Abstract
Context:
The optimal frequency for on-treatment serum T measurement used for dose adjustment after transdermal T gel application is unknown, especially in older men with thinner skin and slower metabolic clearance.
Objectives:
The objectives of the study was to determine the variability of postgel application serum T concentrations and assess whether single levels are reflective of average serum T concentrations over 24 hours (Cavg0–24).
Design:
This was a double-blinded, placebo-controlled randomized trial.
Setting:
The study was conducted at five academic centers.
Participants:
Forty-seven symptomatic men 65 years old or older with an average of two morning T concentration less than 275 ng/dL participated in the study.
Intervention(s):
Transdermal T or placebo gel was applied for 120 ± 14 days. Monthly dose adjustments were made if necessary to target serum T between 400 and 500 to 800 ng/dL.
Main Outcome Measures:
Variability of serum T 2 hours after the gel application on two outpatient visits and at multiple time points over 24 hours during the inpatient day was measured.
Results:
On-treatment T levels varied substantially on the 2 ambulatory days and over 24 hours during the inpatient day. Ambulatory 2-hour postapplication T levels did not correlate significantly with either 2-hour postapplication serum T or Cavg0–24 measured during the inpatient day. Only 22.2% of men receiving T had a Cavg0–24 within the target range of 500–800 ng/dL; 81.5% had a Cavg0–24 within the broader 300–1000 ng/dL range.
Conclusion:
Large within-individual variations in serum T after T gel application render ambulatory 2-hour postapplication T level a poor indicator of average serum T on another day. Our data point out the limitations of dose adjustments based on a single postapplication serum T measurement.
In symptomatic men with low T, on-treatment dose adjustment is recommended to maintain serum T within the midreference range of adult men (1, 2). Older men have thinner skin and slower metabolic clearance rates (3–5), and thus, the application of transdermal T gels may result in higher serum T concentrations than in younger men. Although high-quality evidence-based data to demonstrate a causal relationship are not available, higher serum T levels could be associated with dose-related adverse events (ie, erythrocytosis and cardiovascular events) (6, 7), especially in frail older men. The association of increased cardiovascular events in T-replaced older hypogonadal men is controversial, as reported in some (7) but not other studies (8). In the absence of definitive evidence of cardiovascular safety (9–14), the current practice guidelines recommend that serum T target concentrations for older men should be within the midreference range of young adult men (generally accepted as 300–1000 ng/dL or 10.4–35 nmol/L) (1).
In light of the variability (exemplified by large differences between mean maximum and minimum T levels) in on-treatment T levels with transdermal gels, patches and lotions in hypogonadal men noted in previous studies (15–18) and anecdotally with clinical use of T gel, the monitoring of T levels is particularly important with transdermal T treatment. The Endocrine Society guideline suggests adjustment of T gel dose based on the measurement of serum T concentrations a few hours after transdermal gel/lotion application (1). No study has demonstrated conclusively that serum T levels drawn a few hours after T gel application reflect the average T concentration over 24 hours (Cavg0–24) on another day in older men with low serum T prior to treatment. In this study, we evaluated whether ambulatory samples drawn 2 hours after transdermal T gel application on 2 different outpatient days were reflective of serum T concentrations 2 hours after the gel application or Cavg0–24 in an inpatient day within 2 weeks of ambulatory sampling while on the same dose of T gel in older men participating in The Testosterone Trials (The TTrials). We also assessed whether monitoring the serum T levels and the systematic adjustment of the T dose was effective in attaining a predetermined serum T target range.
Participants and Methods
Participants and study design
Participants were recruited from older men enrolled in The TTrials at five academic medical centers: Harbor-UCLA Medical Center and Los Angeles Biomedical Research Institute; Boston University Medical Center; Yale University; Veterans Affairs Puget Sound Health Care System; and the University of Florida. The TTrials is a coordinated set of trials to determine whether T treatment of elderly men with low serum T concentrations and symptoms and objective evidence of impaired mobility and/or diminished libido and/or reduced vitality would be efficacious in improving mobility, sexual function, fatigue, cognitive function, hemoglobin, bone density, and coronary artery plaque volume (19). The University of Pennsylvania served as the Data Coordinating Center for the overall The TTtrials and this serum T variability substudy.
To be enrolled into The TTrials, men had to be 65 years old or older and had self-reported sexual dysfunction, diminished vitality, and/or mobility limitation confirmed on objective testing. Excluded were men who were at relatively high risk of having conditions that T treatment may exacerbate, such as prostate cancer, benign prostatic hyperplasia, erythrocytosis, and sleep apnea (19). Men were required to have two early morning serum T values with an initial T concentration less than 275 ng/dL and a second T level less than 300 ng/dL, with an average less than 275 ng/dL prior to randomization. Men were randomized to receive either T gel or placebo gel according to the procedures of The TTrials. Both the T gel (AndroGel III 1%) and identical placebo gel were provided by AbbVie. Each actuation of the pump delivered 1.25 g of gel. All participants were advised to wear protective clothing or shower after application of the gels before having close skin contact with another person. The initial dose of AndroGel was 5.0 g gel applied once a day on shoulders and upper arms. During the course of The TTrial participation, the dose was adjusted (higher or lower) monthly in the first 3 months to attempt to achieve a serum T level within a target range between 400 and 800 ng/dL, later changed to 500–800 ng/dL. The dose adjustment was not based on symptoms. When the gel dose was changed in a man in the T gel arm, the dose of gel of a participant in the placebo arm was simultaneously changed to maintain blinding of the study (19).
