Abstract
Context:
Ectopic Cushing's Syndrome (ECS) can be a diagnostic challenge with the hormonal source difficult to find. This study analyzes the accuracy of imaging studies in ECS localization.
Evidence Acquisition:
Systematic review of medical literature for ECS case series providing individual patient data on at least one conventional imaging technique (computed tomography [CT]/magnetic resonance imaging) and one of the following: 111In-pentetreotide (OCT), 131I/123I-metaiodobenzylguanidine, 18F-fluoro-2-deoxyglucose-positron emission tomography (FDG-PET), 18F-fluorodopa-PET (F-DOPA-PET), 68Ga-DOTATATE-PET/CT or 68Ga-DOTATOC-PET/CT scan (68Gallium-SSTR-PET/CT).
Evidence Summary:
The analysis comprised 231 patients (females, 50.2%; age, 42.6 ± 17 y). Overall, 52.4% (121/231) had “overt” ECS, 18.6% had “occult” ECS, and 29% had “covert” ECS. Tumors were located in the lung (55.3%), mediastinum-thymus (7.9%), pancreas (8.5%), adrenal glands (6.4%), gastrointestinal tract (5.4%), thyroid (3.7%), and other sites (12.8%), and primary tumors were mostly bronchial neuroendocrine tumors (NETs) (54.8%), pancreatic NETs (8%), mediastinum-thymus NETs (6.9%), gastrointestinal NETs (5.3%), pheochromocytoma (6.4%), neuroblastoma (3.2%), and medullary thyroid carcinoma (3.2%). Tumors were localized by CT in 66.2% (137/207), magnetic resonance imaging in 51.5% (53/103), OCT in 48.9% (84/172), FDG-PET in 51.7% (46/89), F-DOPA-PET in 57.1% (12/21), 131/123I-metaiodobenzylguanidine in 30.8% (4/13), and 68Gallium-SSTR-PET/CT in 81.8% (18/22) of cases. Molecular imaging discovered 79.1% (53/67) of tumors unidentified by conventional radiology, with OCT the most commonly used, revealing the tumor in 64%, followed by FDG-PET in 59.4%. F-DOPA-PET was used in only seven covert cases (sensitivity, 85.7%). Notably, 68Gallium-SSTR-PET/CT had 100% sensitivity among covert cases.
Conclusions:
Nuclear medicine improves the sensitivity of conventional radiology when tumor site identification is problematic. OCT offers a good availability/reliability ratio, and FDG-PET was proven useful. 68Gallium-SSTR-PET/CT use was infrequent, despite offering the highest sensitivity.
Ectopic Cushing's syndrome (ECS), or ectopic ACTH syndrome, is responsible for 10–15% of the cases of Cushing's syndrome. It is caused by various extrapituitary tumors, frequently malignant. The most prevalent tumors associated with ECS are small cell lung carcinomas (SCLCs) and neuroendocrine tumors (NETs), including bronchial carcinoids, thymic carcinoids, pancreatic NETs, medullary thyroid carcinomas (MTCs), and pheochromocytomas (1, 2). Although SCLCs are rapidly diagnosed, the localization of other tumors can be difficult. In the largest consecutive series, 9–27% of patients with hormone tests suggesting ECS have the primary lesion not identified even after long-term follow-up, and the condition is defined as occult ECS (1–7).
Localization of the source of ectopic ACTH secretion is crucial. In fact, although nonmalignant conditions, such as mediastinal lipomatosis and congenital adrenal hyperplasia, have been associated with ECS (8), the source of ectopic ACTH/CRH is usually a malignant tumor (3). Early localization and treatment can avoid surgical or pharmacological adrenalectomy and reduce the risk of metastatic disease (9).
The use of multiple imaging techniques with reliable, highly sensitive procedures is recommended in most NET guidelines (10–15), although none have specifically addressed ECS. According to guidelines of The Endocrine Society (16), patients with Cushing's syndrome should be characterized by complete endocrine testing, and discordant results should be handled in tertiary referral centers. The combination of thin-cut multislice imaging with chest, abdominal, and pelvic computed tomography (CT) scanning, plus possibly magnetic resonance imaging (MRI) of the chest and pelvis, and complementary imaging tools, including 111In-pentetreotide (OCT) scintigraphy, positron emission tomography (PET), and PET/CT should be employed and possibly repeated during follow-up (6, 8). However, the role of new molecular imaging techniques in localizing the source of ECS is not well established, given the rarity of ECS and the small number of patients enrolled in most studies.
The aim of our study was to systematically review published data about the diagnostic performance of simultaneous conventional and nuclear medicine imaging in the identification of ectopic ACTH/CRH production, relying on individual patient data (IPD) analysis (17). An evidence-based algorithm was then developed for the use of nuclear medicine imaging in difficult cases of ECS.
Patients and Methods
Study design
The current study is an IPD systematic review of the literature following PRISMA guidelines. All published studies describing single or case series of patients with ECS (ectopic ACTH- and/or CRH-secreting tumors causing Cushing's syndrome) and reporting diagnostic accuracy with at least one conventional and one nuclear medicine investigation were reviewed. A search for English language articles in MEDLINE, EMBASE, Cochrane Library, and SCOPUS was performed between June and September, 2013. Search terms were: ectopic ACTH, Cushing's syndrome, hypercortisolism, octreoscan, pentetreotide, MIBG, FDG-PET, Flurodopa-PET, F-DOPA-PET, Dotatoc, Dotatate, Dotanoc, and Gallium-PET. The search was updated in April 2015.
Study selection
Eligibility criteria included: 1) single case report; 2) case series; and 3) diagnostic studies. The review was limited to studies published since 1995, due to the availability and standardization of imaging techniques and tracers. Inclusion was open to all studies published from any site (oncology, radiotherapy, radiology departments) as long as the diagnosis was confirmed by appropriate endocrine/pathology data. Reviews, editorials, commentaries, letters and animal studies were excluded. Four independent reviewers evaluated all titles based on the abstract; the full text of potentially eligible articles was then analyzed. For series not describing IPD but giving only cumulative figures, an attempt was made to obtain the raw data from authors; otherwise they were excluded. Supplemental Figure 1 describes the evidence acquisition process.
Data collection and quality assessment
Four reviewers independently extracted clinical data (age, gender, clinical status, comorbidities); basal hormonal and biochemical values (plasma ACTH, serum cortisol, urinary 24-h cortisol, serum K+); response to dynamic testing with low-dose dexamethasone suppression test (LDDST), high-dose dexamethasone suppression test (HDDST), CRH test, inferior petrosal sinus sampling (IPSS); conventional radiological investigation (CT, MRI); nuclear medicine imaging, including OCT, 131I/123I-metaiodobenzylguanidine (MIBG), 18F-fluoro-2-deoxyglucose (FDG)-PET, 18F-fluorodopa (F-DOPA)-PET, 68Ga-DOTATATE-PET/CT,68Ga-DOTATOC-PET/CT, and 68Ga-DOTANOC-PET/CT (the last three unitarily referred as 68Gallium-SSTR-PET/CT); pathology (including staining for ACTH/CRH and other neuroendocrine markers and NET grading); types of treatment; and follow-up.
Data analysis
Analyses were performed using the IPD approach by developing a line-by-line PC-based database for statistical processing (17). The software used for all statistical analyses was SPSS version 17 (SPSS Inc). Data were presented as mean ± SD. Logarithmic transformation was applied for non-normally distributed data before analysis. Unpaired t-test was used for comparison of tumor sizes. Sensitivity with 95% confidence interval (CI) for each technique or imaging combination was calculated for individual patients (not lesions). Sensitivity was determined from the number of true positives and false negatives. To test the influence of single case reports, subgroup analysis was performed comparing diagnostic accuracy in the subset of articles providing less than five cases to larger series or the entire set. Confidence intervals for proportions were calculated according to the Wilson score method without continuity correction (18). Statistical significance was set at P < .05.
Results
Characteristics of the study population
A total of 1165 studies were identified as potentially relevant. Of these, 826 were excluded based on the title and abstract content, and 232 were excluded after full-text analysis due to non-English language, nonhuman studies, review, editorial, or no individual outcomes being accessible. At the end of the process, 107 studies (2, 6, 19–119) were eligible and were included in the review (Supplemental Figure 1), for a total of 231 patients with a mean age of 42.6 ± 17 years, evenly distributed for sex (females, 50.2%).
Positive cross-sectional imaging on presentation was reported for half of the study population (121 patients, 52.4%) (overt ECS group). In 67 patients (29%), the tumor source was detected only on follow-up conventional imaging or by nuclear medicine functional imaging (covert ECS group). For the remaining 43 patients (18.6%), ECS was diagnosed on the basis of multiple biochemical tests and clinical features, but the primary source was never found (occult ECS group). The general characteristics of the patients according to clinical classification are reported in Table 1.
Table 1.
Patient Characteristics According to Clinical Classification
| All | Overt | Covert | Occult | |
|---|---|---|---|---|
| n | 231 | 121 | 67 | 43 |
| Size, mm | 26.6 ± 26.9 | 30.0 ± 30.4 | 17.8 ± 9.9 | n.a. |
| Median (min–max) | 16 (4–132) | 17 (4–132) | 15 (5–50) | |
| Age, y | 42.6 ± 17.0 | 41.2 ± 17.3 | 40 ± 15.5 | 51.3 ± 15.9 |
| Median (min–max) | 43 (3–82) | 41 (3–75) | 41 (9–75) | 53 (20–82) |
| Sex | ||||
| Female | 108/215 (50.2%) | 61/111 (55.0%) | 26/62 (42.0%) | 21/41 (51.2%) |
| Male | 107/215 (49.8%) | 50/111 (45.0%) | 36/62 (58.0%) | 20/41 (48.8%) |
| ACTH, pg/mL | 295.6 ± 457.1 | 283 ± 296.9 | 416.2 ± 775.9 | 174.9 ± 139.2 |
| Median (min–max) | 172 (18.2–4425) | 204 (18.2–1704) | 169.5 (32–4425) | 133 (25–639) |
| Plasma cortisol, μg/dL | 66.5 ± 58.0 | 58.1 ± 46.6 | 80 ± 79.4 | 66.7 ± 31.6 |
| Median (min–max) | 50.7 (11.5–390) | 45.5 (11.5–298) | 64 (21–390) | 56 (22–130) |
| Urinary cortisol, nmol/24 h | 9046.9 ± 14 059.2 | 7938.6 ± 15 754.3 | 11 920.2 ± 11 586.2 | 8392.6 ± 12 055.7 |
| Median (min–max) | 2946.3 (28.1–69 777) | 2464 (28.1–69 777) | 7515.5 (28.1–32 078) | 2530.9 (189–45 747) |
| K, mEq/L | 2.7 ± 0.6 | 2.62 ± 0.6 | 2.8 ± 0.7 | 2.6 ± 0.6 |
| Median (min–max) | 2.7 (1.1–3.9) | 2.6 (1.8–3.8) | 3.1 (1.1–3.9) | 2.3 (1.8–3.9) |
| Staining for ACTH and/or CRH | 119/137 (86.9%) | 78/86 (90.7%) | 39/41 (95.1%) | On metastasis, 2/10 (20%) |
| Both ACTH and CRH | 10 | 9 | 0 | On metastasis, 1 |
| Only CRH | 4 | 3 | 0 | On metastasis, 1 |
| Staining for chromogranin A | 55/77 (71.4%) | 36/46 (78.3%) | 16/24 (66.7%) | On metastasis, 3/7 (42.9%) |
| Staining for synaptophysin | 40/75 (53.3%) | 29/45 (64.4%) | 10/24 (41.7%) | On metastasis, 1/6 (16.7%) |
| Staining for neuron-specific enolase | 12/73 (16.4%) | 7/43 (16.3%) | 5/24 (20.8%) | On metastasis, 1/6 (16.7%) |
| LDDST (no suppression) | 65/65 (100%) | 37/37 (100%) | 17/17 (100%) | 11/11 (100%) |
| HDDST (no suppression) | 108/114 (94.7%) | 51/53 (96.2%) | 31/33 (93.9%) | 25/27 (92.6%) |
| CRH test (lack of stimulation) | 48/59 (81.4%) | 23/28 (82.1%) | 13/16 (81.3%) | 12/15 (80%) |
| IPSS (absence of gradient) | 85/92 (92.4%) | 30/34 (88.2%) | 32/33 (97.0%) | 22/24 (91.7%) |
Abbreviations: min–max, minimum–maximum; n.a., not applicable. Data are expressed as mean ± SD or number (percentage), unless stated otherwise.
