Abstract
Context:
Vitamin D (25-hydroxyvitamin D [25OHD]) deficiency (<20 ng/mL) and insufficiency (20–29 ng/mL) are common in primary hyperparathyroidism (PHPT), but data regarding their skeletal effects in PHPT are limited.
Objective:
The objective was to evaluate the association between 25OHD levels and PHPT severity.
Design, Settings, and Participants:
This is a cross-sectional analysis of 100 PHPT patients with and without 25OHD insufficiency and deficiency from a university hospital setting.
Outcome Measures:
We measured calciotropic hormones, bone turnover markers, and bone mineral density (BMD) by dual x-ray absorptiometry.
Results:
Lower 25OHD was associated with some (PTH: r = −0.42; P < .0001; 1,25-dihydroxyvitamin D: r = −0.27; P = .008; serum PO4: r = 0.31; P = .002) but not all (serum/urine calcium) indicators of PHPT severity. Lower 25OHD was also associated with younger age, higher body mass index, male gender, better renal function, and lower vitamin D intake. Comparison of those with deficient (<20 ng/mL; 19%) vs insufficient (20–29 ng/mL; 35%) vs replete (≥30 ng/mL; 46%) 25OHD demonstrated more severe PHPT as reflected by higher PTH (mean ± SEM, 126 ± 10 vs 81 ± 7 vs 72 ± 7 pg/mL; P < .0001) but no difference in nephrolithiasis, osteoporosis, fractures, serum or urinary calcium, bone turnover markers, or BMD after adjustment for age and weight. In women, T-scores at the 1/3 radius were lower in those with 25OHD of 20–29 ng/mL, compared to those who were vitamin D replete (P = .048). In multiple regression modeling, 25OHD (but not PTH) was an independent predictor of 1/3 radius BMD.
Conclusion:
Vitamin D deficiency is associated with more severe PHPT as reflected by PTH levels, but effects on BMD are limited to the cortical 1/3 radius and are quite modest. These data support international guidelines that consider PHPT patients with 25OHD <20 ng/mL to be deficient. However, in this cohort with few profoundly vitamin D-deficient patients, vitamin D status did not appear to significantly impact clinical presentation or bone density.
Vitamin D deficiency (25-hydroxyvitamin D [25OHD], <20 ng/mL) and insufficiency (25OHD, 20–29 ng/mL) are both common in primary hyperparathyroidism (PHPT) (1, 2). It has been generally accepted that PHPT disease severity is heightened when coexisting severe vitamin D deficiency is present, with reports of higher PTH and calcium, increased adenoma weight, lower bone mineral density (BMD) with increased bone turnover, and even increased fracture and risk for osteitis fibrosa cystica (3). There are limited data on the effects of vitamin D deficiency in the mainly asymptomatic PHPT populations seen today. Because patients with PHPT may be observed for years without intervention (4, 5), the effects of vitamin D deficiency may be of particular importance to skeletal health in those who do not have parathyroidectomy.
The third and fourth International Workshops on Asymptomatic PHPT recommended measuring 25OHD in all patients and replenishing vitamin D in those with levels <20 ng/mL before medical or surgical treatment is recommended (6, 7). However, the definitions of vitamin D deficiency and insufficiency are controversial, even in the general population, and there are no data on the applicability of these cutpoints to patients with PHPT. The workshops highlighted the need for further data. This study was designed to evaluate the clinical, biochemical, and skeletal consequences of low vitamin D levels in a modern American PHPT cohort and to explore the relevance of various commonly employed vitamin D clinical thresholds (<20, 20–29, and ≥30 ng/mL).
Subjects and Methods
This was a cross-sectional study of clinical characteristics, biochemical findings, bone turnover markers (BTMs), and areal BMD (aBMD) by dual x-ray absorptiometry (DXA) in PHPT patients with and without vitamin D deficiency or insufficiency. Patients gave written, informed consent. This study was approved by the Columbia University Medical Center (CUMC) Institutional Review Board.
Subjects
Participants were recruited from the Metabolic Bone Diseases Unit and the Endocrine Surgery and General Endocrinology Clinics at CUMC. Of 297 PHPT patients who agreed to be screened (eligibility determined and study procedures described) for participation, 193 were eligible. By study design, 100 consecutive PHPT patients who met eligibility criteria and agreed to participate were enrolled (December 2010 to February 2014). Those who enrolled and those who were eligible but declined participation were similar in demographic features (82% female; mean ± SD age, 60.7 ± 12.9 y) and biochemistries (PTH, 95 ± 55 pg/mL; calcium, 10.9 ± 0.5 mg/dL).
Participants had PHPT, diagnosed by both hypercalcemia (calcium >10.2 mg/dL) and an elevated or inappropriately normal PTH level on more than one occasion before enrollment. None had thiazide-induced hyperparathyroidism or familial hypocalciuric hypercalcemia (excluded on the basis of family history and 24-h urine calcium). Of the 100 patients enrolled, 28% were referred from Endocrine Surgery and 69% from Endocrinology, and 3% were recruited from the internet. Exclusion criteria included bisphosphonate use within 2 years; current use of cinacalcet or denosumab; current or previous use of prednisone 7.5 mg >6 months; current or past use of carbamazepine, phenytoin, or phenobarbital >3 months; malignancy within 5 years other than nonmelanomatous skin cancer; granulomatous diseases; HIV; serum creatinine level ≥1.5 mg/dL; liver disease; gastrointestinal diseases known to affect calcium metabolism such as Crohn's disease, celiac disease, or gastric bypass; and pregnancy. Both symptomatic (ie, those with nephrolithiasis) and asymptomatic PHPT patients were enrolled, and participants were enrolled regardless of meeting third or fourth International Workshop guidelines for parathyroidectomy.
Clinical and biochemical evaluation
Demographic data, medical history, and medication use were obtained from participants. Calcium and vitamin D intake and sun exposure were assessed by validated questionnaire (8, 9). Information regarding nephrolithiasis and fragility fracture (atraumatic fracture or trauma involving a fall from a standing height or less) was based on history and included current as well as past events. No renal or fracture imaging was performed as part of this investigation. We used the standard National Institutes of Health classification system for self-identified race/ethnicity, which categorizes individuals into five different racial groups (American Indian/Alaska Native, Asian, Native Hawaiian/Pacific Islander, Black, and White) and two ethnic groups (Hispanic and non-Hispanic).