The current serum T variability study was conducted after 16 ± 2 weeks of gel application (ie, between 106 and 134 d of the treatment phase). Serum T was measured at three visits while the subjects were taking the same dose of T/placebo gel, but symptoms were not separately assessed during the three visits in the variability component of the study described in this manuscript. Men attended two ambulatory visits (visits A and B) during which gel was applied under supervision on the shoulders and upper arms at 8:00 am, and blood was drawn 2 hours later. In addition, they were admitted to a clinical research unit on a different day other than visits A and B for a 24-hour multiple blood sampling study (visit C). Serial blood samples were collected at −15, 0 minutes before, and 1, 2, 4, 8, 12, 16, and 24 hours after gel application at 8:00 amin the unit. A total of 47 men (27 randomized to T and 20 to placebo gel) participated in the serum T variability study.
Measurement of serum T by liquid chromatography tandem mass spectrometry
All serum T samples collected at the ambulatory visits A and B and the inpatient admission visit C from a participant were measured in the same assay. Serum T was measured by liquid chromatography tandem mass spectrometry at The TTrials Central Laboratory at Quest Diagnostics Clinical Trials, Inc. using a validated method (20). The lower limit of quantitation of the assay was 1 ng/dL. For serum T levels between 10 and 1200 ng/dL, the within-run and between-run imprecision was less than 11 and less than 14%, respectively, and the accuracy of the T measured was from 88% to 104%.
Statistical analysis
The variance component model was used to estimate the day-to-day variance (over three visits) within each participant relative to the total variances using the 2-hour postapplication T levels. The within-subject variance is defined as the variability of three 2-hour postapplication serum T levels, and the between-subject variance is defined as the variability among different subjects. The intraclass correlation coefficient was computed to measure the internal consistency of 2-hour postapplication serum T levels over three visit days relative to the total variability. To evaluate how 2-hour postapplication T levels were predictive of for Cavg at visit C, we used a multiple linear regression model. The 2-hour postapplication T levels at visit C and 2-hour postapplication T levels at visits A and B were included in the aforementioned model and a step-wise variable selection procedure was carried out. Correlation analyses were performed for 2-hour postapplication T levels for visits A, B, and C using the Pearson's correlation coefficient. Similar analyses, as described above, were performed using the serum T profile from men on placebo gel. For visit C during which serial blood samples were drawn for T measurements, the area under the 24-hour T concentration curve on the multiple sampling day was calculated using the trapezoidal method. The average concentration over 24 hours (Cavg) was calculated as the area under the 24-hour T concentration curve divided by 24 hours. The maximum and minimum serum concentrations (Cmax, Cmin) and time to reach Cmax were obtained and/or derived from measured serum T concentration levels. The fluctuation index (a summary of variability) was calculated as (Cmax − Cmin)/Cavg. Descriptive statistics were computed for each pharmacokinetics (PK) parameter using means, SDs, and frequencies. A two-sample t test was used to compare primary PK parameters between the T gel and placebo groups. All data analyses were conducted using SAS version 9.3, and the PK parameters were computed using Matlab 2011b.
Results
Characteristics of the participants
There were no significant differences in the participants' baseline characteristics across sites. Data from all five sites were combined for analysis. As shown in Table 1, men randomized to receive T or placebo gel did not differ in terms of age; racial groups; height, weight, or body mass index (BMI); pretreatment baseline serum T concentrations; number of pump actuations at the time of the study (reflecting the dose of gels applied); and the number of dose adjustments made prior to this study. The mean, median and range of the number of dose adjustments before this study was 1.6 ± 1, 1, and 0–3 in the T gel group and 1.5 ± 0.6, 1, and 1–3 in the placebo group. The mean, median, and range of the number of pump actuations per day was 5.1 ± 1.8, 5, and 2–8 for the T gel group and 4.6 ± 1.9, 5, and 1–5 in the placebo group without significant differences between the groups. In the T gel group, the mean dose of 1% T gel applied was 6.38 ± 2.4 g (1.25 g gel per actuation) containing 63.8 ± 2.4 mg of T.
Table 1.
