Abstract
Context:
Intrauterine HIV/antiretroviral (ARV) and postnatal ARVs are known to perturb energy metabolism and could have permanent effects on future metabolic health. Such maladaptive effects could be mediated by changes in mitochondrial function and intermediary metabolism due to fetal and early-life ARV exposure in HIV/ARV-exposed uninfected (HEU) infants.
Objective:
The objective of the study was to understand the relationship(s) between mitochondrial fuel use (assessed via acylcarnitines and branched chain amino acids) and preprandial insulin in infants exposed to in utero HIV/ARV plus postnatal zidovudine or nevirapine compared with HIV/ARV-unexposed uninfected (HUU) infants.
Design:
This was a prospective cohort study with the following three groups: 1) intrauterine HIV/ARV/postnatal zidovudine-exposed (HEU-A), 2) intrauterine HIV/ARV/postnatal nevirapine-exposed (HEU-N), and 3) HUU infants. Principal component analysis and linear regression modeling were performed to assess the association between in utero HIV/ARV exposure and infant insulin.
Setting:
The study was conducted at Cameroonian urban antenatal centers.
Participants:
HIV-infected and -uninfected pregnant woman/infant dyads participated in the study.
Main Outcome:
Preprandial insulin was the main outcome measured.
Results:
Of 366 infants, 38 were HEU-A, 118 HEU-N. Forty intermediary metabolites were consolidated into seven principal components. In a multivariate analysis, both HEU-A (β = −.116, P= .012) and HEU-N (β = −.070, P= .022) demonstrated lower insulin compared with HUU infants. However, at high levels of plasma metabolites, HEU-A (β = .027, P= .050) exhibited higher insulin levels than HEU-N or HUU infants. A unique array of short-chain acylcarnitines (β = .044, P= .001) and branched-chain amino acids (β = .033, P= .012) was associated with insulin.
Conclusion:
HEU-A and HEU-N infants have lower preprandial insulin levels at 6 weeks of age and appear to use metabolic fuel substrates differently than HUU infants. Future studies are warranted to determine whether observed differences have lasting metabolic implications, such as later insulin resistance.
Developing theories on the origins of disease have implicated fetal programming as an important mechanism whereby changes in the intrauterine environment may have profound effects on future cellular processes, including those leading to the development of obesity, insulin resistance, type 2 diabetes mellitus, and cardiovascular disease later in life (1). In utero fetal metabolic programming could affect mitochondrial function and/or pathways of intermediary metabolism (2, 3). Exposure to intrauterine HIV/antiretrovirals (ARVs) and postnatal ARVs are known to perturb energy metabolism and provide a model of early environmental exposure that could impact short- and long-term metabolic health in HIV-exposed uninfected (HEU) children (4). Several studies have demonstrated links between insulin resistance, mitochondrial dysfunction (5, 6), and biomarkers of intermediary energy metabolism, such as acylcarnitines (ACs) (7) and branched-chain amino acids (BCAAs) (8, 9). Few studies (10) have evaluated ACs and BCAAs in HEU infants as indicators of changes in fuel use and insulin sensitivity. In our study, we assessed the association between in utero HIV/ARV plus postnatal zidovudine (AZT) or nevirapine (NVP) exposure on preprandial insulin levels at 6 weeks of age and evaluated ACs and BCAAs to determine the relationship of specific pathways of mitochondrial fuel use to preprandial insulin levels.
Materials and Methods
Study population
HIV-infected and -uninfected pregnant woman/infant dyads were enrolled at the Cameroon Baptist Convention Health Services and followed up until 6 weeks postpartum from 2011 through 2014. Pregnancies with multiple gestations or ending in spontaneous/therapeutic abortions or intrauterine fetal demise were excluded. Infants with documented HIV infection by DNA PCR at 6 weeks of life were also excluded. All infants were breast-fed. During the study period, changes in national guidelines for the Prevention of Mother-To-Child Transmission of HIV followed updated World Health Organization Guidelines (11, 12) (Supplemental Table 1) At the start of the study, pregnant women with a CD4 cell count of 350 cells/mm3 or less were eligible for lifetime combination ARV treatment (cART); their infants received AZT or NVP until 4–6 weeks postpartum. Pregnant women with a CD4 greater than 350 cells/mm3 received prenatal AZT monotherapy plus single-dose NVP intrapartum and AZT/lamivudine for 7 days postpartum, whereas their infants received NVP until breast-feeding ceased. During the last year of the study, national guidelines expanded and all HIV-infected pregnant women were eligible for lifetime cART, regardless of maternal CD4 count; their infants received NVP for 6 weeks. Pregnant women receiving cART prior to pregnancy were continued on their prepregnancy regimens. All participants provided written informed consent. This study was approved by the Institutional Review Boards of Cameroon Baptist Convention Health Services and the Icahn School of Medicine at Mount Sinai.
Primary outcome
We measured infant preprandial insulin levels at 6 weeks of life using filter paper-derived dried blood spot (DBS) specimens via a direct sandwich ELISA technique (13). DBS specimens were shipped at 0°C and stored at −80°C until the time of biochemical assessments. As secondary outcomes, we calculated the homeostasis model assessment for insulin resistance (HOMA-IR) (HOMA-IR = insulin [microinternational units per milliliter] × fasting glucose [millimoles per liter]/22.5) (14) and homeostasis model of β-cell function (homeostasis model of β-cell function = 20 × insulin [microinternational units per milliliter]/[fasting glucose [millimoles per liter] − 3.5) to assess insulin sensitivity and β-cell function, respectively.
Primary exposure of interest
The primary exposure of interest was in utero/postnatal ARVs. Infants were classified as the following: 1) in utero HIV/ARV and postnatal AZT-HEU (HEU-A), 2) in utero HIV/ARV and postnatal NVP-HEU (HEU-N), and 3) HIV/ARV-unexposed uninfected (HUU) as determined by maternal serological HIV ELISA testing and ARV treatment (ART) history per self-report and medical and pharmacy record review. Infants were considered exposed to postnatal AZT if they received 4 weeks or more of AZT or exposed to postnatal NVP if they received 4 weeks or more of NVP after birth prior to insulin assessment at 6 weeks of life.
