Abstract
Context:
There are conflicting reports of increased vs decreased hypothalamic-pituitary-adrenal (HPA) activation in obesity; the most consistent finding is an inverse relationship between body mass index (BMI) and morning cortisol. In anorexia nervosa (AN), a low-BMI state, cortisol measures are elevated.
Objective:
This study aimed to investigate cortisol measures across the weight spectrum.
Design and Setting:
This was a cross-sectional study at a clinical research center.
Participants:
This study included 60 women, 18–45 years of age: overweight/obese (OB; N = 21); AN (N = 18); and normal-weight controls (HC; N = 21).
Measures:
HPA dynamics were assessed by urinary free cortisol, mean overnight serum cortisol obtained by pooled frequent sampling every 20 minutes from 2000–0800 h, 0800 h serum cortisol and cortisol-binding globulin, morning and late-night salivary cortisol, and dexamethasone-CRH testing. Body composition and bone mineral density (BMD) were assessed by dual-energy x-ray absorptiometry.
Results:
Cortisol measures demonstrated a U-shaped relationship with BMI, nadiring in the overweight-class I obese range, and were similarly associated with visceral adipose tissue and total fat mass. Mean cortisol levels were higher in AN than OB. There were weak negative linear relationships between lean mass and some cortisol measures. Most cortisol measures were negatively associated with postero-anterior spine and total hip BMD.
Conclusions:
Cortisol measures are lowest in overweight-class I obese women—lower than in lean women. With more significant obesity, cortisol levels increase, although not to as high as in AN. Therefore, extreme underweight and overweight states may activate the HPA axis, and hypercortisolemia may contribute to increased adiposity in the setting of caloric excess. Hypercortisolemia may also contribute to decreased BMD and muscle wasting in the setting of both caloric restriction and excess.
Activation or suppression of the hypothalamic-pituitary-adrenal (HPA) axis is associated with alterations in body composition. Pathologic hypercortisolemia, as in Cushing's syndrome, results in weight gain, increased intra-abdominal fat, decreased lean body mass, and bone loss (1, 2). In contrast, patients with hypocortisolemia due to adrenal insufficiency lose weight (3). More subtle changes in HPA function may be present in women at the extremes of the weight spectrum, but such abnormalities are not well characterized, particularly in severely obese individuals, and their association with body composition and bone mineral density (BMD) in both underweight and overweight states is unclear.
There are conflicting reports of HPA dynamics in overweight and obese subjects. The most consistent finding in some, but not all, studies is a negative linear relationship between body mass index (BMI) and morning serum or salivary cortisol (4–8). Moreover, previous studies have used waist circumference or waist-to-hip ratio to evaluate the relationship between abdominal adiposity and cortisol measures with inconsistent results (9–11). Only one prior study radiographically measured adipose tissue, and showed no significant association between visceral adipose tissue, as measured by computed tomography scan, and 24-hour urinary free cortisol (UFC) (12). Whether abnormalities in HPA function contribute to weight gain, adiposity, or their metabolic consequences is therefore unknown.
Anorexia nervosa (AN), a psychiatric disorder characterized by food restriction despite extremely low body weight and fat mass, is associated with elevated UFC (13, 14) and late-night salivary cortisol (LNSC) (15), as well as lack of cortisol suppression on 1 mg overnight dexamethasone suppression testing (13, 16) and dexamethasone suppression-CRH stimulation (dex-CRH) testing (14). Despite hypercortisolemia, these women do not appear Cushingoid, presumably due to lack of substrate. With weight gain, however, there is a positive correlation between baseline UFC and percent increase in truncal fat (17). Some studies have shown normalization of cortisol measures with weight gain (13, 18), but no study has evaluated the association of BMI or body composition with comprehensive cortisol measures in AN.
