Abstract
Context:
Roux-en-Y gastric bypass (RYGB) is the most effective treatment for morbid obesity and resolution of diabetes. Over the last decade, it has become well accepted that this resolution of diabetes occurs before significant weight loss; however, the mechanisms behind this effect remain unknown and could represent novel therapeutic targets for obesity and diabetes. Bile acids have been identified as putative mediators of these weight loss-independent effects.
Objective:
To identify the longitudinal changes in bile acids after RYGB, which may provide mechanistic insight into the weight loss-independent effects of RYGB.
Design:
Observational study before/after intervention.
Setting:
Academic medical center.
Patients/Participants:
Samples were collected from morbidly obese patients (n = 21) before and after RYGB.
Intervention:
RYGB.
Main Outcome Measures:
Seventeen individual bile acid species were measured preoperatively and at 1, 6, 12, and 24 months postoperatively. Anthropometric, hormonal, and hyperinsulinemic-euglycemic clamp data were also examined to identify physiological parameters associated with bile acid changes.
Results:
Fasting total plasma bile acids increased after RYGB; however, increases were bimodal and were observed only at 1 (P < .05) and 24 months (P < .01). One-month increases were secondary to surges in ursodeoxycholic acid and its glycine and taurine conjugates, bacterially derived bile acids with putative insulin-sensitizing effects. Increases at 24 months were due to gradual rises in primary unconjugated bile acids as well as deoxycholic acid and its glycine conjugate. Plasma bile acid changes were not significantly associated with any anthropometric or hormonal measures, although hepatic insulin sensitivity was significantly improved at 1 month.
Conclusions:
Overall findings suggest that bacterially derived bile acids may mediate the early improvements at 1 month after RYGB. Future studies should examine the changes in specific bile acid chemical species after bariatric procedures and bile acid-specific signaling changes.
As the obesity and diabetes epidemics worsen, so does the need for more effective therapies. Roux-en-Y gastric bypass (RYGB) and other forms of bariatric surgery are becoming increasingly used to meet this need. Randomized clinical trials, meta-analyses, and a number of smaller clinical studies have demonstrated that bariatric surgery is the most effective treatment for morbid obesity and its associated diabetes (1–4). However, the resolution of diabetes and other comorbid conditions typically occurs before significant weight loss. Over the last several years, it has become well accepted that weight loss-independent mechanisms are at work in these patients, mechanisms that may play even more important roles than the gastric restriction and/or malabsorption induced by the surgical procedures (5). Despite bariatric surgery being the most effective treatment for morbid obesity and growing in popularity, the mechanisms of the weight-independent effects of these operations remain poorly understood.
One class of putative compounds involved in these weight-independent effects of bariatric surgery is bile acids. Bile acid homeostasis is a tightly regulated process, with the enterohepatic circulation allowing minimal daily fecal bile acid losses (∼5%) that are closely balanced by hepatic bile acid synthesis from cholesterol. The total bile acid pool consists of several varieties of bile acids, including conjugated and unconjugated versions of primary bile acids synthesized within hepatocytes as well as secondary bile acids, which are bile acids that are chemically modified by the gut microbiome to unique bile acid species. The discovery that bile acids are endogenous signaling molecules began the era of a new understanding of bile acid physiology, and bile acids have become increasingly recognized for their roles in lipid and glucose metabolism (6, 7), as well as energy expenditure (8, 9). Recent evidence has demonstrated that different bile acids have varying degrees of antagonistic or agonistic activities at the two major bile acid receptors, the farnesoid X receptor (FXR) and the G protein-coupled bile acid receptor (TGR5) (10). Animal models of bariatric surgery, including RYGB and ileal interposition (11, 12), have demonstrated that fasting total bile acids are markedly increased by these procedures through unknown mechanisms, although these effects have been corroborated clinically as well.
Although total bile acid concentrations appear to be increased by an unknown mechanism after RYGB, the changes of individual bile acid species and the time course for those effects are largely unknown. Understanding the postoperative changes in bile acid concentrations could reveal important clues about their potential effects after RYGB. The patient cohort examined in this study was recruited as part of a randomized, singly blinded trial to determine whether removal of the omentum was associated with short- and/or long-term metabolic improvements after RYGB. Those results, which included assessment of body weight, diabetes remission, cardiovascular risk factors, and markers of inflammation have been reported elsewhere (13–15). In the current analysis, we aimed to identify a time course for the increases in plasma bile acid concentrations and determine whether this was a generalized effect on all bile acids or whether specific bile acid species were increased. Additionally, we aimed to determine whether the changes in bile acids were significantly associated with other endpoints by using a database of anthropometric, hormonal, and hyperinsulinemic-euglycemic clamp data from this cohort to help better understand the mechanism of these changes.
Subjects and Methods
Study participants and approvals
The Vanderbilt University Institutional Review Board approved all study protocols and consent and study documents. Informed consent was obtained from all subjects before voluntary study participation. For full details of these subjects, see the reports in Refs. 13 and 15. Briefly, obese men or women 18–60 years old with or without type 2 diabetes were randomized to have RYGB with omentectomy or not at the time of their operation. These individuals included subjects with a body mass index (BMI) > 40 kg/m2 or > 35 kg/m2 with one or more comorbid conditions.