Hormonal profile and biochemical diagnosis of ECS
Mean ACTH levels were 295.6 ± 457.1 pg/mL, with much lower levels in occult cases (174.9 ± 139.2 pg/mL) than in overt cases (283 ± 296.9 pg/mL; P = .015) and highly variable levels in covert cases (416.2 ± 775.9 pg/mL). Potassium levels were similar in the three ECS categories. The response to dynamic hormonal testing is reported in Table 1: 100% of patients failed to respond to LDDST and 94.7% to HDDST; 81.4% had no response to CRH, whereas 92.4% showed no gradient on IPSS.
Discrepancies among tests used in the differential diagnosis of ECS were found in eight of 49 (16.3%) cases when comparing HDDST against CRH: one patient responded to HDDST, seven patients responded to CRH, and one patient responded to both HDDST and CRH but did not show any gradient on IPSS, and the location of the primary tumor remained unknown. Conversely, discrepancies were found in 10 of 70 (14.3%) cases when comparing HDDST against IPSS: six responded to HDDST, and four showed a gradient on IPSS. Finally, discrepancies were found in seven of 28 (25%) cases when comparing CRH against IPSS: five responded to CRH, and two showed a gradient on IPSS. In particular, two patients responded to CRH and showed a gradient on IPSS, but they failed to respond to HDDST, and follow-up imaging revealed two lung nodules of 17 and 30 mm, which were subsequently found to be positive on ACTH immunostaining (Table 1). None of the CRH-secreting tumors that had IPSS (6/14) showed a gradient.
Tumor site and pathology
In the entire series of 231 cases, the most frequent tumor sites were the lungs (45%), followed by mediastinum-thymus (6.5%), pancreas (6.9%), adrenal glands (5.3%), and gastrointestinal tract (4.3%). Less frequent sites were the thyroid (3%), abdomen (2.6%), pelvis (1.3%), cranium (5.2%), and chest (in sites other than the lungs) (1.3%). The site of the primary tumor remained unknown in 18.6% of cases (occult ECS). Pathological typing revealed: bronchial NET (54.8%), pancreatic NET (8%), mediastinum-thymus NET (6.9%), gastrointestinal NET (5.3%), pheochromocytoma (6.4%), neuroblastoma (3.2%), MTC (3.2%), paraganglioma (2.1%), and other histologies (10.1%). The latter included SCLCs, which were underrepresented, probably because they are rapidly diagnosed and do not undergo nuclear imaging for source localization or therapy. The prevalence of metastatic disease at diagnosis was 44 of 231 (19%), whereas in the subgroup of pancreatic lesions, metastases at diagnosis were detected in four of 16 (25%) patients, reflecting a prevalence similar to other reports (10, 11, 13–15). Neuroendocrine immunostaining was available for most tumors (147/231; 63.6%), with positive results on ACTH/CRH staining in 86.9%, chromogranin A in 71.4%, synaptophysin in 53.3%, and neuron-specific enolase in 16.4%. A total of 36.5% of ACTH-positive tumors were also positive to chromogranin A and 22.1% to synaptophysin. Neuroendocrine grading according to recent guidelines (15, 120) was reported in only 14 cases, with a relatively high prevalence of G2 (3/14; 21.4%) and G3 (2/14; 14.3%). Atypical bronchial carcinoids were reported in six of 18 cases (33.3%) of bronchial carcinoids.
Sensitivity of localization techniques
The ability of conventional and nuclear medicine investigations to localize the source of ACTH is presented in Table 2 and Supplemental Figure 2. The subgroup of patients for whom pathology was diagnostic was divided according to clinical presentation as detected immediately on first imaging (overt ECS) or discovered subsequently on follow-up (covert ECS). In the set of patients as a whole, 68Ga-DOTATATE-PET/CT, 68Ga-DOTATOC-PET/CT, and 68Ga-DOTANOC-PET/CT (unitarily referred to as 68Gallium-SSTR-PET/CT) had the highest sensitivity (81.8%; 18/22), followed by CT (66.2%; 137/207) and MRI (51.5%; 53/103). Considering only patients with a histologically confirmed primary tumor, CT had the highest sensitivity (81.1%; 137/169), similar to 68Gallium-SSTR-PET/CT (81.8%; 18/22), followed by MRI with a sensitivity of 73.4% (52/71). In overt cases, CT showed the greatest sensitivity (98.3%; 113/115), followed by MRI (92.9%; 39/42), FDG-PET (71.1%; 27/38), 68Gallium-SSTR-PET/CT (70%; 9/13), and OCT (63.5%; 54/85). In covert cases, 68Gallium-SSTR-PET/CT showed the greatest sensitivity (100%; 9/9), followed by F-DOPA-PET (85.7%; 6/7), OCT (64%; 32/50), FDG-PET (59.4%; 19/32), MRI (44.8%; 13/29), and CT (43.6%; 24/55). Subgroup analysis comparing the sensitivities in small or large series is reported in Supplemental Table 1. No significant difference was found, suggesting that the inclusion of single cases had not biased the overall findings.
Table 2.
Diagnostic Accuracy for Each Imaging Technique in All Patients, in Those With Proven Histology, and in Overt and Covert Patients
| CT | MRI | OCT | FDG-PET | F-DOPA-PET | MIBG | 68Gallium-SSTR-PET/CT | |
|---|---|---|---|---|---|---|---|
| All patients (n = 231) | |||||||
| Sensitivity, % (95% CI) | 66.2% (59.5–72.3) | 51.5% (41.9–60.9) | 48.9% (41.5–56.3) | 51.7% (41.5–61.8) | 57.1% (36.6–75.5) | 30.8% (12.7–57.6) | 81.8% (61.5–92.7) |
| n | 137/207 | 53/103 | 84/172 | 46/89 | 12/21 | 4/13 | 18/22 |
| True positive | 63.7% | 50.5% | 48.3% | 51.1% | 54.5% | 26.7% | 78.3% |
| n | 137/215 | 53/105 | 84/174 | 46/90 | 12/22 | 4/15 | 18/23 |
| False negative | 33.6% | 47.6% | 50.6% | 47.8% | 40.9% | 60% | 17.4% |
| n | 70/215 | 50/105 | 88/174 | 43/90 | 9/22 | 9/15 | 4/23 |
| False positive | 3.7% | 1.9% | 1.1% | 1.1% | 4.5% | 13.3% | 4.3% |
| n | 8/215 | 2/105 | 2/174 | 1/90 | 1/22 | 2/15 | 1/23 |
| Histologically confirmed (n = 188) | |||||||
| Sensitivity, % (95% CI) | 81.1% (74.5–86.3) | 73.4% (61.9–82.2) | 62.9% (54.6–70.7) | 65.7% (54–75.8) | 65.0% (43.3–81.9) | 40% (16.8–68.7) | 81.8% (61.5–92.7) |
| n | 137/169 | 52/71 | 85/135 | 46/70 | 13/20 | 4/10 | 18/22 |
| True positive | 77.4% | 71.2% | 62.0% | 64.8% | 61.9% | 33.3% | 78.3% |
| n | 137/177 | 52/73 | 85/137 | 46/71 | 13/21 | 4/12 | 18/23 |
| False negative | 18.1% | 26% | 36.5% | 33.8% | 33.3% | 50% | 17.4% |
| n | 32/177 | 19/73 | 50/137 | 24/71 | 7/21 | 6/12 | 4/23 |
| False positive | 4.5% | 2.7% | 1.5% | 1.4% | 4.8% | 16.7% | 4.3% |
| n | 8/177 | 2/73 | 2/137 | 1/71 | 1/21 | 2/12 | 1/23 |
| Overt (n = 121) | |||||||
| Sensitivity % (95% CI) | 98.3% (93.9–99.5) | 92.9% (81.0–97.5) | 63.5% (52.9–72.9) | 71.1% (55.2–83) | 53.9% (29.1–76.8) | 37.5% (13.7–69.4) | 70% (39.7–89.2) |
| n | 113/115 | 39/42 | 54/85 | 27/38 | 7/13 | 3/8 | 9/13 |
| True positive | 97.4% | 92.9% | 62.1% | 71.1% | 50,0% | 30% | 69.2% |
| n | 113/116 | 39/42 | 54/87 | 27/38 | 7/14 | 3/10 | 9/13 |
| False negative | 1.7% | 7.1% | 35.6% | 28.9% | 42.9% | 50% | 30.8% |
| n | 2/116 | 3/42 | 31/87 | 11/38 | 6/14 | 5/10 | 4/13 |
| False positive | 0.9% | 2.3% | 7.1% | 20% | |||
| n | 1/116 | 2/87 | 1/14 | 2/10 | |||
| Covert (n = 67) | |||||||
| Sensitivity, % (95% CI) | 43.6% (31.4–56.7) | 44.8% (28.4–62.4) | 64.0% (50.1–75.9) | 59.4% (42.3–74.5) | 85.7% (48.7–97.4) | 50% (9.5–90.6) | 100% (61–100) |
| n | 24/55 | 13/29 | 32/50 | 19 /32 | 6/7 | 1/2 | 9/9 |
| True positive | 39.3% | 41.9% | 64.0% | 57.6% | 85.7% | 50% | 90.0% |
| n | 24/61 | 13/31 | 32/50 | 19/33 | 6/7 | 1/2 | 9/10 |
| False negative | 50.8% | 51.6% | 36.0% | 39.4% | 14.3 | 50% | |
| n | 31/61 | 16/31 | 18/50 | 13/33 | 1/7 | 1/2 | |
| False positive | 9.8% | 6.5% | 3.0% | 10.0% | |||
| n | 6/61 | 2/31 | 1/33 | 1/10 |
Analysis of false positives by localization technique
False-positive CTs were reported in eight of 215 (3.7%) cases. Subsequent imaging techniques identified the true ECS source in the following sites: four in the lung (two identified by OCT, one by bronchoscopy, and one by F-DOPA-PET), one para-aortic lesion (identified by OCT and FDG-PET), one in the right atrium (identified by MRI and FDG-PET), one within the thyroid gland (identified by ultrasound), and one in the paranasal sinus (identified by 68Gallium-SSTR-PET/CT).