Pathologic hypercortisolemia is a known risk factor for metabolic bone disease. Cushing's syndrome is associated with increased bone resorption, decreased BMD, and increased risk of fractures (2, 19). AN is also associated with increased bone resorption, decreased BMD, and increased fracture risk (20, 21). Of note, UFC and mean overnight serum cortisol are negatively correlated with BMD in AN (22, 23). Although there is increasing recognition that obesity is associated with increased fracture risk (24), the relationship between cortisol measures and BMD in overweight/obese individuals remains unknown.
We hypothesized that although mean cortisol levels would be higher in women with AN compared with normal-weight controls, they would be comparable between overweight/obese women and normal-weight controls. However, we predicted that higher cortisol levels would be associated with visceral adiposity in overweight/obese women, similar to patients with Cushing's syndrome, and that cortisol levels would be negatively associated with BMD across the weight spectrum.
Materials and Methods
This study was approved by the Institutional Review Board of Partners Health Care, Inc. Written informed consent was obtained from all subjects prior to procedures. We studied 60 premenopausal women, 18–45 years old; 21 overweight or obese (OB), 18 AN, and 21 normal-weight healthy controls (HC). Patient characteristics and mean overnight serum cortisol every 20 minutes between 2000 and 0800 h and its relationship to BMD in AN, but not OB, were previously reported (23, 25–27). Morning serum cortisol, cortisol-binding globulin (CBG), UFC, morning and LNSC, and dex-CRH testing results have not been previously published, and the relationship between these HPA measures and body composition or BMD has not been reported.
All women in the OB group had BMIs between 25 and 40 kg/m2. Overweight was defined as BMI 25–29.9 kg/m2; class I obesity, BMI 30–34.9 kg/m2; and class II obesity, BMI 35–39.9 kg/m2, as categorized by the World Health Organization (WHO) (28). Subjects with AN met Diagnostic and Statistical Manual of Mental Disorders IV criteria, including intense fear of gaining weight, body image disturbance, weight < 85% of ideal body weight (IBW), and amenorrhea for at least 3 consecutive months. Percent IBW was calculated using 1983 Met Life tables taking into account frame size. HC were at least 90% of IBW with a BMI < 25 kg/m2. Exclusion criteria for all subjects included diabetes mellitus, drug or alcohol abuse, abnormal thyroid function, use of medications known to affect cortisol levels (including estrogen or depot medroxyprogesterone), and pregnancy or breastfeeding. Additional exclusion criteria for the OB and HC groups included significant medical problems, amenorrhea (current or past), disordered eating, or significant anxiety or depression.
BMD and body composition were assessed by dual-energy x-ray absorptiometry (Hologic 4500, Hologic, Inc), with a precision of 0.01 g/cm2 at the lumbar spine and a precision of 3% for fat mass (29). Fat mass index (FMI) was calculated by dividing fat mass in kilograms by height in meters2 (30). Kelly et al (30) developed sex-specific FMI categories using the population prevalence of each of the WHO BMI categories described above to generate FMI categories with similar prevalence. The classification scheme for FMI in women is as follows: normal FMI, 5–9 kg/m2; excess fat, 9–13 kg/m2; class I obese, 13–17 kg/m2; class II obese, 17–21 kg/m2; and class III obese, at least 21 kg/m2 (30).
Participants were asked to collect 24-hour urine samples, as well as 2300 and 0700 h salivary samples within a week of an inpatient, overnight visit. Creatinine clearance (CrCl) was measured for each sample, and UFC/CrCl was calculated for each sample to correct for the decreased creatinine and filtered cortisol associated with AN (31). At the inpatient visit, an IV catheter was placed and subjects were allowed to acclimate to their rooms for at least 2 hours, followed by frequent sampling of blood every 20 minutes from 2000 to 0800 h. Subjects fasted starting at 2000 h and were allowed to sleep through the night. Fasting cortisol and CBG levels were obtained at 0800 h. Serum samples were pooled for mean overnight serum cortisol levels. OB and HC presented for overnight visits during the follicular phase of the menstrual cycle.