Chemicals
Cholic acid (5β-cholanic acid-3α,7α,12α-triol) [CA]; α-muricholic acid (5β-cholanic acid-3α,6β,7α-triol) [αMCA]; β-muricholic acid (5β-cholanic acid-3α,6β,7β-triol) [βMCA]; chenodeoxycholic acid (5β-cholanic acid-3α,7α-diol) [CDCA]; deoxycholic acid (5β-cholanic acid-3α,12α-diol) [DCA]; hyodeoxycholic acid (5β -cholanic acid-3α,6α-diol) [HDCA]; ursodeoxycholic acid (5β-cholanic acid-3α, 7β-diol) [UDCA]; taurocholic acid (5β -cholanic acid-3α,7α,12α-triol-N-[2-sulfoethyl]-amide) [TCA]; tauro-α-muricholic acid (5β-cholanic acid-3α,6β,7α-triol-N-[2-sulfoethyl]-amide) [TαMCA]; lithocholic acid (5β-cholanic acid-3α-ol) [LCA]; tauro-β-muricholic acid (5β-cholanic acid-3α,6β,7β-triol-N-[2-sulfoethyl]-amide) [TβMCA]; tauro-ω-muricholic acid (5β-cholanic acid-3α,6α,7β-triol-N-[2-sulfoethyl]-amide) [TωMCA]; taurochenodeoxycholic acid (5β-cholanic acid-3α,7α-diol-N-[2-sulfoethyl]-amide) [TCDCA]; taurodeoxycholic acid (5β-cholanic acid-3α, 12α-diol-N-[2-sulfoethyl]-amide) [TDCA]; taurohyodeoxycholic acid (5β-cholanic acid-3α,6α-diol-N-[2-sulfoethyl]-amide]) [THDCA]; taurolithocholic acid (5β-cholanic acid-3α-ol-N-[2-sulfoethyl]-amide) [TLCA]; tauroursodeoxycholic acid (5β-cholanic acid-3α,7β-diol-N-[2-sulfoethyl]-amide) [TUDCA]; glycocholic acid (5β-cholanic acid-3α,7α,12α-triol-N-[carboxymethyl]-amide) [GCA]; glycochenodeoxycholic acid (5β-cholanic acid-3α,7α-diol-N- [carboxymethyl]-amide) [GCDCA]; glycodeoxycholic acid (5β-cholanic acid-3α,12α-diol-N-(carboxymethyl)-amide) [GDCA]; glycohyodeoxycholic acid (5β-cholanic acid-3α,6α-diol-N-(carboxymethyl)-amide) [GHCA]; glycoursodeoxycholic acid (5β-cholanic acid-3α,7β-diol-N-(carboxymethyl)-amide) [GUDCA]; and glycolithocholic acid (5β-cholanic acid-3α-ol-N-(carboxymethyl)-amide) [GLCA] were purchased from Steraloids, Inc.
Cholic-2,2,4,4-d4 acid (5β-cholanic acid-3α,7α,12α-triol –2,2,4,4-d4) [d4-CA]; taurocholic-2,2,4,4-d4 acid (5β-cholanic acid-3α,7α,12α-triol-N-[2-sulfoethyl]-amide) [TCA-d4]; chenodeoxycholic-2,2,4,4-d4 acid (5β-cholanic acid-3α,7α-diol-2,2,4,4-d4) [CDCA-d4]; glycocholic-2,2,4,4-d4 acid (5β-cholanic acid-3α,7α,12α-triol-N-[carboxymethyl]-amide-2,2,4,4-d4) [GCA-d4]; and glycochenodeoxycholic-2,2,4,4-d4 acid (5β-cholanic acid-3α,7α-diol-N- [carboxymethyl]-amide-2,2,4,4-d4) [GCDCA-d4] were purchased from C/D/N Isotopes Inc.
Tauro-β-muricholic-2,2,4,4-d4 acid (5β-cholanic acid-3α,6β,7β-triol-N-[2-sulfoethyl]-amide-2,2,4,4-d4) [TβMCA-d4] was purchased from United States Biological Corp.
HPLC grade water, acetonitrile, ethanol, and methanol were used for analyses. Ammonium acetate and ammonia were purchased from Sigma Chemicals, and formic acid was purchased from Thermo Scientific.
Hyperinsulinemic-euglycemic clamps
To assess hepatic and peripheral insulin sensitivity, subjects underwent a hyperinsulinemic-euglycemic clamp. For detailed methods, see Refs. 13–15. Briefly, participants were admitted to the Vanderbilt Clinical Research Center the evening before the clamp, ate a standardized meal, and subsequently fasted overnight. The following morning, catheters were placed for infusions and blood sampling as part of the clamp procedure.
Body composition analysis and energy expenditure
Dual-energy x-ray absorptiometry was used to measure both fat mass and fat free mass at each of the study time points except for the 1-month visit (16). Energy expenditure was measured using a metabolic cart.
Blood collection and hormone measurements
Blood was collected for bile acid, and other hormone measurements were collected at the indicated time points before or after RYGB after a 12-hour fast. Blood was centrifuged at 10 000 × g for 10 minutes, and plasma was frozen at −80°C for further analysis. Human fibroblast growth factor 19 (FGF19) plasma concentrations were measured using a quantitative sandwich ELISA (R&D Systems).