Fasting samples for serum calcium, phosphate, albumin, and creatinine and urinary calcium were measured by an automated chemistry analyzer. PTH was measured by immunoradiometric assay for intact PTH (Scantibodies Laboratory Inc). Serum 25OHD and 1,25-dihydroxyvitamin D were measured by liquid chromatography/tandem mass spectrometry (Quest Diagnostics). Bone-specific alkaline phosphatase (BSAP) was measured by ELISA (Quidel Corp), and carboxy-terminal telopeptides of type 1 collagen (CTX) by ELISA (Immunodiagnostics Systems). C-terminal fibroblast growth factor 23 (FGF-23) was measured by ELISA (Immutopics) because we anticipated between-group differences in PTH, 1,25-dihydroxyvitamin D, and phosphate, all regulators of FGF-23. Estimated glomerular filtration rate (eGFR) was calculated using the modification of diet in renal disease equation (10).
Dual-energy x-ray absorptiometry
aBMD was measured at the lumbar spine (LS; L1–L4), total hip (TH), femoral neck (FN), and 1/3 radius (Hologic Inc). T-scores and Z-scores were obtained using the manufacturer's reference norms. In vivo precision, determined according to the standard method at this facility, is 1.28% at the LS, 1.36% at the hip, and 0.70% for the distal radius (1/3 site) (11).
Statistical analysis
Between-group differences in demographic and skeletal indices were evaluated with ANOVA, χ2, or Fisher's exact test as appropriate. Values are expressed as least squares means ± SEM or percentages. Analysis of covariance models were used to assess between-group differences in absolute BMD and T-scores adjusting for covariates (age and weight). Z-scores were only adjusted for weight. Relationships between 25OHD and skeletal indices were assessed with Pearson or Spearman correlation as appropriate. A penalized B-spline function was used to create a smooth curve for the relationship between PTH and 25OHD levels. No data transformations were necessary, and the default penalized B-spline fit criterion was used, which fits a cubic spline with 100 evenly spaced knots and three evenly spaced exterior knots with degree = 3. The inflection point was estimated using regression modeling.
Stepwise multiple regression was used to evaluate independent predictors of 1/3 radius BMD (age, gender, weight, serum PTH, serum calcium, eGFR, and 25OHD). The stepwise selection process criterion for entry to the model was a univariate P < .3, and the criterion for retention in the model was a multivariate P < .10. For all analyses, a two-tailed P < .05 was considered to indicate statistical significance. Statistical analysis was performed using SAS version 9.3 (SAS Institute Inc).
Results
Overall cohort characteristics
Participants were predominantly female (78%) and had evidence of mild hypercalcemia (mean ± SEM: serum calcium, 10.8 ± 0.1 mg/dL; PTH, 85 ± 4.8 pg/mL). Uncorrected calcium levels ranged from 9.5–13.2 mg/dL on the day of study enrollment. Mean 25OHD was 29 ± 1.0 ng/mL, but ranged from 6–54 ng/mL. Vitamin D deficiency (25OHD <20 ng/mL) and insufficiency (20–29 ng/mL) were present in 19 and 35% of patients, respectively, whereas 46% were vitamin D sufficient (≥30 ng/mL). Only 4% were severely vitamin D deficient (25OHD <10 ng/mL). Vitamin D supplement use was reported in 65% of patients. A history of nephrolithiasis was found in 19%. No one had osteitis fibrosa cystica, but 39% had osteoporosis, and 15% had a fragility fracture history (at the spine, humerus, forearm, femur, tibia/fibula, and foot). Mean T-scores were normal at the LS (−1.0 ± 0.2) and TH (−1.0 ± 0.1) but were in the osteopenic range at the FN (−1.3 ± 0.1) and 1/3 radius (−1.3 ± 0.2). There were no within-person differences in aBMD between the 1/3 radius and LS (P = .10) or FN (P = .88). Osteoporosis was found at the LS (16.8%), FN (14.8%), and radius (23.8%), but the radius was no more likely to be affected by osteoporosis than other sites. Nonskeletal indications for surgery included a serum calcium ≥1 mg/dL above the upper limit of normal in 27%; 11% were <50 years old; 8% had an eGFR <60 mL/min; and 17% had a urine calcium ≥400 mg/d. Overall, 69% and 77% met one or more parathyroidectomy guidelines based on 2008 and 2013 recommendations, respectively (although imaging for occult nephrolithiasis and vertebral fractures was not available).