Participant Characteristics
| T Gel (n = 27) | Placebo Gel (n = 20) | |
|---|---|---|
| Age, y | 71.6 ± 1.1 | 69.8 ± 0.6 |
| BMI, kg/m2 | 30.4 ± 0.8 | 31.2 ± 0.8 |
| Race | ||
| Asian | 0 (0%) | 1 (5%) |
| Black | 3 (11.1%) | 1 (5%) |
| White | 24 (88.9%) | 16 (85%) |
| Other | 0 (0%) | 1 (5%) |
| Multiracial | 0 (0%) | 1 (5%) |
| Weight, kg | 92.4 ± 2.7 | 95.2 ± 3.0 |
| Height, cm | 174.5 ± 1.5 | 174.6 ± 1.5 |
| Baseline serum T, ng/dLa | 211.2 ± 10.7 | 197.9 ± 8.9 |
| Number of pump actuations | 5.1 ± 1.8 | 4.6 ± 1.9 |
| Number of dose adjustments prior to serum T variability study | 1.6 ± 1.0 | 1.5 ± 0.6 |
Abbreviation: BMI, body mass index.
Serum T in nanograms per deciliter = 0.03467 nmol/L.
Variability of serum T
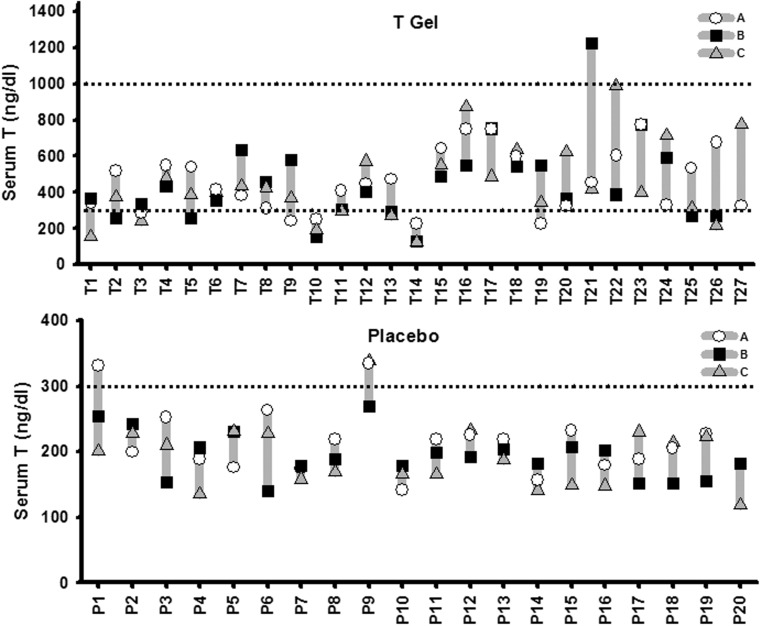
The variability of serum T in the three samples drawn at the two ambulatory visits (A and B) and the inpatient day (visit C) 2 hours after transdermal gel application is shown in Figure 1. Serum T concentrations attained 2 hours after the gel application at visits A and B were not consistently higher or lower than those obtained at the same time after gel application at visit C. In the T gel-treated men (n = 27), two (7.4%) had all three samples consistently less than 300 ng/dL, three (11.1%) had two samples less than 300 ng/dL, and eight had one sample less than 300 ng/dL. Only one man had one sample greater than 1000 ng/dL, whereas the remaining 14 men (51.9%) had all three 2-hour postapplication samples within 300–1000 ng/dL.
Figure 1.
Large variation of serum T levels 2 hours after gel application at the ambulatory visits (A, open circles, or B, closed squares) and the inpatient day (C, shaded triangle) in the T gel (upper panel, participants T1 to T27) and placebo (lower panel, participants P1 to P20) groups. Note the difference in y-axis scale for serum T concentration in the T vs the placebo group. Shaded vertical lines between highest and lowest T value for each man indicates within-subject variation. The dashed lines represent the adult men reference range of 300–1000 ng/dL (10.4–34.7 nmol/L).