Calibrators and controls
Stock solutions of 2.5 mmof all bile acids (THCA, hyocholic acid [HCA], TαMCA, TβMCA, TωMCA, HDCA, THDCA; 10 mm) were used to prepare calibrators with concentrations of 100 μmin methanol. For the preparation of calibrators, bile acids were mixed to achieve final concentrations of 20, 2.5, 0.75, 0.25, 0.05, 0.015, and 0.005 μm. To prepare 20 mL of a 2.0 nminternal standard, 250 μL each of d4-CDCA, d4-TCA, and d4-GCDCA, and 500 μL each of d4-CA, d4-TβMCA, and d4-GCA were added to 20% (v/v) acetonitrile.
Sample preparation for UPLC/ESI-MS
To 50 μL of plasma, the following was added: 200 μL of 100 mmaqueous sodium hydroxide and 50 μL of internal standard. The sample was heated at 64°C for 30 minutes and centrifuged for 10 minutes at 14 400 × g, and the supernatant was acidified to pH 7.0 with 50 μL of 0.1 mhydrochloric acid. The sample was brought to a final volume of 1 mL with water and applied to a 1-cc (30 mg) Oasis HLB cartridge (Waters) previously equilibrated first with 1 mL of methanol, then 1 mL of water (17). The column-bound bile acids were washed with 1 mL of 5% (v/v) aqueous methanol, then 1 mL of 2% (v/v) aqueous formic acid. Bile acids were eluted from the column with 1 mL of 2% (v/v) ammonia in methanol, and the eluent was evaporated to dryness using a rotary evaporator at 30°C for 2 hours. Samples were resuspended in 100 μL of 25% (v/v) acetonitrile in water.
Liquid chromatography
An Acquity ultra performance liquid chromatography system (Waters) employing a Luna C18(2) 50 × 2.0 mm, 3 mm column, C18 4 × 2.0 mm precolumn, both from Phenomenex, was heated to 50°C, and a binary solvent system of 20% (v/v) acetonitrile in water (mobile phase A) and 80% (v/v) acetonitrile in water (mobile phase B) both containing 1 mmammonium acetate were used to resolve plasma bile acids. The injection volume onto the column was 15 μL. The flow rate was 400 μL/min into the mass spectrometry (MS). Chromatography was similar to a published method and started with a solvent mixture of 95% A that decreased to 85% A at 15 minutes, to 75% at 20 minutes, and to 25% at 22 minutes, after which it was increased back to 95% at 24 minutes where it was held for 3 minutes (18).
Mass spectrometry
MS analysis was performed using a TSQ Quantum mass spectrometer (ThermoFinnigan) equipped with an electrospray ionization probe in negative-ion mode. Quantitation was done in a multiple reaction monitoring mode with a collision energy of 10 V. The following (optimized) parameters were used for the detection of the analytes and the internal standard: N2 sheath gas, 49 psi; N2 auxiliary gas, 25 psi; spray voltage, 3.0 kV; source collision-induced dissociation, 25 V; capillary temperature, 300°C; capillary offset, −35 V; tube lens voltage, 160 V; Q2 gas pressure, 1.5 mTorr; Q3 scan width, 1 m/z; Q1/Q3 peak widths at half-maximum, 0.7 m/z. Calibration curves and concentration of individual bile acids were calculated by LCQuan 2.5.5 software (ThermoFinnigan). Concentrations of individual bile acids were calculated from peak area in the chromatogram detected with select reaction monitoring relative to the appropriate internal standard.
Statistical analysis
Data are reported as mean ± SEM unless otherwise indicated. Repeated subject measurements and hormone concentrations over time were compared using mixed-effects modeling with Bonferroni corrections for pairwise comparisons. A priori determination of covariates to be independently examined for associations with changes in bile acids included: BMI, homeostatic model assessment (HOMA), hepatic insulin sensitivity index (HISI), glycated hemoglobin (HbA1c), endogenous glucose production (EGP), and energy expenditure. Data analysis and visualization were performed using IBM SPSS version 22 (IBM Corp) and GraphPad Prism version 6 (GraphPad Software Inc). Because this was an exploratory study using previously collected patient samples, there was no specific power or sample size calculation for the data examined herein. In an attempt to increase our statistical power, we aimed to have a sample size of at least 20 individuals at each time point because most other studies examining similar endpoints have been published with fewer individuals (n = 6–12). Differences were considered statistically significant using α = 0.05.