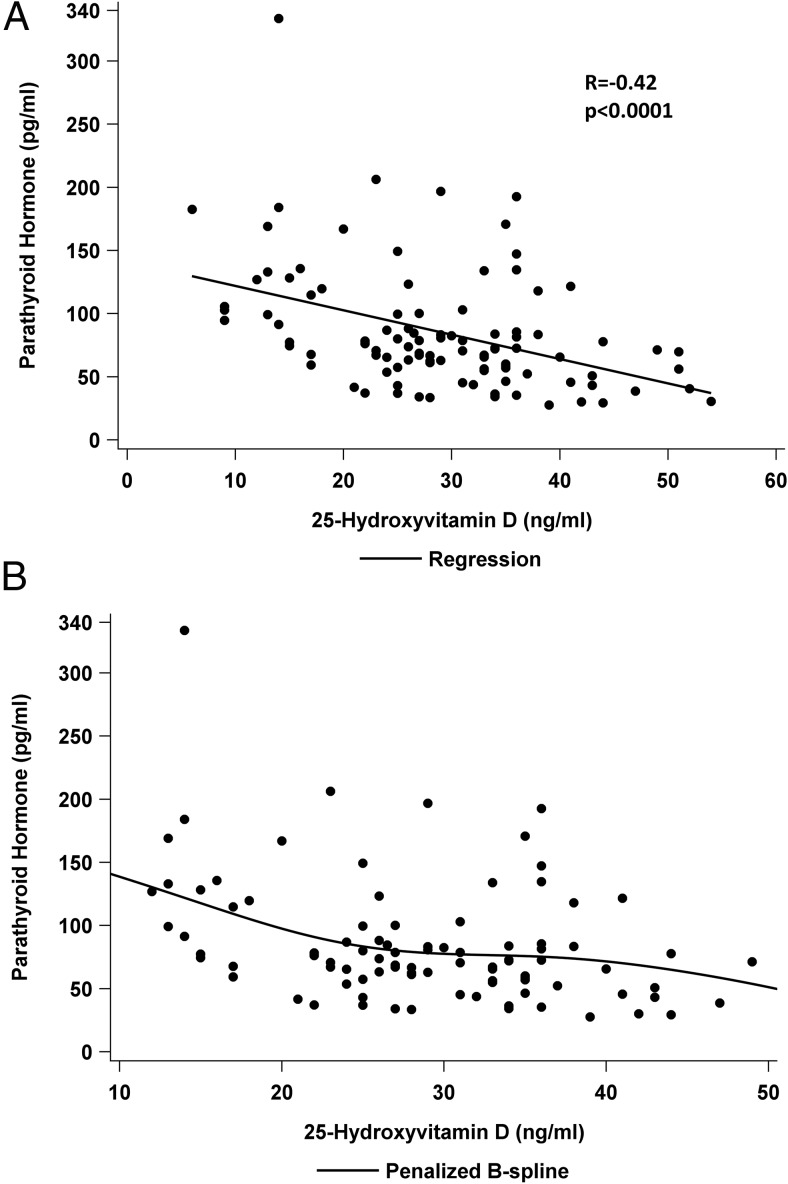
Levels of 25OHD correlated with PTH (r = −0.42; P < .0001; Figure 1A). A spline regressive fit for PTH and 25OHD levels suggested an inflection point for PTH at a 25OHD level of approximately 22 ng/mL (Figure 1B). Levels of 25OHD also correlated with some other indicators of PHPT disease severity, including 1,25-dihydroxyvitamin D (r = −0.27; P = .008) and serum phosphate (r = 0.31; P = .002), but not with serum calcium (r = −0.09; P = .40), urine calcium excretion (r = −0.13; P = .21), or PHPT duration (r = 0.02; P = .81). Renal function was also associated with 25OHD (serum eGFR: r = −0.33; P = .0009; FGF-23: r = 0.21; P = .04). Numerous demographic/behavioral factors correlated with 25OHD, including age (r = 0.31; P = .002), body mass index (BMI) (r = −0.23; P = .02), male sex (r = 0.2; P = .049), and vitamin D intake (r = 0.65; P < .0001). There was no association of 25OHD with race (r = −0.15; P = .15) or ethnicity (r = −0.02; P = .84).
Figure 1.
Relationship between 25OHD and serum PTH. A, Linear relationship between 25OHD as a continuous variable; B, regression spline analysis.
The expected positive associations between the biochemical hallmarks of PHPT, PTH, and calcium (r = 0.43; P < .001) and among these indices and BTMs were observed (CTX with PTH: r = 0.30; P = .0003; CTX with calcium: r = 0.39; P < .0001; BSAP with PTH: r = 0.20; P = .048; and BSAP with calcium: r = 0.31; P = .0018). We found no association of 25OHD with BTMs (CTX: r = −0.17; P = .10; BSAP: r = −0.15; P = .13). 25OHD was negatively associated with absolute BMD by DXA at the TH (r = −0.24; P = .02) and FN (r = −0.21; P = .04), but not at the LS (r = −0.05; P = .59) or 1/3 radius (r = −0.11; P = .28).
Investigation of PHPT by vitamin D status
In addition to the above associations with 25OHD levels (as a continuous variable), we were interested in the utility of commonly used clinical cutpoints for vitamin D status (25OHD <20 vs 20–29 vs ≥30 ng/mL). Those who were vitamin D replete were older than those with 25OHD <20 ng/mL and 20–29 ng/mL (Table 1; both P < .01) and more likely to be postmenopausal. Those with vitamin D deficiency were heavier than those who were vitamin D replete (P = .02). There were no between-group differences (<20 vs 20–29 vs ≥30 ng/mL) in gender, height, BMI, age of menopause, racial/ethnic composition, or current smoking. Daily calcium intake did not differ between the groups (Table 1). As expected, vitamin D supplementation was more common (85 vs 60 vs 26.3%; P < .0001) in those with higher Vitamin D. Daily vitamin D intake was higher in those who were vitamin D replete compared to those in the deficient (P = .048) and insufficient groups (P = .01). Sun exposure did not differ (Table 1).
Table 1.
Demographic, PHPT, and Lifestyle Characteristics by Vitamin D Status
| 25OHD, ng/mL |
P Value | |||
|---|---|---|---|---|
| <20 | 20–29 | ≥30 | ||
| n | 19 | 35 | 46 | |
| Age, y | 56.6 ± 2.7 | 58.5 ± 2.0 | 66.8 ± 1.7a,b | .0009 |
| Females, n/% | 14/17.7 | 25/31.7 | 40/50.6 | .19 |
| Postmenopausal women, n/% | 10/71.4 | 20/80.0 | 40/100 | <.01 |
| Age at menopause, y | 47.6 ± 2.2 | 49.0 ± 1.5 | 50.9 ± 1.1 | .33 |
| Height, cm | 166.4 ± 2.0 | 165.9 ± 1.5 | 162.6 ± 1.3 | .17 |
| Weight, kg | 83.3 ± 4.4 | 79.2 ± 3.2 | 71.1 ± 2.8c | .04 |
| BMI, kg/m2 | 29.5 ± 1.4 | 28.7 ± 1.0 | 26.8 ± 0.9 | .16 |
| White, n/% | 14/87.5 | 30/85.7 | 42/93.3 | .58 |
| Hispanic, n/% | 5/26.3 | 5/14.3 | 7/15.2 | .48 |
| PHPT duration, y | 3.0 ± 1.4 | 3.6 ± 1.0 | 5.2 ± 0.9 | .30 |
| Meets surgical guidelines, n/% | 16/84.2 | 20/57.1 | 33/71.7 | .10 |
| Calcium intake, mg/d | 867 ± 185 | 1033 ± 139 | 1197 ± 119 | .31 |
| Taking vitamin D supplements, n/% | 5/26.3 | 21/60.0 | 39/84.8 | <.0001 |
| Daily supplement use, IU/d | 711 ± 631 | 1056 ± 308 | 2059 ± 226c,d | .01 |
| Current tobacco use, n/% | 1/5.3 | 1/2.9 | 4/8.7 | .83 |
| Sun exposure score | 8.3 ± 1.4 | 8.5 ± 1.0 | 9.1 ± 0.9 | .84 |
Data are expressed as means ± SEM, unless stated otherwise.