Measurements
Potential maternal and infant confounders for infant preprandial insulin were collected and included: maternal sociodemographics, infant sex, gestational age (GA) at delivery per reported last menstrual period, and birth weight. Information regarding maternal immunological status and ART history was also collected.
Physiological measurements
Maternal blood pressure was measured using a manual sphygmomanometer at 24–32 weeks' GA. At the same visit, the presence of gestational diabetes mellitus was assessed by a 75-g oral glucose tolerance test (15). Infant length, weight, and head circumference were measured using a recumbent stadiometer, manual scale, and paper tape measures, respectively, by one of two trained research assistants.
Biochemical measurements
We measured 37 ACs and three BCAAs (leucine, isoleucine, and valine) from DBS specimens at 6 weeks of life using Agilent 6460 tandem mass spectrometry coupled with an Agilent 1260 liquid chromatography system. ACs and BCAAs were extracted with methanol containing stable isotope internal standards of ACs and BCAAs. Dried AC and BCAA extracts were derivatized with 3N-butanolic HCl to form their respective butyl esters. Butyl esters of ACs were analyzed in the positive ion mode using a flow injection program and selective reaction monitoring (16). Butyl esters of BCAAs were separated on Zorbax Eclipse Plus C8 column and analyzed in the positive ion mode and selective reaction monitoring. Preprandial glucose concentrations were measured from capillary blood specimens and analyzed within 30 minutes of the specimen draw.
Statistical analysis
Characteristics of pregnant women and their infants were compared among groups using Kruskal-Wallis, χ2, or Fisher exact tests as appropriate. Weight-for-age z, length-for-age z (LAZ), weight-for-length z (WLZ), and head circumference-for-age z (HCAZ) scores were calculated from World Health Organization child growth standards (17). Insulin and HOMA-IR were quarter-root transformed to approximate a normal distribution. Principal component (PC) analysis (PCA) using an orthogonal rotation was performed as a means of reducing the complexity of the AC and BCAA metabolite variables. In general, PCA is a variable reduction technique that converts a set of possibly correlated variables (in our case, 40 analytes) into a set of values of uncorrelated variables defined as PCs. The number of PCs is less than (usually) the number of original variables. This transformation is defined in such a way that the first PC explains most the variability in the data (variance), and each successive PC in turn explains the next highest variance possible, under the constraint that the PCs are orthogonal (uncorrelated) with the other PCs. The number of components was chosen based on scree plots, eigen values greater than 1, and theoretical congruence with prior knowledge of intermediate metabolic pathways. Metabolites with a component load greater than 0.40 were assigned to a given component. PC scores representing the level of activity for different intermediate metabolic pathways were calculated for each subject as a linear combination of the optimally weighted metabolites (based on standardized scoring coefficients). Finally, linear regression modeling was applied to the entire cohort sample to assess the association of in utero HIV/ARV plus either postnatal AZT or NVP with infant insulin levels and HOMA-IR while adjusting for confounders and PCA-derived PC scores of the metabolites.
We tested for interactions between infant in utero HIV/ARV and postnatal ARV exposure and the PCs on insulin levels by performing regressions of all metabolite PC scores on insulin stratified by exposure group. Baseline characteristics were compared between infants with and without missing insulin measurements. We did not impute missing data because only 5% had missing data on the primary outcome. All statistical analyses were performed using SAS version 9.3.
Results
After excluding four HIV-infected infants, six twin pregnancies, one infant with an implausible glucose level, and 23 infants with missing insulin/glucose measurements, 366 singleton newborns were included for analysis: 38 HEU-A, 118 HEU-N, and 210 HUU infants. Median age of HIV-infected women was higher (30 vs 28 years, P ≤ .001) than that of HIV-uninfected women (Table 1). Among HIV-infected women, 15 (9.6%) received no ARVs, 33 (21.2%) AZT monotherapy, and 108 (69.2%) cART antenatally. Of women receiving antenatal cART, all but two received a nonnucleoside reverse transcriptase inhibitor-based regimen, and all but one received AZT for 2 weeks or longer during the pregnancy. HEU-A and HEU-N infants did not differ significantly in antenatal ARV exposure. Among term infants, HEU-A infants had significantly lower birth weights than HEU-N and HUU infants (3200 g vs 3400 g vs 3300 g, P = .045, respectively). HEU-A infants had the lowest LAZ at the time of insulin assessment, whereas HUU infants had the highest (0.08 vs 0.68 vs 0.92, P = .018). Both HEU-A and HEU-N infants had lower HCAZ than HUU infants (0.30 and 0.31 vs 0.75, P = .05). In addition, HEU-A infants had the highest WLZ (0.36 vs −0.80 in HEU-N vs −0.63 in HUU, P = .002). Infant insulin and glucose levels at 6 weeks of life were positively correlated (Spearman ρ = 0.23, P < .001). In a univariate analysis, infant insulin, glucose to insulin ratio, and HOMA-IR were significantly different among groups (2.7 vs 4.0 vs 4.9 μIU/mL, P < .001 for insulin; 36 vs 24 vs 19, P < .001 for glucose to insulin ratio; and 0.65 vs 0.91 vs 1.12, P < .001 for HOMA-IR) in which the HEU-A infants had the lowest insulin levels, highest glucose to insulin ratios, and lowest HOMA-IR, and HUU infants were the least insulin sensitive. Conversely, homeostasis model of β-cell function was lowest in HEU-A and highest in HUU infants (28.2% vs 38.1% vs 52.4%, P < .001).
Table 1.