Results
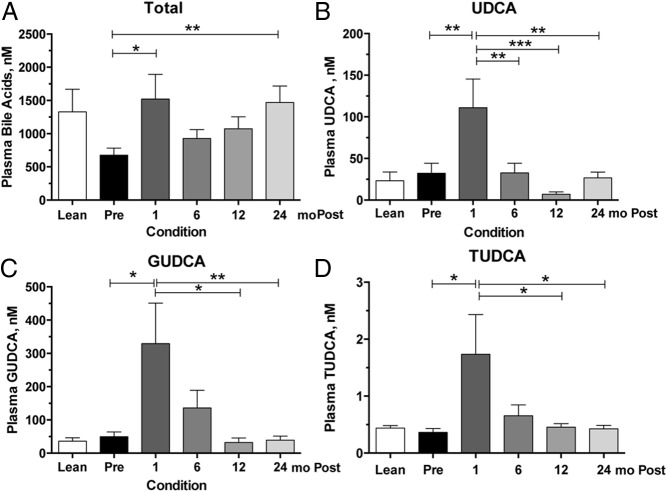
A brief overview of the selected patient cohort (n = 21) from this clinical study can be found in Table 1. All patients underwent RYGB, with approximately half (nine of 21) of the patients having a diagnosis of type 2 diabetes at the time of surgery, and approximately half (12 of 21) were nondiabetic, obese subjects. Using mixed-effects modeling to compare the changes in fasting plasma bile acid concentrations over time, total bile acid concentration (Figure 1A) exhibited a biphasic rise, being significantly elevated at 1 month (P < .05) and 24 months (P < .01) postoperatively when compared to preoperative levels. Of the entire bile acid complement quantified, this increase was due to significant increases in only three bile acids at 1 month—ursodeoxycholic acid (UDCA), glycoursodeoxycholic acid (GUDCA), and tauroursodeoxycholic acid (TUDCA). UDCA is a secondary bile acid and stereoisomer of chenodeoxycholic acid (CDCA), which results from microbial isomerization of CDCA in the gastrointestinal tract. UDCA concentration (Figure 1B) at 1 month was significantly different than every other time point and returned to near-baseline concentration by 6 months. GUDCA and TUDCA are the glycine- and taurine-conjugated forms of UDCA, respectively, that result from gastrointestinal reabsorption of UDCA via the enterohepatic circulation and subsequent hepatic conjugation. Similar to UDCA, GUDCA/TUDCA concentrations were also elevated at 1 month (Figure 1C) and then trended down to baseline values by 12 months. No significant differences in GUDCA or TUDCA were detected between 1 and 6 months or between 6 and 12 months. Because UDCA and its conjugates are non-12-α-hydroxylated bile acids, their significant increases at 1 month were associated with a trend for a decrease in the 12-α/non-12-α ratio, although this was not significantly different. No other bile acid concentrations were significantly changed at 1 month.
Table 1.
Selected Anthropometric Characteristics of Study Cohort (n = 21)
| Variable | Preoperative |
1 mo |
6 mo |
12 mo |
24 mo |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age, y | 44.1 | 9.9 | ||||||||
| Weight, kg | 133.4 | 27.4 | 118.0 | 25.0 | 99.1 | 22.7 | 91.8 | 21.7 | 93.4 | 21.8 |
| BMI, kg/m2 | 47.4 | 8.5 | 42.0 | 8.5 | 35.2 | 7.2 | 32.7 | 7.0 | 33.3 | 7.0 |
| HbA1c, % | 6.56 | 1.42 | 5.83 | 0.58 | 5.61 | 0.66 | 5.82 | 1.05 | 5.74 | 0.85 |
| HOMA | 3.20 | 1.30 | 1.94 | 1.20 | 1.14 | 0.70 | 1.90 | 0.52 | 1.21 | 0.60 |
| EGP, mg/kg/min | 1.03 | 0.32 | 0.91 | 0.22 | 1.13 | 0.18 | 1.24 | 0.28 | 1.21 | 0.26 |
| HISI | 0.42 | 0.20 | 1.10 | 0.78 | 1.76 | 1.17 | 2.10 | 2.18 | 1.37 | 0.56 |
| Energy expenditure | 2062.5 | 334.9 | 1820.5 | 292.0 | 1775.0 | 295.7 | 1686.6 | 299.4 | 1640.2 | 235.9 |
| Fat mass, g | 65 511 | 17 196 | 40 400 | 14 456 | 33 789 | 14 137 | 36 094 | 15 260 | ||
| Lean mass, g | 64 650 | 13 484 | 55 084 | 12 827 | 54 985 | 12 625 | 54 806 | 12 153 | ||
HISI was calculated as 103·min·mL·mg−1·μU−1. HOMA is expressed in arbitrary “resistance” units.
Figure 1.
Plasma concentrations of total, UDCA, and conjugated UDCA species preoperatively and after RYGB. Serial plasma samples were collected for bile acid measurements. A, Total bile acid concentration, which represents the sum of 17 different bile acid species, are significantly increased at 1 and 24 months after RYGB. B—D, Increases in total bile acid concentrations seen at 1 month are due to increases in UDCA (B), GUDCA (C), and TUDCA (D). Sample size for each time point in the surgical cohort is 19 to 21 individuals. Lean individuals (n = 8) are included for visual comparison only. Asterisks represent significant differences of indicated time points: *, P < .05; **, P < .01; ***, P < .001, using mixed-effects modeling with Bonferroni corrections for pairwise comparisons.