P < .01 compared to 25OHD <20 ng/mL.
P < .01 compared to 25OHD 20–29 ng/mL.
P < .05 compared to 25OHD <20 ng/mL.
P < .05 compared to 25OHD 20–29 ng/mL.
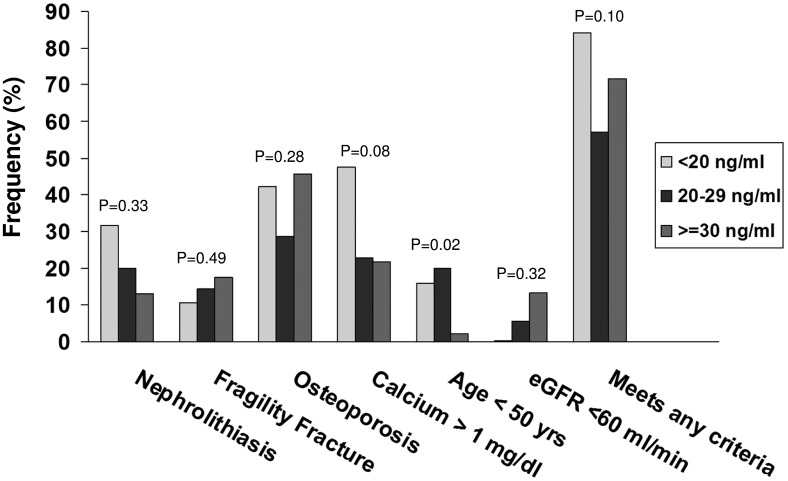
With regard to the features of PHPT, there were no differences in clinical indices reflecting disease severity by vitamin D status (Figure 2). There were no between-group differences in overt signs of symptomatic disease, such as nephrolithiasis (P = .33) or fracture (P = .49). Those who were vitamin D deficient tended to meet more 2008 surgical criteria (P = .10) due to meeting the age <50 criterion (P = .02). There were no between-group differences in other PHPT characteristics (Figure 2), including PHPT duration (P = .30) or the presence of osteoporosis (P = .28).
Figure 2.
Frequency of symptoms and meeting surgical criteria in patients with PHPT using different 25OHD thresholds: <20 (light gray), 20–29 (dark gray), and ≥30 (medium gray) ng/mL.
On the other hand, there were some biochemical differences by vitamin D status. Those with 25OHD <20 ng/mL had evidence of more severe hyperparathyroidism, with PTH levels that were 55 and 75% higher compared to those who were vitamin D insufficient (P = .006) and replete (P < .0001), respectively (Table 2). PTH levels did not differ among the insufficient and replete groups (P = .33). There was a stepwise decrease in serum phosphate with each decrease in vitamin D category and a trend toward higher 1,25-dihydroxyvitamin D levels in those with lower vitamin D (P = .06; Table 2). There were no differences in serum or urine calcium, albumin, renal function, BTMs, or FGF-23, although levels of FGF-23 were elevated.
Table 2.
Biochemical Evaluation by Vitamin D Status
| Normal | 25OHD, ng/mL |
P Value | |||
|---|---|---|---|---|---|
| <20 | 20–29 | ≥30 | |||
| n | 19 | 36 | 45 | ||
| 25OHD, ng/mL | 30–100 | 13.5 ± 1.1 | 25.5 ± 0.8 | 37.9 ± 0.7 | N/A |
| 1,25-dihydroxyvitamin D, pg/mL | 18–72 | 77.3 ± 5.5 | 73.2 ± 4.1 | 63.3 ± 3.6 | .06 |
| Serum calcium, mg/dL | 8.6–10.2 | 10.8 ± 0.1 | 10.7 ± 0.1 | 10.6 ± 0.1 | .32 |
| Serum PTH, pg/mL | 14–66 | 126 ± 10 | 81 ± 7a | 72 ± 7b | <.0001 |
| Phosphate, mg/dL | 2.7–4.5 | 2.8 ± 0.1 | 3.0 ± 0.1c | 3.2 ± 0.1a,d | .0004 |
| Albumin, mg/dL | 3.5–5.2 | 4.5 ± 0.1 | 4.5 ± 0.1 | 4.5 ± 0.1 | .99 |
| eGFR, mL/min | ≥60 | 94 ± 6 | 86 ± 4 | 79 ± 4 | .11 |
| Urine calcium, mg/24 h | 35–250 | 265 ± 37 | 277 ± 25 | 222 ± 22 | .25 |
| BSAP, U/L | 14.2–42.7 | 40.3 ± 4.6 | 44.5 ± 3.3 | 37.4 ± 2.9 | .28 |
| CTX, ng/mL | 0.142–1.351 | 0.738 ± 0.08 | 0.631 ± 0.06 | 0.604 ± 0.05 | .39 |
| FGF-23, RU/mL | 43.6 ± 14.3* | 90.3 ± 26.2 | 103.7 ± 19.4 | 143.6 ± 16.1 | .13 |
Abbreviation: N/A, not applicable. Results represent means ± SEM.
Normal mean published value (Ref. 34).
P < .001 compared to 25OHD <20 ng/mL.
P < .0001 compared to 25OHD <20 ng/mL.
P < .05 compared to 25OHD <20 ng/mL.
P < .05 compared to 25OHD 20–29 ng/mL.
