cells from melanoma SLN and control LN (not shown), 17 were upregulated, and 8 were downregulated in SLNs (Table 2). Notably, there was increased expression of the Th2 cytokines IL-13 (5-fold increased), IL-13R (20 fold increased), IL-4 (3-fold increase), IL-6R (4-fold increase), TGF-β (15-fold increased), and TNF-α (7-fold increase) in SLNs. Likewise, there was a decrease in Th1 cytokines and transcription factors in SLNs; IFNγ (3-fold reduction), IL-12B (5-fold reduction), CCL5 (RANTES) (8-fold reduction), IRF4 (5-fold reduction), SOCS1 (3-fold reduction) and SOCS2, a negative regulator of Th2 differentiation (8-fold reduction) (Table 2).
Table 3.
Immune cell phenotype of the control lymph node, non-SLN and SLN. Mean and standard deviation is reported. Due to multiple comparisons only a p-value ≤ 0.008 is considered statistically significant (Bonferroni correction).
| Immune cell type | Control LN | Non-SLN | SLN | Control vs. Non-SLN p value | Non-SLN vs. SLN p value | Control vs. SLN p value |
|---|---|---|---|---|---|---|
| CD3+ CD8+ (effector T cell) | 19.51 ± 7.36 | 15.74 ± 7.93 | 9.22 ± 4.98 | ns | 0.005 | 0.0005 |
| CD3+ CD62L+ (naive T cell) | 9.19 ± 10.52 | 26.82 ± 23.27 | 32.42 ± 15.43 | ns | ns | 0.0002 |
| CD3+ CD69+ (activated T cell) | 16.86 ± 13.38 | 17.63 ± 7.38 | 17.21 ± 10.72 | ns | ns | ns |
| CD3+ CD152 (CTLA-4) | 7.62 ± 5.90 | 7.38 ± 5.51 | 7.79 ± 3.32 | ns | ns | ns |
| CD3+ CD279 (PD-1) | 0.78 ± 0.93 | 2.25 ± 1.31 | 2.20 ± 1.13 | 0.005 | ns | 0.004 |
| CD11c+ CD86+ (mature dendritic cell) | 9.74 ± 8.70 | 7.59 ± 7.82 | 8.01 ± 6.29 | ns | ns | ns |
| CD141+ CD86+ (myeloid dendritic cell) | 23.91 ± 12.32 | 13.32 ± 15.78 | 15.02 ± 6.01 | ns | ns | ns |
| CD123+ (plasmacytoid dendritic cell) | 6.62 ± 8.79 | 3.49 ± 2.74 | 14.74 ± 18.35 | ns | ns | ns |
| CD20+ (B cell) | 20.73 ± 2.76 | 27.63 ± 2.85 | 31.14 ± 5.40 | s | ns | 0.008 |
| CD14+ (Macrophage) | 7.72 ± 5.56 | 12.69 ± 10.09 | 22.66 ± 18.70 | ns | ns | 0.009 |
| CD68+ (macrophage) | 2.70 ± 1.79 | 5.12 ± 3.58 | 9.26 ± 6.79 | ns | ns | 0.001 |
| CD4+ CD25+ FOXp3+ (Treg) | 25.38 ± 13.04 | 51.12 ± 26.39 | 35.1 ± 20.97 | 0.007 | ns | ns |
Figure 2.
(A) Box-plot illustrating the difference in median percentage of CD3+ CD8+ T cells. (B) Box-plot demonstrating differences in median percentage of CD3+ CD4+ and CD3+ and CD8+ T cells. (C) Box-plot demonstrating differences in median CD3+ CD62L+ cells (Controls n = 13, non-SLN n = 9 and melanoma SLN n = 13). (D) Box-plot illustrating differences in median percentage of CTLA-4 and PD-1 expression.
The neighboring non-SLN is affected but to a lesser degree
The neighboring non-SLN had an immune cell profile that was intermediary to the SLN and the control lymph node. These profiles are evident in Fig. 1A where the percentage of CD8+ T cells within the non-SLN is intermediate to the control and SLN. Similarly, the non-SLN had expression of CD20+ B cells, CD14+ and CD68+ macrophages that was intermediate to the controls and SLN (Table 3). The non-SLN also had an increase in the expression of PD-1 compared to controls (2.3 ± 1.3 vs. 0.7 ± 0.9, p = 0.005), as well as a two-fold increase in regulatory T cells (51.1 ± 26.4 vs. 25.4 ± 13.0, p = 0.007, respectively) (Table 3). In contrast, the number of regulatory T cells within the SLN was comparable to the control lymph nodes. Interestingly, there were no differences observed between non-SLN and SLN (except effector T cells).