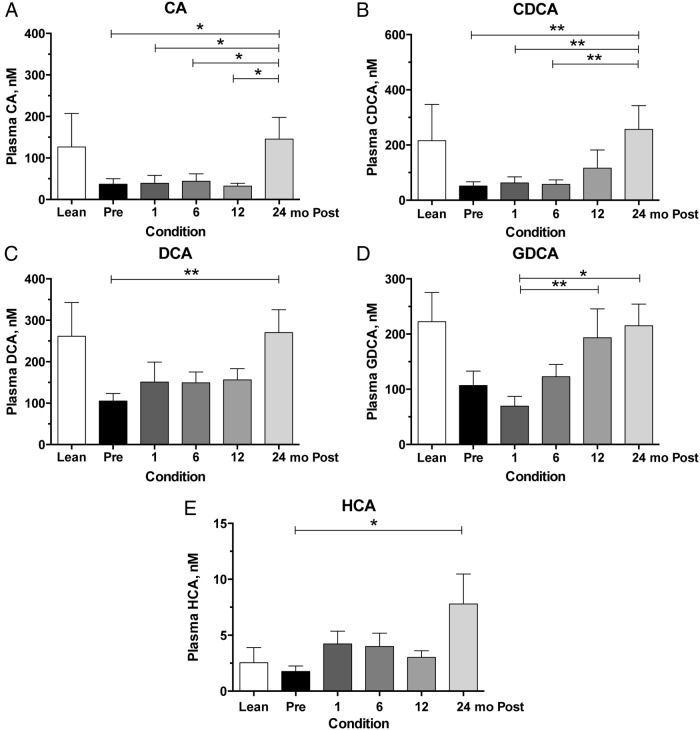
Because the increases in UDCA and its conjugates waned to baseline levels after 1 month, we further examined what bile acid species contributed to the significant increases at 24 months. Of the individual bile acids quantified, there was a trend for a gradual rise in four bile acids over time that resulted in significant elevations at 24 months compared to preoperative values. These were the primary bile acids, cholic acid (CA) and CDCA. CA (Figure 2A) was significantly elevated at 24 months compared to every other time point (P < .05), and CDCA (Figure 2B) was significantly elevated at 24 months compared to every other time point (P < .01) except 12 months. Besides the 24-month time points, no other pairwise differences were detected between the primary bile acids. Similarly, there was a trend for a gradual increase in the secondary bile acid deoxycholic acid (DCA), the dehydroxylated form of CA, with time. DCA concentration (Figure 2C) was significantly elevated at 24 months when compared to preoperative concentration (P < .01). There was also a significant increase in the glycine-conjugated form of DCA (Figure 2D), glycodeoxycholic acid (GDCA), which was observed at 12 months (P < .01) and 24 months (P < .05), compared to the 1-month postoperative concentrations. There were no significant differences between any of the other time points. The only other significant difference at the 24-month time point was a small but significant increase in hyocholic acid (Figure 2E), which is a derivative of CDCA typically found in higher concentrations in murine species, but also present in low concentrations in human plasma. Post hoc dichotomization of the cohort on preoperative diabetes status (ie, comparing diabetic vs nondiabetic), however, did show that diabetic patients tended to have slight nonsignificant trends for higher mean bile acid concentrations overall.
Figure 2.
Plasma concentrations of selected primary and secondary bile acid concentrations preoperatively and after RYGB. Serial plasma samples were collected for bile acid measurements preoperatively and at 1, 6, 12, and 24 months postoperatively. A—D, CA (A) and CDCA (B) have increases observed at 24 months, whereas DCA (C) and its glycoconjugate GDCA (D) have gradual increases at 12 and 24 months compared to preoperative levels, respectively. E, Hyocholic acid (HCA) had a small but significant increase at 24 months compared to preoperative levels. Sample size for each time point in the surgical cohort is 20 or 21 individuals. Lean individuals (n = 8) are included for visual comparison only. Asterisks represent significant differences of indicated time points: *, P < .05; **, P < .01, using mixed-effects modeling with Bonferroni corrections for pairwise comparisons.
To determine whether other variables might be associated with the changes in bile acids over time, we measured circulating FGF19 levels and looked for significant interactions between anthropomorphic and hormonal variables that we collected as part of our original study. FGF19 is secreted via the enteroendocrine cells of the distal ileum in response to bile acids, and we posited that the increases we see in bile acids acutely and long-term may be associated with increased FGF19 levels. Compared to preoperative plasma concentrations, FGF19 increased over the study time course (Figure 3), with gradual increases over time becoming significant in pairwise comparisons at 12 and 24 months relative to preoperative concentrations. However, the increase in FGF19 was not significantly associated with any other study variable that we examined over time (ie, BMI, HOMA, HISI, EGP).
Figure 3.
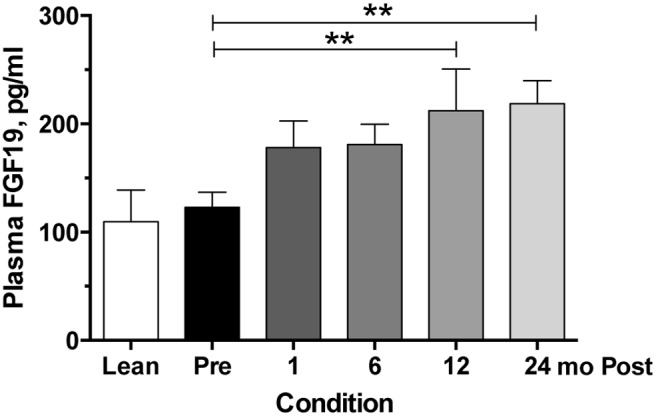
Circulating FGF19 concentrations preoperatively and after RYGB. Serial plasma samples were analyzed for FGF19 concentrations preoperatively and at 1, 6, 12, and 24 months postoperatively. Sample size for each time point in the surgical cohort is 20 or 21 individuals. Lean individuals (n = 8) are included for visual comparison only. Asterisks represent significant differences of indicated time points compared to preoperative levels: **, P < .01, using mixed-effects modeling with Bonferroni corrections for pairwise comparisons.