Characteristics of Women and Infants
| HEU-A Infants (n = 38) | HEU-N Infants (n = 118) | HUU Infants (n = 210) | P Value | |
|---|---|---|---|---|
| Women | ||||
| Age of mother, y | 30 (27–33) | 30 (27–33) | 28 (24–31) | <.001 |
| Gravida | 3 (2–4) | 3 (2–4) | 3 (2–4) | .019a |
| Marital status | .070 | |||
| Single | 8 (21.0) | 28 (23.5) | 26 (12.3) | |
| Living with partner | 2 (5.0) | 3 (2.5) | 12 (5.7) | |
| Married | 28 (74.0) | 87 (74.0) | 172 (82.0) | |
| Highest level of education | .023 | |||
| Secondary school or lower | 26 (68.0) | 78 (66.0) | 110 (52.0) | |
| High school or higher | 12 (32.0) | 40 (34.0) | 100 (48.0) | |
| Gainful employment | 6 (19.0) | 28 (26.9) | 50 (24.5) | .640 |
| Water source | .270 | |||
| Tap water in the home | 4 (10.5) | 20 (17.0) | 44 (20.9) | |
| Community water | 34 (89.5) | 98 (83.0) | 166 (79.1) | |
| Gestational diabetes | 1 (2.6) | 8 (6.8) | 10 (4.8) | .552 |
| Systolic blood pressure, mm Hg | 109 (92–116) | 105 (98–112) | 104 (97–110) | .787 |
| Diastolic blood pressure, mm Hg | 65 (61–68) | 65 (61–69) | 64 (60–70) | .742 |
| Maternal BMI, kg/m2, 6 wk postpartum | 24.7 (22.8–27.0) | 26.1 (23.7–28.8) | 25.3 (23.1–29.3) | .383 |
| CD4 cell count at enrollment, cells/mm3b | .133 | |||
| 0–200 | 5 (13.2) | 32 (27.1) | NA | |
| 201–350 | 12 (31.6) | 26 (22.0) | NA | |
| 351–500 | 11 (28.9) | 21 (17.8) | NA | |
| >500 | 10 (26.3) | 39 (33.1) | NA | |
| ART regimen during pregnancy | .465 | |||
| No ARV | 2 (5.3) | 13 (11.0) | NA | |
| AZT monotherapy | 7 (18.4) | 26 (22.0) | NA | |
| cART | 29 (76.3) | 79 (67.0) | NA | |
| Cesarean section delivery | 7 (18.4) | 14 (12.3) | 32 (15.6) | .585 |
| Infants | ||||
| Female Infant | 16 (42.1) | 60 (51.7) | 109 (52.2) | .512 |
| Preterm (<37 wk GA) | 6 (15.8) | 15 (12.7) | 29 (13.8) | .887 |
| Infant birth weight, gb | 3200 (2800–3400) | 3400 (3000–3600) | 3300 (3050–3600) | .045 |
| Infant LBW | 3 (8.0) | 6 (5.1) | 7 (3.3) | .404 |
| Infant WAZ at 6 wk visit | 0.24 (−0.25, +0.85) | 0.10 (−0.57, +0.90) | 0.35 (−0.38, +0.91) | .180 |
| Infant LAZ at 6 wk visit | 0.08 (−0.64, +0.87) | 0.68 (−0.43, +1.73) | 0.92 (−0.12, +1.73) | .018 |
| Infant HCAZ at 6 wk visit | 0.30 (−0.46, +1.17) | 0.31 (−0.42,+1.29) | 0.75 (−0.27, +1.77) | .050 |
| Infant WFLZ at 6 wk visit | 0.36 (−0.31, +1.31) | −0.80 (−1.89, +0.50) | −0.63 (−1.55, +0.51) | .002 |
| Infant insulin at 6 wk visit, μIU/mL | 2.7 (0.9–5.0) | 4.0 (2.6–5.8) | 4.9 (3.5–6.6) | <.001 |
| Infant glucose to insulin ratio at 6 wk visit | 36 (19–86) | 24 (17–38) | 19 (14–28) | <.001 |
| Infant glucose, mg/dL | 95 (83–104) | 98 (90–109) | 97.0 (89–106) | .248 |
| Infant HOMA-IR at 6 wk visit | 0.65 (0.23–1.34) | 0.91 (0.60–1.45) | 1.12 (0.83–1.7) | <.001 |
| Infant HOMA-β at 6 wk visit, % | 28.2 (15.7–47.7) | 38.1 (24.6–74.3) | 52.4 (35.1–77.6) | <.001 |
Abbreviations: BMI, body mass index; LBW, low birth weight; NA, not applicable; WAZ, weight-for-age z score. All continuous variables shown as median (interquartile range) and categorical variables as n (percentage).
P value confirmed by comparing means between groups via ANOVA (mean gravida = 3.13 among mothers of HEU-A infants, 3.58 among mothers of HEU-N infants, and 3.07 among mothers of HUU infants).
Comparison among term infants only (n = 350).
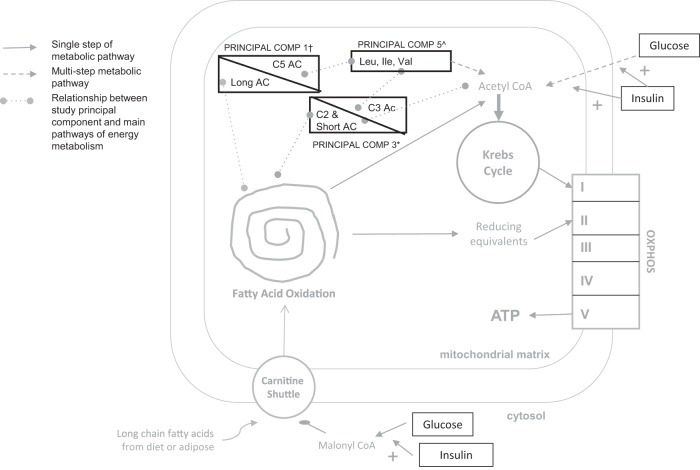
Because of the large number of individual AC and BCAA metabolites, we used PCA to consolidate and partition the AC and BCAA metabolites into evaluative measures. Eight components displayed eigen values greater than 1, but only seven components were retained for rotation based on the following: 1) scree tests, 2) the proportion of cumulative variance accounted for by the seven components (69%), and 3) optimal interpretability of the results (Table 2). In general, PC1 was comprised of long-chain ACs, free carnitine (C0), and isovaleryl-/2-methylbutyrylcarnitine (C5), PC2 of 3-hydroxy long-chain ACs, PC3 of short-chain and BCAA-related ACs, PC4 of unsaturated medium and long-chain ACs, PC5 of BCAAs, PC6 of adipoyl/3-methylglutarylcarnitine (C6-DC) and dodecanoylcarnitine (C12) ACs, and PC7 of medium-chain ACs and tetradecenoylcarnitine (C14:1) (Figure 1).