Two false-positive MRIs were reported (2/105; 1.9%). Subsequent imaging identified the true tumor site in the pancreas and mediastinum lymph nodes; both lesions negative on MRI were positive on CT and OCT.
OCT had the lowest false-positive rate, with two false uptakes (2/174; 1.1%) described in the lung hilum, whereas the true source was an MTC identified on neck ultrasound. This focal lesion was negative on CT and MIBG; the patient also had small locoregional metastases in the liver that stained positive for ACTH, calcitonin, carcinoembryonic antigen, and synaptophysin. The other case was a patient with an OCT-positive pancreatic NET that, however, was not causing ECS because the true ectopic source was then identified in a lung NET seen at CT scan (117).
A false-positive FDG-PET was described in one case (1/90; 1.1%) of spleen uptake where the ACTH secretion actually originated from a pancreatic NET positive on ACTH staining. The lesion was retrospectively identified by MRI, but not by CT scan.
A false-positive F-DOPA-PET was described in one case (1/22; 4.5%) of uptake in the left ventricle, whereas the true source was actually a 16-mm lung NET identified by CT and MRI. This lesion was positive on ACTH staining but failed to show uptake during OCT.
A false-positive 68Gallium-SSTR-PET/CT of the adrenal glands was described in one case (1/23; 4.3%), whereas the true source of ACTH was a lung NET, positive on F-DOPA-PET but not detected on CT.
Analysis of occult tumors associated with ECS
A total of 43 patients had occult ECS, in which clinical signs and biochemical tests were suggestive of ECS but all imaging was negative. The prevalence of occults (43/231; 18.6%; 95% CI, 14.1–24.1) in our series was comparable to that of the five largest consecutive series (50/383; 13.1%; 95% CI, 10–16.8; P = .072; data from Ref. 7). In our series, 13 (5.6%) had partial occult ECS, defined as subsequent identification of metastasis from an unknown primary tumor. With regard to occult ECS, 37 patients had undergone CT; 31 patients, MRI; 39 patients, OCT; 20 patients, FDG-PET; two patients, F-DOPA-PET; and three patients, MIBG investigation. No occult cases were found among patients undergoing 68Gallium-SSTR-PET/CT scan, suggesting that this approach had no false negatives, at least in this collection of ECS patients. Among occults, eight had undergone a pituitary surgical exploration (26%; 8/30; if excluding the partial occults), reducing the remote, but still possible, chance that these patients had an invisible Cushing's disease with biochemical response typical of ECS (121).
Sensitivity according to tumor site
The ability to localize the tumor according to its primary site is reported in Table 3. Lung lesions were identified with the greatest sensitivity by CT (79.4%), 68Gallium-SSTR-PET/CT (77.8%), F-DOPA-PET (71.4%), and MRI (66.7%). Thymus-mediastinum tumors were identified by OCT (85.7%), CT (85%), MRI (62.5%), and FDG-PET (62.5%). Pancreatic lesions were found by FDG-PET (100%), MRI (87.5%), and CT (85.7%). Adrenal gland tumors were found by CT, MRI, and FDG-PET with a sensitivity of 100%. Gastrointestinal tract lesions were found by CT (90%) and MRI (71.4%). Thyroid tumors were found by CT with a sensitivity of 80% and by OCT (66.7%). Carotid glomus, atrium, and para-aortic region tumors were found by OCT (80%). Head tumors were found by MRI (87.5%), OCT (80%), and FDG-PET (71.4%). Rare abdominal sites were found by MRI (66.7%). A detailed analysis on the combination of various conventional and nuclear medicine imaging techniques is described in Supplemental Table 2.
Table 3.
Sensitivity (95% CI) of Diagnostic Techniques in Primary Source Localization According to Tumor Site
| Site (Positive Finding) | CT + | MRI + | OCT + | FDG-PET + | F-DOPA-PET + | MIBG + | 68Gallium-SSTR-PET/CT + |
|---|---|---|---|---|---|---|---|
| Lung | 79.4% (70.3–86.2) | 66.7% (48.8–80.8) | 60.9% (50.2–70.8) | 54.6% (38.0–70.2) | 71.4% (45.4–88.3) | 50% (9.5–90.6) | 77.8% (45.3–93.7) |
| n | 77/97 | 20/30 | 50/82 | 18/33 | 10/14 | 1/2 | 7/9 |
| Thymus, mediastinum | 85% (63.9–94.8) | 62.5% (30.6–86.3) | 85.7% (60.1–96.0) | 62.5% (30.6–86.3) | 33.3% (6.2–79.2) | nd | 50% (15.0–85.0) |
| n | 17/20 | 5/8 | 12/14 | 5/8 | 1/3 | 2/4 | |
| Pancreas | 85.7% (60.1–96.0) | 87.5% (52.9–97.8) | 66.7% (35.4–88) | 100% (61–100) | nd | Out of 1 case: 0 TP, 1 FN | 100% (34.2–100) |
| n | 12/14 | 7/8 | 6/9 | 6/6 | 2/2 | ||
| Adrenal gland | 100% (72–100) | 100% (57–100) | 60% (23.1–88.2) | 100% (44–100) | 100% (20.7–100) | 50% (15–85) | nd |
| n | 10/10 | 5/5 | 3/5 | 3/3 | 1/1 | 2/4 | |
| Gastrointestinal tract | 90% (59.6–98.2) | 71.4% (35.9–91.8) | 50% (21.5–78.5) | 57.1% (25.1–84.2) | 100% (20.7–100) | nd | 100% (34.2–100) |
| n | 9/10 | 5/7 | 4/8 | 4/7 | 1/1 | 2/2 | |
| Thyroid | 80% (37.6–96.4) | 100% (20.7–100) | 66.7% (20.8–93.9) | 100% (43.9–100) | nd | Out of 3 cases: 0 TP, 1 FP, 2 FN | 100% (34.2–100) |
| n | 4/5 | 1/1 | 2/3 | 3/3 | 2/2 | ||
| Carotid glomus, atrium, para-aortic region | 33.3% (6.2–79.2) | 33.3% (6.2–79.2) | 80% (37.6–96.4) | 100% (34.2–100) | nd | nd | nd |
| n | 1/3 | 1/3 | 4/5 | 2/2 | |||
| Head: ethmoidal-paranasal-sphenoid- sinus, olfactory bulb, skull base, etc | 57.1% (25.1–84.2) | 87.50% (52.9–97.8) | 80% (37.6–96.4) | 71.4% (35.9–91.8) | Out of 1 case: 0 TP, 1 FN | nd | 100% (43.9–100) |
| n | 4/7 | 7/8 | 4/5 | 5/7 | 3/3 | ||
| Abdomen/other (abdominal paraganglioma, ovary) | 60% (23.1–88.2) | 66.7% (20.8–93.9) | 20% (3.6–62.5) | 100% (20.7–100) | nd | 100% (34.2–100) | nd |
| n | 3/5 | 2/3 | 1/5 | 1/1 | 2/2 |
Abbreviations: TP, true positive; FP, false positive; FN, false negative; nd, not done; +, positive. Data are expressed as percentage (95% CI), unless stated otherwise.
Analysis of false positives by tumor site
In 101 lung tumors, four (4/101; 3.9%) false positives (two in the adrenal glands, one in the pancreas, and one in the liver) were found with CT, whereas MRI and FDG-PET did not show any false positives. In contrast, one patient (1/83; 1.2%) showed a false OCT uptake in the pancreas, one patient (1/15; 6.7%) showed a false F-DOPA-PET uptake in the left ventricle, and one (1/10; 10%) showed a false 68Gallium-SSTR-PET/CT uptake in the adrenal gland.
In thymus-mediastinum tumors, there was one false-positive MRI finding (1/9; 11.1%), with an apparent nodule in the left subclavicular region. In contrast, no false positives were reported for CT, OCT, FDG-PET, F-DOPA-PET, or 68Gallium-SSTR-PET/CT.
Among patients with proven pancreatic lesions, MRI suggested one (1/9; 11.1%) false pituitary tumor, whereas no false positives were described for CT, OCT, or FDG-PET.
No false-positive CT, MRI, OCT, FDG-PET, or MIBG findings were reported in the groups of adrenal gland tumors or gastrointestinal tumors.
Among thyroid lesions, there was one false positive in the lung with CT (1/6; 16.7%), OCT (1/4; 25%), and MIBG (1/3; 33.3%).
In the carotid glomus, atrium, and para-aortic region, CT showed two of five (40%) false positives with a false uptake described in the adrenal gland and thymus, whereas there were no false positives for MRI and OCT. In less frequent lesions, for head tumors (ethmoidal-paranasal- sphenoid sinus, olfactory bulb, skull base), there were no false positives with CT, MRI, OCT, or FDG-PET; for abdominal lesions (abdominal paraganglioma, ovary), there were no false positives with CT, MRI, or OCT.
Data on false-negative results at conventional radiology are described in the Supplemental Data, along with diagnostic accuracy of the combination of cross-sectional (CT/MRI) and nuclear medicine scans (Supplemental Table 2).