Subjects also presented at a morning outpatient visit for a dex-CRH test as previously described (32). Participants were instructed to take oral dexamethasone (0.5 mg every 6 h) for 48 hours to reduce endogenous variability in cortisol levels. At 0800 h, 2 hours after the final dexamethasone dose, corticorelin (1 μg/kg Acthrel; max dose, 100 μg; Ferring Pharmaceuticals) was administered iv. Serum cortisol levels were measured 5 minutes before and 15 minutes after corticorelin administration. Baseline dexamethasone levels were measured. The iv was placed at least 15 minutes prior to drawing blood.
Biochemical analysis
Urinary free cortisol was measured by immunoassay (Immulite, Diagnostics Product Corp), with a sensitivity of 0.20 μg/dL and reference range of 20–70 μg/day. Serum cortisol was measured by chemiluminescent microparticle immunoassay (Architect System, Abbot Diagnostics), with a sensitivity of 0.8 μg/dL. Serum-free cortisol was measured by liquid chromatography tandem mass spectrometry, equilibrium dialysis (LC/MS/equilibrium dialysis) (Quest Diagnostics), with a sensitivity of 0.1 μg/dL. Salivary cortisol was measured using EIA (Salimetrics), with a sensitivity of 0.003 mg/dL and reference range of 0.007–0.115 μg/dL. In Table 1 and Figure 1, a normal range was calculated and reported as mean ± 2 SD of the HC group for all cortisol measures.
Table 1.
Subject Characteristics and Cortisol Measures
| Subject Characteristics | AN (n = 18) | HC (n = 21) | OB (n = 21) | Combined (n = 60) | ANOVA P Value | AN v HC P Value | AN v OB P Value | HC v OB P Value |
|---|---|---|---|---|---|---|---|---|
| Age, y | 26 ± 6 | 27 ± 7 | 30 ± 8 | 28 ± 7 | NS | |||
| BMI, kg/m2 | 18.2 ± 1.0 | 22.3 ± 1.4 | 31.2 ± 4.3 | 24.1 ± 6.0 | <.0001 | <.0001 | <.0001 | <.0001 |
| Visceral adipose tissue, g | 90 ± 39 | 215 ± 85 | 481 ± 185 | 255 ± 196 | <.0001 | .002 | <.0001 | <.0001 |