VEGF A as a soluble mediator of SLN Th2 polarization
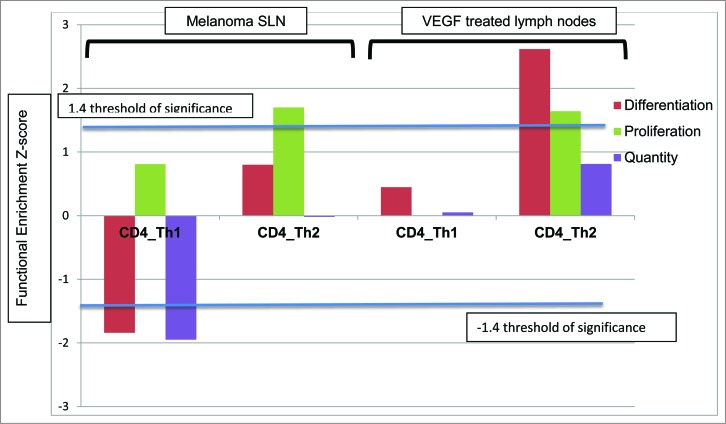
We have previously identified VEGF A as a potential mediator of systemic Th2 polarization within metastatic melanoma.6 Pathway analysis revealed several downstream mediators of VEGF A signaling (STAT3, MAPK8, and CD40-LG) to be upregulated within CD4+ T cells of melanoma SLNs, suggesting that VEGF signaling may be active in localized melanoma. To further investigate the role of VEGF A on Th2 polarization of the SLN, we treated lymph nodes from healthy controls with VEGF A. This resulted in a repolarization from Th1 to a Th2 bias (Fig. 2). Fig. 2 shows the similar decrease in Th1 differentiation, proliferation and quantity and simultaneously an increase in Th2 differentiation, proliferation and quantity in both the melanoma SLN and the control lymph nodes treated with VEGF A. Gene expression profiles of VEGF A treated control lymph node were dramatically different than the untreated control lymph node. VEGF A alone was sufficient for repolarization of a Th1 biased control lymph node to a Th2 biased lymph node.
VEGF A expression of primary melanoma
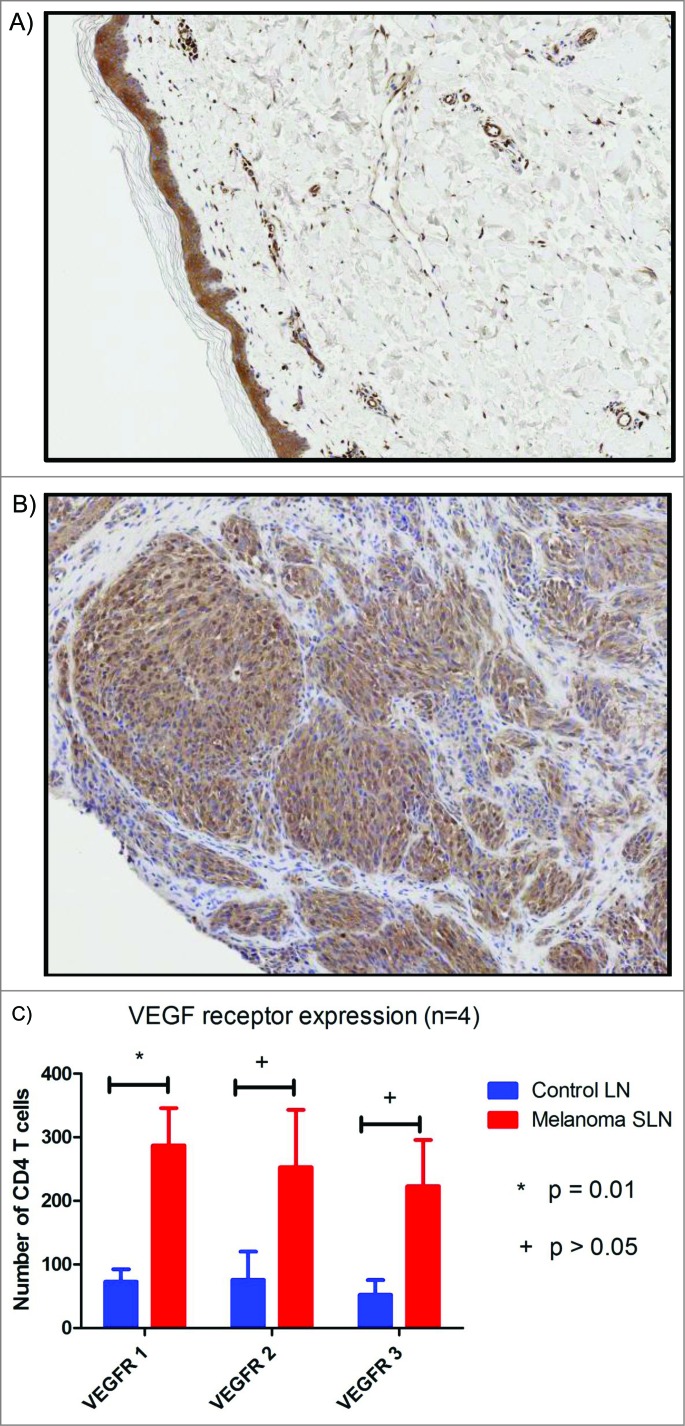
Of the 24 melanoma patients enrolled into the study only six had archived, formalin-fixed, paraffin-embedded blocks of the primary tumor available for study. Immunohistochemical analysis of the primary melanoma demonstrated strongly positive, homogenous cytoplasmic VEGF A staining in four of the six (67%) samples. One (17%) primary melanoma had weak staining and the other one (17%) was negative. There were no differences in the expression of VEGF A with respect to Breslow level. In contrast, no VEGF A expression was identified in the benign nevi (Fig. 3A and B).