Table 2.
Results of Principal Component Analysis
| Principal Component | Metabolites Within Component | Description of Component | Eigen Value | Proportion of Variance Explained by Component | Cumulative Proportion of Variance |
|---|---|---|---|---|---|
| 1 | C0, C14, C16, C16:1, C18, C18:1, C18:2, C5 | Long-chain acylcarnitines, C0, and C5 | 11.30 | 0.30 | 0.30 |
| 2 | C12-OH, C14-OH, C14:1-OH, C16:OH, C16:1-OH, C18:OH, C18:1-OH, C18:2-OH | 3-Hydroxy long-chain acylcarnitines | 4.04 | 0.11 | 0.41 |
| 3 | C2, C3, C4, C4-OH, C5-OH, C3-DC, C4-DC, C5-DC, C8:1 | Short-chain and BCAA-related acylcarnitines | 3.18 | 0.08 | 0.49 |
| 4 | C5:1, C10:1, C10:2, C12:1, C14:2 | Unsaturated medium- and long-chain acylcarnitines | 2.71 | 0.07 | 0.56 |
| 5 | Leucine, isoleucine, valine | BCAAs | 2.11 | 0.06 | 0.62 |
| 6 | C6-DC, C12 | C6-DC and C12 acylcarnitines | 1.57 | 0.04 | 0.66 |
| 7 | C6, C8, C10, C14:1 | Medium-chain acylcarnitines and C14:1 | 1.31 | 0.03 | 0.69 |
Figure 1.
Relationship between principal components. +, PC1, long-chain fatty acylcarnitines, C0, and C5; *, PC3, short-chain fatty acyl carnitines and BCAAs; ^, PC5, BCAAs (isoleucine, leucine, valine). OXPHOS, oxidative phosphorylation.
Comparison of the study sample to those missing insulin or glucose results did not reveal differences in the primary exposure, baseline maternal characteristics at enrollment, infant sex, or WLZ at 6 weeks.
After adjusting for maternal age and highest level of education, gestational diabetes mellitus, infant sex, preterm birth, low birth weight, infant WLZ at 6 weeks, AC/BCAA PCs, and interactions between exposure groups, HEU-A (β = −.116, P = .012) and HEU-N (β = −.070, P = .022) exposure was associated with lower preprandial insulin levels at 6 weeks of life compared with no exposure (Table 3). In addition, PC3 and PC5 were positively associated with higher insulin levels (β = .044, P = .001 and β = .033, P = .012, respectively) in the model including the entire study population. PC3 consisted of ACs related to BCAA catabolism (propionylcarnitine [C3], iso-/butyrylcarnitine [C4], 3-hydroxyisovaleryl/2-methyl-3-hydroxylcarnitine [C5-OH], malonylcarnitine [C3-DC], methylmalonylcarnitine [C4-DC]), glutarylcarnitine (C5-DC) and ACs related to fatty acid oxidation and ketogenesis (acetylcarnitine [C2], C4, 3-hydroxybutyrylcarnitine [C4-OH], and octenoylcarnitine [C8:1]). PC5 consisted of the three BCAAs, leucine, isoleucine and valine (Figure 1). When we tested for interactions between exposure groups and individual PCs, we found that PC4 was positively associated with higher insulin levels in both the HEU-A group (β = .166, P = .001) and the HEU-N group (β = .095, P = .002). This positive relationship between PC4 and insulin was, however, not present in the overall cohort (β = −.029, P = .101), meaning that PC4 has a different association with insulin and may have different physiological significance among HEU infants compared with HUU infants. These findings were similar when evaluating HOMA-IR as an outcome in multivariate analysis except that the association between HEU-N infants and HOMA-IR only approached significance (β = −.045), P = .060), trending in the same direction as in the insulin model. The association between HEU-A exposure and HOMA-IR remained statistically significant (β = −.080), P = .022).
Table 3.