| Total fat mass, kg | 9.3 ± 2.3 | 16.9 ± 3.6 | 32.5 ± 9.1 | 19.4 ± 11.0 | <.0001 | .0001 | <.0001 | <.0001 |
| Lean body mass, kg | 40.7 ± 3.6 | 43.4 ± 4.8 | 51.7 ± 7.3 | 45.1 ± 7.0 | <.0001 | NS | <.0001 | <.0001 |
| Cortisol measures | ||||||||
| UFC, μg/d (13.5–88.7)a | 82.0 ± 42.3 | 51.1 ± 18.8 | 67.0 ± 35.2 | 63.7 ± 33.0 | .04 | .01 | NS | NS |
| UFC/CrCl, μg · kg/mg · d (0.15–1.23)a | 1.16 ± 0.52 | 0.69 ± 0.27 | 0.61 ± 0.25 | 0.76 ± 0.39 | .0004 | .0007 | .0002 | NS |
| Overnight serum cortisol, μg/dL (4.0–11.2)a | 11.6 ± 3.3 | 7.6 ± 1.8 | 6.2 ± 1.7 | 8.1 ± 3.1 | <.0001 | <.0001 | <.0001 | .05 |
| Overnight serum unbound cortisol, ng/mL (3.7–12.7)a | 14.2 ± 6.7 | 8.2 ± 2.3 | 6.8 ± 2.3 | 9.2 ± 4.8 | <.0001 | <.0001 | <.0001 | NS |
| Morning serum cortisol, μg/dL (4.1–39.7)a | 26.9 ± 8.1 | 21.9 ± 8.9 | 18.3 ± 6.8 | 22.0 ± 8.6 | .01 | NS | .003 | NS |
| Morning serum unbound cortisol, ng/mL (0–98.0)a | 51.0 ± 21.1 | 44.7 ± 26.7 | 34.9 ± 21.6 | 42.9 ± 24.1 | NS | |||
| LNSC, μg/dL (0.01–0.09)a | 0.06 ± 0.03 | 0.05 ± 0.02 | 0.04 ± 0.01 | 0.05 ± 0.02 | .08 | |||
| Morning salivary cortisol, μg/dL (0–1.01)a | 0.71 ± 0.43 | 0.43 ± 0.29 | 0.29 ± 0.15 | 0.48 ± 0.35 | .005 | .02 | .001 | NS |
| CBG, μg/mL (11.7–31.7)a | 24.4 ± 3.9 | 21.7 ± 5.0 | 20.2 ± 3.0 | 21.9 ± 4.4 | .02 | NS | .006 | NS |
| Dex-CRH 0-min cortisol, μg/dL (0.05–0.73)a | 1.86 ± 3.85 | 0.39 ± 0.17 | 0.34 ± 0.22 | 0.81 ± 2.17 | .07 | |||
| Dex-CRH 15-min cortisol, μg/dL (0–1.4)a | 2.05 ± 4.22 | 0.61 ± 0.41 | 0.46 ± 0.21 | 0.61 ± 0.51 | .09 | |||
| Dex level prior to CRH stimulation, ng/dL (237–857)a | 386 ± 99 | 547 ± 155 | 554 ± 204 | 499 ± 174 | .004 | .004 | .003 | NS |
Abbreviations: dex, dexamethasone; NS, not significant.
Values are mean ± 2 sdof the HC group.
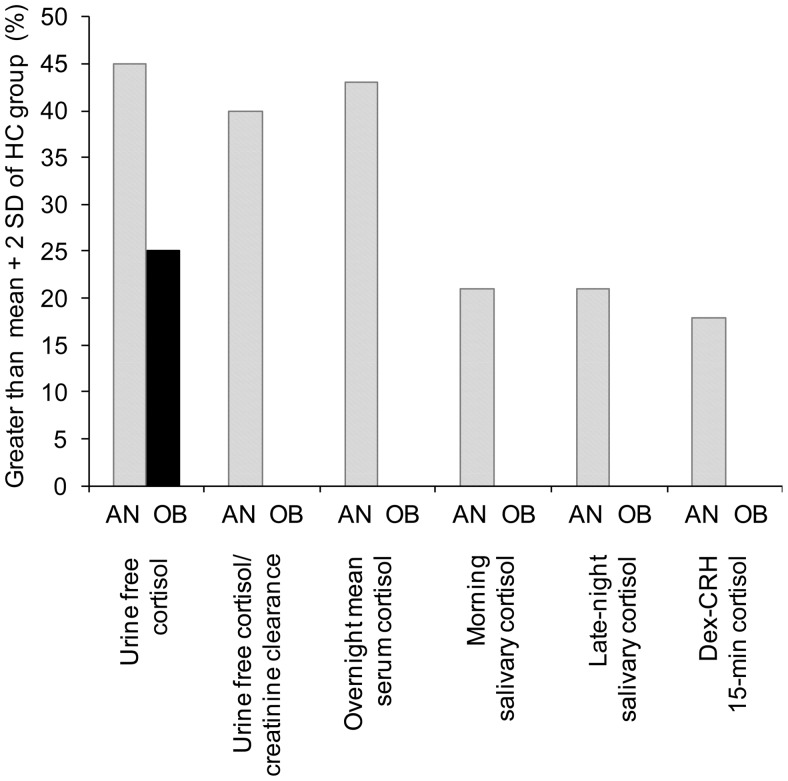
Figure 1.