Figure 3.
Histogram demonstrating the functional enrichment z scores of Th1/Th2 pathways within CD4+ T cells of SLN draining melanoma compared to control LNs, respectively (n = 4) on the left and CD4+ T cells of control lymph nodes treated with VEGF compared to untreated control lymph nodes on the right (n = 3).
VEGF receptor expression within the SLN
In order to investigate potential downstream VEGF signaling within the SLN, we performed intracellular staining with flow cytometry. We compared four SLN and four control lymph nodes and found a significant increase in the number of VEGFR1 expressed by CD4+ T cells within the SLN (Fig. 3C). VEGFR2, VEGFR3 or neuropilin expression between melanoma and control lymph nodes was not statistically significant (p = 0.13 VEGF2; p = 0.07 VEGF3) and this could be secondary to type 2 error. VEGFR1 is known to potentiate VEGFR2 activation, suggesting that VEGFR signaling is occurring within the SLN. Further investigation of phosphorylated VEGFR expression on CD4+ T cells, as well as other cell types within the SLN, is warranted.
Discussion
Our data builds off our previous immunohistochemical study demonstrating marked alterations in the innate and humoral immunity within the first draining lymph node of melanoma.5 These alterations in immune composition and function precede nodal metastasis and may be important in preparing the SLN to tolerate metastatic cells. We have demonstrated a decrease of cytotoxic CD8+ T cells within the SLN of patients with melanoma, regardless of metastatic involvement using both immunohistochemistry and flow cytometry.5 The CD8+ depletion could possibly be the result of dysfunctional T cell trafficking with preferential increase in naive CD62L expressing T cells and fewer CD62L-effector T cells. Downregulation of CD62L expression is necessary to retain the T cells within the lymph node to facilitate antigen presentation, co-stimulation, and T cell proliferation.15 Once naive T cells become effector T cells, they upregulate CD69 expression and egress from the lymph node to traffic to the peripheral tissue. However, we found fewer CD62L-T cells within the SLN, suggesting that the T cells are not retained within the LN for maximum stimulation and rapidly egress from the lymph node; potentially, this egress of T cells may explain why we found a reduction in CD8+ T cells. The T cells do not appear to be trafficking to the primary tumor as only one melanoma primary had a brisk TIL response and the TIL response within the primary was not associated with decreased CD8+ T cell expression within the SLN. Alternatively, fewer naive T cells may become activated within the SLN due to impaired activation of naive T cells or exhaustion of activated T cells. Theses change in immune profile may explain the paradox of melanoma proliferating and residing within the immune environment of the lymph node without eliciting an effective antitumor immune response.
The CD8+ T cells that are present within the SLN appear to be less functional as illustrated by the increased expression of PD-1 mRNA, a negative T cell regulatory protein on T cells compared to controls. PD-L1 the ligand for PD-1 is expressed on melanoma cells and has correlates with an increased risk of lymph node metastasis and decreased overall survival.16 PD-1/PD-L1 mediated T cell exhaustion begins early in the progression of melanoma and continues to increase with progression of the melanoma.16 Because PD-1/PD-L1 mediated T cell exhaustion occurs early and blockade of PD-1 signaling appears to suppress production of Th2 cytokines, shifting toward a pro-inflammatory Th1 response, PD-1 immune therapies hold promise as an early adjuvant treatment for melanoma.16–18 In addition, we found an increase in the RNA expression of the negative T cell regulatory protein CTLA-4 within the CD8+ T cells residing within the SLN of melanoma patients. CTLA-4-mediated T cell exhaustion is an important checkpoint for preventing exaggerated immune responses. However, in melanoma CTLA-4 appears to be upregulated on the T cell surface, providing inhibitory signals and thereby limiting the antitumor immune response.19 Blockade of CTLA-4 with the monoclonal antibody ipilimumab enhances T cell activation and improves overall survival compared to traditional cytotoxic chemotherapy.20
Furthermore, we demonstrated an influx of macrophages and B cells within the SLN compared to controls. These cell types are suggestive of a chronic inflammatory, Th2 immune response. This finding is consistent with our gene expression analysis which demonstrated Th2 polarity within the SLN. Table 2 and Fig. 2 illustrate that Th2 polarization of the SLN is primarily the result of Th2 activation and to a lesser degree Th1 suppression. Similar to previous gene expression analysis of SLN and non-SLN, we found an increase in Th2 polarizing cytokines including TNF and a decrease in Th1 polarizing cytokines IFN and IL-12B within the SLN.21 However, perhaps because our comparison was to a “normal” lymph node in patients without cancer or inflammation instead of a neighboring non-SLN, we also identified differences in the expression of genes involved in Th1/Th2 differentiation. We have previously demonstrated a systemic Th2 bias in patients with stage IV but not earlier stage melanoma.6 This is the first time to our knowledge anyone has demonstrated a Th2 bias within the SLN of patients with melanoma. The results of our study suggest that the inflammatory cytokine profile of the SLN is different from normal lymph nodes and predisposes to Th2 bias.
