Abstract
Although the profile of safety of tumor-targeted oncolytic virus (TOV) is encouraging, the antitumor efficacy of TOV alone is disappointing. Interleukin-10 (IL-10) plays an important role in carcinogenesis and anti-virus immunity. Here we report that tumor-targeted oncolytic vaccinia virus (VV) armed with IL10 shows promising potential for treatment of pancreatic cancer (PaCa).
Keywords: oncolytic virus, vaccinia virus, pancreatic cancer, immunotherapy, IL-10
PaCa is the fourth leading cause of cancer-related death worldwide1 and remains consistently lethal with a five-year survival rate of less than 5%. This situation signifies a need for radically new therapeutic strategies that are not subject to cross-resistance with conventional therapies.
An appropriate antitumor immune response is known to be vital for effective tumor elimination and prevention of disease recurrence, however natural cytotoxic T lymphocyte (CTL)-mediated immunity against tumors often falls short in preventing the development of malignancies. TOV have emerged as attractive, non-cross-resistant therapeutic candidates for cancer as TOV have the ability to specifically target and lyse tumor cells and importantly induce systemic antitumor effects. TOV as a treatment modality for cancer have been shown in a number of clinical trials to be acceptably safe, however antitumor efficacy has so far been limited. This is likely due to their rapid clearance by the host immune system, preventing oncolytic effects and an effective immune-stimulating release of tumor-associated antigens (TAA). VV has strong potential for exploitation as an oncolytic agent as it has several inherent features that make it particularly suitable, including fast and efficient replication with rapid cell-to-cell spread, natural tropism for tumors, a well-documented safety record and an ability to replicate in many different cell types. We recently demonstrated that hypoxia, which contributes to the aggressive and treatment-resistant phenotype of PaCa,2 does not inhibit and may even enhance the potency of VV.3 VV has also recently been shown to be effective at human tumor targeting after intravenous delivery.4
IL-10, first described as a factor produced by Th2 clones capable of inhibiting Th1 cytokine production, is a potent inhibitor of T cell-mediated anti-viral responses by prevention of activation of the CD4+Th1 inflammatory pathway.5 IL-10 is a key player in the establishment and perpetuation of viral clearance or persistence in vivo.6 However, although the long-held paradigm of IL-10 suggests it to be an immunosuppressive cytokine, recent evidence demonstrates that IL-10 can also function as an immune stimulant, inducing antitumor activities in the host.7 It has been reported to enhance the therapeutic effectiveness of a VV-based vaccine against murine cancer cells,8 which may be connected to its ability to enhance the growth and proliferation of T cells.9 Thus, given its pleiotropic effects, IL-10 may be an effective agent with which to improve the antitumor potential of VV.
A Lister strain, thymidine kinase (TK)-deleted replicating VV armed with murine IL-10 (VVLΔTK-IL-10) was examined for antitumor efficacy using two murine models of PaCa. In a subcutaneous model, DT6606 allografts, derived from a genetically engineered LSL-KrasG12D/+; Pdx-1-Cre (KC) mouse model devised by Tuveson and colleagues,10 were established in immune competent mice. Tumors were treated daily for 5 d with a low dose (1×108) of IL-10-armed, or unarmed VV. Treatment with VVLΔTK-IL-10 more than doubled the number of survivors in the study, with 87.5% of mice responding to treatment by complete tumor clearance, compared to 40% of those treated with unarmed virus. Using the more pathophysiologically relevant transgenic mouse model (Genetically engineered LSL-KrasG12D/+, Trp53R172H/+, Pdx-Cre (KPC)), in which mice spontaneously develop lethal pancreatic carcinomas, three intraperitoneal (IP) treatments of 2 × 108 pfu/dose VVLΔTK-IL-10 resulted in specific viral tumor targeting and drastic improvement in survival times of 138.5 d following commencement of treatment, vs. 69.7 d after treatment with unarmed virus. Analysis of viral persistence in tumors suggested that inclusion of IL-10 resulted in delayed viral clearance, suggesting that arming VV with IL-10 perpetuated the oncolytic potential of the virus. However, treatment also resulted in rejection of tumors after rechallenge, confirming the development of effective long-term immunity against tumor antigens and thus activation by IL-10 of an effective antitumor immune response.
Analysis of T cell populations in spleens and tumors of both spontaneous and subcutaneous tumor models revealed that treatment with both unarmed and IL-10-armed viruses induced a high level of adaptive immunity in mice compared to untreated mice. However, an interesting finding was that the magnitude of the activated splenic CD4+ and CD8+ population response in VVLΔTK-IL-10 treated mice was lower compared to the unarmed virus. This difference correlated with a reduction in virus-specific CD8+ T cells and T cell-mediated IFNγ recovery from tumors, which accounted for the delayed viral clearance from tumors. Interestingly, although VVLΔTK-IL-10 treatment reduced anti-viral CD8+ populations, IL-10 had no inhibitory effect on production of antitumor CD8+ cells. Indeed, at certain timepoints an increase in antitumor CD8+ cells was observed, which we postulate is a result of the increased oncolysis occurring after VVLΔTK-IL-10 treatment, improving TAA release.
These results suggest that IL-10 improves the efficacy of OV by modulation of the early immune response to infection, resulting in dampening of antiviral, but not antitumor immunity (Fig. 1). However, the mechanism by which IL-10 elicits this alteration remains unclear. Our investigations also revealed that local IL-10 expression results in modification of the tumor macrophage population. While we found that VVLΔTK-IL-10 treatment increased macrophage infiltrate into tumors in both models, we found that VVLΔTK-IL-10 treatment resulted in significant downregulation of MHC II expression. Thus, it is feasible that in our model tumor macrophages are responsible for viral antigen presentation to T cells and a reduction in macrophage activation by IL-10 leads to reduced cross-priming of the anti-viral immune response. VV uptake and processing by tumor macrophages remains to be proven and it will be informative to investigate the phenomenon in the context of IL-10 expression. A further consideration is that this model suggests distinct pathways of viral and tumor antigen presentation, which are the subject of ongoing investigation in our laboratory.