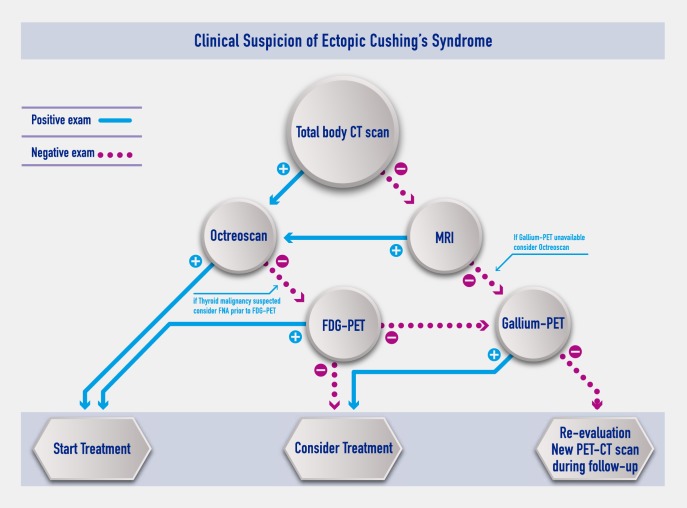
Integrated approach
A diagnostic flowchart has been developed on the basis of the findings collected on these 231 patients with ECS (Figure 1) and compared to available guidelines for non-ECS NETs (11–14, 122–125) (see Supplemental Data). Most tumors causing ECS were in the lungs, mediastinum, or neck. For this reason, a CT body scan was the most useful first examination. If CT was successful in identifying the lesion (137/215 in our series), OCT was found to be the best confirmatory and the most requested examination (lowest false-positive rate). When CT and OCT findings were concordant, most clinicians started treatment, normally opting for surgery (63.1%; 65/103 in our series). In contrast, where the OCT was negative (36.9%; 38/103), FDG-PET was the best performing third examination (36.4%). With FDG-PET confirming a previous investigation, treatment was started. With only one positive CT scan, clinicians were cautious in performing treatment (an option selected in 15.8% of cases; 34/215). An alternative could have been to perform a highly sensitive 68Gallium-SSTR-PET/CT scan. Where CT failed to reveal any lesion (78/215 in our series), MRI was successful in 35.3% of cases (6/17) when performed. The subsequent confirmatory examination was OCT (five cases, 40% positive), whereas with both negative MRI and CT scan (16.6%; 11/66), patients should have a 68Gallium-SSTR-PET/CT (positive, 2/2). Most guidelines on NETs do not indicate FDG-PET in their algorithm; however, ECS can be sustained by aggressive pancreatic tumors or SCLC frequently positive to FDG-PET. In addition, up to one-third of bronchial carcinoids were described with a pathology of atypical carcinoids. Interestingly, thyroid malignancies (seven cases) were frequently missed at CT or OCT. Therefore, if these lesions were suspected, fine-needle aspiration with measurement of calcitonin (or other neuroendocrine markers) provides confirmatory findings and should be followed by a FDG-PET scan. In addition, our analyses also revealed that FDG-PET was more sensitive than somatostatin analog tracers for detection of abdominal lesions. Overall, the sensitivity shown by 68Gallium-SSTR-PET/CT scans found in our collection is similar to that reported in most recent guidelines for functioning and nonfunctioning NETs. The herein reviewed collection of difficult ECS cases clearly shows that two concordant exams are desirable to correctly identify the tumor source. 68Gallium-SSTR-PET/CT appears promising but is much less available than OCT; FDG-PET use seems justified by the higher prevalence of aggressive and/or less differentiated tumors associated with ECS. For patients with unclear or discordant findings, a re-evaluation during follow-up with a 68Gallium-SSTR-PET/CT scan could be a reasonable add-on strategy.
Figure 1.
Clinical suspicion of ectopic CS.
Discussion
ECS has been well known for many decades, but it is still a diagnostic conundrum for the clinical endocrinologist. When not overtly detected, ECS can remain undiagnosed due to difficulties in localizing ectopic ACTH-secreting tumors, despite impressive advances in imaging techniques.
The introduction of molecular imaging has greatly expanded the tools for the diagnosis of neuroendocrine and endocrine-related tumors, including those causing ECS (11–14, 122–129). This systematic review gathers evidence that helps to clarify the efficacy of nuclear medicine imaging techniques most frequently used for the diagnosis of ECS sources, highlighting their advantages and limitations compared to conventional cross-sectional imaging. Because the findings of diagnostic studies are occasionally inflated by including patients with a clearly evident diagnosis, particular attention was paid to the separate analysis of data on patients with subtle or hidden disease on presentation. In fact, this series is the largest collection of patients with covert and occult ECS.
Clinical and hormonal characteristics
Patients were mainly diagnosed in the fourth decade of life, with no sex differences, but wide age distribution (130). Potassium levels do not seem to differ between the overt and covert ECS cases. In occult cases, ACTH levels are much lower than in the other ECS. Another point emerging from these cases was the association of a smaller diameter in covert ECS tumors with a greater mean ACTH and cortisol secretion compared to overt cases. These results suggest that covert cases need a longer workup to be identified, meaning that they will progress much more before a diagnosis can be made.
In keeping with previous literature (1, 2), up to one-fourth of patients had discordant dynamic hormonal test results, confirming the need for multiple testing (9). In overt tumors, HDDST was found to have a good sensitivity (Table 1), confirming that no additional test was necessary in the presence of unquestionable radiological findings. This could also explain the underrepresentation of SCLC in our series (see Limitations), because SCLCs are generally diagnosed without the need for further imaging. Overall, HDDST performed better than in other series (88–91%) (1, 2, 131, 132); this could have been inflated by the stringent inclusion criteria for coverts and occults. In contrast, IPSS was found to be the most sensitive in covert ECS cases (97%), superior to HDDST (93.9%) and CRH (81.3%). As expected, test performances in occult ECS were low. Peripheral whole-body catheter venous sampling was found to be less sensitive than CT, MRI, scintigraphy, or PET in the localization of ACTH-producing extrapituitary tumors (2, 6, 133, 134).
Covert and occult ECS
This systematic analysis shows that whereas approximately half of ECS sources are readily diagnosed, extensive investigations are needed to discover the hidden sources in up to 30% of cases (coverts). In these patients, nuclear imaging identified 79.1% of tumors not seen on conventional imaging. This is an encouraging result, given the severity of comorbidities associated with hypercortisolism (135, 136). However, despite intensive investigations, 18.6% of the tumor sources of ACTH remain occult, indicating the need for further improvement in available imaging techniques. It should be highlighted, however, that no tumor remained occult in patients who underwent 68Gallium-SSTR-PET/CT, suggesting its possible superiority over all other imaging techniques with which a significant number of tumors remained occult (Table 2). 68Gallium-SSTR-PET/CT showed the highest sensitivity in localizing covert ECS and always helped identify its source. However, only a minority of studies report its use (15% of coverts), indicating limited availability. The prevalence of occults in our collection was similar to that reviewed by Ejaz et al (7) in the largest series of consecutive patients: Ilias et al, 19% (1); Isidori et al, 14% (2); Aniszewski et al, 7% (5); Ejaz et al, 9% (7); and Zemskova et al, 27% (6).
False-negative imaging
In identifying the tumor site where the CT was falsely negative, MRI was helpful in 35% of cases. After false-negative CT or MRI scans, OCT detected the source in 66.7% of cases, and subsequent FDG-PET detected it in 72.2%, suggesting that these exams could be performed as second- and third-level investigations after a negative CT or MRI. Where CT and OCT were both falsely negative (six cases), FDG-PET found the ACTH source in two cases (lung/sacrococcygeal lesion), MRI in one case (pancreatic), F-DOPA-PET in one case (lung), FDG-PET and F-DOPA-PET in one case (lung), and 68Gallium-SSTR-PET/CT in one case (sphenoid sinus).
False-positive imaging
The various discrepancies between the different diagnostic techniques prompted us to focus on false-positive findings. In fact, the urgent need to control severe hypercortisolism exposes patients to the risk of multiple or inconclusive surgeries when the results are inconsistent (135). In this respect, OCT imaging had the lowest false-positive rate (1.1%)—only two cases, one of MTC (identified on neck ultrasound) that showed a false uptake in the lung, and one pancreatic lesion that was a pancreatic NET coexisting with a ACTH-secreting lung NET (identified by CT). False-positive uptakes were described in the spleen for FDG-PET. However, FDG-PET was particularly helpful in reducing the number of falsely positive lung CT findings. In contrast, MIBG was associated with the highest percentage of false positives (13.3%). In the much smaller group of patients who underwent 68Gallium-SSTR-PET/CT, there was one false positive in the adrenal gland (whereas the true source was a bronchial NET, negative on CT, and eventually identified by F-DOPA-PET). False-positive ventricle uptake was described for F-DOPA-PET. The issue of false positivity should always be taken into account for techniques that are increasingly able to detect small lesions. For this reason, a double-step approach is advocated, with sequential use of detection and confirmatory exams (Figure 1). Unique cases of the coexistence of two distinct NETs, one secreting and the other non-secreting (117), or different secretory activity by primary source vs metastases and, finally, 68Gallium-SSTR-PET/CT positivity related to degree of hypercortisolism (113), further complicate the scenario.
Integrated approach
None of the imaging techniques employed showed a 100% sensitivity in diagnosing lung lesions, the most frequent in both overt and covert cases (9). All techniques employed give both false-positive and false-negative results. Multiple imaging techniques are necessary for the correct diagnosis. Site-specific differences occur, the detection of thymus-mediastinum tumors being more difficult than for pancreatic lesions for which MRI and FDG-PET appear to be highly sensitive. An approach based on the data reviewed herein is presented in Figure 1. The usefulness of FDG-PET in NETs has been recently reviewed (128). The presence of uptake on FDG-PET may suggest that pancreatic tumors responsible for ECS have more aggressive features than other pancreatic NETs generally considered not to be sensitive to this technique. However, FDG-PET is very sensitive for the detection of NETs with a proliferation index of >15% (137, 138). It is unclear whether ACTH-secreting tumors behave more aggressively or show poor differentiation in comparison with other pancreatic NETs; interestingly, the current series found a relatively higher frequency of G2 and G3 compared to the other series (139). The need for multiple investigations, including conventional imaging, FDG-PET, and 68Gallium-SSTR-PET/CT is confirmed by a recent series of seven patients with aggressive NET causing ectopic mixed ACTH and CRH secretion (140). The role of nuclear imaging in neuroendocrine lung lesion has been recently meta-analyzed (141, 142). The conflicting results with a FDG-PET detection of bronchial carcinoid ranged from 14% to >90%, partially related to inappropriate cutoff values given the low standardized uptake value exhibited by most carcinoids. They also found a much higher detection rate and standardized uptake value in atypical carcinoids, which in our series were up to one-third of lung tumors.
Our study also shows that repeated imaging, in some cases over several months, enabled the tumor source to be identified and the condition to be resolved. However, drugs to control adrenal function are required in cases of severe hypercortisolism. Recent advances in old medications (143–145), the development of new drugs (43, 146, 147), and greater understanding of end-organ complications (135, 136) have made it more feasible to adopt a watchful waiting approach for these patients, who were previously invariably treated by bilateral adrenalectomy.
Limitations
This study presents advantages and limitations. First, the current case series does not reflect the epidemiology of ECS, because SCLCs were underrepresented. Most SCLCs do not undergo nuclear imaging for source localization; they are generally negative at octreoscan and rapidly growing, and access to third-level procedures such as 68Gallium-SSTR-PET/CT or F-DOPA-PET is limited. Nevertheless, the aim of the current study was not to describe the epidemiology of ECS, which only national and international tumor registries can provide, but rather to produce an updated analysis of all cases in which source localization was performed using a combination of conventional and nuclear imaging. Second, we systematically included all reports matching the predefined criteria, limiting reviewer selection biases; however, this approach led to the inclusion of a significant number of single/small series. The associated risk of bias was assessed by comparing small vs large published series as reported in Supplemental Table 1. No major differences in sensitivities were found. The prevalence of occult ECS and, conversely, the frequency of metastatic disease at presentation were compared to that reported for the largest ECS published series (1, 2, 4–7) and NETs in general (11–14, 122–125, 127–129). Overall, no major differences were found, suggesting that our collection was sufficiently representative of the scenario encountered in most referral centers. Third, positive results are more prone to be published, and this could have inflated the findings for newer techniques. In this respect, however, it should be noted that in most studies, three different imaging modalities were available for within-subject comparisons; some studies revealed positive findings with one tracer but also negative findings with another tracers. Because these negative data have also been counted, our approach should have minimized publication bias. Finally, occult ECS is a condition that is difficult to prove. Some patients with occult ECS may still have a subtle Cushing's disease (121). However, all measures to prevent inclusion of non-ECS were taken: we performed an analysis based on IPD with extensively documented clinical and hormonal testing, pathology data (for 100% of coverts), and negative pituitary surgical exploration for one-fourth of occults.