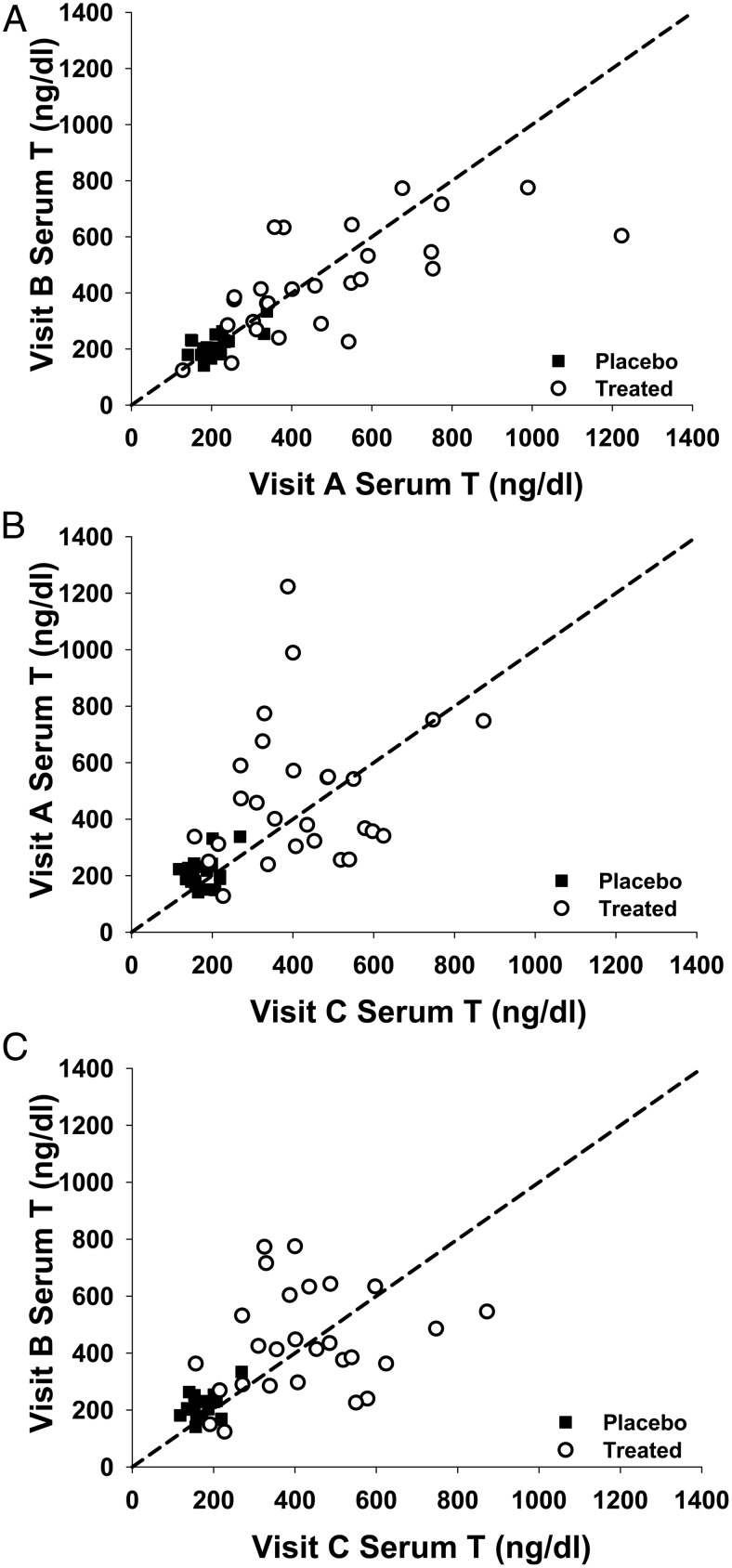
There was relatively good concordance of serum T levels 2 hours after T gel application between the two ambulatory samples at visits A and B (Figure 2 A) but lack of concordance between ambulatory serum T level at visits A or B and visit C, the inpatient day (Figure 2, B and C). The lack of concordance was mainly due to the large within-subject variability as shown by the intraclass correlation (ICC) analyses described below (Supplemental Table 1). For the 2-hour postgel application serum T levels, 37% [ICC = 0.37, 95% confidence interval (CI) 0.13, 0.61] and 36% (ICC = 0.36, CI 0.08, 0.64) of the total variance were accounted for by the between-subject variability in the T gel and placebo, respectively. Thus, 63% (CI 0.51, 0.87) and 64% (CI 0.36, 0.92) of the total variance are due to the within-subject variability in the T and placebo gel groups, respectively (Supplemental Table 1). Comparing the serum T variance between the two ambulatory days showed that the ICC was 0.64, indicating the variance was mainly due to between-subject variance, whereas the variance of serum T levels between two ambulatory and the inpatient day 2-hour postgel application sample, the ICC was 0.19–0.22, suggesting the variance was mainly contributed by within-subject variance. For the 24-hour serum T level on the inpatient day, 49% (95% CI 0.13, 0.61) and 40% (CI 0.08, 0.64) of the total variance were accounted for the between-subject variability; thus, 51% (CI 0.51, 0.87) and 60% (CI 0.36, 0.92) of the total variance was due to the within-subject variability in the T and placebo gel groups, respectively (Supplemental Table 1). The variability of serum T concentration was also shown by the large fluctuation index ([Cmax − Cmin]/Cavg) after the T gel application. The fluctuation index was higher in the T gel group (88% ± 42%) compared with the placebo gel group (52% ± 18%, P < .001) during multiple samplings during visit C (Table 2).
Figure 2.
Concordance of serum T levels 2 hours after T or placebo gel application between visits A and B (A); visits A and C (inpatient day) (B); and between visits A and C (C). The diagonal line (x = y) shown is the concordance line or line of agreement.
Table 2.
Serum T PK Parameters After Transdermal T Gel Application in Older Men
| T PK Parameters (mean ± SEM) | T Gel (n = 27) | Placebo Gel (n = 20) |
|---|---|---|
| Cavg0–24, ng/dL | 453 ± 178a | 182 ± 34 |
| Cmax, ng/dL | 680 ± 297 | 230 ± 40 |
| Cmin, ng/dL | 281 ± 143 | 134 ± 18 |
| Tmax, h | 9.7 ± 9.1 | 17.3 ± 9.0 |
| Fluctuation, % | 88 ± 42 | 52 ± 18 |
Abbreviations: Cavg0–24, area under the 24-hour T concentration curve (AUC0–24) divided by 24 hours; fluctuation index (a summary of variability), (Cmax − Cmin)/Cavg; Tmax, time to reach Cmax.