Percentage of subjects in the AN and OB groups with elevated cortisol measures as defined as greater than the mean + 2 SDs of the HC group. Cortisol measures were elevated in 15–45% of AN subjects. UFC was the only cortisol measure elevated in any OB subjects.
CBG was measured by ELISA (BioVendor), with a sensitivity of 0.01 ng/mL. Unbound serum cortisol was estimated using Coolens equation (33). Dexamethasone was measured using HPLC-tandem mass spectrometry (Esoterix, Inc), with a sensitivity of 5 ng/dL. Coefficients of variation were < 10% for all assays.
Data analysis
JMP Statistical Discoveries (SAS Institute, Inc) was used for statistical analyses. Three outliers for morning salivary cortisol and LNSC were excluded (1 AN, 2 OB) using a quantile analysis. Clinical characteristics and hormone levels were compared using Fisher's Least Significant Difference Test. Additional correction for multiple comparisons is not indicated when this method is used for three-group comparisons (34). Data modeling was performed to determine the best fit between cortisol measures and BMI or body composition measurements. Multivariate least-square analyses were constructed to control for potential confounders. Statistical significance was defined as a two-tailed P ≤ .05. Data are reported as mean ± SD or as a correlation coefficient (R) with an associated P value.
Results
Baseline characteristics
The groups did not differ in mean age (Table 1). Per study design, BMI was highest in the OB group, lowest in the AN group, and intermediate in the HC group (Table 1). BMI ranged from 16.0 to 39.8 kg/m2. As expected, visceral adipose tissue and total fat mass were also highest in the OB group, lowest in the AN group, and intermediate in the HC group (Table 1). Lean body mass was significantly greater in the OB group compared with the AN or HC groups (Table 1).
UFC
Mean UFC was significantly higher in the AN group compared with the HC group (P = .01); there was no difference in mean UFC between the OB and HC groups (Table 1). Mean UFC/CrCl was significantly higher in the AN group compared with the OB (P = .0002) and HC (P = .0007) groups; there was no difference in mean UFC/CrCl between the OB and HC groups (Table 1).
Overnight serum cortisol
Mean overnight serum cortisol was also significantly higher in the AN group compared with the OB (P < .0001) and HC (P < .0001) groups (Table 1). These differences remained significant when estimating mean overnight serum unbound cortisol (P < .0001), which was calculated given a significantly higher CBG level in the AN group compared with the OB group (Table 1). Although the OB group had significantly higher mean overnight serum cortisol than the HC group (P = .05), there was no significant difference between the two groups in mean overnight serum unbound cortisol (Table 1).
LNSC
The differences in LNSC between the three groups did not reach significance (P = .08) (Table 1).
Morning cortisol
In addition to having higher UFC and overnight serum cortisol measures, the AN group also had significantly higher morning serum and salivary cortisol measures compared with the OB group (P = .003 and .001, respectively) (Table 1). Morning serum unbound cortisol was not significantly different between the three groups (Table 1).
dex-CRH stimulation testing results
Morning serum cortisol after dexamethasone suppression and after subsequent CRH administration trended toward being significantly different between the three groups (Table 1). The AN group had significantly lower baseline dexamethasone levels compared with the OB (P = .003) and HC (P = .004) groups (Table 1). After controlling for differences in CBG levels and baseline dexamethasone levels, morning serum cortisol after dexamethasone suppression and after subsequent CRH administration was not significantly different between the three groups.
Elevated cortisol measures in AN and OB groups
The percentage of subjects in the AN and OB groups with elevated cortisol measures, as defined as greater than the mean + 2 SD in the HC group, was determined. The mean + 2 SD of UFC in the HC group was 89 μg/day. The mean + 2 SD of LNSC in the HC group was 0.09 μg/dL. The mean + 2 SD of dex-CRH 15-minute serum cortisol in the HC group was 1.4 μg/dL, which is the same as the published cutoff for the dex-CRH test (32).
