Abstract
Context:
Cardiovascular risk increases in women after menopause. Mounting evidence demonstrates a role of cardiovascular fat (CF) in the pathogenesis of coronary heart disease, but no research has examined CF in relation to sex hormones or menopausal status in women.
Objective:
The objective was to determine the relationship between CF depots, menopausal status, and endogenous sex hormones.
Design:
Cross-sectional and longitudinal study designs were used.
Setting:
The setting included the Study of Women's Health Across the Nation (SWAN) Heart and Cardiovascular Fat Ancillary Study.
Participants:
A total of 456 women (mean age, 50.75 y); 62% premenopausal/early perimenopausal, and 38% late peri-/postmenopausal.
Intervention:
Menopausal status, endogenous sex hormones measured simultaneously with CF volumes, and circulating estradiol available 4.80 years (median) before CF measures.
Main Outcome Measures:
Volumes of CF (epicardial adipose tissue [EAT], paracardial adipose tissue [PAT], total heart adipose tissue [TAT = EAT + PAT], and aortic perivascular adipose tissue [PVAT]).
Results:
In final models, late peri-/postmenopausal women had 9.88% more EAT, 20.72% more PAT, and 11.69% more TAT volumes than pre-/early perimenopausal women (P < .05). PVAT was not associated with menopausal status. In final models, lower estradiol concentrations were associated with greater volumes of PAT and TAT (P < .05). Women with the greatest reduction in estradiol since baseline had greater volumes of PAT compared to women with the least reduction (P = .02).
Conclusions:
Late peri-/postmenopausal women have greater volumes of heart fat compared with pre-/early perimenopausal women independent of age, obesity, and other covariates. Endogenous sex hormones are associated with CF. Perhaps CF plays a role in the higher risk of coronary heart disease reported in women after menopause.
Increased weight gain in women over the menopausal transition has been a matter of long-term debate (1). Most of the cross-sectional and longitudinal studies evaluating weight gain and menopause have concluded that weight gain at midlife is due to aging rather than to menopause (1–3). However, this conclusion does not consider changes in body composition during the menopausal transition. Several cross-sectional studies and a few longitudinal studies found that postmenopausal women have greater abdominal visceral fat and/or waist circumference when compared to premenopausal women (4–8). Moreover, older and postmenopausal women tend to have less sc fat in the abdomen and/or legs (7, 8). The changes in body fat composition and distribution may be due to hormonal fluctuations that occur during the menopausal transition (9, 10).
The accumulation of abdominal visceral fat during the menopausal transition (4–8) may play a key role in explaining the higher rates of coronary heart disease (CHD) among postmenopausal women (11, 12). Visceral fat is metabolically active and produces several inflammatory markers with significant atherogenic features (13). The redistribution of fat deposition in women at midlife is not limited to visceral and sc fat depots. Increasing visceral fat and decreasing fat storage capability in sc adipose tissue are often indications of increased fat deposition and infiltration into other visceral tissues such as the heart (14).
The excess of fat around the heart and aorta, known as cardiovascular fat (CF), may be more detrimental for cardiovascular risk than visceral fat given its close anatomical location (15). Increasing evidence supports a role of CF in the pathogenesis of CHD (16–18). Whether CF increases in women transitioning through menopause parallels the increase in visceral abdominal fat is unknown. Evaluating the potential association between CF and menopause may reveal CF as a novel risk factor in women at midlife.
To the best of our knowledge, no previous study has evaluated whether CF is associated with menopausal status or endogenous sex hormone concentrations in women at midlife. Studies were mainly limited to either postmenopausal women or hysterectomized women with or without bilateral oophorectomy. Premenopausal women were not included for comparisons in any of these studies; therefore, the above question has not been addressed (19, 20). The SWAN CF study, an ancillary study to the Study of Women's Health Across the Nation (SWAN), was specifically designed to: 1) determine the relationship between CF depots and menopausal status; and 2) investigate the associations between CF depots and concentrations of endogenous sex hormones in a sample of women at midlife. We hypothesize that postmenopausal women will have greater volumes of CF compared to premenopausal women and that lower concentrations of estradiol (E2) and SHBG and higher concentrations of free androgen index (FAI) will be associated with higher volumes of CF in midlife women.
Subjects and Methods
Study population
SWAN is an ongoing, community-based, longitudinal study of the menopausal transition (21). Briefly, 3302 participants aged 42–52 years were recruited in 1996–1997 from seven designated sites (Boston, Massachusetts; Detroit, Michigan; Oakland and Los Angeles, California; Pittsburgh, Pennsylvania; Chicago, Illinois; and Newark, New Jersey). The eligibility criteria for the SWAN study were: 1) an intact uterus and at least one ovary; 2) at least one menstrual period within the past 3 months; and 3) no hormone therapy (HT) use within the past 3 months. At the Pittsburgh and Chicago sites, subclinical measures of atherosclerosis were collected as part of the SWAN Heart ancillary study. The SWAN CF ancillary study was designed to measure CF among SWAN Heart study participants. To be part of the SWAN CF ancillary study, participants needed to have electron beam computed tomography scans performed at the SWAN Heart baseline visit. Of 608 SWAN Heart participants, 564 had computed tomography scans to measure CF depots. For the current analyses, women were excluded if they were surgically menopausal, with undetermined menopausal status due to HT use, or missing menopausal status (n = 42). An additional 66 women were excluded due to missing covariates data or hormone data or due to use of HT, leaving 456 women in the final analyses.