VEGF has been associated with a Th2 bias in stage IV melanoma.6 Our data suggests that increased levels of VEGF derived from melanoma could play a role in the Th2 polarization of the SLN in localized melanoma. Immunohistochemical studies demonstrated that about two-thirds of all primary melanomas express VEGF. Increased levels of VEGF expression are found in the vertical growth phase within thicker melanomas and are associated with the propensity for metastasis.22 Increased serum levels of VEGF have been detected in patients with nodal and visceral metastasis, but not in patients with localized melanoma.6,22 However, VEGF may be active within the regional lymph nodes in early stage melanoma as supported by our findings of increased expression of VEGF1 receptors within the CD4+ T cells of the SLN compared to controls. VEGFR-1 signaling has been implicated in inducing T cell tolerance, mainly by inhibiting the maturation of dendritic cells and their migration into lymph nodes23 and VEGFR2 has been demonstrated to be a critical regulator of the Th2-mediated inflammation.23,24 Our group has previously demonstrated that patients with metastatic melanoma have high serum VEGF levels and corresponding systemic Th2 polarization. The tumor was not the source of the Th2 cytokines but was the source of the elevated VEGF levels. In vitro, exposure of normal peripheral blood monocytic cells to VEGF leads to repolarization from Th1 to Th2. In this study, we have similarly, demonstrated that treatment of normal lymph nodes with VEGF leads to repolarization from Th1 to Th2 as well. We are currently measuring VEGF levels in afferent lymphatic channels as we hypothesize that melanoma releases VEGF into the lymphatic channels in early stages resulting in immune modulation and only produces elevated serum VEGF when the melanoma has gained access to the blood stream in stage IV melanoma.
Furthermore, single agent anti-VEGF antibody bevacizumab has been demonstrated to have clinical activity in randomized phase 2 clinical trials of patients’ with advanced metastatic melanoma.25 Neoadjuvant bevacizumab has demonstrated safety and promising clinical efficacy as demonstrated by a decline in S100 and evidence of nodal necrosis26 Currently, a randomized controlled trial of adjuvant bevacizumab given for one year after resection of stage IIB, IIC, and III melanoma is open and enrolling.27 Pre-clinical models also demonstrate that blockade of VEGF signaling enhances the infiltration of adoptively transferred T cells into the tumor, leading to inhibition of melanoma and improved survival with adoptive cell transfer-based immunotherapies.28 The authors postulated that this trafficking of T cells may be due to normalization of the tumor vasculature; however, an alternative hypothesis may be that blockade of VEGF signaling reverses the regional immunosuppression allowing a more robust immunological response. Translational investigations of this cohort may lead to a better understanding of the impact of anti-VEGF therapy on the Th1/Th2 immune balance and are currently underway.
The strength of our study is the novel use of control lymph nodes. Our control lymph nodes were obtained from healthy female patients without evidence of infection or malignancy undergoing an elective procedure. This allowed a true evaluation of a “normal” resting lymph node. Studies utilizing nearby non-SLN from the same nodal basin in melanoma patients may miss key immunological differences. Our comparison demonstrated the non-SLN to have an immunological profile that was more similar to the SLN than the control LN, suggesting that neighboring non-SLNs are being similarly influenced by the melanoma, albeit to a lesser degree.
Despite our limited sample size we identified key immunological changes that are consistent with previous investigations of both systemic and regional immunity. Although, largely descriptional, these findings illustrate the important immunological changes that occur within the SLN prior to lymphatogneous metastasis and identifies VEGF as a potential candidate mediator of these immunological changes. By investigating human lymph nodes, we are able to avoid the pitfalls of key immunologic distinctions between species. For example, it has been demonstrated previously that VEGF has no immunological role in mice; however, we have demonstrated a substantial role in humans.29 The mechanisms responsible for these immunological changes within the SLN are likely multifactorial and additional soluble mediators likely play a role. Thus, further investigation elucidating these mechanisms and evaluating the response within the SLN to inhibition of these mediators is warranted
In conclusion, the initial draining lymph node appears to be critical for priming of the CD8+ T cell response against melanoma. Our data are consistent with previous studies suggesting that the SLN is suppressed in comparison to healthy normal lymph nodes.5,21,30 Melanoma is capable of promoting regional metastasis through poorly understood mechanisms by which melanoma suppresses the adaptive immune response. Our data demonstrates that fewer CD8+ T cells, upregulation of co-inhibitory signals, and evidence of chronic inflammation with Th2 polarization of the SLN may be necessary in order for melanoma to successfully evade the immune system and develop lymphatogenous metastasis. Potential mechanisms identified for these changes include impaired T cell trafficking and/or activation. We have identified VEGF as a potential mediator of these changes as VEGF is widely expressed within most primary melanomas and there appears to be an upregulation of VEGFR1 within the SLN. Lastly, treatment of control lymph nodes with VEGF produces similar Th2 polarization of the control LN.