Linear Regression Models for Infant Preprandial Insulin and HOMA-IR
| Effect | Insulin Model |
HOMA-IR Model |
||
|---|---|---|---|---|
| Coefficient | P Value | Coefficient | P Value | |
| Infant HIV/ARV exposure status | ||||
| HIV/ARV unexposed | 0 | 0 | ||
| HIV/ARV exposed to postnatal NVP | −0.070 | .022 | −0.045 | .060 |
| HIV/ARV exposed to postnatal AZT | −0.116 | .012 | −0.080 | .022 |
| Maternal age, per year | −0.001 | .941 | −0.001 | .776 |
| Highest level of education | ||||
| Secondary school or lower | −0.027 | .325 | −0.013 | .520 |
| High school or higher | 0 | 0 | ||
| Maternal gestational diabetes status | ||||
| Gestational diabetes | 0.065 | .261 | 0.057 | .186 |
| No gestational diabetes | 0 | 0 | ||
| Infant gender | ||||
| Female | −0.010 | .709 | −0.011 | .582 |
| Male | 0 | 0 | ||
| Low birth weight (<2500 g) | −0.039 | .595 | −0.011 | .849 |
| Preterm birth (<37 wk) | −0.001 | .982 | 0.006 | .832 |
| Infant WLZ at 6-week visit | 0.003 | .623 | 0.001 | .873 |
| PC1, long-chain ACs, C0, and C5 | 0.003 | .831 | 0.005 | .642 |
| PC2, 3-hydroxy long-chain ACs | −0.009 | .535 | −0.005 | .673 |
| PC3, short-chain and BCAA-related ACs | 0.044 | .001 | 0.037 | .002 |
| PC4, unsaturated medium- and long-chain ACs | −0.029 | .101 | −0.025 | .061 |
| PC5, BCAAs | 0.034 | .012 | 0.023 | .024 |
| PC6, C6-DC and C12 ACs | 0.010 | .496 | 0.006 | .568 |
| PC7, Medium-chain ACs and C14:1 | 0.001 | .991 | −0.001 | .949 |
| HIV/ARV exposed to postnatal NVP PC4 | 0.095 | .002 | 0.078 | .001 |
| HIV/ARV exposed to postnatal AZT PC4 | 0.166 | .001 | 0.118 | .001 |
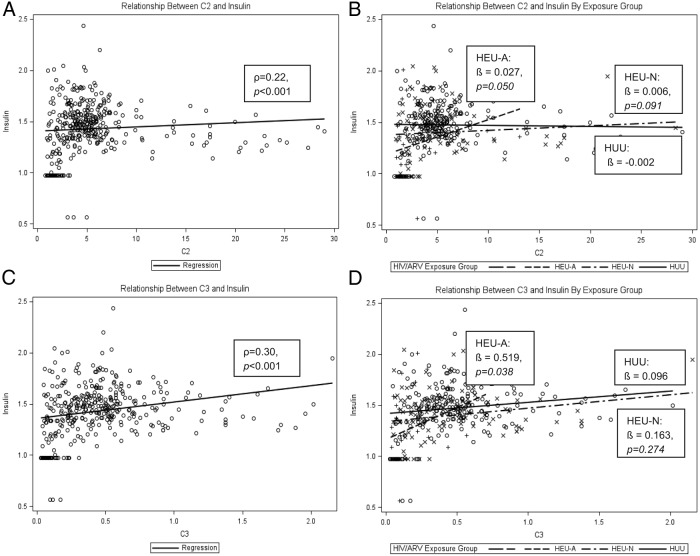
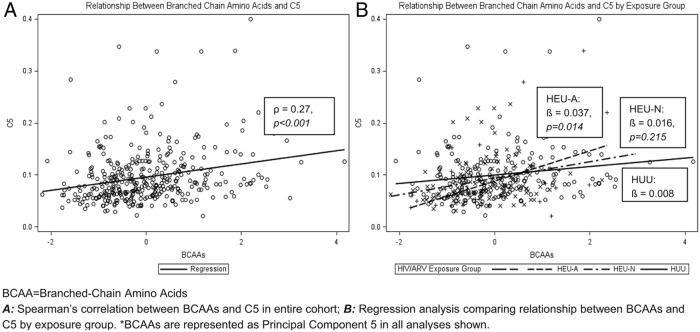
The general trend for individual ACs showed the lowest levels in HEU-A infants, intermediate levels in HEU-N infants, and the highest in HUU infants (Supplemental Table 2). Valine and leucine levels showed similar trends. Figure 2, A and C, shows overall linear associations between C2 (reflecting body stores of acetyl CoA) as well as C3 (reflecting, in part, BCAA metabolism) and preprandial insulin (ρ = 0.22, P < .001, and ρ = 0.3, P < .001, respectively). The overall correlation factors are strongly influenced by the HEU-A subpopulation. HEU-A infants demonstrated a stronger positive association between C2 and preprandial insulin compared with HUU infants (β = .027 vs β = −.002, P = .05) (Figure 2B). Findings were similar when evaluating the relationship between C3 and preprandial insulin (β = .519 vs β = .096, P = .038) (Figure 2D). The relationship between C5 and preprandial insulin was positive (ρ = 0.23, P < .001) but did not show differences between exposure groups. Lastly, plasma BCAA levels were positively correlated with C5 (ρ = 0.27, P < .001), and the small HEU-A subpopulation demonstrated the strongest linear dependence between BCAA and C5 AC (β = .037 vs β = .008, P = .014) (Figure 3).
Figure 2.
Relationships between C2 and insulin and C3 and insulin. A, Spearman's correlation between C2 and preprandial insulin in entire cohort. B, Regression analysis comparing relationship between C2 and preprandial insulin by exposure group. C, Spearman's correlation between C3 and preprandial insulin in entire cohort. D, Regression analysis comparing relationship between C3 and preprandial insulin by exposure group. *, Insulin is quarter-root-transformed for all analyses shown.
Figure 3.
Relationship between BCAAs and C5. A, Spearman's correlation between BCAAs and C5 in entire cohort. B, Regression analysis comparing relationship between BCAAs and C5 by exposure group. *, BCAAs are represented as PC5 in all analyses shown.
Discussion
In this cohort of Cameroonian HIV-infected and -uninfected woman/infant dyads, we found in utero HIV/ARV plus either postnatal AZT or NVP exposure to be associated with lower infant preprandial insulin levels at 6 weeks of life compared with no HIV/ARV exposure, even after adjusting for confounders. Moreover, HEU-A infants exhibited higher insulin at higher levels of fuel input (C2 or C3 ACs; see Figure 2) than HEU-N or HUU infants, suggesting that these infants use metabolic fuel substrates differently that may affect their future metabolic health.
The associations between postnatal AZT exposure and lower preprandial insulin, as well as lower HOMA-IR at 6 weeks of life, are novel findings. No established standards for normal serum insulin concentrations or measures of insulin sensitivity exist in infants. The median insulin values we report in the HUU group are consistent with other published studies in normal appropriate for GA (AGA) newborns (18, 19). However, median insulin and HOMA-IR levels in the HEU-A group correspond to the fifth percentile levels for these same variables in a Mexican study of AGA newborns (19). In adults, lower fasting insulin and HOMA-IR reflect increased insulin sensitivity (20).