Finally, skeletal indices did not differ by vitamin D status. There were no between-group differences in BSAP or CTX, even after adjusting for age and weight. Nor did LS or 1/3 radius aBMD differ before or after adjustment for covariates (Table 3). At the hip, absolute aBMD and T-scores were lower in those with 25OHD >30 ng/mL compared to those with lower vitamin D levels, but Z-scores did not differ, and differences were not significant after adjusting for age and weight. There were no interactions between weight and vitamin D status (25OHD <20, 20–29, or ≥30 ng/mL) at any site (data not shown).
Table 3.
BMD by Vitamin D Status
| 25OHD, ng/mL |
P Value | Age- and Weight- Adjusted P Value | |||
|---|---|---|---|---|---|
| <20 | 20–29 | ≥30 | |||
| n | 19 | 36 | 45 | ||
| LS, g/cm2 | 0.976 ± 0.04 | 0.957 ± 0.03 | 0.944 ± 0.03 | .81 | .87 |
| LS T-score | −0.8 ± 0.4 | −1.0 ± 0.3 | −1.1 ± 0.2 | .83 | .89 |
| LS Z-score | 0.4 ± 0.4 | 0.2 ± 0.3 | 0.7 ± 0.3 | .54 | .34* |
| FN, g/cm2 | 0.772 ± 0.03 | 0.747 ± 0.02 | 0.684 ± 0.02a,b | .02 | .89 |
| FN T-score | −1.0 ± 0.2 | −1.1 ± 0.2 | −1.5 ± 0.1a,b | .04 | .95 |
| FN Z-score | 0.1 ± 0.2 | 0.1 ± 0.2 | 0.0 ± 0.1 | .77 | .99* |
| TH, g/cm2 | 0.909 ± 0.03 | 0.880 ± 0.02 | 0.796 ± 0.02c,d | .004 | .50 |
| TH T-score | −0.6 ± 0.2 | −0.7 ± 0.2 | −1.3 ± 0.1b,c | .009 | .59 |
| TH Z-score | 0.2 ± 0.2 | 0.1 ± 0.2 | −0.1 ± 0.1 | .41 | .87* |
| 1/3 radius, g/cm2 | 0.663 ± 0.02 | 0.651 ± 0.02 | 0.630 ± 0.02 | .46 | .15 |
| 1/3 radius T-score | −1.1 ± 0.4 | −1.3 ± 0.3 | −1.4 ± 0.2 | .81 | .08 |
| 1/3 radius Z-score | 0.2 ± 0.3 | 0.0 ± 0.2 | 0.5 ± 0.2 | .24 | .12* |
Results represent means ± SEM.
Z-scores adjusted only for weight.
P < .05 vs 25OHD <20 ng/mL.
P < .05 vs 25OHD 20–29 ng/mL.
P < .01 vs 25OHD <20 ng/mL.
P < .01 vs 25OHD 20–29 ng/mL.
Subgroup analysis of women only
Because the cohort was overwhelmingly female (n = 79), we assessed this group separately. In this subgroup, 17.7% had vitamin D deficiency, 31.7% had insufficiency, and 50.6% were vitamin D replete. Mean calcium (LS mean ± SEM, 10.7 ± 0.1 mg/dL), PTH (86 ± 6 pg/mL), and 25OHD (29.8 ± 1.2 ng/mL) levels were comparable to the overall cohort. The pattern of biochemical findings was similar in this subgroup. Serum PTH levels were higher in those with 25OHD <20 ng/mL (PTH, 139 ± 12 pg/mL) compared to those with 25OHD levels in the insufficient (PTH, 86 ± 9 pg/mL; P = .003) and replete (PTH, 68 ± 7 pg/mL; P < .0001) ranges. Serum calcium levels did not differ (P = .65).
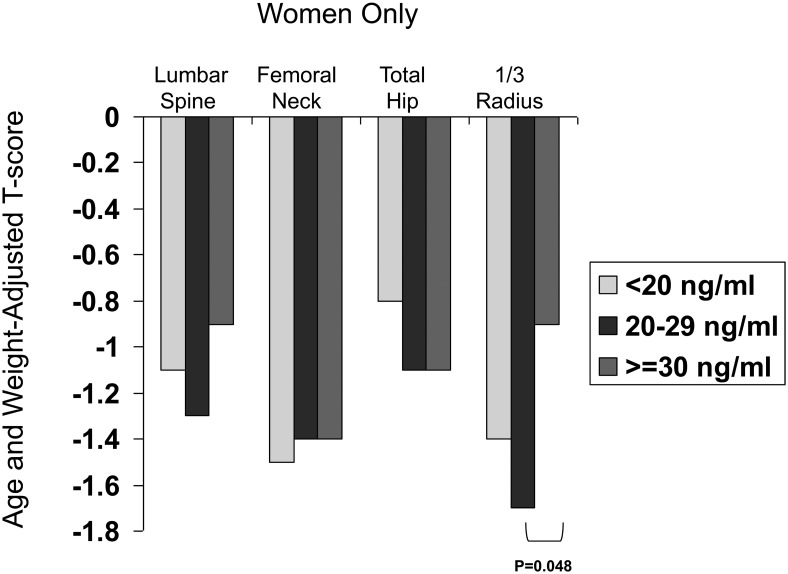
There were no differences in aBMD at the LS, TH, or FN before or after adjustment for covariates (data not shown). At the 1/3 radius, absolute aBMD, T-score, and Z-score did not differ between groups before adjustment for covariates. After adjustment for age and weight, only T-scores were significantly lower (−1.7 ± 0.3 vs −0.9 ± 0.2; P = .048) in those with vitamin D insufficiency compared to those who were vitamin D replete at the 1/3 radius (Figure 3). Although their numbers were small, women with vitamin D deficiency (n = 14) did not differ from either group in adjusted aBMD, T-score, or Z-score at the 1/3 radius.
Figure 3.
Comparison of age- and weight-adjusted T-scores in women using different 25OHD thresholds: <20 (light gray), 20–29 (dark gray), and ≥30 (medium gray) ng/mL.