The institutional review board at each site approved the study protocol, and all participants signed informed consent forms before participation.
CF depots
Computerized tomographic scans (GE-Imatron C150 EBCT) were used to quantify CF depots. Four CF depots were measured including: 1) epicardial adipose tissue (EAT), the adipose tissue within the pericardial sac; 2) paracardial adipose tissue (PAT), the adipose tissue outside the pericardial sac; 3) total heart adipose tissue (TAT), the sum of EAT and PAT; and 4) perivascular adipose tissue (PVAT), the adipose tissue surrounding the descending thoracic aorta Supplemental Figure 1. EAT, PAT, and TAT were quantified at the Biomedical Research Institute, Harbor-UCLA Medical Center (Torrance, California), as described previously (18). In brief, EAT, PAT, and TAT volumes were determined from 15 mm above to 30 mm below the superior extent of the left main coronary artery. This region of the heart was selected because it includes the epicardial fat located around the proximal coronary arteries. The anterior border of the TAT volume was the chest wall, and the posterior borders were the aorta and the bronchus. Using the volume analysis software (GE Healthcare), fat was distinguished from other heart tissue by a threshold of −190 to −30 Hounsfield units. EAT was measured by manually tracing out the pericardium every two or three slices below the start point and then using the software to automatically trace out the segments between these selected slices. PAT was measured by subtracting EAT from TAT volume. EAT and TAT measures have excellent reproducibility. Spearman correlation coefficients between-reader and within-reader were ≥ 0.97. Of the 456 women in the final analysis, 32 women did not have EAT, PAT, or TAT due to technical issues, leaving 424 women for analyses of these measures. PVAT was quantified at the University of Pittsburgh Ultrasound Research Lab. Briefly, using an image analysis workstation equipped with Slice-O-Matic version 4.3 (Tomovision), PVAT was distinguished from other tissues by using the same Hounsfield thresholds as above. The pulmonary bifurcation served as the proximal border, and the initial image of the first lumbar vertebrae marked the distal border. The borders surrounding the descending thoracic aorta were manually traced for every slice. The anterior borders included a horizontal line through the left bronchus, esophagus, and eventually the interior border of the crus of the diaphragm. The posterior border was a horizontal line tangent to the anterior border of the vertebral foramen. A similar protocol has been used before with excellent intra-reader and inter-reader intraclass coefficients of at least 0.99 (22). All women in the final analysis had PVAT measures (n = 456).
Menopausal status
Menopausal status was determined based on frequency and regularity of menstrual bleeding as follows: 1) premenopause, no perceived change in bleeding; 2) early perimenopause, perceived change in cycle interval, but at least one menstrual period within the past 3 months; 3) late perimenopause, 3 consecutive months of amenorrhea; and 4) postmenopause, 12 consecutive months of amenorrhea. Due to the small sample sizes of premenopausal (n = 48) and late perimenopausal (n = 53) categories, and similar to previous publications from the SWAN Heart study (23, 24), both premenopausal and early perimenopausal women were combined in one group, whereas late peri- and postmenopausal women were combined in a second group.
Endogenous sex hormones
Women provided fasting blood samples during the early follicular phase (d 2–5 of the menstrual cycle) at each visit. Fasting samples were obtained within 90 days of the recruitment anniversary date if a timed sample could not be obtained. Accordingly, the cycle day of blood draw was reported as either days 2–5 or outside that period. Blood was prepared and serum was shipped to the Clinical Ligand Assay Satellite Services Central Laboratory at the University of Michigan. Endogenous sex hormones were measured using the Automated Chemiluminescence System-180 automated analyzer (Bayer Diagnostics Corp). E2 was measured using a modified, off-line Automated Chemiluminescence System: 180 (E2–6). The lower limit of detection (LLD) was between 1 and 7 pg/mL. The inter- and intra-assay coefficients of variation were 10.6 and 6.4%, respectively. FSH was measured by a modified manual assay kit (Bayer Diagnostics) utilizing two monoclonal antibodies directed to different regions on the β-subunit. The LLD was between 0.4 and 1.0 mIU/mL. The inter- and intra-assay coefficients of variation were 11.4 and 3.8%, respectively. Serum T concentration was evaluated with the Automated Chemiluminescence System: 180 total T assay, modified to increase precision in the low ranges. The LLD was between 2 and 2.2 ng/dL. The inter- and intra-assay coefficients of variation were 10.5 and 8.5%, respectively. SHBG was measured with a two-site chemiluminescent immunoassay. The LLD was between 1.9 and 3.2 nm. The inter- and intra-assay coefficients of variation were 9.9 and 6.1%, respectively. The FAI was used to estimate the amount of T unbound by SHBG and thus, immediately biologically active. FAI was calculated as 100 × T/(28.84 × SHBG). Only E2 assays were conducted in duplicate. The average for the duplicate measures was calculated and reported (coefficients of variation of 3 to 12%). Endogenous sex hormone values between zero and the LLD were replaced with a random value between zero and the LLD.