Alterations in insulin sensitivity seen in our cohort of HEU infants may have long-term clinical implications. It would appear that HEU-A and, to some degree, HEU-N infants in our cohort are more insulin sensitive than HUU infants, consistent with a more metabolically efficient or thrifty phenotype as described in the hypothesis by Barker (21). This phenotype was also observed in a longitudinal study of small for GA (SGA) and AGA infants evaluated from birth to 3 years wherein SGA infants also had lower fasting insulin, higher glucose to insulin ratios, and increased insulin sensitivity at birth than AGA infants. However, by 3 years, these trends had significantly reversed with these same SGA children now exhibiting higher fasting insulin and decreased insulin sensitivity compared with their AGA counterparts (22, 23). A French study found similar longitudinal trends between birth and 4 years using oral glucose tolerance tests in which SGA infants began life as more insulin sensitive but, by age 4 years, were less insulin sensitive than their AGA counterparts (24). In addition, a British study evaluating fetal insulin levels by cordocentesis reported lower fetal insulin and higher fetal glucose to insulin ratios in SGA fetuses between 20 and 38 weeks of GA compared with AGA fetuses (25).
The apparent increased metabolic efficiency of the HEU-A infants seems to mirror that of SGA infants early in life. Although we do not have longitudinal data on whether this increased insulin sensitivity reverses later in life as in SGA infants, our cross-sectional data do reveal some concerning associations indicative of a possible risk for worsening insulin sensitivity later in life. In exploring the relationship between C2 and preprandial insulin, we see that the HEU-A infants use fuel differently than HEU-N and HUU infants (Figure 2B). C2 may be described as a general marker of fuel input/production because fat, carbohydrate, and protein metabolism all result in the formation of acetyl CoA, a precursor for C2. C2 thus reflects whole-body acetyl CoA stores. At lower levels of fuel input into the tricyclic acid cycle (reflected by C2), HEU-A infants handle fuel more efficiently with less amounts of insulin than HEU-N or HUU infants. However, as fuel input (proportional to C2) increases, we see that HEU-A infants actually require more insulin than their HEU-N and HUU counterparts, raising the notion that perhaps insulin resistance may be provoked later in life, perhaps with a diabetogenic diet that would create a fuel overload situation. In addition, we see similar findings when we observe the association between C3 and preprandial insulin in our cohort (Figure 2D). C3 may be described as a marker of BCAA load as C3 (along with C5) reflects the levels of BCAA metabolites.
One mechanism that might explain the changes in fuel use we see in the HEU-A infants is mitochondrial dysregulation. AZT is a known mitochondrial toxin. AZT inhibits mitochondrial DNA (mtDNA) polymerase-γ, resulting in the production of defective mtDNA (3), which, in turn, diminishes levels of mtDNA/RNA and disrupts oxidative phosphorylation, leading to mitochondrial dysfunction (2, 26). A recent study demonstrated mitochondrial dysfunction in leukocytes caused by ART toxicity in 52 pregnant woman/infant dyads (27). All of the HIV-infected women received cART, which included nucleoside reverse transcriptase inhibitors, and 33 of 35 of the HEU infants received AZT monotherapy. It is reasonable to speculate that the initial apparent thrifty phenotype we see among HEU-A infants may be reflective of altered mitochondria, which have adapted to maximally produce energy from low levels of fuel (low C2 associated with low insulin). However, once HEU-A infants age or gain increased fuel input in their diet, decreased insulin sensitivity due to mitochondrial-related deficiencies may be provoked later in life. Furthermore, the relationship that we observe between C3 and preprandial insulin (Figure 2D) may suggest a predisposition for mitochondrial overload in HEU-A infants. BCAA metabolism via anapleurotic flux into the succinyl CoA pool, if excessive, can overload the tricyclic acid cycle, resulting in the accumulation of acetyl-CoA (28). The relationship between C3 and insulin (Figure 2D) may be considered a contributing cause, whereas the relationship between C2 and insulin (Figure 2B) may be considered as an effect. Lastly, C5 levels increase faster with BCAA levels for HEU-A infants than for HEU-N and HUU infants (Figure 3B), again suggesting HEU-A infants may be predisposed to mitochondrial fuel overload.
We also observed a positive association between a signature array of BCAAs and short-chain ACs involved in fatty acid oxidation and both infant preprandial insulin levels and HOMA-IR in the entire cohort. (Table 3) This reflects a diminished flux of protein and fat substrates through their catabolic pathways, a scenario with potential implications for metabolic gene regulation and the development of chronic metabolic disorders. Our results are consistent with adult studies linking ACs, BCAAs, and aromatic amino acids (phenylalanine and tyrosine) to increased HOMA-IR (7, 8, 29–32). A nested case-control study in the Framingham offspring cohort reported a significant association between a metabolite cluster including the BCAAs and aromatic amino acids and future type 2 diabetes (30). Another study found a metabolite group including the BCAAs, the aromatic amino acids, C3 and C5 ACs, methionine, and glutamate/glutamine to be highly correlated with HOMA-IR and increased in obese compared with lean adults (29). Furthermore, the large European Prospective Investigation into Cancer and Nutrition-Potsdam cohort study reported a metabolite cluster containing BCAAs, aromatic amino acids, diacyl-phosphatidylcholines, propionylcarnitine (C3), and hexose was associated with a 3.8-fold increased risk of type 2 diabetes (32). Few studies evaluating these associations in children exist (9). A longitudinal study of 25 healthy children aged 8–18 years reported that baseline BCAAs were positively associated with HOMA-IR measured 18 months later. A positive relationship between BCAAs and insulin has also been demonstrated in intrauterine growth retardation (IUGR) twin fetuses with discordant growth in which IUGR fetuses displayed lower insulin levels than their AGA twins (33).
