Abstract
Pancreatic stellate cells (PSCs) are key components of pancreatic ductal adenocarcinoma (PDAC). We recently demonstrated that IP-10/CXCL10 is highly expressed by PSCs in the presence of pancreatic cancer cells (PCCs) and its expression correlates with infiltration by regulatory T cells (Tregs) and poor survival. Thus, stromal cells in pancreatic cancer can promote immunosuppression and tumor progression, through the expression of IP-10.
Keywords: CXCL10, IP-10, pancreatic cancer, pancreatic stellate cells, regulatory T cells
PDAC continues to be a difficult disease to treat. Standards of care have not changed significantly in the last decade and the 5-y survival-rate remains less than 5%. Despite of the introduction of more effective chemotherapeutic regimens, combined gemcitabine and nab-paclitaxel and FOLFIRINOX, median survival still is not above one year in advanced disease.1,2 In order to develop more effective therapeutic strategies, we need to better understand the underlying pancreatic cancer biology.
The histology of pancreatic cancer is notable for a prominent desmoplastic reaction that in addition to PCCs, is predominantly characterized by the presence of PSCs. PSCs can be activated by inflammatory stimuli, injury and cancer and when activated, they proliferate and produce large amounts of extracellular proteins. We identified cytokines and growth factors produced by PSCs alone or in the presence of PCCs.3 Activated PSCs secrete many pro-inflammatory cytokines such as IL-8, IL-6, SDF-1α and GROα that have the potential to favor tumor growth by recruiting pro-tumorigenic leukocytes such as tumor-associated macrophages and myeloid-derived suppressor cells. We reported in our recent study that PCCs induce PSCs to express and secrete interferon-γ inducible protein 10 (IP-10), also called CXCL10.3
IP-10 is a chemokine implicated in many inflammatory diseases and often acts as a chemoattractant for T cells. Moreover, IP-10 signaling via its cognate receptor CXCR3 was shown to promote tumor growth, migration and invasion of cancer cells in several tumor types.4 Interestingly, despite the fact that PCCs express CXCR3, IP-10 did not affect PCC proliferation or migration in our experiments. Instead, we found an association between the expression of IP-10 and CXCR3 with the presence of Tregs and of an immunosuppressed microenvironment. In view of the fact that Tregs are known to express CXCR3, we hypothesized that PCCs could stimulate PSCs to produce IP-10 leading to the recruitment of CXCR3+ Tregs that are involved in mediating tumor immunosuppression. Indeed, we found that IP-10 attract CXCR3+ Tregs as well as CD8+ and CD4+ CXCR3+ T cells in peripheral blood mononuclear cells (PBMCs) obtained from PDAC patients.3 Moreover, PBMCs in these patients contained more Tregs than PBMCs from healthy volunteers, suggesting that circulating Tregs may be preferentially recruited into PDAC by IP-10 compared to other types of T cell subsets.
CXCR3+ Tregs selectively accumulate in ovarian cancer tumors and contribute to a reduction in the activity of Th1 lymphocytes.5 However, in other tumor types such as breast cancer6 and melanoma,7 IP-10 and CXCR3 expression have been associated with an antitumoral response driven by CXCR3+ Th1 lymphocytes. Hence, IP-10 may have a divergent effect on the immune response to a cancer in a tumor-specific and patient-specific manner. Depending on the equilibrium between CXCR3+ Th1 lymphocytes and CXCR3+ Tregs, the immune system could shift from an immune activating to an immune suppressing state. Based on our findings, we propose a model in which stromal expression of IP-10, induced by PCCs, preferentially recruits immunosuppressive CXCR3+ Tregs to PDAC (Fig. 1).
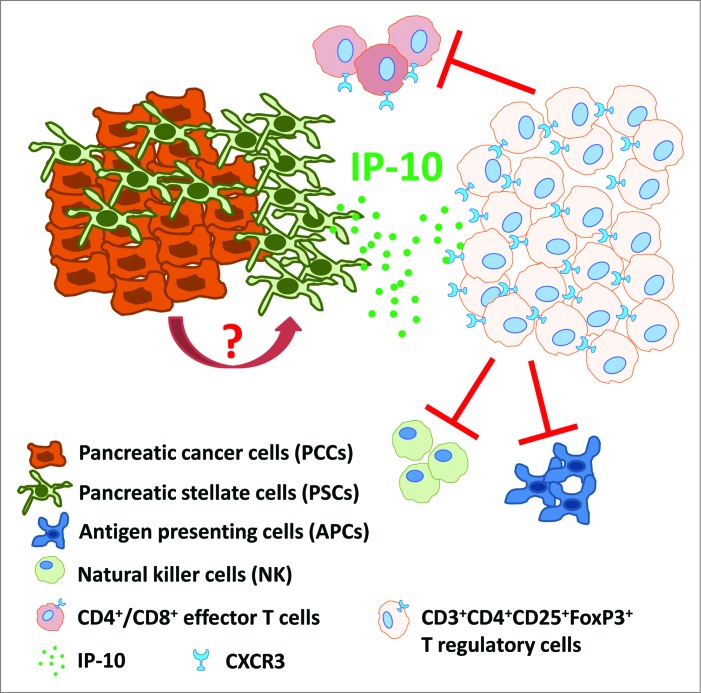
Figure 1.