Study covariates
Weight and height were measured to calculate body mass index (BMI), and obesity was defined as BMI ≥ 30 kg/m2. Race/ethnicity and educational level were self-reported. Age, smoking status, and alcohol consumption were derived from questionnaires. Physical activity was self-reported and was assessed via a modified Baecke score of habitual physical activity (25), with higher scores indicating more physical activity. Morbidity was defined as “yes” if a participant reported a history of hypertension, diabetes, angina, stroke, or myocardial infarction. Medication use was defined as “yes” if a participant reported use of medications for hypertension, diabetes, or high cholesterol.
Statistical analyses
CF volumes and hormone values were log-transformed to achieve normality. Separate linear regression models were developed to evaluate the associations between each log-transformed CF depot as an outcome, with menopausal status or each log-transformed hormone as the main independent variable. For ease of interpretation, percentage differences/changes and 95% confidence interval (CI) in each CF volume were calculated (26, 27). For multivariable analyses, all variables that were found to be significantly associated with study outcomes in the univariate analyses were considered as potential covariates. Because E2 was found to be associated with PAT in the current study, and in order to better understand the potential role of changes in E2 over the menopausal transition on PAT, E2 concentrations at the SWAN parent baseline visit (median [Q1, Q4], 4.80 [4.09, 5.10] y before the SWAN CF ancillary study) were available for 417 participants and were utilized to calculate and evaluate quartiles of E2 relative change since baseline in relation to log-transformed PAT volume. Similar analyses were conducted with other CF depots, and results were not significant (data not shown). Statistical tests were two-sided, with a significance level of 0.05 unless otherwise specified. SAS Version 9.03 (SAS Institute Inc) and STATA 13 (StataCorp) software were used for the analysis.
Results
Participants' characteristics in the total sample and by menopausal status are presented in Table 1. Participants were 50.75 ± 2.83 years old, 38% were African American, and 62% were premenopausal/early perimenopausal. In unadjusted analyses, late peri-/postmenopausal women had greater volumes of all CF depots (Figure 1). Late peri-/postmenopausal women had 9.88% more EAT, 20.72% more PAT, and 11.69% more TAT compared to premenopausal/early perimenopausal women (P < .05). These differences were independent of study covariates. Menopausal status was not associated with PVAT in final models (Table 2). Volumes of CF depots were very similar between premenopausal and early perimenopausal women (P ≥ .05 for all) as well as between late peri- and postmenopausal women (P ≥ .05 for all) (Supplemental Table).
Table 1.
Characteristics of Participants by Menopausal Status
| Characteristics | Total | Premenopausal/Early Perimenopausal | Late Peri-/Postmenopausal | P Value |
|---|---|---|---|---|
| n (%) | 456 | 282 (61.84) | 174 (38.16) | |
| Age, y | 50.75 ± 2.83 | 49.5 ± 2.21 | 52.63 ± 2.70 | <.001 |
| African American | 173 (37.94) | 99 (35.11) | 74 (42.53) | .11 |
| Educational level | .08 | |||
| ≤ High school | 65 (14.77) | 33 (12.13) | 32 (19.05) | |
| Some college/vocational | 229 (52.05) | 151 (55.51) | 78 (46.43) | |
| College degree or higher | 146 (33.18) | 88 (32.35) | 58 (34.52) | |
| Income, $ US | .16 | |||
| Low, ≤20K–34K | 63 (13.88) | 33 (11.74) | 30 (17.34) | |
| Medium, 35K–75K | 179 (39.43) | 109 (38.79) | 70 (40.46) | |
| High, ≥76K | 212 (46.70) | 139 (49.47) | 73 (42.20) | |
| Alcohol consumption | .9 | |||
| 0 to 1/mo | 170 (37.28) | 105 (37.23) | 65 (37.36) | |
| >1/mo to 1/wk | 175 (38.38) | 110 (39.01) | 65 (37.36) | |
| >2/wk | 111 (24.34) | 67 (23.76) | 44 (25.29) | |
| Morbiditya | 191 (41.89) | 108 (38.30) | 83 (47.70) | .048 |
| Medicationb | 91 (19.96) | 52 (18.44) | 39 (22.41) | .3 |
| BMI, kg/m2 | 29.52 ± 6.45 | 29.23 ± 6.57 | 29.98 ± 6.24 | .22 |
| Obesity (BMI ≥30 kg/m2) | 184 (40.35) | 106 (37.59) | 78 (44.83) | .12 |
| Physical activity scores | 7.93 ± 1.76 | 8.04 ± 1.75 | 7.76 ± 1.75 | .09 |
| Smoker | 76 (16.67) | 47 (16.67) | 29 (16.67) | .99 |
| E2, median (Q1, Q3), pg/mL | 29.88 (16.25, 77.85) | 46.05 (25.00, 109.10) | 16.45 (11.90, 27.25) | <.001 |
| FSH, median (Q1, Q3), mIU/mL | 32.55 (14.05, 83.65) | 17.60 (11.00, 35.90) | 85.55 (57.30, 106.10) | <.001 |
| FAI, median (Q1, Q3) | 3.14 (1.76, 5.24) | 2.71 (1.63, 4.68) | 3.47 (2.25, 6.04) | .0004 |
| SHBG, median (Q1, Q3), nm | 41.80 (28.10, 61.55) | 43.80 (28.90, 63.30) | 38.35 (26.30, 56.30) | .039 |
Data are expressed as number (percentage) or mean ± SD, unless specified otherwise.