Cortisol measures were elevated in 15–45% of AN subjects (Figure 1). Three AN subjects “failed” the dex-CRH test with a 15-minute serum cortisol > 1.4 μg/dL (Figure 1). The dexamethasone levels in these three cases were 282, 332, and 386 ng/dL, and none of these subjects were receiving medications that are inducers of cytochrome P450 variants 3A4, 5, and 7, of which dexamethasone is a substrate. UFC was the only elevated cortisol measure in OB subjects; it was elevated in 25% of the OB group (Figure 1). These OB subjects had no specific symptoms or signs of Cushing's syndrome, and had normal LNSC and dex-CRH testing results (Figure 1).
Relationship between cortisol measures and BMI
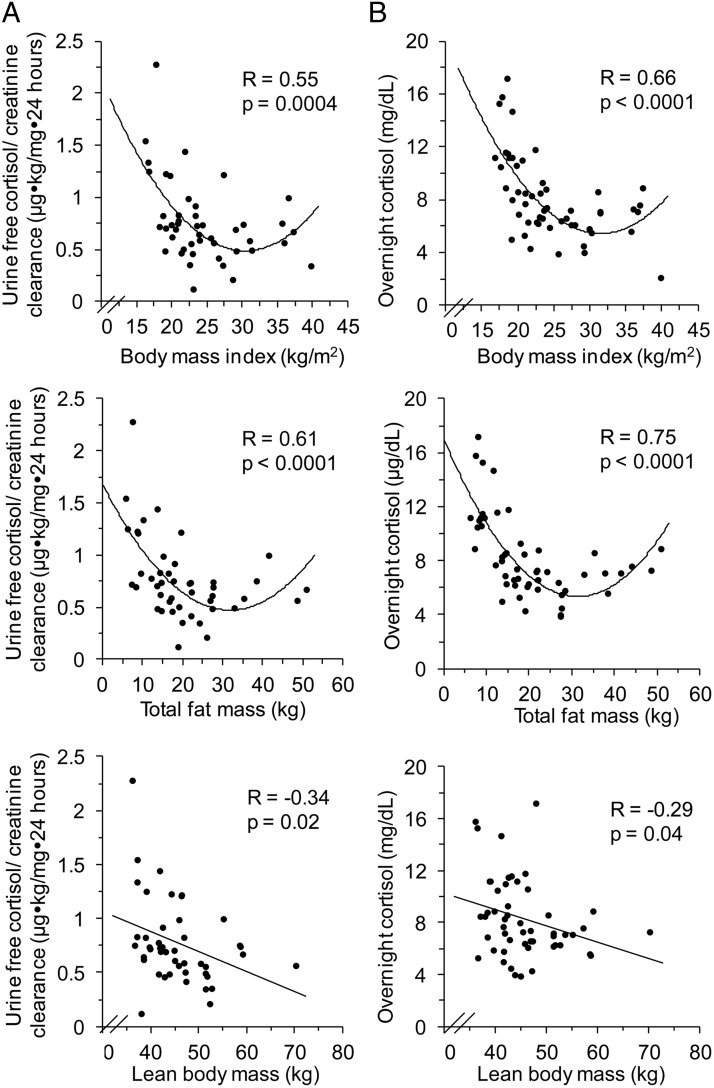
When BMI was evaluated as a continuous rather than a categorical variable, all cortisol measures except morning serum unbound cortisol and dex-CRH testing results demonstrated a U-shaped relationship with BMI (Table 2, Figure 2). Most notable were the U-shaped relationships between BMI and UFC/CrCl (R = 0.55; P = .0004) and between BMI and mean overnight serum cortisol (R = 0.66; P < .0001) (Table 2, Figure 2). Nadir cortisol measures in most of these U-shaped relationships were in the class I obese range (Table 2 and Figure 2).
Table 2.