In conclusion, this extensive collection demonstrates the difficulty of diagnosing ECS when this is not immediately overt. However, nuclear medicine techniques may help greatly in identifying the source of ectopic ACTH production, with sensitivity varying according to the site. This systematic review highlights the potentials and pitfalls of these techniques, advocating a more reasoned use as opposed to their random selection based on local availability. A tentative scheme, based on the evidence gathered in this analysis, is reported, covering an area that was not addressed in previous guidelines. This is far from definitive and is limited by the retrospective nature, selection and publication biases, and uneven number of patients who underwent the most recent techniques. It should be emphasized that imaging alone, even when including molecular, anatomical, biological, and endoscopic imaging, is not sufficient for a correct diagnosis—a process that needs additional information, including clinical characteristics (128), to be integrated in a broad discussion within a multidisciplinary team.
Acknowledgments
* ABC (Altogether to Beat Cushing's syndrome) 2013 Group collaborators: N. Albiger, A. Ambrogio, G. Arnaldi, E. Arvat, R. Berardelli, M. Boscaro, M. Boschetti, S. Cannavò, F. Cavagnini, A. Colao, S. M. Corsello, A. Cozzolino, M. De Leo, C. Di Somma, K. Esposito, D. Ferone, C. Foresta, F. Gatto, C. Giordano, D. Giugliano, C. Graziadio, A. M. Isidori, P. Loli, L. Manetti, M. Mannelli, P. Marzullo, F. Mantero, F. Minuto, R. M. Paragliola, F. Pecori Giraldi, R. Pivonello, G. Reimondo, C. Scaroni, A. Scillitani, C. Simeoli, A. Stigliano, M. Terzolo, F. Tortora, L. Trementino, G. Vitale, and M. C. Zatelli.
Disclosure Summary: A.M.I. has been an occasional consultant for Otsuka, Viropharma, Besins, Menarini. M.C.Z. has been an occasional consultant for Novartis and Genzyme. E.S., M.B., and G.V. have nothing to disclose. A.C. has been principal investigator of research studies sponsored by Novartis, Ipsen, Pfizer and Lilly; has received research grants from Novartis, Ipsen, Pfizer, Ferring, Lilly, Merck-Serono, and Novo Nordisk; has been an occasional consultant for Novartis, Ipsen, and Pfizer; and has received fees and honoraria from Ipsen, Novartis, and Pfizer. R.P. has been Principal Investigator of research studies sponsored by Novartis and HRA Pharma; has received research grants from Novartis, Pfizer, Viropharma, Ipsen, and IBSA; has been an occasional consultant for Novartis, Ipsen, Pfizer, Viropharma, Ferring, and Italfarmaco; and has received fees and honoraria for presentations from Novartis and Shire.
Footnotes
- CI
- confidence interval
- CT
- computed tomography
- ECS
- ectopic Cushing's syndrome
- FDG
- 18F-fluoro-2-deoxyglucose
- F-DOPA
- 18F-fluorodopa
- HDDST
- high-dose dexamethasone suppression test
- IPD
- individual patient data
- IPSS
- inferior petrosal sinus sampling
- LDDST
- low-dose dexamethasone suppression test
- MIBG
- 131I/123I-metaiodobenzylguanidine
- MRI
- magnetic resonance imaging
- MTC
- medullary thyroid carcinoma
- NET
- neuroendocrine tumor
- OCT
- 111In-pentetreotide
- PET
- positron emission tomography
- SCLC
- small cell lung carcinoma.
Contributor Information
Collaborators: * ABC (Altogether to Beat Cushing's syndrome) 2013 Group collaborators, N. Albiger, A. Ambrogio, G. Arnaldi, E. Arvat, R. Berardelli, M. Boscaro, M. Boschetti, S. Cannavò, F. Cavagnini, A. Colao, S. M. Corsello, A. Cozzolino, M. De Leo, C. Di Somma, K. Esposito, D. Ferone, C. Foresta, F. Gatto, C. Giordano, D. Giugliano, C. Graziadio, A. M. Isidori, P. Loli, L. Manetti, M. Mannelli, P. Marzullo, F. Mantero, F. Minuto, R. M. Paragliola, F. Pecori Giraldi, R. Pivonello, G. Reimondo, C. Scaroni, A. Scillitani, C. Simeoli, A. Stigliano, M. Terzolo, F. Tortora, L. Trementino, G. Vitale, and M. C. Zatelli
References
- 1. Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing's syndrome due to ectopic corticotropin secretion: twenty years' experience at the National Institutes of Health. J Clin Endocrinol Metab. 2005;90:4955–4962. [DOI] [PubMed] [Google Scholar]
- 2. Isidori AM, Kaltsas GA, Pozza C, et al. The ectopic adrenocorticotropin syndrome: clinical features, diagnosis, management, and long-term follow-up. J Clin Endocrinol Metab. 2006;91:371–377. [DOI] [PubMed] [Google Scholar]
- 3. Wajchenberg BL, Mendonca BB, Liberman B, et al. Ectopic adrenocorticotropic hormone syndrome. Endocr Rev. 1994;15:752–787. [DOI] [PubMed] [Google Scholar]
- 4. de Herder WW, Lamberts SW. Tumor localization–the ectopic ACTH syndrome. J Clin Endocrinol Metab. 1999;84:1184–1185. [DOI] [PubMed] [Google Scholar]
- 5. Aniszewski JP, Young WF, Jr, Thompson GB, Grant CS, van Heerden JA. Cushing syndrome due to ectopic adrenocorticotropic hormone secretion. World J Surg. 2001;25:934–940. [DOI] [PubMed] [Google Scholar]
- 6. Zemskova MS, Gundabolu B, Sinaii N, et al. Utility of various functional and anatomic imaging modalities for detection of ectopic adrenocorticotropin-secreting tumors. J Clin Endocrinol Metab. 2010;95:1207–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ejaz S, Vassilopoulou-Sellin R, Busaidy NL, et al. Cushing syndrome secondary to ectopic adrenocorticotropic hormone secretion: the University of Texas MD Anderson Cancer Center Experience. Cancer. 2011;117:4381–4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boscaro M, Arnaldi G. Approach to the patient with possible Cushing's syndrome. J Clin Endocrinol Metab. 2009;94:3121–3131. [DOI] [PubMed] [Google Scholar]
- 9. Isidori AM, Lenzi A. Ectopic ACTH syndrome. Arq Bras Endocrinol Metabol. 2007;51:1217–1225. [DOI] [PubMed] [Google Scholar]
- 10. Fendrich V, Waldmann J, Bartsch DK, Langer P. Surgical management of pancreatic endocrine tumors. Nat Rev Clin Oncol. 2009;6:419–428. [DOI] [PubMed] [Google Scholar]
- 11. Vinik AI, Woltering EA, Warner RR, et al. NANETS consensus guidelines for the diagnosis of neuroendocrine tumor. Pancreas. 2010;39:713–734. [DOI] [PubMed] [Google Scholar]
- 12. Singh S, Dey C, Kennecke H, et al. Consensus recommendations for the diagnosis and management of pancreatic neuroendocrine tumors: guidelines from a Canadian National Expert Group [published online November 4, 2014]. Ann Surg Oncol. doi: 10.1245/s10434-014–4145-0. [DOI] [PubMed] [Google Scholar]
- 13. Jensen RT, Cadiot G, Brandi ML, et al. ENETS consensus guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Neuroendocrinology. 2012;95:98–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Falconi M, Bartsch DK, Eriksson B, et al. ENETS consensus guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: well-differentiated pancreatic non-functioning tumors. Neuroendocrinology. 2012;95:120–134. [DOI] [PubMed] [Google Scholar]
- 15. Phan AT, Oberg K, Choi J, et al. NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the thorax (includes lung and thymus). Pancreas. 2010;39:784–798. [DOI] [PubMed] [Google Scholar]
- 16. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing's syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:1526–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340:c221. [DOI] [PubMed] [Google Scholar]
- 18. Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17:873–890. [DOI] [PubMed] [Google Scholar]
- 19. Nikolaou A, Thomas D, Kampanellou C, et al. The value of 11C-5-hydroxy-tryptophan positron emission tomography in neuroendocrine tumor diagnosis and management: experience from one center. J Endocrinol Invest. 2010;33:794–799. [DOI] [PubMed] [Google Scholar]
- 20. Zhang DM, Xue HD, Duan L, Li J, Li NS, Jin ZY. A small solitary pulmonary nodule discovered by (18)F-fluorodeoxyglucose positron emission tomography and CT: rare infection instead of rare tumor. Chin Med Sci J. 2013;27:249–252. [DOI] [PubMed] [Google Scholar]
- 21. Zangeneh F, Young WF, Jr, Lloyd RV, Chiang M, Kurczynski E, Zangeneh F. Cushing's syndrome due to ectopic production of corticotropin-releasing hormone in an infant with ganglioneuroblastoma. Endocr Pract. 2003;9:394–399. [DOI] [PubMed] [Google Scholar]
- 22. Yu J, Koch CA, Patsalides A, et al. Ectopic Cushing's syndrome caused by an esthesioneuroblastoma. Endocr Pract. 2004;10:119–124. [DOI] [PubMed] [Google Scholar]
- 23. Xu H, Zhang M, Zhai G, Zhang M, Ning G, Li B. The role of integrated (18)F-FDG PET/CT in identification of ectopic ACTH secretion tumors. Endocrine. 2009;36:385–391. [DOI] [PubMed] [Google Scholar]
- 24. Willhauck MJ, Pöpperl G, Rachinger W, Giese A, Auernhammer CJ, Spitzweg C. An unusual case of ectopic ACTH syndrome. Exp Clin Endocrinol Diabetes. 2012;120:63–67. [DOI] [PubMed] [Google Scholar]
- 25. Wahlberg J, Ekman B. Atypical or typical adrenocorticotropic hormone-producing pulmonary carcinoids and the usefulness of 11C-5-hydroxytryptophan positron emission tomography: two case reports. J Med Case Rep. 2013;7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Venkitaraman B, Karunanithi S, Kumar A, Bal C, Ammini AC, Kumar R. 68Ga-DOTATOC PET-CT in the localization of source of ectopic ACTH in patients with ectopic ACTH-dependent Cushing's syndrome. Clin Imaging. 2014;38:208–211. [DOI] [PubMed] [Google Scholar]
- 27. Veit JA, Boehm B, Luster M, et al. Detection of paranasal ectopic adrenocorticotropic hormone-secreting pituitary adenoma by Ga-68-DOTANOC positron-emission tomography-computed tomography. Laryngoscope. 2013;123:1132–1135. [DOI] [PubMed] [Google Scholar]