Within the mixed-effects model, we examined whether other anthropomorphic or hormonal variables were significantly associated with the changes in individual bile acid concentrations or groups of structurally similar bile acids (eg, conjugated vs unconjugated, primary vs secondary) over the time course of the study. All individual and grouped plasma bile acid concentrations were examined in the mixed-effects model, and changes in BMI, HOMA, HISI, HbA1c, and EGP were examined independently and for significant interactions with the changes in bile acid groups or individual species. However, none of these variables independently or in combination had significant associations with any of the bile acid changes over the course of the study.
Discussion
The contributions of bile acids to the early and late improvements in insulin sensitivity and other metabolic endpoints after RYGB are incompletely understood. In this study, we examined longitudinal changes in fasting plasma bile acid concentrations in a cohort of RYGB patients preoperatively and up to 24 months after surgery. Increased fasting concentrations of plasma total bile acids appear to be biphasic, with significant increases observed early at 1 month after RYGB and then later at 24 months compared to preoperative levels. The biphasic increases in total bile acids at these early and late time points are secondary to increases in chemically different bile acid species. The early changes are due to surges in the secondary bile acid UDCA and its conjugates. These changes in bacterially derived bile acids wane by 6 months, but then are replaced by gradual increases in primary bile acids as well as the predominate secondary bile acids, DCA and GDCA.
With the increasingly recognized effects of bile acids on metabolism, a number of studies have attempted to establish a causal link between plasma bile acid levels and metabolic improvements after bariatric surgery. Several studies with smaller sample sizes (n = 7–12) have shown that total bile acids are increased after RYGB, compared to preoperative levels, but not after purely restrictive procedures like adjustable gastric banding (19–21). The complicating factor when comparing studies is the time elapsed between the pre- and postoperative measurement(s). Patti et al (19) demonstrated in a cross-sectional study with BMI-matched controls that fasting plasma bile acids were significantly increased at approximately 2.7 years after RYGB (n = 9). Follow-up longitudinal studies have examined plasma concentrations much sooner postoperatively with conflicting results. Some studies described significant increases in total bile acids within weeks to months (21–23), whereas others fail to detect early changes (ie, days/weeks) but do detect increases by 1 year (24, 25). Further complicating these comparisons is that plasma bile acids tend to be higher in severely insulin-resistant subjects (26), and there may be changes in the response to bariatric surgery based on the preoperative degree of insulin resistance of the patient (22) and whether insulin resistance resolves completely or partially after RYGB (27). This natural variation coupled with a relatively smaller sample size may explain why some investigators have not detected differences at various time points. Given these conflicting reports, we aimed to clarify this with more frequent and early (ie, 1 mo) measurements of bile acids from fasting plasma in a group of patients that were nearly equally represented by nondiabetics and persons with diabetes who had complete resolution of diabetes postoperatively (ie, as measured by the need for antidiabetic medications). These findings confirm a number of other studies that have examined total circulating bile acids preoperatively and postoperatively after bariatric surgery. Our data suggest that there is a biphasic increase in total bile acids peaking early and late, and those differences are due to specific bile acid species.
In addition to the fluctuations in total bile acid concentrations after RYGB, we aimed to identify whether there was a particular bile acid or group of bile acids associated with the changes seen postoperatively, or whether the increases were a generalized effect of all bile acid chemical species. Most early reports describing the changes in bile acid concentrations postoperatively do not detail the individual concentrations and tend to group chemically similar bile acids (eg, primary/secondary, conjugated/unconjugated) for analysis. The largest (n = 63) and most comprehensive study to date by Werling et al (28) examined chemical groups and individual bile acids in the fasted and postprandial states. This study showed increases in fasting total and unconjugated bile acids, which is similar to the current study. Comparing our results to theirs, however, is limited because that study only compared one time point postoperatively at 15 months. Additionally, we do not have any data on the bile acid responses to meal-challenge tests that show significant increases in area under the curve for bile acids in that study. In this study, we present only fasting plasma levels of bile acids and cannot comment on how postprandial responses in bile acid species may have differed. Regardless, Ahmad et al (29) showed that even if fasting plasma bile acid concentrations are unchanged after bariatric surgery, the response to a meal appears to be restored to that of a healthy, lean individual. Nonetheless, the increases seen at 15 months by Werling et al (28) in fasting bile acids are similar to our results for total and unconjugated bile acids. The changes reported in primary bile acids are mixed compared to the current study because they report increases in CDCA but not CA, whereas we see increases in both.
With the growing evidence that particular bile acids may have unique antagonistic or even partial agonist effects at bile acid receptors (eg, FXR, TGR5) (30, 31), we wanted to identify whether one of these species may be accountable for the overall changes seen and whether it might affect gastrointestinal hormonal signaling. Bile acid signaling in the distal ileum is known to stimulate ileal production of FGF19, an endocrine factor that improves insulin sensitivity and down-regulates bile acid production through a negative feedback mechanism. Other groups have previously demonstrated that FGF19 levels are increased after bariatric surgery but have not shown any significant evidence that direct FGF19 changes explain the metabolic improvements after RYGB (22, 32). Our findings are consistent with other reports showing increased FGF19 concentrations after RYGB, although the gradual changes in FGF19 levels do not parallel the more rapid metabolic improvements by 1 month postoperatively (22). The data from the current study as well as those of others (22, 33) suggest that the metabolic improvements seen early after RYGB are not secondary to changes in FGF19 levels. However, the early changes in UDCA and its metabolites may contribute to metabolic improvements through a mechanism not yet identified. Several recent studies are consistent with the hypothesis that UDCA and/or its metabolites may be effectors in the early improvements in insulin sensitivity after RYGB (30, 34). Indeed, recent data have identified UDCA as a putative antagonistic bile acid at the FXR receptor, a possible mechanism for improvements to be achieved within the first weeks to months after surgery (30, 35, 36).