History of any of the following conditions: hypertension, diabetes, angina, stroke, or heart attack.
Use of any of the following: lipid-lowering, blood pressure, or diabetic medications.
Figure 1.
Cardiac fat volumes by menopausal status.
Table 2.
Unadjusted and Adjusted Percentage Differences in CF Volumes by Menopausal Status
| Models | EATa (n = 424) |
PATa (n = 424) |
TATa (n = 424) |
PVATa (n = 456) |
||||
|---|---|---|---|---|---|---|---|---|
| % Diff (95% CI) | P | % Diff (95% CI) | P | % Diff (95% CI) | P | % Diff (95% CI) | P | |
| Unadjusted | ||||||||
| Late peri-/postmenopausal | 16.02 (5.91, 27.08) | .002 | 25.86 (8.92, 45.44) | .002 | 17.53 (7.11, 28.95) | .001 | 9.52 (2.11, 7.47) | .01 |
| Premenopausal/early perimenopausal | — | — | — | — | ||||
| Model 1b | ||||||||
| Late peri-/postmenopausal | 9.25 (−0.07, 19.44) | .05 | 20.00 (4.30, 38.06) | .01 | 11.00 (1.72, 21.13) | .02 | 1.78 (−4.80, 8.80) | .60 |
| Premenopausal/early perimenopausal | — | — | — | — | ||||
| Model 2c | ||||||||
| Late peri-/postmenopausal | 9.88 (0.411, 20.25) | .04 | 20.72 (4.73, 39.15) | .009 | 11.69 (2.25, 22.00) | .01 | 1.67 (−4.95, 8.74) | .63 |
| Premenopausal/early perimenopausal | — | — | — | — | ||||
Log transformed. β Coefficients and related 95% CI from linear regression were presented as percentage differences (% Diff) between menopausal status using the following formula: (eβ − 1) ∗ 100 (26).
Model 1: adjusted for study site, race, income, age, current smoking, physical activity, and obesity (BMI ≥ 30 kg/m2).
Model 2: model 1 + alcohol consumption, medication use, and comorbidities.
When evaluating associations between CF depots and endogenous sex hormones (Table 3), higher concentrations of E2 were associated with lower volumes of EAT, PAT, and TAT, but not PVAT in unadjusted analyses. In final models, higher concentrations of E2 remained associated with lower volumes of PAT and TAT. Although higher concentrations of FAI were associated with greater CF volumes, in unadjusted models, only associations with PVAT remained significant in final models. Higher concentrations of SHBG were associated with lower volumes of all CF depots in all models. There were no associations between FSH and CF volumes.
Table 3.
Unadjusted and Adjusted Percentage Change in Cardiovascular Fat Volumes for Each 20% Increment in Endogenous Sex Hormones
| Sex Hormonesa | EATa (n = 424) |
PATa (n = 424) |
TATa (n = 424) |
PVATa (n = 456) |
||||
|---|---|---|---|---|---|---|---|---|
| % Change (95% CI) | P | % Change (95% CI) | P | % Change (95% CI) | P | % Change (95% CI) | P | |
| Unadjusted | ||||||||
| E2 | −0.95 (−1.67, −0.22) | .01 | −1.72 (−2.85, −0.57) | .004 | −1.04 (−1.78, −0.30) | .006 | −0.53 (−1.09, 0.03) | .07 |
| FSH | 0.48 (−0.32, 1.28) | .24 | 0.14 (−1.12, 1.42) | .83 | 0.36 (−0.46, 1.18) | .39 | −0.03 (−0.64, 0.59) | .92 |
| FAI | 2.30 (1.29, 3.31) | <.001 | 3.80 (2.19, 5.43) | <.001 | 2.53 (1.51, 3.56) | <.001 | 2.66 (1.92, 3.41) | <.001 |
| SHBG | −3.25 (−4.43, −2.06) | <.001 | −4.38 (−6.23, −2.48) | <.001 | −3.34 (−4.54, −2.13) | <.001 | −2.97 (−3.86, −2.07) | <.001 |
| Model 1b | ||||||||
| E2 | −0.59 (−1.21, 0.04) | .07 | −1.16 (−2.14, −0.18) | .02 | −0.64 (−1.26, −0.03) | .04 | −0.12 (−0.59, 0.36) | .63 |
| FSH | 0.30 (−0.47, 1.07) | .45 | 0.04 (−1.16, 1.25) | .95 | 0.19 (−0.56, 0.95) | .62 | −0.38 (−0.95, 0.20) | .20 |
| FAI | 0.52 (−0.35, 1.39) | .24 | 0.82 (−0.54, 2.20) | .24 | −0.53 (−0.32, 1.38) | .22 | 1.27 (0.63, 1.92) | .0001 |
| SHBG | −1.29 (−2.35, −0.22) | .02 | −1.91 (−3.56, −0.22) | .03 | −1.27 (−2.31, −0.22) | .02 | −1.56 (−2.35, −0.77) | .0001 |
| Model 2c | ||||||||
| E2 | −0.57 (−1.20, 0.07) | .08 | −1.15 (−2.14, −0.16) | .02 | −0.63 (−1.25, −0.01) | .04 | −0.08 (−0.56, 0.40) | .74 |
| FSH | 0.32 (−0.45, 1.11) | .41 | 0.09 (−1.13, 1.32) | .89 | 0.22 (−0.54, 0.99) | .56 | −0.39 (−0.97, 0.19) | .18 |
| FAI | 0.45 (−0.40, 1.34) | .29 | 0.81 (−0.56, 2.20) | .25 | 0.49 (−0.37, 1.34) | .26 | 1.25 (0.61, 1.90) | .0001 |
| SHBG | −1.21 (−2.28, −0.13) | .03 | −1.89 (−3.56, −0.19) | .03 | −1.20 (−2.25, −0.14) | .03 | −1.56 (−2.35, −0.76) | .0002 |
Log-transformed. Percentage change and 95% CI in each CF volume per 20% increase in each endogenous sex hormone were calculated using the following formula: (eβ ∗ log(1.2) − 1) ∗ 100 (27).
