Abstract
We and others recently discovered HHLA2 as a new B7 family member and transmembrane and immunoglobulin domain containing 2 (TMIGD2) as one of its receptors. Based on a new study we propose that HHLA2 may represent a novel immunosuppressive mechanism within the tumor microenvironment and hence could be a target for cancer therapy. TMIGD2 may be another therapeutic target.
Keywords: angiogenesis, B7 family, CD28 family, HHLA2, immunosuppression, immunotherapy, ligand, receptor, tumor microenvironment, TMIGD2
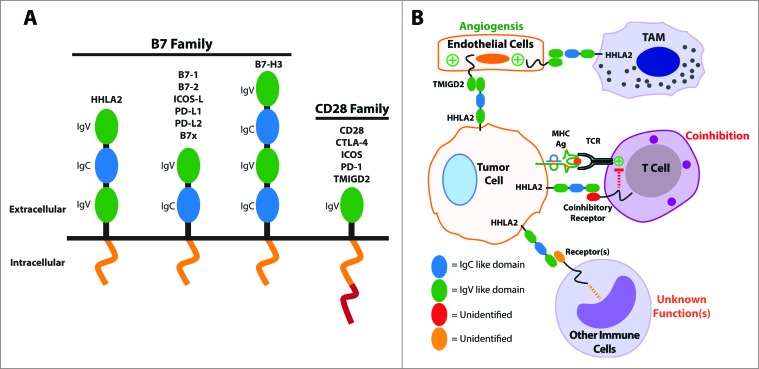
HHLA2 (B7H7/B7-H5/B7y) has recently been identified as a new member of the B7 family member.1-3 HHLA2 was initially discovered as a gene in the Immunoglobulin (Ig) superfamily when screening the human genome for human endogenous retroviral (HERV) long terminal repeat (LTR) sequences which provide polyadenation signals.4 Hence the name, HHLA2, is short for HERV-H LTR-associating 2. HHLA2 orthologs appear to be present in a wide range of species such as fish, frog, giant panda, monkey and human, but not in laboratory mouse and rat strains. The HHLA2 protein has amino acid similarity of 23 to 33% to the other human B7 family molecules and phylogenetically it is most similar to B7-H3 and B7x (B7-H4/B7S1). The predicted structure of HHLA2 is a type I transmembrane molecule with three extracellular Ig domains. This is unique as most other B7 family members contain only two Ig domains while human B7-H3 has four Ig domains (Fig. 1A).
Figure 1.
The B7 and CD28 families and the significance of HHLA2 and TMIGD2 within the tumor microenvironment. (A) A structural representation of the B7 and CD28 family members. (B) A proposed model for the roles of HHLA2 and TMIGD2 within the tumor microenvironment. Tumor-expressed HHLA2 can interact not only with an unidentified receptor on activated T cells that leads to coinhibition, but also with TMIGD2 on endothelium that stimulates tumor angiogenesis. Additionally, tumor-expressed HHLA2 can bind to other immune cells and likely affects their functions in ways that are not yet understood. Finally, tumor-associated macrophages (TAM) may express HHLA2 and interact with TMIGD2 on endothelium.
HHLA2 functions as a T cell coinhibitory molecule as it suppresses proliferation and cytokine production of both human CD4+ and CD8+ T cells.1,5 HHLA2 is constitutively expressed on the surface of human monocytes and is induced on B cells after stimulation.1 Unlike PD-L1 and B7–1 though, HHLA2 is not inducible on T cells. The differences in expression on immune cells suggest that HHLA2 could be involved in immune regulation at a different functional level than other B7 family members. Using immunohistochemistry with an HHLA2 monoclonal antibody, we have recently found that HHLA2 is not expressed in most human tissues, except the placenta, kidney, intestine, gall bladder, and breast.6 Expression of HHLA2 in the placenta and the intestines is interesting as it may help fetal-maternal immune tolerance or control intestinal inflammation, respectively. Importantly, we have shown many human cancers overexpress HHLA2 including cancers from the breast, lung, thyroid, melanoma, pancreas, ovary, liver, bladder, colon, prostate, kidney, and esophagus.6 Moreover, in a small cohort of human triple-negative breast cancer (TNBC) patients, higher expression of HHLA2 on tumor cells was associated with increased lymph node metastases.6 The wide expression of HHLA2 in human cancers and its association with more invasive disease in the TNBC cohort suggest that HHLA2 potentially plays an important role in tumor evolution and metastases through immune suppression.
There are at least two mechanisms upregulating HHLA2 expression. One mechanism is inflammatory stimulation.1,2 HHLA2 expression can be increased on monocytes and macrophages and is induced on B cells by stimulation with LPS and IFN-γ.1,2 The second mechanism is the gene copy-number variation.6 We compared gene dosage in the basal subtype of TNBC using the cBioPortal for the Cancer Genomics database.7 In this subtype, 32% had HHLA2 gene copy-number variations and the majority (95%) of these variants were either amplifications or gains of HHLA2 gene copy number,6 suggesting this could be another mechanism of overexpression.