IP-10 recruits immunosuppressive CXCR3+FoxP3+ regulatory T cells in pancreatic ductal adenocarcinoma. Pancreatic cancer cells (PCCs) induce pancreatic stellate cells (PSCs) to secrete IP-10 by a yet to be characterized mechanism. IP-10 recruits CXCR3 (the cognate receptor)-expressing CD4+/CD8+ effector T cells and FoxP3+ regulatory T cells (Tregs). However, because circulating Treg numbers are highly elevated relative to effector T cells, CXCR3+ Tregs may be preferentially recruited to inhibit adaptive immune responses (via effector T cell, NK cell and APC inhibition), thus contributing to an immunosuppressive and tumor-promoting microenvironment.
We examined pathology specimens from patients who had undergone resection for PDAC. IP-10 was upregulated in those cancers compared to normal pancreatic tissue adjacent to the tumor and its expression also correlated with poor survival. Our results suggest that the use of IP-10 and/or CXCR3 as targets in new multimodal therapeutic approaches might need careful stratification. Inhibiting either IP-10 or CXCR3 may prevent the recruitment of T effector cells as well as Tregs. Thus inhibition of IP-10 and CXCR3, either alone or together, may not be adequate to stimulate an immune response against a tumor and alternative strategies need to be explored. Several clinical trials are implementing immunotherapy for the treatment of PDAC.8 Two different approaches are currently being applied. CTLA4 and PD-1 blocking antibodies alone or in combination with chemo- or radiotherapy are being deployed to inhibit the immunosuppressive activity of Tregs against reactive T cells (NCT01313416, NCT0230518, NCT02303990, NCT00112580; www.clinicaltrials.gov). Cancer vaccines are also being tested in the clinic combined with chemotherapy in patients with locally advanced or metastatic pancreatic cancer (NCT00425360). A phase I clinical trial where adjuvant chemoradiation was combined with tumor cell vaccination have showed an significant increase in median survival.9 Finally, CTLA4 and PD-1 inhibitors are being combined with cancer vaccines to further potentiate the immune reaction stimulated by the vaccine against tumor (NCT02243371, NCT00836407, NCT01896869).
Overall, recent studies have shed light on PDAC biology and the importance of the tumor microenvironment in the response to therapy. Our work points toward a detrimental role of PSCs by promoting immunosuppression while another new study suggests that the presence of PSCs-related fibrosis could prevents the recruitment of Tregs into PDAC.10 Now, further basic and clinical investigations are needed to clarify the PSCs-immune system interplay in pancreatic cancer in order to find the right multi-targeting strategy for improving current treatment.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Funding was provided by Cancer Research UK and Medical Research Council (MRC).
References
- 1.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C et al.. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. New Engl J Med 2011; 364:1817-25; PMID:21561347; http://dx.doi.org/ 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 2.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN et al.. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. New EnglJ Med 2013; 369:1691-703; PMID:24131140; http://dx.doi.org/ 10.1056/NEJMoa1304369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lunardi S, Jamieson NB, Lim SY, Griffiths KL, Carvalho-Gaspar M, Al-Assar O, Yameen S, Carter RC, McKay CJ, Spoletini G et al.. IP-10/CXCL10 induction in human pancreatic cancer stroma influences lymphocytes recruitment and correlates with poor survival. Oncotarget 2014; 5:11064-80; PMID:25415223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billottet C, Quemener C, Bikfalvi A. CXCR3, a double-edged sword in tumor progression and angiogenesis. Biochim Biophys Acta 2013; 1836:287-95; PMID:23994549; http://dx.doi.org/ 10.1016/j.bbcan.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 5.Redjimi N, Raffin C, Raimbaud I, Pignon P, Matsuzaki J, Odunsi K, Valmori D, Ayyoub M. CXCR3+ T regulatory cells selectively accumulate in human ovarian carcinomas to limit type I immunity. Cancer Res 2012; 72:4351-60; PMID:22798340; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-0579 [DOI] [PubMed] [Google Scholar]
- 6.Mulligan AM, Raitman I, Feeley L, Pinnaduwage D, Nguyen LT, O'Malley FP, Ohashi PS, Andrulis IL. Tumoral lymphocytic infiltration and expression of the chemokine CXCL10 in breast cancers from the Ontario Familial Breast Cancer Registry. Clin Cancer Res 2013; 19:336-46; PMID:23213058; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muthuswamy R, Berk E, Junecko BF, Zeh HJ, Zureikat AH, Normolle D, Luong TM, Reinhart TA, Bartlett DL, Kalinski P. NF-kappaB hyperactivation in tumor tissues allows tumor-selective reprogramming of the chemokine microenvironment to enhance the recruitment of cytolytic T effector cells. Cancer Res 2012; 72:3735-43; PMID:22593190; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-4136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodson LF, Hawkins WG, Goedegebuure P. Potential targets for pancreatic cancer immunotherapeutics. Immunotherapy 2011; 3:517-37; PMID:21463193; http://dx.doi.org/ 10.2217/imt.11.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, Goemann M, Coleman J, Grochow L, Donehower RC et al.. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol 2001; 19:145-56; PMID:11134207 [DOI] [PubMed] [Google Scholar]
- 10.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV et al.. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014; 25:719-34; PMID:24856586; http://dx.doi.org/ 10.1016/j.ccr.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]