Methods
Patient population
After approval by the Institutional Review Board, consecutive patients diagnosed with primary cutaneous melanoma ≥1.0 mm Breslow depth or those with Breslow depth > 0.75 mm – 1.0 mm and at least one high risk feature (young age, mitotic rate or ulceration) who were undergoing lymphatic mapping with radiolabeled colloid and methylene blue were prospectively enrolled into the clinical study. Patients with primary or secondary immunodeficiency, including continuous use of NSAIDs were excluded. Patients underwent lymphatic mapping and SLN biopsy using our institution's technique of dual agent mapping.31 Each SLN was identified by observing the path of methylene blue dye and by using a hand-held gamma counter to measure radioactive counts. In addition to the SLN, a single neighboring non-SLN (non-blue and non-radioactive) was removed and sent to the frozen section laboratory. Control lymph nodes were obtained from consecutive females undergoing prophylactic mastectomy. Patients with primary or secondary immunodeficiency, including continuous use of NSAIDs were excluded as were patients who were found to harbor occult cancer in the breast specimen. The entire breast specimen was sent to pathology where the specimen was serially sectioned and examined by a pathology assistant for intramammary or low lying axillary lymph nodes. If any lymph nodes were found, two non-contiguous shavings were sent for study and the remaining lymph node was examined by pathology to exclude occult malignancy. All fresh lymph nodes were dissected free of surrounding fat immediately after retrieval from the patient, cut into 2 mm sections and placed on a cryotome where they were frozen from the bottom up (Fig. 4). Prior to freezing, the tops of two non-continuous sections were shaved off from each 2 mm section and placed in RPMI media and then processed into a single cell suspension using a Milltenyi gentleMACS dissociator (Milltenyi Biotech, Bergisch Gladbach, Germany) according to the manufacturer's directions.
Figure 4.
Representative immunohistochemistry slides (5x) stained with anti-VEGF antibody from (A) benign nevi with no VEGF staining and (B) melanoma with diffuse cytoplasmic staining. (C) Histogram comparing VEGF receptor expression within melanoma SLN and control lymph nodes.
Immunophenotyping
The following extracellular, anti-human monoclonal antibodies were used for lymph node immunophenotyping: anti-CD3 PE-Cy 7, anti-CD8+ PE-Cy 5, anti-CD4 FITC, anti-CD62L PE-Cy 5, anti-CD69 FITC, anti-CD152 (CTLA-4) PE, anti-CD11c PE-Cy 5, anti-CD86 PE-Cy 7, anti-HLA-DR PE, anti-CD3 FITC, anti-CD14 FITC, anti-CD16 FITC, anti-CD19 FITC, anti-CD123 PE-Cy 7, anti-CD141 PE, anti-CD68 PE-Cy 5, anti-CD20 PE, anti-CD56 PE, anti-CD279 (PD-1) APC, anti-CD11b PE, anti-CD64 FITC (BD Pharmingen, San Jose, CA).
The human monoclonal antibodies, anti-CD4 PE-Cy 5 and anti-CD25 PE, were purchased from Biolegend (San Diego, CA) and used in conjunction with intracellular staining with anti-FoxP3 FITC for the quantification of regulatory T cells. Cell death was determined by propidium iodine staining (eBioscience, San Diego, CA USA). Four-color flow cytometry was performed on a Guava 8HT flow cytometer (EMD Millipore, Billerica, MA USA) capturing 25,000 events for all samples. Results were analyzed using Guava Soft Incyte (EMD Millipore, Billerica, MA USA).
Lymph Node RNA Extraction and RT-qPCR Array
Lymph nodes were processed into a single cell suspension using a Milltenyi gentleMACS dissociator (Bergish Gladbach, Germany) according to the manufacturer's directions. Lymphocytes were then stimulated with PMA (5 ng/ml) and Ionomycin (0.1 µg/ml) and incubated for 4 h at 37°C with 5% CO2. After stimulation, the cells were positively selected for CD4+ and CD8+ T cells using Miltenyi magnetic beads as prescribed by the manufacturer (Miltenyi Bergish Gladbach, Germany). The purity of each T cell population was validated using flow cytometry demonstrating 96–98% isolation of the desired population.