Model 1: adjusted for study site, cycle day of the blood draw, race, income, age, current smoking, physical activity, and obesity (BMI ≥ 30 kg/m2).
Model 2: model 1 + alcohol consumption, medication use, and comorbidities.
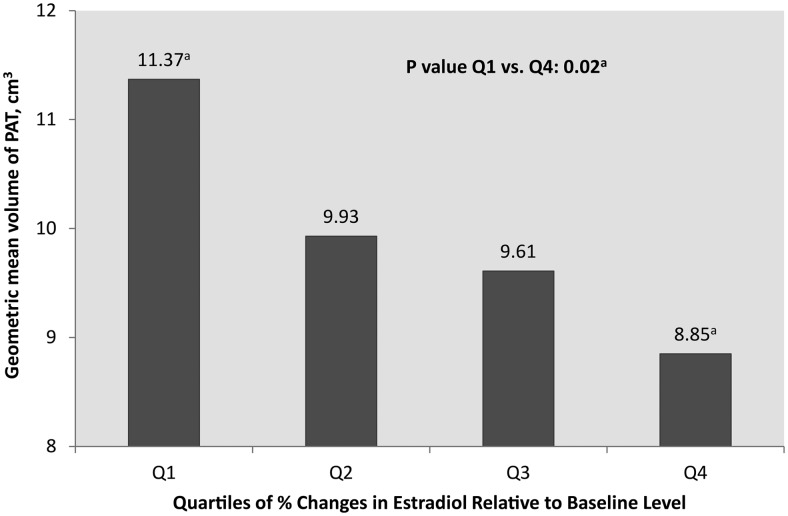
Quartiles of E2 relative change to the baseline visit of the SWAN parent study were evaluated in relation to PAT to better understand the association between change in E2 and PAT. Women with the greatest reduction in E2 since baseline had significantly greater volumes of PAT compared to women with the least reduction in E2 since baseline (Q1 vs Q4). These differences were independent of study covariates, including age (Figure 2).
Figure 2.
Geometric means of volumes of paracardial fat by quartiles of percentage changes in E2 relative to SWAN parent baseline level. a, Model adjusted for age, site, ethnicity, income, smoking, physical activity, and obesity. P value is adjusted for multiple comparisons.
Discussion
Using data from a large sample of midlife women, the current study showed for the first time that late peri-/postmenopausal women have significantly greater volumes of heart fat depots independent of age, race, obesity, physical activity, smoking, alcohol consumption, medications use, and comorbidity. These differences may be attributed to concentrations of endogenous sex hormones at midlife. We were able to show that lower concentrations of E2, measured concomitantly with CF volume, and a decline in E2 concentrations over 4.80 years were significantly associated with greater PAT volumes in midlife women. Additionally, concentrations of FAI and SHBG were associated with volumes of certain CF depots.
CF is a metabolically active organ that releases substances with known vascular actions and proinflammatory factors (15). Therefore, CF may locally modulate the function and morphology of the heart and vasculature, which could play a key role in adiposity-related atherosclerosis (15). Due to the absence of fascial boundaries, CF may have a local effect and modulate coronary and aortic arteries as well as myocardium via both paracrine and endocrine release of anti- and proinflammatory adipokines (15). Higher CF has been associated with multiple CHD risk factors, the presence of coronary artery disease, and coronary and aortic artery calcification (16–18, 28). Our findings of greater volumes of all CF depots in late peri-/postmenopausal women suggest a potential role of menopause on body fat distribution to visceral organs such as the heart and highlight CF as a possible risk factor for women at midlife. Interestingly, data from the Framingham study showed that the associations between both pericardial (TAT in our study) and periaortic fat and CHD risk factors are significantly stronger in women than men (16, 17).
Although BMI and weight increase throughout the menopausal transition, abdominal visceral fat increases at least three times faster than BMI (10); in one study, it was the only fat depot that was significantly greater in postmenopausal than premenopausal women, independent of age (4). Researchers have evaluated associations between menopause and other visceral fat depots including liver fat. Interestingly, postmenopausal women showed greater liver fat compared with premenopausal women, which was found to be related to their lower concentrations of serum E2 (29, 30). These findings suggest that the menopausal transition is associated with changes in fat deposition location and not overall obesity. No previous study has evaluated associations between menopause and CF in humans. In animal models, 12 weeks after ovariectomization, the weight of periaortic fat was significantly higher in the oophorectomized female rats compared to a sham group (31). Our findings are consistent with these results and extend them to the human population.