Serum T in nanograms per deciliter = 0.03 467 nmol/L.
Correlation of two outpatient ambulatory serum T concentrations with serum T and Cavg during the inpatient day
The correlations of the serum T concentrations between the two ambulatory blood samples drawn 2 hours after gel application at visits A and B were statistically significant (T gel, r = 0.68, P < .01; placebo gel, r = 0.66, P = .002) and much stronger than between T levels on either ambulatory day (visit A and B) with serum T 2 hours (neither statistically significant) after gel application at visit C (Table 3). Serum T concentrations at visit A or B were not significantly correlated with any of the postapplication T concentrations (data not shown) but were significantly correlated with the preapplication serum T concentration (time 0, r = 0.53, P = .004 for visit A and r = 43, P = .023 for visit B) on the inpatient C day. The serum samples drawn 2 hours after T or placebo gel application on the inpatient day predicted the Cavg0–24 on the same day (regression coefficient = 0.70, P < .001 for both for T and placebo groups). However, individual or average serum T levels 2 hours after T gel application on ambulatory visits A and B were not significantly correlated with Cavg0–24 on visit C (multiple blood sampling day) in men receiving the T gel (Table 3).
Table 3.
Correlation Coefficients Between Serum T Concentrations 2 Hours After Gel Application at Visits A, B, and C and Cavg0–24 on PK Day (Visit C)
| Serum T Concentration 2 Hours After Gel Application | Correlation Coefficient |
|
|---|---|---|
| T Gel | Placebo Gel | |
| Visit A vs B | 0.67a | 0.72a |
| Visit A+B vs C | 0.20 | 0.36 |
| Visit A vs Visit C Cavg0–24 | 0.17 | 0.51b |
| Visit B vs Visit C Cavg0–24 | 0.25 | 0.74a |
| Visit A+B vs Visit C Cavg0–24 | 0.20 | 0.54c |
| Visit C vs Visit C Cavg0–24 | 0.70a | 0.70a |
P < .001.
P < .05.
P < .01.
Using a stepwise variable selection procedure, serum T concentration at 8 hours after T gel application on the inpatient day predicted 80% of the variability of Cavg0–24 (R2 = 0.8), and adding the serum T level at 12 hours after T gel application increased R2 by 10%. Therefore, the serum T level at 8 hours and 12 hours after T gel application explained 90% of the Cavg variability (R2 = 0.9). Serum T concentration 8 hours after the T gel application significantly correlated with serum T concentrations at 1, 2, 4, 12, 16, and 24 hours after gel application (P < .001 for all time points when serum T was measured); the 8-hour value did not correlate with T concentrations at visits A or B. In the placebo group, the serum T level at 16 hours after placebo gel application accounted for 81% of the variability of Cavg, and adding the serum T level at 4 hours increased this to 91%.
Serum T PK parameters after gel applications
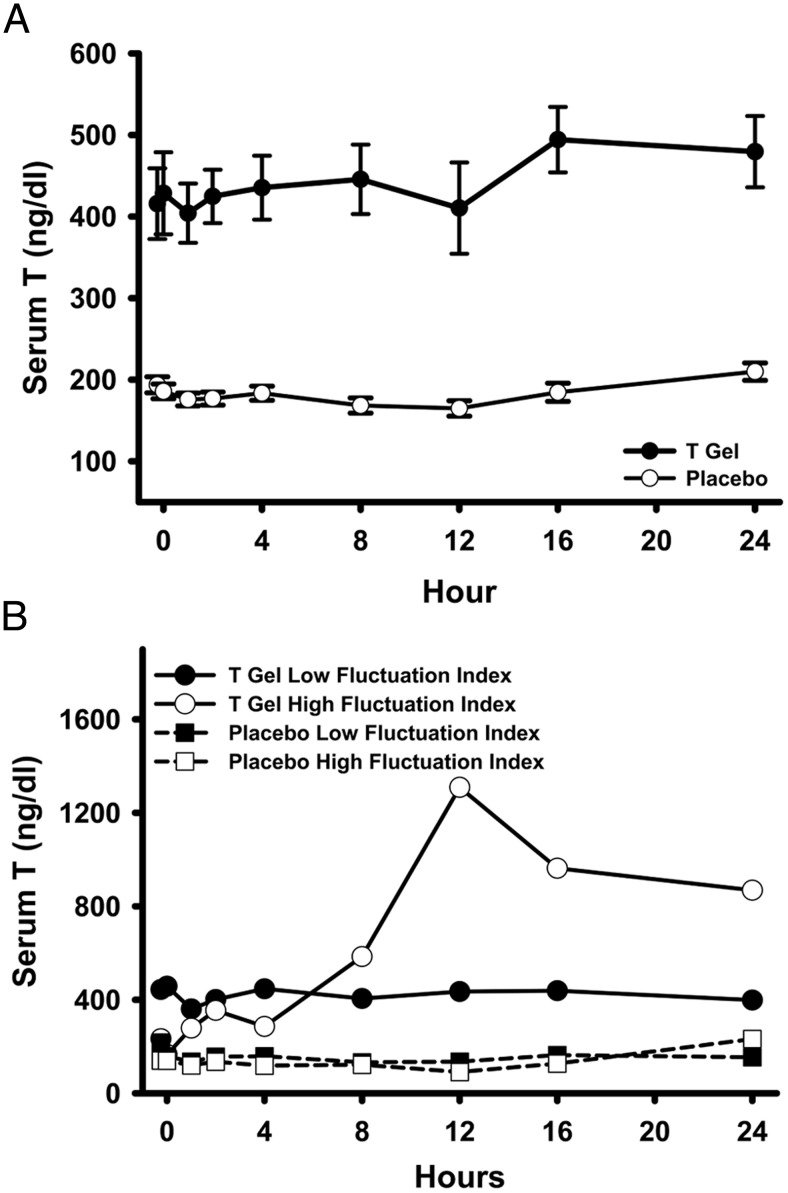
The mean serum T concentration attained in the T gel group on the multiple-sampling inpatient day is shown in Figure 3A and the PK parameters are shown in Table 2. In the T gel group, even with dose adjustment based on ambulatory serum T concentrations, 24 participants (88.9%) attained the initial target T level between 400 and 800 ng/dL sometime during the inpatient day, 15 (55.6%) had Cavg0–24 within this target range and one remained within the target range all the time. Using the target range of 500–800 ng/dL, 19 (70.4%) attained this range sometime during the day, six (22.2%) had Cavg0–24 within this range, but there was no participant whose serum T remained between 500 and 800 ng/dL throughout the 24 hours. Using the generally accepted nominal adult male reference range of 300–1000 ng/dL, 25 participants (92.5%) ever reached this range, 22 (81.5%) had Cavg0–24 within this range, but only nine participants (33.3%) remained within the range throughout the 24 hours after T gel application. Only three participants (11.1%) applying the T gel had serum T levels above 1000 ng/dL during the 24-hour sampling period. The serum T Cavg0–24 in participants randomized to the T arm was not significantly related either to the pretreatment serum T levels or to the number of dose adjustment each participant had prior to the PK day.