- 28. Tsagarakis S, Christoforaki M, Giannopoulou H, et al. A reappraisal of the utility of somatostatin receptor scintigraphy in patients with ectopic adrenocorticotropin Cushing's syndrome. J Clin Endocrinol Metab. 2003;88:4754–4758. [DOI] [PubMed] [Google Scholar]
- 29. Treglia G, Salomone E, Petrone G, Giaccari A, Rindi G, Rufini V. A rare case of ectopic adrenocorticotropic hormone syndrome caused by a metastatic neuroendocrine tumor of the pancreas detected by 68Ga-DOTANOC and 18F-FDG PET/CT. Clin Nucl Med. 2013;38:e306–e308. [DOI] [PubMed] [Google Scholar]
- 30. Tani Y, Sugiyama T, Hirooka S, Izumiyama H, Hirata Y. Ectopic ACTH syndrome caused by bronchial carcinoid tumor indistinguishable from Cushing's disease. Endocr J. 2010;57:679–686. [DOI] [PubMed] [Google Scholar]
- 31. Sugiyama M, Sugiyama T, Yamaguchi M, et al. Successful localization of ectopic ACTH-secreting bronchial carcinoid by selective pulmonary arterial sampling. Endocr J. 2010;57:959–964. [DOI] [PubMed] [Google Scholar]
- 32. Mondello S, Fodale V, Cannavò S, et al. Hypophosphatemia as unusual cause of ARDS in Cushing's syndrome secondary to ectopic CRH production. A case report. ScientificWorldJournal. 2008;8:138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sofka S, Jackson T. Bronchopulmonary carcinoid presenting as dexamethasone suppressible Cushing's syndrome. W V Med J. 2013;109:26–28. [PubMed] [Google Scholar]
- 34. Singer J, Werner F, Koch CA, et al. Ectopic Cushing's syndrome caused by a well differentiated ACTH-secreting neuroendocrine carcinoma of the ileum. Exp Clin Endocrinol Diabetes. 2010;118:524–529. [DOI] [PubMed] [Google Scholar]
- 35. Silye R, Rieger R, Topakian R, Dunzinger A, Aigner RM, Pichler R. Cushing syndrome due to ectopic adrenocorticotropin secretion by oncocytic thyroid nodule. J Clin Endocrinol Metab. 2012;97:39–40. [DOI] [PubMed] [Google Scholar]
- 36. Sharma ST, Raff H, Nieman LK. Prolactin as a marker of successful catheterization during IPSS in patients with ACTH-dependent Cushing's syndrome. J Clin Endocrinol Metab. 2011;96:3687–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shahani S, Nudelman RJ, Nalini R, Kim HS, Samson SL. Ectopic corticotropin-releasing hormone (CRH) syndrome from metastatic small cell carcinoma: a case report and review of the literature. Diagn Pathol. 2010;5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schalin-Jäntti C, Asa SL, Arola J, Sane T. Recurrent acute-onset Cushing's syndrome 6 years after removal of a thymic neuroendocrine carcinoma: from ectopic ACTH to CRH. Endocr Pathol. 2013;24:25–29. [DOI] [PubMed] [Google Scholar]
- 39. Salgado LR, Fragoso MC, Knoepfelmacher M, et al. Ectopic ACTH syndrome: our experience with 25 cases. Eur J Endocrinol. 2006;155:725–733. [DOI] [PubMed] [Google Scholar]
- 40. Ruggeri RM, Ferraù F, Campennì A, et al. Immunohistochemical localization and functional characterization of somatostatin receptor subtypes in a corticotropin releasing hormone- secreting adrenal phaeochromocytoma: review of the literature and report of a case. Eur J Histochem. 2009;53:1–6. [PubMed] [Google Scholar]
- 41. Rodrigues P, Castedo JL, Damasceno M, Carvalho D. Ectopic Cushing's syndrome caused by a pulmonary ACTH-secreting tumor in a patient treated with octreotide. Arq Bras Endocrinol Metabol. 2012;56:461–464. [DOI] [PubMed] [Google Scholar]
- 42. Rod A, Voicu M, Chiche L, et al. Cushing's syndrome associated with a nested stromal epithelial tumor of the liver: hormonal, immunohistochemical, and molecular studies. Eur J Endocrinol. 2009;161:805–810. [DOI] [PubMed] [Google Scholar]
- 43. Pivonello R, Ferone D, Lamberts SW, Colao A. Cabergoline plus lanreotide for ectopic Cushing's syndrome. N Engl J Med. 2005;352:2457–2458. [DOI] [PubMed] [Google Scholar]
- 44. Perakakis N, Laubner K, Keck T, et al. Ectopic ACTH-syndrome due to a neuroendocrine tumour of the appendix. Exp Clin Endocrinol Diabetes. 2011;119:525–529. [DOI] [PubMed] [Google Scholar]
- 45. Parenti G, Nassi R, Silvestri S, et al. Multi-step approach in a complex case of Cushing's syndrome and medullary thyroid carcinoma. J Endocrinol Invest. 2006;29:177–181. [DOI] [PubMed] [Google Scholar]
- 46. Bhatia PD, Fung K, Edmonds M, Driedger AA, Malthaner RA. A case of bronchopulmonary carcinoid tumor: the role of octreotide scanning in localization of an ectopic source of ACTH. J Hosp Med. 2006;1:312–316. [DOI] [PubMed] [Google Scholar]
- 47. Özkan ZG, Kuyumcu S, Balköse D, et al. The value of somatostatin receptor imaging with In-111 octreotide and/or Ga-68 DOTATATE in localizing ectopic ACTH producing tumors. Mol Imaging Radionucl Ther. 2013;22:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Otsuka F, Miyoshi T, Murakami K, et al. An extra-adrenal abdominal pheochromocytoma causing ectopic ACTH syndrome. Am J Hypertens. 2005;18:1364–1368. [DOI] [PubMed] [Google Scholar]
- 49. Oosterhuis JK, van den Berg G, Monteban-Kooistra WE, et al. Life-threatening Pneumocystis jiroveci pneumonia following treatment of severe Cushing's syndrome. Neth J Med. 2007;65:215–217. [PubMed] [Google Scholar]
- 50. Netea-Maier RT, Nieuwlaat WA, Sweep CG, Wesseling P, Massuger L, Hermus AR. Virilization due to ovarian androgen hypersecretion in a patient with ectopic adrenocorticotrophic hormone secretion caused by a carcinoid tumour: case report. Hum Reprod. 2006;21:2601–2605. [DOI] [PubMed] [Google Scholar]
- 51. Al Brahim NY, Rambaldini G, Ezzat S, Asa SL. Complex endocrinopathies in MEN-1: diagnostic dilemmas in endocrine oncology. Endocr Pathol. 2007;18:37–41. [DOI] [PubMed] [Google Scholar]
- 52. Moreno-Fernández J, Gutiérrez-Alcántara C, Gálvez Moreno MA, Jiménez-Reina L, Castaño JP, Benito-López P. Corticotrophin-dependent Cushing syndrome due to sacrococcygeal teratoma detected by [18F]fluorodeoxyglucose positron emission tomography. J Clin Endocrinol Metab. 2008;93:3282–3283. [DOI] [PubMed] [Google Scholar]
- 53. More J, Young J, Reznik Y, et al. Ectopic ACTH syndrome in children and adolescents. J Clin Endocrinol Metab. 2011;96:1213–1222. [DOI] [PubMed] [Google Scholar]
- 54. Molina Garrido MJ, Guillén Ponce C, Maciá Escalante S, Pons Sanz V, Carrato Mena A. Cushing's paraneoplastic syndrome as first manifestation of an adenocarcinoma of unknown origin. Clin Transl Oncol. 2006;8:621–623. [DOI] [PubMed] [Google Scholar]
- 55. Miehle K, Tannapfel A, Lamesch P, et al. Pancreatic neuroendocrine tumor with ectopic adrenocorticotropin production upon second recurrence. J Clin Endocrinol Metab. 2004;89:3731–3736. [DOI] [PubMed] [Google Scholar]
- 56. Meinardi JR, van den Berg G, Wolffenbuttel BH, Kema IP, Dullaart RP. Cyclical Cushing's syndrome due to an atypical thymic carcinoid. Neth J Med. 2006;64:23–27. [PubMed] [Google Scholar]
- 57. McDermott JH, Thabit H, Hickey N, et al. ACTH-secreting bronchial carcinoid: a diagnostic and therapeutic challenge. Ir J Med Sci. 2008;177:269–272. [DOI] [PubMed] [Google Scholar]
- 58. Matarazzo P, Tuli G, Tessaris D, et al. Cushing syndrome due to ectopic adrenocorticotropic hormone secretion in a 3-year-old child. J Pediatr Endocrinol Metab. 2011;24:219–222. [DOI] [PubMed] [Google Scholar]
- 59. Markou A, Manning P, Kaya B, Datta SN, Bomanji JB, Conway GS. [18F]fluoro-2-deoxy-D-glucose ([18F]FDG) positron emission tomography imaging of thymic carcinoid tumor presenting with recurrent Cushing's syndrome. Eur J Endocrinol. 2005;152:521–525. [DOI] [PubMed] [Google Scholar]
- 60. Machado MC, Sá SV, Goldbaum TS, et al. In vivo response to growth hormone-releasing peptide-6 in adrenocorticotropin-dependent Cushing's syndrome by lung carcinoid tumor is associated with growth hormone secretagogue receptor type 1a mRNA expression. J Endocrinol Invest. 2007;30:334–340. [DOI] [PubMed] [Google Scholar]
- 61. Lutgers HL, Vergragt J, Dong PV, et al. Severe hypercortisolism: a medical emergency requiring urgent intervention. Crit Care Med. 2010;38:1598–1601. [DOI] [PubMed] [Google Scholar]
- 62. de Matos LL, Trufelli DC, das Neves-Pereira JC, Danel C, Riquet M. Cushing's syndrome secondary to bronchopulmonary carcinoid tumor: report of two cases and literature review. Lung Cancer. 2006;53:381–386. [DOI] [PubMed] [Google Scholar]
- 63. Luca PD, Oren A, Somers GR, Urbach SL. The search for ectopic ACTH production in a 9-year-old boy. J Pediatr Endocrinol Metab. 2013;26:781–783. [DOI] [PubMed] [Google Scholar]
- 64. Lee T, Karl M, Solorzano CC. Adrenocorticotropic hormone-secreting pancreatic islet cell carcinoma. J Am Coll Surg. 2004;199:336–337. [DOI] [PubMed] [Google Scholar]
- 65. La Rosa S, Marando A, Ghezzi F, Colombo P, Finzi G, Capella C. Cushing's syndrome due to a pancreatic neuroendocrine tumor metastatic to the ovaries: a clinicopathological description of a case. Endocr Pathol. 2011;22:118–124. [DOI] [PubMed] [Google Scholar]
- 66. Kumar J, Spring M, Carroll PV, Barrington SF, Powrie JK. 18Flurodeoxyglucose positron emission tomography in the localization of ectopic ACTH-secreting neuroendocrine tumours. Clin Endocrinol Oxf. 2006;64:371–374. [DOI] [PubMed] [Google Scholar]
- 67. Kondo T, Matsuyama R, Ashihara H, et al. A case of ectopic adrenocorticotropic hormone-producing pancreatic neuroendocrine tumor with multiple liver metastases. Endocr J. 2010;57:229–236. [DOI] [PubMed] [Google Scholar]