Over the last several years, much has been learned about the intricate relationship between bile acid metabolism and the gut microbiome because both fields independently have become areas of great interest for their potential therapeutic benefits (reviewed in Refs. 37 and 38). Bariatric procedures in rodents are also associated with changes in gut microbiome and are tightly coupled to bile acid metabolic and signaling changes as well (35, 39, 40). In the current study, it is tempting to speculate on the mechanism of the interaction between bile acids and the gut microbiome, although our findings demonstrate selective increases in UDCA and its conjugates that can only result from microbial transformation of CDCA to UDCA because humans lack the 7-α-epimerase responsible for this transformation (41). Thus, the increased UDCA and conjugate concentrations must be the result of either: 1) increased microbial conversion of CDCA to UDCA; or 2) retention of UDCA in the bile acid pool by an unknown mechanism. Our study cannot address this further; however, animal models of bariatric surgery from our group (42) and others (32, 43) have demonstrated that bile acid synthesis is increased, and the increases in UDCA observed here are likely the result of conversion of newly synthesized CDCA. Further studies are needed to determine whether the gut microbiome is subsequently driving these bile acid changes, whether bile acid concentrations are directly altering the gut microbiome, or whether the adaptive microbiome changes are unrelated to bile acids or other metabolic improvements.
Overall, this study strengthens evidence that total plasma bile acid concentrations increase after RYGB. Furthermore, those plasma increases are biphasic, with early increases being secondary to surges in the insulin-sensitizing and putative FXR antagonistic bile acids, UDCA and GUDCA. The increases in plasma bile acids observed at 2 years are due to nonspecific rises in the concentrations of the primary and major secondary bile acids. The early increases in UDCA and its glycine and taurine conjugates at 1 month are associated with improved hepatic insulin sensitivity and do not appear to be acting through changes in FGF19. At later time points, it is likely that the gradual weight loss that typically ensues after surgery maintains this improved hepatic insulin sensitivity after RYGB (13). Future studies should focus on individual bile acid changes and mechanistic studies examining these changes after RYGB and other bariatric procedures.
Acknowledgments
The authors thank Drs Joseph Antoun and Wade Calcutt as well as Pamela Marks-Shulman and Reem Sidani, Vanderbilt University Medical Center, Nashville, Tennessee, for their invaluable technical and administrative efforts related to this project.
The National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (NIH) supported the research reported in this publication, specifically by NIH Grants DK020593 (to the Vanderbilt Diabetes Research and Training Center), Clinical and Translational Science Award (CTSA) Grant (UL1 TR000445), DK058404 (to the Vanderbilt Digestive Disease Research Center), R01 DK091748 (to N.N.A.), R01 DK070860 (to N.N.A.), R01 DK096527 (to N.N.A.), F32 DK103474 (to V.L.A.), R01 DK105847 (to N.N.A. and C.R.F.), P30 DK020593 (to C.R.F.), and P30 DK058404 (to C.R.F.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CA
- cholic acid
- CDCA
- chenodeoxycholic acid
- DCA
- deoxycholic acid
- EGP
- endogenous glucose production
- FGF19
- fibroblast growth factor 19
- FXR
- farnesoid X receptor
- GDCA
- glycodeoxycholic acid
- GUDCA
- glycoursodeoxycholic acid
- HbA1c
- glycated hemoglobin
- HISI
- hepatic insulin sensitivity index
- HOMA
- homeostatic model assessment
- MS
- mass spectrometry
- RYGB
- Roux-en-Y gastric bypass
- TUDCA
- tauroursodeoxycholic acid
- UDCA
- ursodeoxycholic acid.