Figure 3.
A, Serum T levels (mean ± SE) over 24 hours during the inpatient day (visit C) in the T gel (closed circles) and the placebo gel (open circles) groups. B, Serum T levels over 24 hours during the inpatient day (visit C) in representative participants with a low (fluctuation index 23%, closed circles) and high (fluctuation index 147%, open circles) fluctuation index in the T gel group and in another participant with a low (fluctuation index 21%, closed squares) and high (fluctuation index 103%, open squares) fluctuation index in the placebo group.
None of the participants in the placebo group had Cavg0–24 within either the target range of 400–800 or 500–800 or the nominal physiological range of 300–1000 ng/dL in healthy young men. Serum T concentrations over 24 hours were significantly higher in the group applying the T gel (Cavg 453 ± 178 ng/dL) compared with those applying the placebo gel (Cavg 182 ± 34 ng/dL) (P < .001) (Figure 3 and Table 2). Both the Cmax and the Cmin were also significantly higher in the group applying the T gel vs placebo gel (both P < .001). Examination of 24-hour serum T levels in a participant with a high and another with a low fluctuation index from each of the T and the placebo group showed the range of within-subject variations in serum T levels in T-treated and untreated older men within a day (Figure 3B).
Discussion
Our results demonstrate large variability in 2-hour postgel application serum T concentration collected on two different outpatient visits and one inpatient day in older symptomatic men with unequivocally low pretreatment serum T concentrations. This variability was observed despite the samples being drawn at the same time after the same dose of transdermal T gel application and measured in the same T assay. About 63% of the total variance was due to the within-subject variability in the T gel group. It is unlikely that such large variability was due to the imprecision of serum T measurements because both within- and between-run imprecision were less than 11% and 14%, respectively. In clinical practice, the samples drawn for the monitoring of on-treatment serum T may show even larger variations because the time relative to application and different laboratory methods may contribute to the differences in serum T levels. It is not known whether the variability in responsiveness of endogenous T to the negative feedback of exogenous T contributed to the within-individual variability in serum T observed in this study. The impact of endogenous T fluctuation might be reflected by associated serial multiple sampling for gonadotropins to gauge the variability of the suppression of the hypothalamic-pituitary-testis axis. Associated serum LH levels were not assessed in this study. There were also large fluctuations of serum T within a 24-hour period both in the T and placebo groups. The large fluctuation of serum T was due to the within-subject variability, which accounted for 51% of the variation of serum T levels after T gel application within a 24-hour period. After gel application, the fluctuation index (index of overall variability within 24 h) reached 88% in the T gel group compared with 52% in the placebo group because there appeared to be less variation in the baseline T levels over 24 hours in hypogonadal men. Prior studies in younger hypogonadal men (mean age 51 y) using the same T gel formulation (Androgel 1% III) for 180 days showed that the average fluctuation index ranged from 86% after 90 days of T gel application (dose ranging from 5 to 10 g/d of gel) to about 60% at baseline (15), similar to the results in older men in the current study.