Receptors for HHLA2 can be found on a wide variety of immune cells, including T cells, B cells, monocytes, and dendritic cells.1 We and others have independently identified one of receptors for HHLA2, TMIGD2,6 also called CD28 homolog (CD28H),2 through a bioinformatics analysis/immunological approach and a high-throughput screening, respectively. Like HHLA2, this molecule is expressed in humans and monkeys but not in mice or rats. This molecule was initially reported as an endothelial adhesion molecule which was renamed Immunoglobulin-containing and Proline-rich Receptor-1 (IGPR-1).8 TMIGD2 protein can be detected in cells of epithelial and endothelial origins, and is able to enhance angiogenesis in vitro when overexpressed by endothelial cell lines.8 Furthermore, TMIGD2 is reported as a stimulatory receptor expressed primarily on naive T cells.2 Like other CD28 family members, TMIGD2 is an Ig superfamily member with an extracellular IgV-like domain, a transmembrane region, and a cytoplasmic tail.6 The cytoplasmic tail contains tyrosine residues which can be phosphorylated2 and a proline-rich domain which associates with multiple Src homology 3 (SH3)-containing signaling molecules.8 Together, these studies suggest that TMIGD2 has multiple functions depending on the cell type and signaling pathways.
In summary, we have shown that the HHLA2 pathway could represents a novel immunosuppressive mechanism within the tumor microenvironment and is an attractive target for human cancer therapy. HHLA2's overexpression may be advantageous to cancer growth and survival through different mechanisms (Fig. 1B). Tumor-expressed HHLA2 could protect the tumor from immune surveillance via its interaction with unidentified receptors on activated T cells and other immune cells, and it may also promote angiogenesis within the microenvironment via its interaction with endothelial-expressed TMIGD2. The blockade of the B7–1/B7–2/CTLA-4 and PD-L1/PD-L2/PD-1 pathways within the B7 and CD28 families to enhance anti-tumor immunity has been exploited with therapeutic success.9,10 Interestingly, therapies targeting HHLA2 could not only enhance anti-tumor immune responses, but may also inhibit tumor angiogenesis. Further studies are required to dissect TMIGD2's expression patterns and functions in order to develop new therapies targeting TMIGD2.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Zhao R, Chinai JM, Buhl S, Scandiuzzi L, Ray A, Jeon H, Ohaegbulam KC, Ghosh K, Zhao A, Scharff MD et al.. HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function. Proc Natl Acad Sci U S A 2013; 110:9879-84; PMID:23716685; http://dx.doi.org/ 10.1073/pnas.1303524110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu Y, Yao S, Iliopoulou BP, Han X, Augustine MM, Xu H, Phennicie RT, Flies SJ, Broadwater M, Ruff W et al.. B7-H5 costimulates human T cells via CD28H. Nat Commun 2013; 4:2043; PMID:23784006; http://dx.doi.org/ 10.1038/ncomms3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flajnik MF, Tlapakova T, Criscitiello MF, Krylov V, Ohta Y. Evolution of the B7 family: co-evolution of B7H6 and NKp30, identification of a new B7 family member, B7H7, and of B7's historical relationship with the MHC. Immunogenetics 2012; 64:571-90; PMID:22488247; http://dx.doi.org/ 10.1007/s00251-012-0616-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mager DL, Hunter DG, Schertzer M, Freeman JD. Endogenous retroviruses provide the primary polyadenylation signal for two new human genes (HHLA2 and HHLA3). Genomics 1999; 59:255-63; PMID:10444326; http://dx.doi.org/ 10.1006/geno.1999.5877 [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Manick B, Wu G, Hao R. Biofunctions of three new B7 family members (IRM7P.486). 2014. AAI Annual Meeting Abstracts126.11. [Google Scholar]
- 6.Janakiram M, Chinai J, Fineberg S, Fiser A, Montagna C, Medaverepu R, Castano E, Jeon H, Ohaegbulam KC, Zhao R et al.. Expression, clinical significance, and receptor identification of the newest B7 family member HHLA2 protein. Clin Cancer Res 2015; 21:2359-66; PMID:25549724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E et al.. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012; 2:401-4; PMID:22588877; http://dx.doi.org/ 10.1158/2159-8290.CD-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahimi N, Rezazadeh K, Mahoney JE, Hartsough E, Meyer RD. Identification of IGPR-1 as a novel adhesion molecule involved in angiogenesis. Mol Biol Cell 2012; 23:1646-56; PMID:22419821; http://dx.doi.org/ 10.1091/mbc.E11-11-0934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zang X, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res 2007; 13:5271-9; PMID:17875755; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-1030 [DOI] [PubMed] [Google Scholar]
- 10.Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med 2015; 21:24-33; PMID:25440090; http://dx.doi.org/ 10.1016/j.molmed.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]