No previous study has directly evaluated associations between E2 concentrations and CF in midlife women. There is evidence from animal studies and studies of HT on other visceral fat depots that support our findings. Oophorectomized rats receiving 17-β E2 supplementation were found to have less weight and area of periaortic fat (31) and were protected from developing fatty liver (32). Furthermore, aromatase gene knockout mice, which cannot synthesize endogenous E2, exhibit a marked increase in gonadal and infrarenal fat pads that were reduced after receiving E2 therapy (33). In humans, women without fatty liver had significantly higher E2 concentrations (29). Additionally, HT was found to be associated with a reduction in central adiposity in randomized controlled trials (34). Our findings of significant and independent associations between lower concentrations and decline of E2, and higher volumes of PAT, suggest a potential role of E2 in explaining why late peri-/postmenopausal women showed greater volumes of CF depots compared to premenopausal/early perimenopausal women in the current study. This hypothesis should be confirmed using a longitudinal study design.
Estrogen plays a significant role in regulating adipocyte metabolism and sexual dimorphism of particular adipose depots via estrogen receptors ER-α (35) expressed in human sc and visceral adipose tissues (36). E2 can directly increase the number of antilipolytic α2A-adrenergic receptors in sc adipocytes (37) and lipolytic β-adrenergic expression in visceral adipocytes (38), which maintain the typical female type of fat distribution resulting in greater accumulation of fat in sc fat depot with minimal deposition in abdominal visceral fat depot (39). Therefore, it is possible that lower concentrations of E2 and the decline in E2 over time in our study were associated with higher volumes of fat deposition around the heart through lowering antilipolytic and lipolytic receptors in sc and visceral fat, respectively. This in turn, would reduce the ability of sc fat to deposit fat and potentially increase the deposition and infiltration of fat in visceral tissues, including the heart and aorta.
The current study showed that higher concentrations of FAI and lower concentrations of SHBG were associated with greater volumes of certain CF depots. FAI concentrations were significantly associated with fat around the aorta, whereas concentrations of SHBG were significantly associated with fat in all evaluated CF depots in women at midlife. Other studies in populations known to have excess androgen, such as women with polycystic ovary syndrome and women with idiopathic hirsutism, showed that these women have significantly greater EAT compared to controls (40). These findings are in line with our findings and suggest that androgens might also influence adipose tissue deposition.
The incidence of CHD, the leading cause of death in women, increases after the age of 50 (11, 12). Identifying potential risk factors for CHD development in women at midlife will enhance our understanding of the reasons that women after menopause are subjected to a higher risk of CHD. Doubling of or a 100% increase of EAT has been associated with a 54% increase in coronary events, adjusting for cardiovascular risk factors (hazard ratio, 1.54; 95% CI, 1.09 to 2.19) (41). The current study found an average 20% higher PAT in late peri-/postmenopausal than in premenopausal/early perimenopausal women, which could correspond to an 11% increased risk of coronary events. Identifying possible prevention strategies to reduce CF in women at midlife may reduce CHD risk associated with excess CF. Weight management could be a potential prevention strategy. Weight-loss interventions of equal energy deficit with/without aerobic exercise significantly reduced pericardial fat (TAT in the current study) by 17% in postmenopausal women (42).
The current study has some limitations, including the cross-sectional design, which did not allow us to assess the temporality of the evaluated associations: 1) whether CF volumes progress over time in midlife women; and 2) whether the dynamic changes in sex hormones are associated with greater progression in CF over the menopausal transition. Due to small sample sizes of the premenopausal and late perimenopausal categories, we were not able to assess study aims in each of these categories separately. The late perimenopausal stage would be of great interest because women are subjected to significant vascular remodeling during this period (43). Although levels of E2 and FSH may not be similar in late peri- and postmenopausal women, they were close. The same applies to levels of E2 and FSH in premenopausal and early perimenopausal women. Additionally, volumes of CF were very similar between premenopausal and early perimenopausal women as well as between late peri- and postmenopausal women. Another limitation of the current analyses was the inability to adjust for total body fat because we did not have this measure in SWAN Heart participants. Despite these limitations, this study has several strengths, which include being the first to evaluate whether volumes of CF are associated with menopausal status and endogenous sex hormones in women at midlife. It utilized a well-characterized cohort, the SWAN Study. Subclinical CHD measures and related risk factors are available through the SWAN parent study and will be utilized to assess the potential role of CF on CHD risk factors and subclinical measures in women at midlife.
In conclusion, late peri-/postmenopausal women have greater volumes of EAT, PAT, and TAT depots compared with premenopausal/early perimenopausal women independent of age, obesity, and other covariates. Endogenous sex hormones are associated with the CF volumes, with E2 being associated with TAT driven by the significant association with paracardial heart fat. Perhaps CF plays a role in the higher risk of CHD reported in women after menopause.
SWAN Investigators
Clinical Centers: University of Michigan, Ann Arbor, MI—Siobán Harlow, Principal Investigator (PI) 2011 to present; MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA—Joel Finkelstein, PI 1999 to present; Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL—Howard Kravitz, PI 2009 to present; Lynda H. Powell, PI 1994–2009; University of California, Davis/Kaiser, CA—Ellen Gold, PI; University of California, Los Angeles, CA—Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY—Carol Derby, PI 2011 to present; Rachel Wildman, PI 2010–2011; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry-New Jersey Medical School, Newark, NY—Gerson Weiss, PI 1994–2004; and University of Pittsburgh, Pittsburgh, PA—Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD—Winifred Rossi, 2012 to present; Sherry Sherman, 1994–2012; Marcia Ory, 1994–2001; National Institute of Nursing Research, Bethesda, MD—Program Officers.