Sentinel and non-SLNs processed to a single cell suspension were cultured (2 × 106 cells/mL) with RPMI + 10% FBS with a 1:1 ratio of CD3:CD28 Human T-activator beads (Invitrogen, Oslo, Norway). The cells were cultured in the presence and absence of recombinant human VEGF A (R&D systems Minneapolis, MN) (200 ng/mL) at 37°C. After 48 h incubation, cells were stimulated with PMA (5 ng/mL) and ionomycin (0.1 µg/mL) for an additional 4 h prior to harvesting of the cells. Total RNA was isolated from each of the treatments using the Qiagen RNA extraction kit (Qiagen, Germantown MD USA). The quality of the RNA was evaluated by obtaining electropherograms on Agilent 2100 Bioanalyzer and RNA integrity number using 2100 Expert software (Agilent Technologies, Santa Clara, CA USA). cDNA was reverse transcribed from a total of 0.3 µg of RNA using RT2 First strand Kit (Qiagen) per manufacturer's instructions coupled with the RT2 SYBR Green ROX qPCR Mastermix (Qiagen). The Th1/Th2/Th3 RT-qPCR arrays (SA Bioscience, Valencia CA USA) was used to quantify RNA expression of the following genes: CCR5, CD28, CSF2, CXCR3, HAVCR2, IFNG, IGSF6, IL12B, IL12RB2, IL18, IL18R1, IL2, IL2RA, IRF1, SOCS1, SOCS5, STAT1, STAT4, TBX21, TLR4, TLR6, TNF, CCL11, CCL5, CCL7, CCR2, CCR3, CCR4, CEBPB, NFATC2IP (FLJ14639), GATA3, GFI1, GPR44, ICOS, IL10, IL13, IL13RA1, IL1R1, IL1R2, IL4, IL4R, IL5, IL9, IRF4, JAK1, MAF, NFATC1, NFATC2, NFATC2IP (FLJ14639), PCGF2 (RNF110), STAT6, TMED1, CD4, CD40LG (TNFSF5), CD80, CD86, CREBBP, CTLA4, FASLG, IL15, IL6, IL6R, IL7, JAK2, LAG3, LAT, MAP2K7, MAPK8, PTPRC, TFCP2, TGFB3, CD27, TNFRSF8, TNFRSF9, TNFSF4, TYK2, YY1, IL17A, IL23A, GLMN, SFTPD, SPP1, CD27, INHA, INHBA, CD27, GLMN, SFTPD, SPP1, IL13, CSF2, MAF, TFCP2, SOCS2. There were seven PCR controls included as well as the following housekeeping genes: ribosomal protein large P1, hypoxanthine phosphoribosyltransferase 1, ribosomal protein L13A, lactate dehydrogenase A and β actin. The qPCR was run on an ABI7900HT (Life Technologies, Grand Island, NY) and analyzed on SDS v. 2.4 (Applied Biosystems, Carlsbad, CA USA). Differential expression of RNA was calculated using Partek Genomics Suite v. 6.5 (Partek, St. Louis, MO USA).
Immunohistochemistry
Six primary melanoma tumors from the 13 patients whose SLN were analyzed and six benign nevi from healthy controls were identified from a prospectively maintained clinical database. Archival, formalin-fixed, paraffin-embedded primary melanoma blocks were sectioned into 3 to 4 μm slices and affixed on glass slides. Immunohistochemistry was performed by Histoserv (Germantown, MD USA). Briefly, samples were heated for 30 min at 56°C, deparaffinized in xylene, rehydrated in a graded alcohol series, and washed in water. Heat-induced epitope retrieval was performed. Endogenous peroxidase activity was quenched in a bath of methanol and hydrogen peroxide. Only one antibody per slide was evaluated. Samples were incubated overnight with primary antibody. Peroxidase activity was localized for all samples with 3, 3′-diaminobenzidine and counterstained with hematoxylin and mounted. Negative controls were made by omitting the primary antibody; otherwise specimens were processed identically. Each slide was automatically scanned at 20x using a continuous scan technology involving a 4096 × 64 pixel charged-couple device sensor (NanoZoomer 2.0; Olympus America, Center Valley, PA USA) to produce a virtual slide of each section. Digitized images of control tissues were examined to standardize thresholds of each stain. Standardized thresholds were then applied to determine the positively stained area of each section for each stain using an IHCScore (Olympus America, San Jose, CA). Immunoreactivity was graded as intensely positive (>75%), strongly positive (50–75%), moderately positive (25–50%), weakly positive (less than 25%) and negative. The entire area of the primary tumor or benign nevi was scanned at 5x and scored by a dermatopathologist blinded to the specimen.