We also demonstrated that after T gel replacement, serum T concentrations obtained by ambulatory blood draws at a specified time (2 h post application of T gel) were not correlated with the serum T drawn at the same postapplication time or with any other time point or the Cavg0–24 on another day when the participants were confined to a clinical research unit for 24-hour multiple blood draws. The timed two ambulatory serum T levels correlated significantly with each other. There are no prior reports of how well timed 2-hour post-T gel application serum T levels during two different inpatient days with multiple sampling for the PK study correlate with each other, but Cavg, Cmax, and Cmin were reported to be very similar on the two different sampling days (15, 18). Concentrations of serum T obtained during the inpatient day were not consistently higher or lower than those obtained during the ambulatory visits. These data indicate that postgel application serum T concentrations during ambulatory visits do not reflect serum T levels when participants in a clinical trial are confined to a research unit with a controlled environment and standardized medical center-provided meals. The differences in post-T gel application serum T levels between ambulatory and inpatient settings may be due to the timing of the breakfast, upright vs recumbent posture, and limited physical activity in the inpatient environment.
Our data demonstrate the limitations of using ambulatory T levels to reflect either a timed serum T concentration or Cavg over a 24-hour period in an inpatient setting and suggest that dose adjustment based on a single ambulatory sample will not predictably bring 24-hour serum T levels within a narrow desired target range. Using the study serum T target range of 500–800 ng/dL, despite the opportunity for three dose adjustments, only 30% of participants had Cavg within that range, but 81.5% had Cavg between 300 and 1000 ng/dL, meeting the criteria that T gel was effective in achieving serum T range for adult men. Despite dose adjustments, none of the participants had serum T levels stay within the target range between 500 and 800 ng/dL throughout the 24-hour sampling period of the inpatient day. The relative ineffectiveness of dose adjustment to provide uniform in-range serum T levels within a narrower target (500–800 ng/dL) reflected the large within-subject variation of serum T within the same day and between sampling days. A narrower target range for T replacement may be desired in older men who may be more susceptible to erythrocytosis (21) and when other adverse effects, for example, cardiovascular events seem to suggest that targeting serum T to the low midadult male range may be prudent (7). However, dose adjustments were successful in bringing the three 2-hour post-T gel application to within the reference range of adult men (300–1000 ng/dl) consistently in 52% and inconsistently in 40.7% of T-treated men. Only 7.4% had consistently low T concentration (<300 ng/mL). Dose adjustment based on one or two ambulatory serum T measurements should be sufficient to establish that the serum T will fall within the wider, generally accepted adult male range of serum T between 300 and 1000 ng/dL.
Our study did show that applying T gel for 120 days in older hypogonadal men increases serum T levels 2.5-fold over that of placebo gel and within the adult range in most men. The serum T levels in the placebo group remained in the hypogonadal range. There were only three participants whose serum T level ever exceeded the upper limit of the generally accepted serum T concentration greater than 1000 ng/dL. Thus, the concern that doses of T gel commonly used in younger men would lead to excessively high serum T levels in older men was not observed in this study. The earlier T gel PK study also reported that downward adjustment was effective in reducing the serum T levels attained in hypogonadal men (15).
We conclude that after T gel application, the between- and within-subject variations in the serum T levels were large. The 2-hour ambulatory serum T level after T gel application was neither a good indicator of timed serum T concentration nor Cavg with the same dose on another inpatient day. In older men administered a given dose of T gel, one or two measurements of serum T at any time after T gel application may be inadequate for attaining a serum concentration within a target range much narrower but within the population reference range (eg, in the midadult male range). However, our results do suggest that one or two T measurements may be sufficient for monitoring of treatment aimed at maintaining serum concentrations within the broader normal reference range.
Acknowledgments
The testosterone trials were supported by a grant from the National Institute on Aging, National Institutes of Health (Grant U01 AG030644), supplemented by support from the National Heart, Lung, and Blood Institute, National Institute of Neurological Diseases and Stroke, and Eunice Kennedy Shriver National Institute of Child Health and Human Development. AbbVie (formerly Solvay and Abbott Laboratories) generously provided support and donated the AndroGel and placebo gel.