Multiple regression modeling of 1/3 radius BMD
In a stepwise multiple linear regression model (whole cohort), among potential risk factors for low 1/3 radius aBMD (age, gender, weight, PTH, calcium, eGFR, and 25OHD), age was a negative predictor, whereas male sex, weight, and higher 25OHD were positively associated with aBMD. Calcium, PTH, and eGFR were not independent predictors. Each 10-year age increment was associated with 0.0444 g/cm2 lower aBMD at the forearm, whereas male sex was associated with 0.0823 g/cm2 higher aBMD. Each 10-kg increase in weight was associated with a 0.0264 g/cm2 increase in aBMD. Each 10-ng/mL increase in 25OHD was associated with 0.0206 g/cm2 higher aBMD. The model accounted for 56% of the variance in 1/3 radius aBMD, but 25OHD accounted for only 3% of the explained variance.
Discussion
Our hypothesis that vitamin D insufficiency and deficiency would be associated with more severe clinical, biochemical, and skeletal manifestations of PHPT was not uniformly confirmed in this cohort, which had few profoundly vitamin D-deficient subjects. Only the extent of hyperparathyroidism, as reflected by degree of PTH elevation, was associated with vitamin D deficiency.
It has long been accepted that coexisting vitamin D deficiency is associated with more severe PHPT. Vitamin D-deficient PHPT patients have been reported to have higher PTH levels, greater adenoma weight, lower aBMD particularly at cortical sites, higher bone turnover, and increased fracture risk (1, 2, 12–19). Our disparate findings may be explained by the fact that these earlier studies were performed in settings (geographic or temporal) where long-standing, severe vitamin D deficiency and perhaps low calcium intake were commonplace. Such an environment, uncommon in today's Western world, could predispose to more severe PHPT. Consistent with this theory, more severe and symptomatic PHPT remains the common phenotype of the disease in those parts of the world where vitamin D deficiency remains endemic today (20). In the United States, vitamin D deficiency is becoming less common as supplement use becomes more widespread (21). These secular trends in vitamin D supplementation are apparent in PHPT as well, and vitamin D deficiency was less common in this cohort than in previous cohorts (22).
Unlike prior reports, clinical characteristics reflecting the severity of PHPT (nephrolithiasis, osteoporosis, fracture; meeting 2008 and 2013 guidelines for parathyroidectomy [other than renal and vertebral imaging, which were not available]) were not associated with low 25OHD levels. Only age <50 years was associated with lower vitamin D levels, which likely reflects the common practice in our area for older people, particularly postmenopausal women, to supplement their vitamin D intake.
Our results regarding the biochemical effects of vitamin D insufficiency and deficiency upon PHPT confirmed prior findings that lower vitamin D is associated with higher PTH levels (1, 2, 12, 16, 23). Similarly, lower 25OHD was linearly associated with lower serum phosphate and higher 1,25-dihydroxyvitamin D levels (13). We did not find previously reported associations between lower 25OHD and higher serum calcium and/or BTMs (1, 12, 16, 23). In older studies, these differences are likely due to more severe vitamin D deficiency.
The effect of low vitamin D on aBMD in our study was modest and inconsistent, seen only at the 1/3 radius site in women with vitamin D insufficiency. It is notable that overall BMD in this cohort was remarkably preserved—T-scores were normal at the LS and hip and in the osteopenic range at the FN and radius. Also, in contrast to prior cohorts (24), aBMD at the 1/3 radius site was not lower than at other sites. Whether the higher vitamin D levels in this cohort vs prior cohorts may have mitigated the preferential loss of aBMD at the 1/3 radius is unknown.
Interpretation of the effect of vitamin D deficiency and insufficiency on aBMD was somewhat confounded by the association of lower 25OHD with younger age, higher weight, and male gender. Again, this association is likely explained by patterns of vitamin D supplement use. Because of these demographic factors, those with lower vitamin D levels had better (unadjusted) aBMD at the FN and hip despite having more biochemically severe PHPT (higher PTH). After controlling for demographics, we found that neither vitamin D deficiency nor insufficiency was associated with aBMD differences at the LS or hip. T-scores were only modestly reduced at the cortical 1/3 radius, and this finding was apparent only in women with 25OHD insufficiency (and not 25OHD deficiency). We suspect that BMD differences may also be present in those with 25OHD <20 ng/mL and in men, but we had lower statistical power to detect them.
Our regression model suggests that the effect of vitamin D on cortical BMD may not be due to higher PTH, but is due instead to independent effects. The mechanism by which vitamin D might have a direct effect on aBMD at the 1/3 radius is unclear and was not investigated in this study. It is, however, consistent with our prior finding that 25OHD <20 ng/mL was associated with lower cortical width on bone biopsy, whereas PTH levels did not show this association (25). The model indicated, however, that significantly more of the variance in forearm aBMD was accounted for by male sex, age, and weight, rather than 25OHD.
In our prior retrospective analysis of the effects of vitamin D deficiency on PHPT in a cohort from the same geographic area, adverse skeletal sequelae by DXA were similarly observed only at the 1/3 radius, but only in patients with 25OHD <9 ng/mL, the laboratory lower limit of normal at that time (16). Divergent results were found in a recent retrospective study of Italian patients, in which those with 25OHD <20 ng/dL had lower aBMD at all sites (23). However, those vitamin D-deficient patients had severe PHPT in which osteitis fibrosa cystica was common, making it impossible to compare the two cohorts. Data from other studies are conflicting, with some indicating positive associations between vitamin D and aBMD at the FN and radius only, or the LS only, whereas other smaller studies have found no association between 25OHD and aBMD at any site (1, 18, 26).
Thus, it seems clear that the findings of this study cannot be generalized to populations with more severe PHPT or more widespread vitamin D deficiency. Severe or prolonged vitamin D deficiency in PHPT accompanied by higher PTH elevations is likely to have a greater impact upon the skeleton. It is also possible that there is a threshold 25OHD level below which deleterious effects occur (25OHD <9 ng/mL in our prior report), and the lack of frequent profound vitamin D deficiency in our cohort may have prevented us from observing an effect. As such, these data may not be applicable to other countries or populations where vitamin D supplementation is not widespread.