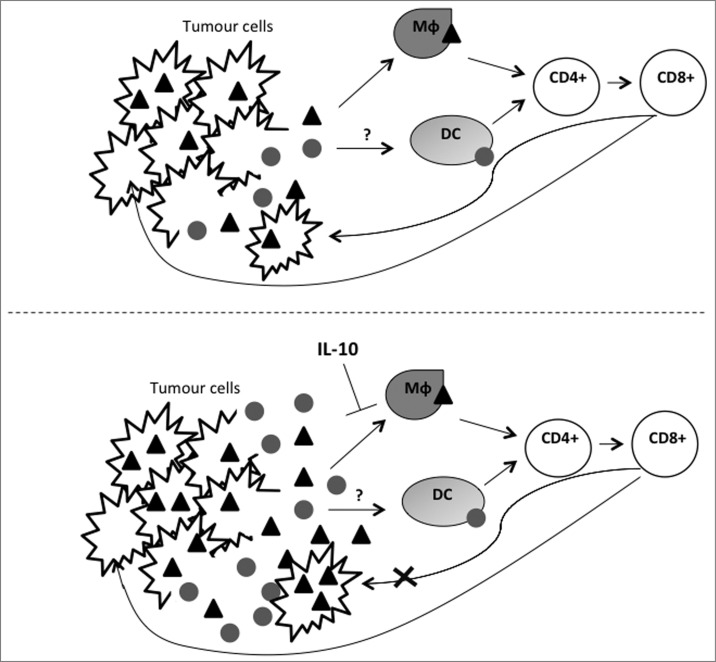
Figure 1.
Schematic of proposed effect of VVΔTK-IL10 on tumor cells and viral clearance. After administration, virus particles specifically infect, replicate in and lyse tumor cells. Tumor cell lysis releases both viral () and tumor (and bull;) antigens. Experimental data suggests that these are presented to T cells via different antigen presenting cells. Data indicates that macrophages (Mφ) are responsible for presentation of viral antigens and it is this process that is disrupted by IL-10, which causes downregulation of macrophage MHC II markers, inhibiting macrophage antigen display function. As a result, virus is cleared less rapidly, resulting in increased tumor cell infection and tumor cell antigen release. Tumor antigen recognition is unaffected by IL-10, therefore, this model suggests a distinct, possibly dendritic cell (DC)-mediated presentation of tumor antigens.
These findings demonstrate that IL-10 armed VV shows great promise as a novel therapeutic for PaCa and that IL-10 in combination with oncolytic virotherapy is clearly able to enhance tumor rejection through delicate modulation of the innate and adaptive immune responses.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The UK Charity Pancreatic Cancer Research Fund, National Natural Science Foundation of China (81101608, 81201792), Ministry of Sciences and Technology, China (2013DFG32080).
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin 2005; 55(1):10-30; PMID:15661684 [DOI] [PubMed] [Google Scholar]
- 2.Yokoi K, Fidler IJ. Hypoxia increases resistance of human pancreatic cancer cells to apoptosis induced by gemcitabine. Clin Cancer Res 2004; 10(7):2299-306; PMID:15073105; http://dx.doi.org/ 10.1158/1078-0432.CCR-03-0488 [DOI] [PubMed] [Google Scholar]
- 3.Hiley CT, Yuan M, Lemoine NR, Wang Y. Lister strain vaccinia virus, a potential therapeutic vector targeting hypoxic tumours. Gene Ther 2009; 17(2):281-7; PMID:19890355; http://dx.doi.org/ 10.1038/gt.2009.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ, Nieva J, Hwang TH, Moon A, Patt R et al.. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature 2011; 477(7362):99-102; PMID:21886163; http://dx.doi.org/ 10.1038/nature10358 [DOI] [PubMed] [Google Scholar]
- 5.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol 2008; 180(9):5771-7; PMID:18424693; http://dx.doi.org/ 10.4049/jimmunol.180.9.5771 [DOI] [PubMed] [Google Scholar]
- 6.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nature medicine 2006; 12(11):1301-9; PMID:17041596; http://dx.doi.org/ 10.1038/nm1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berman RM, Suzuki T, Tahara H, Robbins PD, Narula SK, Lotze MT. Systemic administration of cellular IL-10 induces an effective, specific, and long-lived immune response against established tumors in mice. J Immunol 1996; 157(1):231-8; PMID:8683120 [PubMed] [Google Scholar]
- 8.Kaufman HL, Rao JB, Irvine KR, Bronte V, Rosenberg SA, Restifo NP. Interleukin-10 enhances the therapeutic effectiveness of a recombinant poxvirus-based vaccine in an experimental murine tumor model. J Immunother 1999; 22(6):489-96; PMID:10570747; http://dx.doi.org/ 10.1097/00002371-199911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emmerich J, Mumm JB, Chan IH, LaFace D, Truong H, McClanahan T, Gorman DM, Oft M. IL-10 directly activates and expands tumor-resident CD8(+) T cells without de novo infiltration from secondary lymphoid organs. Cancer Res 2012; 72(14):3570-81; PMID:22581824; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-0721 [DOI] [PubMed] [Google Scholar]
- 10.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer cell 2005; 7(5):469-83; PMID:15894267; http://dx.doi.org/ 10.1016/j.ccr.2005.04.023 [DOI] [PubMed] [Google Scholar]