This work was supported by the National Center for Advancing Translational Sciences through the University of California, Los Angeles, Clinical and Translational Science Institute (Grant UL1TR000124) and Diabetes and Endocrinology Training Grant T32 DK007571 (to the Los Angeles site). A.M.M. is supported by the Department of Veterans Affairs Puget Sound Health Care System. T.M.G. was the recipient of Academic Leadership Award K07AG3587 from the National Institute on Aging. The Yale Field Center was partially supported by the Claude D. Pepper Older Americans Independence Center (Grant P30-AG021342) and Clinical and Translational Science Institute Grant UL1TR000142. Additional support was provided by resources of the Boston Claude D. Pepper Older Americans Independence Center Grant 5P30AG031679 (to S.B.) and Clinical and Translational Science Institute Grant 1UL1RR025771 from Boston University.
Disclosure Summary: R.S.S. received grant support from Clarus Therapeutics, Antares, and Novartis and consultant fees from Clarus Therapeutics, Quest Diagnostics, and TesoRx. C.W. received grant support from Clarus Therapeutics, Lipocine, and Prolor and consultant fees from Lipocine and TesoRx. S.B. received research grants from AbbVie, Regeneron, and Takeda Pharmaceuticals and consultant fess from Allergan, Sanofi, and Lilly Pharmaceuticals and has equity interest in FPT, LLC. A.M.M. received research support from AbbVie and GlaxoSmithKline; consultation fees from AbbVie, Lilly, Endo, and Clarus Therapeutics; and royalties from UpToDate. P.J.S. received research support from AbbVie and consultant fees from Watson. Y.P., P.Y.L., T.M.G., and M.P. have nothing to disclose.
Footnotes
- Cavg0–24
- average T concentration over 24 hours
- CI
- confidence interval
- Cmax
- maximum serum concentration
- Cmin
- minimum serum concentration
- ICC
- intraclass correlation
- PK
- pharmacokinetics
- The TTrials
- The Testosterone Trials.
References
- 1. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–2559. [DOI] [PubMed] [Google Scholar]
- 2. Wang C, Nieschlag E, Swerdloff R, et al. Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. J Androl. 2009;30:1–9. [DOI] [PubMed] [Google Scholar]
- 3. Baker HW, Burger HG, de Kretser DM, et al. Changes in the pituitary-testicular system with age. Clin Endocrinol (Oxf). 1976;5:349–372. [DOI] [PubMed] [Google Scholar]
- 4. Wang C, Catlin DH, Starcevic B, et al. Testosterone metabolic clearance and production rates determined by stable isotope dilution/tandem mass spectrometry in normal men: influence of ethnicity and age. J Clin Endocrinol Metab. 2004;89:2936–2941. [DOI] [PubMed] [Google Scholar]
- 5. Coviello AD, Lakshman K, Mazer NA, Bhasin S. Differences in the apparent metabolic clearance rate of testosterone in young and older men with gonadotropin suppression receiving graded doses of testosterone. J Clin Endocrinol Metab. 2006;91:4669–4675. [DOI] [PubMed] [Google Scholar]
- 6. Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90:678–688. [DOI] [PubMed] [Google Scholar]
- 7. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–650. [DOI] [PubMed] [Google Scholar]
- 9. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9:e85805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97:2050–2058. [DOI] [PubMed] [Google Scholar]
- 11. Vigen R, O'Donnell CI, Baron AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–1836. [DOI] [PubMed] [Google Scholar]
- 12. Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013;11:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fernandez-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95:2560–2575. [DOI] [PubMed] [Google Scholar]
- 14. Corona G, Maseroli E, Rastrelli G, et al. Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin Drug Saf. 2014;13:1327–1351. [DOI] [PubMed] [Google Scholar]
- 15. Swerdloff RS, Wang C, Cunningham G, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85:4500–4510. [DOI] [PubMed] [Google Scholar]
- 16. Steidle C, Schwartz S, Jacoby K, Sebree T, Smith T, Bachand R. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab. 2003;88:2673–2681. [DOI] [PubMed] [Google Scholar]
- 17. Wang C, Ilani N, Arver S, McLachlan RI, Soulis T, Watkinson A. Efficacy and safety of the 2% formulation of testosterone topical solution applied to the axillae in androgen-deficient men. Clin Endocrinol (Oxf). 2011;75:836–843. [DOI] [PubMed] [Google Scholar]
- 18. Mazer N, Bell D, Wu J, Fischer J, Cosgrove M, Eilers B. Comparison of the steady-state pharmacokinetics, metabolism, and variability of a transdermal testosterone patch versus a transdermal testosterone gel in hypogonadal men. J Sex Med. 2005;2:213–226. [DOI] [PubMed] [Google Scholar]
- 19. Snyder PJ, Ellenberg SS, Cunningham GR, et al. The Testosterone Trials: seven coordinated trials of testosterone treatment in elderly men. Clin Trials (London, England). 2014;11:362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salameh WA, Redor-Goldman MM, Clarke NJ, Reitz RE, Caulfield MP. Validation of a total testosterone assay using high-turbulence liquid chromatography tandem mass spectrometry: total and free testosterone reference ranges. Steroids. 2010;75:169–175. [DOI] [PubMed] [Google Scholar]
- 21. Calof OM, Singh AB, Lee ML, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60:1451–1457. [DOI] [PubMed] [Google Scholar]