These data also cannot address whether vitamin D repletion could have beneficial skeletal effects even in so mildly affected patients. Vitamin D repletion in most studies reduces PTH in PHPT patients, and some have shown declines in alkaline phosphatase levels (27–31), but few treatment studies have evaluated aBMD. Uncontrolled studies have shown variable effects of vitamin D repletion upon aBMD in PHPT (29, 32). A recent, small but well-executed, double-blind, randomized controlled trial found an increase in 25OHD from a mean of 20 to 38 ng/mL, whereas PTH and CTX decreased and LS aBMD increased by 2.5% with treatment vs placebo (33). There were no between-group differences in BMD changes at the hip or 1/3 radius.
Finally, a goal of this study was to investigate the clinical utility of currently used 25OHD thresholds in PHPT. This was undertaken because of the limited data for these thresholds in PHPT as opposed to the general population and because international guidelines (2008 and 2013) suggest repletion of 25OHD to 20 ng/mL in PHPT. First, our data suggest that PTH elevations are clearly heightened when 25OHD falls below 20 ng/mL in PHPT (6, 7). Although no PTH difference was observed between those with 25OHD levels of 20–29 vs ≥30 ng/mL, data from our spline curve suggest that the increase in PTH begins in this insufficient range. On the other hand, our data suggest that commonly used cutpoints for vitamin D status do not appear to have a major effect upon clinical indices of disease severity or on skeletal health in PHPT; BTMs did not differ according to these cutpoints, and the modestly lower T-score at the 1/3 radius was present in women with vitamin D insufficiency but not deficiency. The PTH data support the current international guidelines recommendation to consider PHPT patients with 25OHD <20 ng/mL to be vitamin D deficient, but overall these data do not make a strong argument for clinical differences in disease severity or for repletion at one or another cutpoint.
Our study has several limitations. Most notably, few participants had very low 25OHD levels, likely because many subjects were self-supplementing with vitamin D. This may have impaired our ability to detect between-group differences. Furthermore, a single 25OHD level may not be reflective of chronic exposure, and we were unable to ascertain accurate information regarding total duration and past dosing of vitamin D treatment. Despite these limitations, our study has important strengths, including the relatively large group of PHPT patients, extensive historical and demographic data including factors that may affect vitamin D status, and the investigation of various common vitamin D thresholds to assess the effects of vitamin D on the presentation and clinical characteristics, as well as the biochemical and skeletal profile of PHPT.
In summary, our findings demonstrate that low vitamin D, using a threshold of <20 but not <30 ng/mL, is associated with more biochemically severe PHPT, as manifested by higher PTH levels. Although vitamin D insufficiency was associated with modest cortical effects upon the skeleton, low vitamin D levels using the current thresholds were not associated with evidence of more severe disease as reflected by symptoms or meeting criteria for parathyroidectomy. As secular trends in vitamin D supplementation extend into PHPT populations, cohorts such as this one, with less vitamin D deficiency, are likely to become more common. We conclude that in this PHPT cohort with few profoundly vitamin D-deficient patients, vitamin D status did not appear to significantly impact clinical presentation or aBMD.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 DK084986 and K24 DK074457, as well as the Joseph Weintraub Family Foundation.
Author Contributions: M.D.W., acquisition of data, data analysis and interpretation, drafting of manuscript; E.C., J.A.L., and A.K., acquisition of data, manuscript revision; C.Z. and D.J.M., analysis of data, manuscript revision; and S.J.S., design, acquisition of data, interpretation of data, and manuscript drafting.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aBMD
- areal BMD
- BMD
- bone mineral density
- BMI
- body mass index
- BSAP
- bone-specific alkaline phosphatase
- BTM
- bone turnover marker
- CTX
- carboxy-terminal telopeptides of type 1 collagen
- DXA
- dual x-ray absorptiometry
- eGFR
- estimated glomerular filtration rate
- FGF-23
- fibroblast growth factor 23
- FN
- femoral neck
- LS
- lumbar spine
- 25OHD
- 25-hydroxyvitamin D
- PHPT
- primary hyperparathyroidism
- TH
- total hip.
References
- 1. Moosgaard B, Vestergaard P, Heickendorff L, Melsen F, Christiansen P, Mosekilde L. Vitamin D status, seasonal variations, parathyroid adenoma weight and bone mineral density in primary hyperparathyroidism. Clin Endocrinol (Oxf). 2005;63:506–513. [DOI] [PubMed] [Google Scholar]
- 2. Boudou P, Ibrahim F, Cormier C, Sarfati E, Souberbielle JC. A very high incidence of low 25 hydroxy-vitamin D serum concentration in a French population of patients with primary hyperparathyroidism. J Endocrinol Invest. 2006;29:511–515. [DOI] [PubMed] [Google Scholar]
- 3. Rao DS, Agarwal G, Talpos GB, et al. Role of vitamin D and calcium nutrition in disease expression and parathyroid tumor growth in primary hyperparathyroidism: a global perspective. J Bone Miner Res. 2002;17(suppl 2):N75–N80. [PubMed] [Google Scholar]
- 4. Rubin MR, Bilezikian JP, McMahon DJ, et al. The natural history of primary hyperparathyroidism with or without parathyroid surgery after 15 years. J Clin Endocrinol Metab. 2008;93(9):3462–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999;341:1249–1255. [DOI] [PubMed] [Google Scholar]
- 6. Eastell R, Arnold A, Brandi ML, et al. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the Third International Workshop. J Clin Endocrinol Metab. 2009;94:340–350. [DOI] [PubMed] [Google Scholar]