Central Laboratory: University of Michigan, Ann Arbor, MI—Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA—Maria Mori Brooks, PI 2012 to present; Kim Sutton-Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, MA—Sonja McKinlay, PI 1995–2001.
Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair.
Acknowledgments
We thank the study staff at each site and all the women who participated in SWAN.
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), Department of Health and Human Services, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR), and the NIH Office of Research on Women's Health (ORWH) (Grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). SWAN Heart was supported by the National Heart, Lung, and Blood Institute (Grants HL065581, HL065591). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
The SWAN Cardiovascular Fat ancillary study was supported by an award from the American Heart Association (AHA) Great River Affiliation Clinical Research Program (12CRP11900031).
Disclosure Summary: S.R.E.K. reports grants from the AHA during the conduct of the study. I.J. reports grants from Rush University Medical Center during the conduct of the study. M.J.B. reports grants from the NIH during the conduct of the study and grants from General Electric outside the submitted work. E.B.-M. reports grants from the AHA during the conduct of the study. S.A.E.-R. reports grant no. MD003422 from the National Institute of Minority Health and Health Disparities and the Applied Clinical Research Program and Program in Health Disparities Research at the University of Minnesota. K.A.M. reports grants from NIH during the conduct of the study. K.J.S., C.H., and L.H.P. have nothing to disclose.
Footnotes
- BMI
- body mass index
- CF
- cardiovascular fat
- CHD
- coronary heart disease
- CI
- confidence interval
- E2
- estradiol
- EAT
- epicardial adipose tissue
- FAI
- free androgen index
- HT
- hormone therapy
- LLD
- lower limit of detection
- PAT
- paracardial adipose tissue
- PVAT
- perivascular adipose tissue
- TAT
- total heart adipose tissue.
References
- 1. Davis SR, Castelo-Branco C, Chedraui P, et al. Understanding weight gain at menopause. Climacteric. 2012;15:419–429. [DOI] [PubMed] [Google Scholar]
- 2. Wing RR, Matthews KA, Kuller LH, Meilahn EN, Plantinga PL. Weight gain at the time of menopause. Arch Intern Med. 1991;151:97–102. [PubMed] [Google Scholar]
- 3. Guthrie JR, Dennerstein L, Dudley EC. Weight gain and the menopause: a 5-year prospective study. Climacteric. 1999;2:205–211. [DOI] [PubMed] [Google Scholar]
- 4. Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond). 2008;32:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abdulnour J, Doucet E, Brochu M, et al. The effect of the menopausal transition on body composition and cardiometabolic risk factors: a Montreal-Ottawa New Emerging Team group study. Menopause. 2012;19:760–767. [DOI] [PubMed] [Google Scholar]
- 6. Guthrie JR, Dennerstein L, Taffe JR, Lehert P, Burger HG. The menopausal transition: a 9-year prospective population-based study. The Melbourne Women's Midlife Health Project. Climacteric. 2004;7:375–389. [DOI] [PubMed] [Google Scholar]
- 7. Enzi G, Gasparo M, Biondetti PR. Subcutaneous and visceral fat distribution according to sex, age, and overweight, evaluated by computed tomography. Am J Clin Nutr. 1986;44:739–746. [DOI] [PubMed] [Google Scholar]
- 8. van der Leeuw J, Wassink AM, van der Graaf Y, et al. Age-related differences in abdominal fat distribution in premenopausal and postmenopausal women with cardiovascular disease. Menopause. 2013;20:409–417. [DOI] [PubMed] [Google Scholar]
- 9. Guthrie JR, Dennerstein L, Taffe JR, et al. Central abdominal fat and endogenous hormones during the menopausal transition. Fertil Steril. 2003;79:1335–1340. [DOI] [PubMed] [Google Scholar]
- 10. Janssen I, Powell LH, Jasielec MS, Kazlauskaite R. Covariation of change in bioavailable testosterone and adiposity in midlife women. Obesity. 2015;23:488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. [DOI] [PubMed] [Google Scholar]
- 12. Gorodeski GI. Impact of the menopause on the epidemiology and risk factors of coronary artery heart disease in women. Exp Gerontol. 1994;29:357–375. [DOI] [PubMed] [Google Scholar]
- 13. Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–1241. [DOI] [PubMed] [Google Scholar]
- 14. Ravussin E, Smith SR. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Ann NY Acad Sci. 2002;967:363–378. [DOI] [PubMed] [Google Scholar]
- 15. Iacobellis G, Gao YJ, Sharma AM. Do cardiac and perivascular adipose tissue play a role in atherosclerosis? Curr Diab Rep. 2008;8:20–24. [DOI] [PubMed] [Google Scholar]
- 16. Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. [DOI] [PubMed] [Google Scholar]