Whereas several studies have evaluated metabolic outcomes in HIV-infected children (34), few studies have demonstrated relationships between insulin sensitivity and intermediary metabolism using HEU and HUU children as comparator groups. One Brazilian cross-sectional study of 76 prepubertal children described differences in metabolic measures between HEU and HUU children (35). No differences in insulin or HOMA-IR were reported, although this study was limited by its small sample size and inability to adequately adjust for confounders. Another US study reported higher rates of abnormal newborn metabolic screens in HIV-exposed vs -unexposed infants (36). In addition, among HIV-exposed infants, those exposed to in utero ARVs were significantly more likely than ARV-unexposed infants to exhibit an abnormal AC (>99th percentile compared with age matched references). Lastly, a recent US study evaluated a cohort of HEU children for rates of abnormal AC profiles found that GA as well as antenatal alcohol and protease inhibitor exposure were associated with an increased risk of abnormal ACs (37). This study, however, did not evaluate other metabolic outcomes as ours did and lacked a comparison group of HUU children.
Our study is limited by the lack of longitudinal data on insulin in our infant cohort, restricting our ability to draw conclusions about the long-term impact of our findings. Insulin and glucose, although more commonly measured in serum and plasma samples, respectively, from venous blood, were measured in our study from DBS and capillary blood specimens. However, both fasting and nonpreprandial insulin levels measured from DBS have been shown to have high correlation (ρ = 0.93 and 0.97, respectively) in other studies (38, 39), and appropriately obtained capillary glucose specimens are comparable with those obtained by venous sampling (40). In addition, we acknowledge that fasting/preprandial insulin and HOMA-IR have not been well validated in infants, but performing the gold standard hyperinsulinemic-euglycemic clamp in infants would have been impractical. Because of this, we are cautious not to overinterpret our findings without additional data on normal changes in glucose metabolism throughout infancy. We also were unable to assess other domains of metabolic health such as infant adiposity because this would not have been feasible in our study setting. Although we hypothesize that mitochondrial toxicity from AZT may play a role in readjusting β-cell function, resulting in lower infant insulin levels, we were unable to assess mitochondrial function or β-cell damage, but we do observe possible alterations in β-cell responsiveness for the HEU-A vs HEU-N and HUU infants groups. Lastly, birth length and head circumference were not available on all infants due to differing practices at different birthing sites. We were not able to evaluate for the presence of SGA or IUGR as obstetrical ultrasounds are not part of routine clinical care at our site.
In conclusion, in utero HIV/ARV and postnatal ARV exposure affects relationships between insulin sensitivity and intermediary metabolism which appear to alter the way in which HEU infants (in particular, HEU-A) use metabolic fuel substrates. Our data support a hypothesis that in utero and postnatal HIV/ARV exposure affects relationships between insulin sensitivity and intermediary metabolism, which may manifest itself later in life as a predisposition to the development of insulin resistance. Because in utero and postnatal ARVs are the key to preventing mother-to-child HIV transmission, it is critical to understand the long-term health implications of the metabolic alterations noted in our cohort. Further studies are warranted to monitor HEU-A and HEU-N infants for the development of insulin resistance in later life, particularly those with low insulin/AC/BCAA levels in early infancy and to assess potential etiological links with mitochondrial and intermediary metabolic regulation.
Acknowledgments
We thank all the study participants and staff at Cameroon Baptist Convention Health Services Nkwen Family Care and Treatment Center without whose involvement and support this study would not have been possible. We also gratefully acknowledge Dr Christopher Newgard (Duke University Medical Center) for his advice and input during the study analysis.
This work was supported by the Icahn School of Medicine at Mount Sinai Global Health Innovation Fund. J.J. is supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grant K23HD070760. B.K. was supported by Grant KL2TR000076 from the National Institutes of Health National Center for Advancing Translational Sciences during the preparation of this manuscript. K.P. is supported by NICHD Grant K23HD070774. Y.Q. is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant P60DK020541. M.G. is partially supported by the Pediatric HIV/AIDS Cohort Study, which is supported by the NICHD with cofunding from the National Institute of Allergy and Infectious Diseases, the National Institute on Drug Abuse, the National Institute of Mental Health, the National Institute on Deafness and Other Communication Disorders, the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard University School of Public Health (Grant U01 HD052102-04) and the Tulane University School of Medicine (Grant U01 HD052104-01). I.J.K. is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant P60DK020541 (Einstein Diabetes Research and Training Center) and National Institute of Allergy and Infectious Diseases Grant 1U19AI091175 (Einstein Center for Medical Countermeasures against Radiation).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AC
- acylcarnitine
- AGA
- appropriate for gestational age
- ART
- antiretroviral treatment
- ARV
- antiretroviral
- AZT
- zidovudine
- BCAA
- branched-chain amino acid
- cART
- combination ART
- DBS
- dried blood spot
- GA
- gestational age
- HCAZ
- head circumference-for-age z
- HEU
- HIV-exposed uninfected
- HEU-A
- HIV/ARV and postnatal AZT-HEU
- HEU-N
- HIV/ARV and postnatal NVP-HEU
- HOMA-IR
- homeostasis model assessment for insulin resistance
- HUU
- HIV/ARV-unexposed uninfected
- IUGR
- intrauterine growth retardation
- LAZ
- length-for-age z
- mtDNA
- mitochondrial DNA
- NVP
- nevirapine
- PC
- principal component
- PCA
- PC analysis
- SGA
- small for GA
- WLZ
- weight-for-length z.