Statistical analysis
Descriptive statistics were applied using mean and standard deviation or number and frequency. Expression of anti-human monoclonal antibodies was reported as percentage of cells and was compared between the two groups using non-parametric testing such as the Mann–Whitney U-test. All tests were two-sided, using a Bonferroni correction for multiple testing with a p value of 0.008 considered statistically significant. JMP 10 was used for statistical analysis (SAS Institute, Cary, NC) and all figures were produced using Prism 6 (GraphPad Software, La Jolla, CA). Pathway analysis of PCR data was done with Ingenuity Pathway Analysis software (Redwood City, CA). Genes with fold change 2 or greater (over- or under-expressed) in tumor relative to control samples were used for pathway analysis. The resulting histogram shows z scores of enrichment of a number of functional groups with genes from the input set. Only functional groups with scores greater than 1.4 or less than –1.4 are shown in the histogram.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Balch CM, Gershenwald JE, Soong SJ, Thompson JF. Update on the melanoma staging system: the importance of sentinel node staging and primary tumor mitotic rate. J Surg Oncol 2011; 104(4):379-85; PMID:21858832; http://dx.doi.org/ 10.1002/jso.21876 [DOI] [PubMed] [Google Scholar]
- 2.Wen DR, Hoon DS, Chang C, Cochran AJ. Variations in lymphokine generation by individual lymph nodes draining human malignant tumors. Cancer Immunol Immunother 1989; 30(5):277-82; PMID:2696592; http://dx.doi.org/ 10.1007/BF01744894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoon DS, Bowker RJ, Cochran AJ. Suppressor cell activity in melanoma-draining lymph nodes. Cancer Res 1987; 47(6):1529-33; PMID:2949828 [PubMed] [Google Scholar]
- 4.Farzad Z, McBride WH, Ogbechi H, Asnong-Holthoff C, Morton DL, Cochran AJ. Lymphocytes from lymph nodes at different distances from human melanoma vary in their capacity to inhibit/enhance tumor cell growth in vitro. Melanoma research 1997; 7(Suppl 2):S59-65; PMID:9578418 [PubMed] [Google Scholar]
- 5.Mansfield AS, Holtan SG, Grotz TE, Allred JB, Jakub JW, Erickson LA, Markovic SN. Regional immunity in melanoma: immunosuppressive changes precede nodal metastasis. Mod Pathol 2011; 4:487-94; PMID:21151098; http://dx.doi.org/ 10.1038/modpathol.2010.227 [DOI] [PubMed] [Google Scholar]
- 6.Nevala WK, Vachon CM, Leontovich AA, Scott CG, Thompson MA, Markovic SN. Evidence of systemic Th2-driven chronic inflammation in patients with metastatic melanoma. Clin Cancer Res 2009; 15(6):1931-9; PMID:19240164; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahmoud FA, Rivera NI. The role of C-reactive protein as a prognostic indicator in advanced cancer. Curr Oncol Rep 2002; 4(3):250-5; PMID:11937016; http://dx.doi.org/ 10.1007/s11912-002-0023-1 [DOI] [PubMed] [Google Scholar]
- 8.Yuan J, Zhou J, Dong Z, Tandon S, Kuk D, Panageas KS, Wong P, Wu X, Naidoo J, Page DB et al.. Pretreatment serum VEGF is associated with clinical response and overall survival in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res 2014; 2(2):127-32; PMID:24778276; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mansfield AS, Nevala WK, Lieser EA, Leontovich AA, Markovic SN. The immunomodulatory effects of bevacizumab on systemic immunity in patients with metastatic melanoma. Oncoimmunology 2013; 2(5):e24436; PMID:23762809; http://dx.doi.org/ 10.4161/onci.24436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodi FS, Lawrence D, Lezcano C, Wu X, Zhou J, Sasada T, Zeng W, Giobbie-Hurder A, Atkins MB, Ibrahim N et al.. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res 2014; 2(7):632-42; PMID:24838938; http://dx.doi.org/ 10.1158/2326-6066.CIR-14-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sebastiani S, Allavena P, Albanesi C, Nasorri F, Bianchi G, Traidl C, Sozzani S, Girolomoni G, Cavani A. Chemokine receptor expression and function in CD4+ T lymphocytes with regulatory activity. J Immunol 2001; 166(2):996-1002; PMID:11145678; http://dx.doi.org/ 10.4049/jimmunol.166.2.996 [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Martin A, Gomez L, Lustgarten J, Mira E, Manes S. Maximal T cell-mediated antitumor responses rely upon CCR5 expression in both CD4(+) and CD8(+) T cells. Cancer Res 2011; 71(16):5455-66; PMID:21715565; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-1687 [DOI] [PubMed] [Google Scholar]