References
- 1. Kashyap SR, Bhatt DL, Wolski K, et al. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care. 2013;36:2175–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Puzziferri N, Roshek TB, 3rd, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312:934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahn SM, Pomp A, Rubino F. Metabolic surgery for type 2 diabetes. Ann NY Acad Sci. 2010;1212:E37–E45. [DOI] [PubMed] [Google Scholar]
- 6. Matsubara T, Li F, Gonzalez FJ. FXR signaling in the enterohepatic system. Mol Cell Endocrinol. 2013;368:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang Y, Lee FY, Barrera G, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci USA. 2006;103:1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watanabe M, Horai Y, Houten SM, et al. Lowering bile acid pool size with a synthetic farnesoid X receptor (FXR) agonist induces obesity and diabetes through reduced energy expenditure. J Biol Chem. 2011;286:26913–26920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. [DOI] [PubMed] [Google Scholar]
- 10. Trauner M, Claudel T, Fickert P, Moustafa T, Wagner M. Bile acids as regulators of hepatic lipid and glucose metabolism. Dig Dis. 2010;28:220–224. [DOI] [PubMed] [Google Scholar]
- 11. Culnan DM, Albaugh V, Sun M, Lynch CJ, Lang CH, Cooney RN. Ileal interposition improves glucose tolerance and insulin sensitivity in the obese Zucker rat. Am J Physiol Gastrointest Liver Physiol. 2010;299:G751–G760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bhutta HY, Rajpal N, White W, et al. Effect of Roux-en-Y gastric bypass surgery on bile acid metabolism in normal and obese diabetic rats. PLoS One. 2015;10:e0122273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dunn JP, Abumrad NN, Breitman I, et al. Hepatic and peripheral insulin sensitivity and diabetes remission at 1 month after Roux-en-Y gastric bypass surgery in patients randomized to omentectomy. Diabetes Care. 2012;35:137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tamboli RA, Hajri T, Jiang A, et al. Reduction in inflammatory gene expression in skeletal muscle from Roux-en-Y gastric bypass patients randomized to omentectomy. PLoS One. 2011;6:e28577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fabbrini E, Tamboli RA, Magkos F, et al. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology. 2010;139:448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tataranni PA, Ravussin E. Use of dual-energy x-ray absorptiometry in obese individuals. Am J Clin Nutr. 1995;62:730–734. [DOI] [PubMed] [Google Scholar]
- 17. Rodrigues CM, Setchell KD. Performance characteristics of reversed-phase bonded silica cartridges for serum bile acid extraction. Biomed Chromatogr. 1996;10:1–5. [DOI] [PubMed] [Google Scholar]
- 18. Hagio M, Matsumoto M, Fukushima M, Hara H, Ishizuka S. Improved analysis of bile acids in tissues and intestinal contents of rats using LC/ESI-MS. J Lipid Res. 2009;50:173–180. [DOI] [PubMed] [Google Scholar]
- 19. Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring). 2009;17:1671–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, Klein S. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab. 2013;98:E708–E712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pournaras DJ, Glicksman C, Vincent RP, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153:3613–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jørgensen NB, Dirksen C, Bojsen-Møller KN, et al. Improvements in glucose metabolism early after gastric bypass surgery are not explained by increases in total bile acids and fibroblast growth factor 19 concentrations. J Clin Endocrinol Metab. 2015;100:E396–E406. [DOI] [PubMed] [Google Scholar]
- 23. Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58:1400–1407. [DOI] [PubMed] [Google Scholar]
- 24. Steinert RE, Peterli R, Keller S, et al. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity (Silver Spring). 2013;21:E660–E668. [DOI] [PubMed] [Google Scholar]
- 25. Simonen M, Dali-Youcef N, Kaminska D, et al. Conjugated bile acids associate with altered rates of glucose and lipid oxidation after Roux-en-Y gastric bypass. Obes Surg. 2012;22:1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haeusler RA, Astiarraga B, Camastra S, Accili D, Ferrannini E. Human insulin resistance is associated with increased plasma levels of 12α-hydroxylated bile acids. Diabetes. 2013;62:4184–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gerhard GS, Styer AM, Wood GC, et al. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care. 2013;36:1859–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Werling M, Vincent RP, Cross GF, et al. Enhanced fasting and post-prandial plasma bile acid responses after Roux-en-Y gastric bypass surgery. Scand J Gastroenterol. 2013;48:1257–1264. [DOI] [PubMed] [Google Scholar]
- 29. Ahmad NN, Pfalzer A, Kaplan LM. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes (Lond). 2013;37:1553–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mueller M, Thorell A, Claudel T, et al. Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity. J Hepatol. 2015;62:1398–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gonzalez FJ, Jiang C, Bisson WH, Patterson AD. Inhibition of farnesoid X receptor signaling shows beneficial effects in human obesity. J Hepatol. 2015;62:1234–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dutia R, Embrey M, O'Brien S, et al. Temporal changes in bile acid levels and 12α-hydroxylation after Roux-en-Y gastric bypass surgery in type 2 diabetes. Int J Obes (Lond). 2015;39:806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kars M, Yang L, Gregor MF, et al. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010;59:1899–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sayin SI, Wahlström A, Felin J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-β-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. [DOI] [PubMed] [Google Scholar]
- 36. Wang YD, Chen WD, Yu D, Forman BM, Huang W. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor κ light-chain enhancer of activated B cells (NF-κB) in mice. Hepatology. 2011;54:1421–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuipers F, Bloks VW, Groen AK. Beyond intestinal soap–bile acids in metabolic control. Nat Rev Endocrinol. 2014;10:488–498. [DOI] [PubMed] [Google Scholar]
- 38. Jones ML, Tomaro-Duchesneau C, Prakash S. The gut microbiome, probiotics, bile acids axis, and human health. Trends Microbiol. 2014;22:306–308. [DOI] [PubMed] [Google Scholar]
- 39. Liou AP, Paziuk M, Luevano JM, Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5:178ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li F, Jiang C, Krausz KW, et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun. 2013;4:2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. [DOI] [PubMed] [Google Scholar]
- 42. Flynn CR, Albaugh VL, Cai S, et al. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nat Commun. 2015;6:7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Myronovych A, Kirby M, Ryan KK, et al. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity (Silver Spring). 2014;22:390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]