- 17. Lehman SJ, Massaro JM, Schlett CL, O'Donnell CJ, Hoffmann U, Fox CS. Peri-aortic fat, cardiovascular disease risk factors, and aortic calcification: the Framingham Heart Study. Atherosclerosis. 2010;210:656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ding J, Kritchevsky SB, Harris TB, et al. The association of pericardial fat with calcified coronary plaque. Obesity (Silver Spring). 2008;16:1914–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cakir E, Ozkaya E, Korkmaz V, Goktas I, Kucukozkan T. Comparison of the effects of surgical and natural menopause on epicardial fat thickness and γ-glutamyltransferase level. Menopause. 2011;18:901–905. [DOI] [PubMed] [Google Scholar]
- 20. Huang G, Wang D, Zeb I, et al. Intra-thoracic fat, cardiometabolic risk factors, and subclinical cardiovascular disease in healthy, recently menopausal women screened for the Kronos Early Estrogen Prevention Study (KEEPS). Atherosclerosis. 2012;221:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sowers M, Crawford S, Sternfeld B, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, eds. Menopause: Biology and Pathology. New York, NY: Academic Press; 2000;175–188. [Google Scholar]
- 22. Shields KJ, Barinas-Mitchell E, Gingo MR, et al. Perivascular adipose tissue of the descending thoracic aorta is associated with systemic lupus erythematosus and vascular calcification in women. Atherosclerosis. 2013;231:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wildman RP, Colvin AB, Powell LH, et al. Associations of endogenous sex hormones with the vasculature in menopausal women: the Study of Women's Health Across the Nation (SWAN). Menopause. 2008;15:414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Janssen I, Powell LH, Kazlauskaite R, Dugan SA. Testosterone and visceral fat in midlife women: the Study of Women's Health Across the Nation (SWAN) fat patterning study. Obesity (Silver Spring). 2010;18:604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med. 1999;28:313–323. [DOI] [PubMed] [Google Scholar]
- 26. Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 27. Benoit K. Linear Regression Models with Logarithmic Transformations. Methodology Institute, London School of Economics; March 17, 2011. http://www.kenbenoit.net/courses/ME104/logmodels2.pdf Accessed January 15, 2015.
- 28. Mahabadi AA, Massaro JM, Rosito GA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gutierrez-Grobe Y, Ponciano-Rodríguez G, Ramos MH, Uribe M, Méndez-Sánchez N. Prevalence of non alcoholic fatty liver disease in premenopausal, postmenopausal and polycystic ovary syndrome women. The role of estrogens. Ann Hepatol. 2010;9:402–409. [PubMed] [Google Scholar]
- 30. Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–1657. [DOI] [PubMed] [Google Scholar]
- 31. Xu J, Xiang Q, Lin G, et al. Estrogen improved metabolic syndrome through down-regulation of VEGF and HIF-1α to inhibit hypoxia of periaortic and intra-abdominal fat in ovariectomized female rats. Mol Biol Rep. 2012;39:8177–8185. [DOI] [PubMed] [Google Scholar]
- 32. Stubbins RE, Najjar K, Holcomb VB, Hong J, Núñez NP. Oestrogen alters adipocyte biology and protects female mice from adipocyte inflammation and insulin resistance. Diabetes Obes Metab. 2012;14:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Misso ML, Murata Y, Boon WC, Jones ME, Britt KL, Simpson ER. Cellular and molecular characterization of the adipose phenotype of the aromatase-deficient mouse. Endocrinology. 2003;144:1474–1480. [DOI] [PubMed] [Google Scholar]
- 34. Davis SR, Walker KZ, Strauss BJ. Effects of estradiol with and without testosterone on body composition and relationships with lipids in postmenopausal women. Menopause. 2000;7:395–401. [DOI] [PubMed] [Google Scholar]
- 35. Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci USA. 2000;97:12729–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pedersen SB, Kristensen K, Hermann PA, Katzenellenbogen JA, Richelsen B. Estrogen controls lipolysis by up-regulating α2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor α. Implications for the female fat distribution. J Clin Endocrinol Metab. 2004;89:1869–1878. [DOI] [PubMed] [Google Scholar]
- 38. Monjo M, Pujol E, Roca P. α2- to β3-Adrenoceptor switch in 3T3–L1 preadipocytes and adipocytes: modulation by testosterone, 17β-estradiol, and progesterone. Am J Physiol Endocrinol Metab. 2005;289:E145–E150. [DOI] [PubMed] [Google Scholar]
- 39. Lovejoy JC. The menopause and obesity. Prim Care. 2003;30:317–325. [DOI] [PubMed] [Google Scholar]
- 40. Cakir E, Doğan M, Topaloglu O, et al. Subclinical atherosclerosis and hyperandrogenemia are independent risk factors for increased epicardial fat thickness in patients with PCOS and idiopathic hirsutism. Atherosclerosis. 2013;226:291–295. [DOI] [PubMed] [Google Scholar]
- 41. Mahabadi AA, Berg MH, Lehmann N, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol. 2013;61:1388–1395. [DOI] [PubMed] [Google Scholar]
- 42. Brinkley TE, Ding J, Carr JJ, Nicklas BJ. Pericardial fat loss in postmenopausal women under conditions of equal energy deficit. Med Sci Sports Exerc. 2011;43:808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton-Tyrrell K. Progression rates of carotid intima-media thickness and adventitial diameter during the menopausal transition. Menopause. 2013;20:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]