- 68. Kenchaiah M, Hyer S. Cushing's syndrome due to ectopic ACTH from bronchial carcinoid: a case report and review. Case Rep Endocrinol. 2012;2012:215038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kishida K, Moriwaki M, Miyagawa J, et al. Successful use of 111In-pentetrotide scintigraphy for localizing ectopic adrenocorticotropin-producing bronchial carcinoid tumor in a patient with Cushing's syndrome. Intern Med. 2003;42:996–1005. [DOI] [PubMed] [Google Scholar]
- 70. Pacak K, Ilias I, Chen CC, Carrasquillo JA, Whatley M, Nieman LK. The role of [(18)F]fluorodeoxyglucose positron emission tomography and [(111)In]-diethylenetriaminepentaacetate-D-Phe-pentetreotide scintigraphy in the localization of ectopic adrenocorticotropin-secreting tumors causing Cushing's syndrome. J Clin Endocrinol Metab. 2004;89:2214–2221. [DOI] [PubMed] [Google Scholar]
- 71. Iwata M, Oki Y, Okazawa T, et al. A rare case of adrenocorticotropic hormone (ACTH)-independent macroadrenal hyperplasia showing ectopic production of ACTH. Intern Med. 2012;51:2181–2187. [DOI] [PubMed] [Google Scholar]
- 72. Oh HC, Koh JM, Kim MS, et al. A case of ACTH-producing pheochromocytoma associated with pregnancy. Endocr J. 2003;50:739–744. [DOI] [PubMed] [Google Scholar]
- 73. Willenberg HS, Feldkamp J, Lehmann R, Schott M, Goretzki PE, Scherbaum WA. A case of catecholamine and glucocorticoid excess syndrome due to a corticotropin-secreting paraganglioma. Ann NY Acad Sci. 2006;1073:52–58. [DOI] [PubMed] [Google Scholar]
- 74. Hodish I, Giordano TJ, Starkman MN, Schteingart DE. Location of ectopic adrenocortical hormone-secreting tumors causing Cushing's syndrome in the paranasal sinuses. Head Neck. 2009;31:699–706. [DOI] [PubMed] [Google Scholar]
- 75. Hernández I, Espinosa-de-los-Monteros AL, Mendoza V, et al. Ectopic ACTH-secreting syndrome: a single center experience report with a high prevalence of occult tumor. Arch Med Res. 2006;37:976–980. [DOI] [PubMed] [Google Scholar]
- 76. Hashemzadeh S, Asvadi Kermani A, Ali-Asgharzadeh A, Halimi M, Soleimani M, Ladan A. Ectopic Cushing's syndrome secondary to pulmonary carcinoid tumor. Ann Thorac Surg. 2013;95:1797–1799. [DOI] [PubMed] [Google Scholar]
- 77. Han JY, Mirsadraei L, Yeh MW, et al. Bilateral adrenalectomy: lifesaving procedure in severe Cushing syndrome. Endocr Pract. 2012;18:e85–e90. [DOI] [PubMed] [Google Scholar]
- 78. Illyés G, Luczay A, Benyó G, et al. Cushing's syndrome in a child with pancreatic acinar cell carcinoma. Endocr Pathol. 2007;18:95–102. [DOI] [PubMed] [Google Scholar]
- 79. Grossrubatscher E, Vignati F, Dalino P, et al. Use of radioguided surgery with [111In]-pentetreotide in the management of an ACTH-secreting bronchial carcinoid causing ectopic Cushing's syndrome. J Endocrinol Invest. 2005;28:72–78. [DOI] [PubMed] [Google Scholar]
- 80. Grossman AB, Kelly P, Rockall A, Bhattacharya S, McNicol A, Barwick T. Cushing's syndrome caused by an occult source: difficulties in diagnosis and management. Nat Clin Pract Endocrinol Metab. 2006;2:642–647. [DOI] [PubMed] [Google Scholar]
- 81. Gani LU, Gianatti EJ, Cheung AS, Jerums G, Macisaac RJ. Failure of functional imaging with gallium-68-DOTA-D-Phe1-Tyr3-octreotide positron emission tomography to localize the site of ectopic adrenocorticotropic hormone secretion: a case report. J Med Case Rep. 2011;5:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gabalec F, Zavrelová A, Havel E, et al. Pneumocystis pneumonia during medicamentous treatment of Cushing's syndrome–a description of two cases. Acta Medica (Hradec Kralove). 2011;54:127–130. [DOI] [PubMed] [Google Scholar]
- 83. Uwaifo GI, Koch CA, Hirshberg B, et al. Is there a therapeutic role for octreotide in patients with ectopic Cushing's syndrome? J Endocrinol Invest. 2003;26:710–717. [DOI] [PubMed] [Google Scholar]
- 84. Arnaldi G, Mancini T, Kola B, et al. Cyclical Cushing's syndrome in a patient with a bronchial neuroendocrine tumor (typical carcinoid) expressing ghrelin and growth hormone secretagogue receptors. J Clin Endocrinol Metab. 2003;88:5834–5840. [DOI] [PubMed] [Google Scholar]
- 85. Corcuff JB, Deminiere C, Trouillas J, Puel O, Perel Y, Barat P. Ectopic Cushing's syndrome due to an adrenal ganglioneuroma. Horm Res Paediatr. 2010;73:405–408. [DOI] [PubMed] [Google Scholar]
- 86. Fasshauer M, Lincke T, Witzigmann H, et al. Ectopic Cushing' syndrome caused by a neuroendocrine carcinoma of the mesentery. BMC Cancer. 2006;6:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Farage M, Costa MA, Godoy-Matos AF. A rare case of Cushing syndrome by cyclic ectopic-ACTH. Arq Bras Endocrinol Metabol. 2012;56:324–330. [DOI] [PubMed] [Google Scholar]
- 88. Esfahani AF, Chavoshi M, Noorani MH, et al. Successful application of technetium-99m-labeled octreotide acetate scintigraphy in the detection of ectopic adrenocorticotropin-producing bronchial carcinoid lung tumor: a case report. J Med Case Rep. 2010;4:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. El Zein M, Vali R, Charron M, Manson D, Perlman K, Shammas A. Neuroendocrine tumor in liver with positive ACTH receptor: a case report. J Pediatr Hematol Oncol. 2014;36:e1–e4. [DOI] [PubMed] [Google Scholar]
- 90. Dutta R, Kumar A, Julka PK, et al. Thymic neuroendocrine tumour (carcinoid): clinicopathological features of four patients with different presentation. Interact Cardiovasc Thorac Surg. 2010;11:732–736. [DOI] [PubMed] [Google Scholar]
- 91. Doi M, Sugiyama T, Izumiyama H, Yoshimoto T, Hirata Y. Clinical features and management of ectopic ACTH syndrome at a single institute in Japan. Endocr J. 2010;57:1061–1069. [DOI] [PubMed] [Google Scholar]
- 92. de Bruin C, Hofland LJ, Nieman LK, et al. Mifepristone effects on tumor somatostatin receptor expression in two patients with Cushing's syndrome due to ectopic adrenocorticotropin secretion. J Clin Endocrinol Metab. 2012;97:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Därr R, Zöphel K, Eisenhofer G, et al. Combined use of 68Ga-DOTATATE and 18F-FDG PET/CT to localize a bronchial carcinoid associated with ectopic ACTH syndrome. J Clin Endocrinol Metab. 2012;97:2207–2208. [DOI] [PubMed] [Google Scholar]
- 94. Granberg D, Sundin A, Janson ET, Oberg K, Skogseid B, Westlin JE. Octreoscan in patients with bronchial carcinoid tumours. Clin Endocrinol (Oxf). 2003;59:793–799. [DOI] [PubMed] [Google Scholar]
- 95. Dahir KM, Gonzalez A, Revelo MP, Ahmed SR, Roberts JR, Blevins LS., Jr Ectopic adrenocorticotropic hormone hypersecretion due to a primary pulmonary paraganglioma. Endocr Pract. 2004;10:424–428. [DOI] [PubMed] [Google Scholar]
- 96. Danilovic DL, Brandão Neto RA, D'Abronzo H, Menezes MR, Lucon AM, Mendonca BB. Ectopic ACTH syndrome caused by pheochromocytoma: computed tomography-guided percutaneous ethanol injection as an alternative treatment. J Endocrinol Invest. 2007;30:780–786. [DOI] [PubMed] [Google Scholar]
- 97. Corsello SM, Fintini D, Lovicu RM, et al. Ectopic ACTH syndrome due to occult bronchial carcinoid. Clin Nucl Med. 2009;34:459–461. [DOI] [PubMed] [Google Scholar]
- 98. Cohade C, Broussaud S, Louiset E, Bennet A, Huyghe E, Caron P. Ectopic Cushing's syndrome due to a pheochromocytoma: a new case in the post-partum and review of literature. Gynecol Endocrinol. 2009;25:624–627. [DOI] [PubMed] [Google Scholar]
- 99. Coe SG, Tan WW, Fox TP. Cushing's syndrome due to ectopic adrenocorticotropic hormone production secondary to hepatic carcinoid: diagnosis, treatment, and improved quality of life. J Gen Intern Med. 2008;23:875–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chrisoulidou A, Pazaitou-Panayiotou K, Georgiou E, et al. Ectopic Cushing's syndrome due to CRH secreting liver metastasis in a patient with medullary thyroid carcinoma. Hormones (Athens). 2008;7:259–262. [DOI] [PubMed] [Google Scholar]
- 101. Carrillo-Muñoz A, Onofre-Borja M, Borrego-Borrego R, Chávez-Mercado L, Navarro-Reynoso FP, Ibarra-Pérez C. Atypical intermediate-grade mediastinal carcinoid. Case presentation. Cir Cir. 2011;79:191–195. [PubMed] [Google Scholar]
- 102. Candrina R, Sleiman I, Zorzi F. ACTH-secreting pituitary adenoma within an ovarian teratoma. Eur J Intern Med. 2005;16:359–360. [DOI] [PubMed] [Google Scholar]
- 103. Burman P, Lethagen A, Ivancev K, Johansson L, Sundin A. Dual bronchial carcinoids and Cushing's syndrome with a paradoxical response to dexamethasone and a false positive outcome of inferior petrosal sinus sampling. Eur J Endocrinol. 2008;159:483–488. [DOI] [PubMed] [Google Scholar]
- 104. Borrero CG, McCook B, Mountz JM. Indium-111 pentetreotide imaging of carcinoid tumor of the thymus. Clin Nucl Med. 2005;30:218–221. [DOI] [PubMed] [Google Scholar]
- 105. Bodelier AG, Groeneveld W, van der Linden AN, Haak HR. Symptomatic epidural lipomatosis in ectopic Cushing's syndrome. Eur J Endocrinol. 2004;151:765–769. [DOI] [PubMed] [Google Scholar]
- 106. Koo BK, An JH, Jeon KH, et al. Two cases of ectopic adrenocorticotropic hormone syndrome with olfactory neuroblastoma and literature review. Endocr J. 2008;55:469–475. [DOI] [PubMed] [Google Scholar]
- 107. Biering H, Pirlich M, Bauditz J, Sandrock D, Lochs H, Gerl H. PET scan in occult ectopic ACTH syndrome: a useful tool? Clin Endocrinol (Oxf). 2003;59:404–405. [DOI] [PubMed] [Google Scholar]