- 7. Eastell R, Brandi ML, Costa AG, D'Amour P, Shoback DM, Thakker RV. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99:3570–3579. [DOI] [PubMed] [Google Scholar]
- 8. Hertzler A, Frary R. A dietary calcium rapid assessment method (RAM). Top Clin Nutr. 1994;9:76–85. [Google Scholar]
- 9. Stein EM, Strain G, Sinha N, et al. Vitamin D insufficiency prior to bariatric surgery: risk factors and a pilot treatment study. Clin Endocrinol (Oxf). 2009;71:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 11. Bonnick SL, Johnston CC, Jr, Kleerekoper M, et al. Importance of precision in bone density measurements. J Clin Densitom. 2001;4:105–110. [DOI] [PubMed] [Google Scholar]
- 12. Rao DS, Honasoge M, Divine GW, et al. Effect of vitamin D nutrition on parathyroid adenoma weight: pathogenetic and clinical implications. J Clin Endocrinol Metab. 2000;85:1054–1058. [DOI] [PubMed] [Google Scholar]
- 13. Moosgaard B, Vestergaard P, Heickendorff L, Melsen F, Christiansen P, Mosekilde L. Plasma 25-hydroxyvitamin D and not 1,25-dihydroxyvitamin D is associated with parathyroid adenoma secretion in primary hyperparathyroidism: a cross-sectional study. Eur J Endocrinol. 2006;155:237–244. [DOI] [PubMed] [Google Scholar]
- 14. Raef H, Ingemansson S, Sobhi S, Sultan A, Ahmed M, Chaudhry M. The effect of vitamin D status on the severity of bone disease and on the other features of primary hyperparathyroidism (pHPT) in a vitamin D deficient region. J Endocrinol Invest. 2004;27:807–812. [DOI] [PubMed] [Google Scholar]
- 15. Ozbey N, Erbil Y, Ademoglu E, Ozarmagan S, Barbaros U, Bozbora A. Correlations between vitamin D status and biochemical/clinical and pathological parameters in primary hyperparathyroidism. World J Surg. 2006;30:321–326. [DOI] [PubMed] [Google Scholar]
- 16. Silverberg SJ, Shane E, Dempster DW, Bilezikian JP. The effects of vitamin D insufficiency in patients with primary hyperparathyroidism. Am J Med. 1999;107:561–567. [DOI] [PubMed] [Google Scholar]
- 17. Moosgaard B, Christensen SE, Vestergaard P, Heickendorff L, Christiansen P, Mosekilde L. Vitamin D metabolites and skeletal consequences in primary hyperparathyroidism. Clin Endocrinol (Oxf). 2008;68:707–715. [DOI] [PubMed] [Google Scholar]
- 18. Inoue Y, Kaji H, Hisa I, et al. Vitamin D status affects osteopenia in postmenopausal patients with primary hyperparathyroidism. Endocr J. 2008;55:57–65. [DOI] [PubMed] [Google Scholar]
- 19. Nordenström E, Westerdahl J, Lindergård B, Lindblom P, Bergenfelz A. Multifactorial risk profile for bone fractures in primary hyperparathyroidism. World J Surg. 2002;26:1463–1467. [DOI] [PubMed] [Google Scholar]
- 20. Silverberg SJ, Clarke BL, Peacock M, et al. Current issues in the presentation of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99:3580–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dickinson A, Blatman J, El-Dash N, Franco JC. Consumer usage and reasons for using dietary supplements: report of a series of surveys. J Am Coll Nutr. 2014;33:176–182. [DOI] [PubMed] [Google Scholar]
- 22. Walker MD, Cong E, Lee JA, et al. Low vitamin D levels have become less common in primary hyperparathyroidism. Osteoporos Int. DOI:10.1007/500198-015-3199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tassone F, Gianotti L, Baffoni C, et al. Vitamin D status in primary hyperparathyroidism: a Southern European perspective. Clin Endocrinol (Oxf). 2013;79:784–790. [DOI] [PubMed] [Google Scholar]
- 24. Silverberg SJ, Shane E, de la Cruz L, et al. Skeletal disease in primary hyperparathyroidism. J Bone Miner Res. 1989;4:283–291. [DOI] [PubMed] [Google Scholar]
- 25. Stein EM, Dempster DW, Udesky J, et al. Vitamin D deficiency influences histomorphometric features of bone in primary hyperparathyroidism. Bone. 2011;48:557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamashita H, Noguchi S, Uchino S, et al. Vitamin D status in Japanese patients with hyperparathyroidism: seasonal changes and effect on clinical presentation. World J Surg. 2002;26:937–941. [DOI] [PubMed] [Google Scholar]
- 27. Shah VN, Shah CS, Bhadada SK, Rao DS. Effect of 25 (OH) D replacements in patients with primary hyperparathyroidism (PHPT) and coexistent vitamin D deficiency on serum 25(OH) D, calcium and PTH levels: a meta-analysis and review of literature. Clin Endocrinol (Oxf). 2014;80(6):797–803. [DOI] [PubMed] [Google Scholar]
- 28. Rao RR, Randeva HS, Sankaranarayanan S, et al. Prolonged treatment with vitamin D in postmenopausal women with primary hyperparathyroidism. Endocr Connect. 2012;1:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grey A, Lucas J, Horne A, Gamble G, Davidson JS, Reid IR. Vitamin D repletion in patients with primary hyperparathyroidism and coexistent vitamin D insufficiency. J Clin Endocrinol Metab. 2005;90:2122–2126. [DOI] [PubMed] [Google Scholar]
- 30. Grubbs EG, Rafeeq S, Jimenez C, et al. Preoperative vitamin D replacement therapy in primary hyperparathyroidism: safe and beneficial? Surgery 2008;144:852–858; discussion 858–859. [DOI] [PubMed] [Google Scholar]
- 31. Tucci JR. Vitamin D therapy in patients with primary hyperparathyroidism and hypovitaminosis D. Eur J Endocrinol. 2009;161:189–193. [DOI] [PubMed] [Google Scholar]
- 32. Kantorovich V, Gacad MA, Seeger LL, Adams JS. Bone mineral density increases with vitamin D repletion in patients with coexistent vitamin D insufficiency and primary hyperparathyroidism. J Clin Endocrinol Metab. 2000;85:3541–3543. [DOI] [PubMed] [Google Scholar]
- 33. Rolighed L, Rejnmark L, Sikjaer T, et al. Vitamin D treatment in primary hyperparathyroidism: a randomized placebo controlled trial. J Clin Endocrinol Metab. 2014;99(3):1072–1080. [DOI] [PubMed] [Google Scholar]
- 34. Laroche M, Boyer JF, Jahafar H, Allard J, Tack I. Normal FGF23 levels in adult idiopathic phosphate diabetes. Calcif Tissue Int. 2009;84:112–117. [DOI] [PubMed] [Google Scholar]