References
- 1. Barker DJ. The developmental origins of insulin resistance. Horm Res. 2005;64(suppl 3):2–7. [DOI] [PubMed] [Google Scholar]
- 2. Dalakas MC, Illa I, Pezeshkpour GH, Laukaitis JP, Cohen B, Griffin JL. Mitochondrial myopathy caused by long-term zidovudine therapy. N Engl J Med. 1990;322:1098–1105. [DOI] [PubMed] [Google Scholar]
- 3. Konig H, Behr E, Lower J, Kurth R. Azidothymidine triphosphate is an inhibitor of both human immunodeficiency virus type 1 reverse transcriptase and DNA polymerase gamma. Antimicrob Agents Chemother. 1989;33:2109–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jao J, Abrams EJ. Metabolic complications of in utero maternal HIV and antiretroviral exposure in HIV-exposed infants. Pediatr Infect Dis J. 2014;33:734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. [DOI] [PubMed] [Google Scholar]
- 6. Wang CH, Wang CC, Wei YH. Mitochondrial dysfunction in insulin insensitivity: implication of mitochondrial role in type 2 diabetes. Ann NY Acad Sci. 2010;1201:157–165. [DOI] [PubMed] [Google Scholar]
- 7. Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes. 2013;62:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu J, Xie G, Jia W. Insulin resistance and the metabolism of branched-chain amino acids. Front Med. 2013;7:53–59. [DOI] [PubMed] [Google Scholar]
- 9. McCormack SE, Shaham O, McCarthy MA, et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr Obes. 2013;8:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jao J, Kirmse K, Yu C, et al. 2015 Lower insulin, acylcarnitines, and branch-chain amino acids in HIV-exposed infants. Abstract presented at Conference on Retroviruses and Opportunistic Infections; February 2015, Seattle, WA. [Google Scholar]
- 11. World Health Organization. Antiretroviral Drugs for treating Pregnant Women and Preventing HIV Infection in Infants. Geneva: WHO Press; 2010. [PubMed] [Google Scholar]
- 12. World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Geneva; WHO Press; 2013. [PubMed] [Google Scholar]
- 13. Kapur S, Zava D. Cardiometabolic risk factors assessed by a finger stick dried blood spot method. J Diabetes Sci Technol. 2008;2:236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 15. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(suppl 1):S11–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rinaldo P, Cowan TM, Matern D. Acylcarnitine profile analysis. Genet Med. 2008;10:151–156. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Geneva: World Health Organization; 2006. [Google Scholar]
- 18. Gesteiro E, Bastida S, Sanchez-Muniz FJ. Insulin resistance markers in term, normoweight neonates. The Merida cohort. Eur J Pediatr. 2009;168:281–288. [DOI] [PubMed] [Google Scholar]
- 19. Simental-Mendia LE, Castaneda-Chacon A, Rodriguez-Moran M, Guerrero-Romero F. Birth-weight, insulin levels, and HOMA-IR in newborns at term. BMC Pediatr. 2012;12:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E26. [DOI] [PubMed] [Google Scholar]
- 21. Barker DJ. Rise and fall of Western diseases. Nature. 1989;338:371–372. [DOI] [PubMed] [Google Scholar]
- 22. Bazaes RA, Salazar TE, Pittaluga E, et al. Glucose and lipid metabolism in small for gestational age infants at 48 hours of age. Pediatrics. 2003;111:804–809. [DOI] [PubMed] [Google Scholar]
- 23. Mericq V, Ong KK, Bazaes R, et al. Longitudinal changes in insulin sensitivity and secretion from birth to age three years in small- and appropriate-for-gestational-age children. Diabetologia. 2005;48:2609–2614. [DOI] [PubMed] [Google Scholar]
- 24. Milovanovic I, Njuieyon F, Deghmoun S, Chevenne D, Levy-Marchal C, Beltrand J. SGA children with moderate catch-up growth are showing the impaired insulin secretion at the age of 4. PLoS One. 2014;9:e100337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Economides DL, Proudler A, Nicolaides KH. Plasma insulin in appropriate- and small-for-gestational-age fetuses. Am J Obstet Gynecol. 1989;160:1091–1094. [DOI] [PubMed] [Google Scholar]
- 26. Lewis W, Simpson JF, Meyer RR. Cardiac mitochondrial DNA polymerase-γ is inhibited competitively and noncompetitively by phosphorylated zidovudine. Circ Res. 1994;74:344–348. [DOI] [PubMed] [Google Scholar]
- 27. Moren C, Noguera-Julian A, Garrabou G, et al. Mitochondrial disturbances in HIV pregnancies. AIDS. 2015;29:5–12. [DOI] [PubMed] [Google Scholar]
- 28. Kurland IJ, Accili D, Burant C, et al. Application of combined omics platforms to accelerate biomedical discovery in diabesity. Ann NY Acad Sci. 2013;1287:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huffman KM, Shah SH, Stevens RD, et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care. 2009;32:1678–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Floegel A, Stefan N, Yu Z, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes. 2013;62:639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bajoria R, Sooranna SR, Ward S, Hancock M. Placenta as a link between amino acids, insulin-IGF axis, and low birth weight: evidence from twin studies. J Clin Endocrinol Metab. 2002;87:308–315. [DOI] [PubMed] [Google Scholar]
- 34. Geffner ME, Patel K, Miller TL, et al. Factors associated with insulin resistance among children and adolescents perinatally infected with HIV-1 in the pediatric HIV/AIDS cohort study. Horm Res Paediatr. 2011;76:386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Claudio CC, Patin RV, Palchetti CZ, Machado DM, Succi RC, Oliveira FL. Nutritional status and metabolic disorders in HIV-exposed uninfected prepubertal children. Nutrition. 2013;29:1020–1023. [DOI] [PubMed] [Google Scholar]
- 36. Kirmse B, Hobbs CV, Peter I, et al. Abnormal newborn screens and acylcarnitines in HIV-exposed and ARV-exposed infants. Pediatr Infect Dis J. 2013;32:146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kirmse B, Yao T, Hofher S, et al. Abstract presented at the Conference on Retroviruses and Opportunistic Infections March 2014 Boston, MA. [Google Scholar]
- 38. Dowlati B, Dunhardt PA, Smith MM, Shaheb S, Stuart CA. Quantification of insulin in dried blood spots. J Lab Clin Med. 1998;131:370–374. [DOI] [PubMed] [Google Scholar]
- 39. Butter NL, Hattersley AT, Clark PM. Development of a bloodspot assay for insulin. Clin Chim Acta. 2001;310:141–150. [DOI] [PubMed] [Google Scholar]
- 40. Cowett RM, D'Amico LB. Capillary (heelstick) versus venous blood sampling for the determination of glucose concentration in the neonate. Biol Neonate. 1992;62:32–36. [DOI] [PubMed] [Google Scholar]