- 108. Bernardi S, Grimaldi F, Finato N, et al. A pheochromocytoma with high adrenocorticotropic hormone and a silent lung nodule. Am J Med Sci. 2011;342:429–432. [DOI] [PubMed] [Google Scholar]
- 109. Moraes AB, Taboada GF, Carneiro MP, et al. Utility of [(18)F] fluoro-2-deoxy-D: -glucose positron emission tomography in the localization of ectopic ACTH-secreting tumors. Pituitary. 2009;12:380–383. [DOI] [PubMed] [Google Scholar]
- 110. Bansal M, Agarwal A, Govindarajan R. Ectopic adrenocorticotropic hormone syndrome due to a pancreatic neuroendocrine tumor [published online June 26, 2012]. J Gastrointest Cancer. doi: 10.1007/s12029-012-9400-7. [DOI] [PubMed] [Google Scholar]
- 111. Ballav C, Naziat A, Mihai R, Karavitaki N, Ansorge O, Grossman AB. Mini-review: pheochromocytomas causing the ectopic ACTH syndrome. Endocrine. 2012;42:69–73. [DOI] [PubMed] [Google Scholar]
- 112. Asha HS, Sudeep K, Alexander M, Korula A, Gnanamuthu BR, Thomas N. Cushing's syndrome in a case of thymic carcinoma. Indian J Endocrinol Metab. 2011;15:346–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Davi' MV, Salgarello M, Francia G. Positive (68)Ga-DOTATOC-PET/CT after cortisol level control during ketoconazole treatment in a patient with liver metastases from a pancreatic neuroendocrine tumor and ectopic Cushing syndrome. Endocrine. 2015;49(2):566–567. [DOI] [PubMed] [Google Scholar]
- 114. Huang YT, Aziz SI, Ravi Kumar AS. Gallium-68 DOTA-TATE positron emission tomography/computed tomography: scintigraphic changes of adrenal glands following management of ectopic Cushing's syndrome by steroidogenesis inhibitors. World J Nucl Med. 2014;13:201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ghazi AA, Abbasi Dezfooli A, Amirbaigloo A, et al. Ectopic Cushing's syndrome secondary to lung and mediastinal tumours – report from a tertiary care centre in Iran. Endokrynol Pol. 2015;66:2–9. [DOI] [PubMed] [Google Scholar]
- 116. Dubois S, Morel O, Rodien P, et al. A pulmonary adrenocorticotropin-secreting carcinoid tumor localized by 6-fluoro-[18F]L-dihydroxyphenylalanine positron emission/computed tomography imaging in a patient with Cushing's syndrome. J Clin Endocrinol Metab. 2007;92:4512–4513. [DOI] [PubMed] [Google Scholar]
- 117. Salvatori R, Fintini D, Westra WH, Cho SY, Schulick RD. Cushing's syndrome attributable to ectopic secretion of corticotropin in a patient with two neuroendocrine tumors. Endocr Pract. 2006;12:656–659. [DOI] [PubMed] [Google Scholar]
- 118. Thomas T, Zender S, Terkamp C, Jaeckel E, Manns MP. Hypercortisolaemia due to ectopic adrenocorticotropic hormone secretion by a nasal paraganglioma: a case report and review of the literature. BMC Res Notes. 2013;6:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kakade HR, Kasaliwal R, Jagtap VS, et al. Ectopic ACTH-secreting syndrome: a single-center experience. Endocr Pract. 2013;19:1007–1014. [DOI] [PubMed] [Google Scholar]
- 120. Rindi G, Petrone G, Inzani F. The 2010 WHO classification of digestive neuroendocrine neoplasms: a critical appraisal four years after its introduction. Endocr Pathol. 2014;25:186–192. [DOI] [PubMed] [Google Scholar]
- 121. Swearingen B, Katznelson L, Miller K, et al. Diagnostic errors after inferior petrosal sinus sampling. J Clin Endocrinol Metab. 2004;89:3752–3763. [DOI] [PubMed] [Google Scholar]
- 122. Naswa N, Sharma P, Kumar A, et al. 68Ga-DOTANOC PET/CT in patients with carcinoma of unknown primary of neuroendocrine origin. Clin Nucl Med. 2012;37:245–251. [DOI] [PubMed] [Google Scholar]
- 123. Koukouraki S, Strauss LG, Georgoulias V, Eisenhut M, Haberkorn U, Dimitrakopoulou-Strauss A. Comparison of the pharmacokinetics of 68Ga-DOTATOC and [18F]FDG in patients with metastatic neuroendocrine tumours scheduled for 90Y-DOTATOC therapy. Eur J Nucl Med Mol Imaging. 2006;33:1115–1122. [DOI] [PubMed] [Google Scholar]
- 124. Caplin ME, Baudin E, Ferolla P, et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids [published online February 2, 2015]. Ann Oncol. doi: 10.1093/annonc/mdv041. [DOI] [PubMed] [Google Scholar]
- 125. Ramage JK, Ahmed A, Ardill J, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut. 2012;61:6–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. de Herder WW. GEP-NETS update: functional localisation and scintigraphy in neuroendocrine tumours of the gastrointestinal tract and pancreas (GEP-NETs). Eur J Endocrinol. 2014;170:R173–R183. [DOI] [PubMed] [Google Scholar]
- 127. Toumpanakis C, Kim MK, Rinke A, et al. Combination of cross-sectional and molecular imaging studies in the localization of gastroenteropancreatic neuroendocrine tumors. Neuroendocrinology. 2014;99:63–74. [DOI] [PubMed] [Google Scholar]
- 128. Bodei L, Sundin A, Kidd M, Prasad V, Modlin IM. The status of neuroendocrine tumor imaging: from darkness to light? Neuroendocrinology. 2015;101(1):1–17. [DOI] [PubMed] [Google Scholar]
- 129. Koopmans KP, Glaudemans AW. Other PET tracers for neuroendocrine tumors. PET Clin. 2014;9:57–62. [DOI] [PubMed] [Google Scholar]
- 130. Chuang CC, Bhurke S, Chen SY, Brulais S, Gabriel S. Clinical characteristics, treatment patterns, and economic burden in patients treated for neuroendocrine tumors in the United States: a retrospective cohort study. J Med Econ. 2015;18(2):126–136. [DOI] [PubMed] [Google Scholar]
- 131. Isidori AM, Kaltsas GA, Mohammed S, et al. Discriminatory value of the low-dose dexamethasone suppression test in establishing the diagnosis and differential diagnosis of Cushing's syndrome. J Clin Endocrinol Metab. 2003;88:5299–5306. [DOI] [PubMed] [Google Scholar]
- 132. Woo YS, Isidori AM, Wat WZ, et al. Clinical and biochemical characteristics of adrenocorticotropin-secreting macroadenomas. J Clin Endocrinol Metab. 2005;90:4963–4969. [DOI] [PubMed] [Google Scholar]
- 133. de Herder WW, Krenning EP, Malchoff CD, et al. Somatostatin receptor scintigraphy: its value in tumor localization in patients with Cushing's syndrome caused by ectopic corticotropin or corticotropin-releasing hormone secretion. Am J Med. 1994;96:305–312. [DOI] [PubMed] [Google Scholar]
- 134. Lamberts SW, de Herder WW, Krenning EP, Reubi JC. A role of (labeled) somatostatin analogs in the differential diagnosis and treatment of Cushing's syndrome. J Clin Endocrinol Metab. 1994;78:17–19. [DOI] [PubMed] [Google Scholar]
- 135. Isidori AM, Graziadio C, Paragliola RM, et al. The hypertension of Cushing's syndrome: controversies in the pathophysiology and focus on cardiovascular complications. J Hypertens. 2015;33(1):44–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Scillitani A, Mazziotti G, Di Somma C, et al. Treatment of skeletal impairment in patients with endogenous hypercortisolism: when and how? Osteoporos Int. 2014;25:441–446. [DOI] [PubMed] [Google Scholar]
- 137. Binderup T, Knigge U, Loft A, et al. Functional imaging of neuroendocrine tumors: a head-to-head comparison of somatostatin receptor scintigraphy, 123I-MIBG scintigraphy, and 18F-FDG PET. J Nucl Med. 2010;51:704–712. [DOI] [PubMed] [Google Scholar]
- 138. Severi S, Nanni O, Bodei L, et al. Role of 18FDG PET/CT in patients treated with 177Lu-DOTATATE for advanced differentiated neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:881–888. [DOI] [PubMed] [Google Scholar]
- 139. Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med. 2010;134:1628–1638. [DOI] [PubMed] [Google Scholar]
- 140. Karageorgiadis AS, Papadakis GZ, Biro J, et al. Ectopic adrenocorticotropic hormone and corticotropin-releasing hormone co-secreting tumors in children and adolescents causing Cushing syndrome: a diagnostic dilemma and how to solve it. J Clin Endocrinol Metab. 2015;100(1):141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Treglia G, Castaldi P, Rindi G, Giordano A, Rufini V. Diagnostic performance of Gallium-68 somatostatin receptor PET and PET/CT in patients with thoracic and gastroenteropancreatic neuroendocrine tumours: a meta-analysis. Endocrine. 2012;42:80–87. [DOI] [PubMed] [Google Scholar]
- 142. Lococo F, Cesario A, Paci M, et al. PET/CT assessment of neuroendocrine tumors of the lung with special emphasis on bronchial carcinoids. Tumour Biol. 2014;35:8369–8377. [DOI] [PubMed] [Google Scholar]
- 143. Sharma ST, Nieman LK. Prolonged remission after long-term treatment with steroidogenesis inhibitors in Cushing's syndrome caused by ectopic ACTH secretion. Eur J Endocrinol. 2012;166:531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Gentilin E, Tagliati F, Terzolo M, et al. Mitotane reduces human and mouse ACTH-secreting pituitary cell viability and function. J Endocrinol. 2013;218:275–285. [DOI] [PubMed] [Google Scholar]
- 145. Donadille B, Groussin L, Waintrop C, et al. Management of Cushing's syndrome due to ectopic adrenocorticotropin secretion with 1,ortho-1, para'-dichloro-diphenyl-dichloro-ethane: findings in 23 patients from a single center. J Clin Endocrinol Metab. 2010;95:537–544. [DOI] [PubMed] [Google Scholar]
- 146. Bertagna X, Pivonello R, Fleseriu M, et al. LCI699, a potent 11β-hydroxylase inhibitor, normalizes urinary cortisol in patients with Cushing's disease: results from a multicenter, proof-of-concept study. J Clin Endocrinol Metab. 2014;99:1375–1383. [DOI] [PubMed] [Google Scholar]
- 147. Ferone D, Pivonello C, Vitale G, Zatelli MC, Colao A, Pivonello R. Molecular basis of pharmacological therapy in Cushing's disease. Endocrine. 2014;46:181–198. [DOI] [PubMed] [Google Scholar]