- 13.Ngai P, McCormick S, Small C, Zhang X, Zganiacz A, Aoki N, Xing Z. Gamma interferon responses of CD4 and CD8 T-cell subsets are quantitatively different and independent of each other during pulmonary Mycobacterium bovis BCG infection. Infect Immun 2007; 75(5):2244-52; PMID:17307945; http://dx.doi.org/ 10.1128/IAI.00024-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Critchley-Thorne RJ, Yan N, Nacu S, Weber J, Holmes SP, Lee PP. Down-regulation of the interferon signaling pathway in T lymphocytes from patients with metastatic melanoma. PLoS Med 2007; 4(5):e176; PMID:17488182; http://dx.doi.org/ 10.1371/journal.pmed.0040176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girard JP, Moussion C, Forster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nature reviews. Immunology 2012; 12(11):762-73; PMID:23018291 [DOI] [PubMed] [Google Scholar]
- 16.Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, Okazaki T, Tokura Y. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 2010; 116(7):1757-66; PMID:20143437; http://dx.doi.org/ 10.1002/cncr.24899 [DOI] [PubMed] [Google Scholar]
- 17.Dulos J, Carven GJ, van Boxtel SJ, Evers S, Driessen-Engels LJ, Hobo W, Gorecka MA, de Haan AF, Mulders P, Punt CJ et al.. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J Immunother 2012; 35(2):169-78; PMID:22306905; http://dx.doi.org/ 10.1097/CJI.0b013e318247a4e7 [DOI] [PubMed] [Google Scholar]
- 18.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014; 20(19):5064-74; PMID:24714771; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009; 114(8):1537-44; PMID:19423728; http://dx.doi.org/ 10.1182/blood-2008-12-195792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ et al.. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364(26):2517-26; PMID:21639810; http://dx.doi.org/ 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 21.Torisu-Itakura H, Lee JH, Scheri RP, Huynh Y, Ye X, Essner R, Morton DL. Molecular characterization of inflammatory genes in sentinel and nonsentinel nodes in melanoma. Clin Cancer Res 2007; 13(11):3125-32; PMID:17545514; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-2645 [DOI] [PubMed] [Google Scholar]
- 22.Redondo P, Sanchez-Carpintero I, Bauza A, Idoate M, Solano T, Mihm MC Jr. Immunologic escape and angiogenesis in human malignant melanoma. J Am Acad Dermatol 2003; 49(2):255-63; PMID:12894074; http://dx.doi.org/ 10.1067/S0190-9622(03)00921-6 [DOI] [PubMed] [Google Scholar]
- 23.Kim YS, Choi SJ, Tae YM, Lee BJ, Jeon SG, Oh SY, Gho YS, Zhu Z, Kim YK. Distinct roles of vascular endothelial growth factor receptor-1- and receptor-2-mediated signaling in T cell priming and Th17 polarization to lipopolysaccharide-containing allergens in the lung. J Immunol 2010; 185(9):5648-55; PMID:20921519; http://dx.doi.org/ 10.4049/jimmunol.1001713 [DOI] [PubMed] [Google Scholar]
- 24.Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, Kang MJ, Cohn L, Kim YK, McDonald DM et al.. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nature medicine 2004; 10(10):1095-103; PMID:15378055; http://dx.doi.org/ 10.1038/nm1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varker KA, Biber JE, Kefauver C, Jensen R, Lehman A, Young D, Wu H, Lesinski GB, Kendra K, Chen HX et al.. A randomized phase 2 trial of bevacizumab with or without daily low-dose interferon alfa-2b in metastatic malignant melanoma. Ann Surg Oncol 2007; 14(8):2367-76; PMID:17534686; http://dx.doi.org/ 10.1245/s10434-007-9389-5 [DOI] [PubMed] [Google Scholar]
- 26.Kruijff S, Bastiaannet E, Brouwers AH, Nagengast WB, Speijers MJ, Suurmeijer AJ, Hospers GA, Hoekstra HJ. Use of S-100B to evaluate therapy effects during bevacizumab induction treatment in AJCC stage III melanoma. Ann Surg Oncol 2012; 19(2):620-6; PMID:21861214; http://dx.doi.org/ 10.1245/s10434-011-2027-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biswas S, Wrigley J, East C, Hern A, Marshall A, Dunn J, Lorigan P, Middleton M, Corrie P. A randomised trial evaluating bevacizumab as adjuvant therapy following resection of AJCC stage IIB, IIC and III cutaneous melanoma: an update. Ecancermedicalscience 2008; 2:108; PMID:22275984; http://dx.doi.org/ 10.3332/ecancer.2008.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res 2010; 70(15):6171-80; PMID:20631075; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Block MS, Nevala WK, Leontovich AA, Markovic SN. Differential response of human and mouse dendritic cells to VEGF determines interspecies discrepancies in tumor-mediated TH1/TH2 polarity shift. Clin Cancer Res 2011; 17(7):1776-83; PMID:21349994; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-2836 [DOI] [PubMed] [Google Scholar]
- 30.Cochran AJ, Huang RR, Lee J, Itakura E, Leong SP, Essner R. Tumour-induced immune modulation of sentinel lymph nodes. Nat Rev Immunol 2006; 6(9):659-70; PMID:16932751; http://dx.doi.org/ 10.1038/nri1919 [DOI] [PubMed] [Google Scholar]
- 31.Jakub JW, Reintgen DS, Shivers S, Pendas S. Regional node dissection for melanoma: techniques and indication. Surg Oncol Clin N Am 2007; 16(1):247-61; PMID:17336247; http://dx.doi.org/ 10.1016/j.soc.2006.10.012 [DOI] [PubMed] [Google Scholar]