U-Shaped Relationships Between Most Cortisol Measures and BMI or Adiposity
| BMI |
Nadir BMI, kg/m2 | Visceral Adipose Tissue |
Total Fat |
||||
|---|---|---|---|---|---|---|---|
| R | P | R | P | R | P | ||
| UFC | 0.47 | .004 | 28 | 0.36 | .05 | 0.32 | .1 |
| UFC/CrCl | 0.55 | .0004 | 34 | 0.50 | .003 | 0.61 | <.0001 |
| Mean overnight serum cortisol | 0.66 | <.0001 | 32 | 0.67 | <.0001 | 0.75 | <.0001 |
| Mean overnight serum unbound cortisol | 0.58 | <.0001 | 31 | 0.58 | <.0001 | 0.65 | <.0001 |
| Morning serum cortisol | 0.45 | .003 | 32 | 0.37 | .03 | 0.44 | .004 |
| Morning serum unbound cortisol | 0.31 | .08 | 34 | 0.24 | .24 | 0.44 | .1 |
| LNSC | 0.42 | .03 | 32 | 0.33 | .11 | 0.33 | .11 |
| Morning salivary cortisol | 0.4 | .02 | 33 | 0.52 | .003 | 0.51 | .002 |
Bold values indicate P < .05.
Figure 2.
A, Significant U-shaped relationship between UFC/CrCl and BMI and between UFC/CrCl and total fat mass. Weak negative linear relationship between UFC/CrCl and lean body mass. B, Significant U-shaped relationship between mean overnight serum cortisol and BMI and between mean overnight serum cortisol and total fat mass. Weak negative linear relationship between mean overnight serum cortisol and lean body mass.
Relationship between cortisol measures and adiposity
There was a U-shaped association between visceral adipose tissue and most cortisol measures, namely UFC/CrCl (R = 0.50; P = .003), mean overnight serum cortisol (R = 0.67; P < .0001), morning serum cortisol (R = 0.37; P = .03), and morning salivary cortisol (R = 0.52; P = .003) (Table 2 and Figure 2). Subsequent analysis demonstrated that this U-shaped relationship was also present between total fat mass and most cortisol measures, namely UFC/CrCl (R = 0.61; P < .0001), mean overnight serum cortisol (R = 0.75; P < .0001), morning serum cortisol (R = 0.44; P < .004), and morning salivary cortisol (R = 0.51; P < .002) (Table 2 and Figure 2). In addition, FMI shared a U-shaped association with most cortisol measures, namely UFC (R = 0.37; P = .03; nadir FMI, 9.9 kg/m2), UFC/CrCl (R = 0.57; P < .0001; nadir FMI, 10.5 kg/m2), mean overnight serum cortisol (R = 0.74; P < .0001; nadir FMI, 10.8 kg/m2), morning serum cortisol (R = 0.41; P = .01; nadir FMI, 11.3 kg/m2), and morning salivary cortisol (R = 0.48; P = .007; nadir FMI, 11.7 kg/m2).
Relationship between cortisol measures and lean body mass
In contrast, there was a weak negative linear relationship between lean body mass and UFC/CrCl (R = −0.34; P = .02), mean overnight serum cortisol (R = −0.29; P = .04), and morning serum cortisol (R = −0.30; P = .03) (Table 3, Figure 2). There was no association between lean body mass and UFC, LNSC, or morning salivary cortisol (Table 3). There were no associations between cortisol measures and lean body mass within BMI groups (AN, HC, OB).
Table 3.
Negative Linear Relationships Between Some Cortisol Measures and Lean Body Mass or BMD
| Lean Body Mass |
PA Spine BMD |
Total Hip BMD |
||||
|---|---|---|---|---|---|---|
| R | P | R | P | R | P | |
| UFC | −0.07 | .66 | −0.15 | .32 | −0.06 | .71 |
| UFC/CrCl | −0.34 | .02 | −0.40 | .006 | −0.39 | .007 |
| Mean overnight serum cortisol | −0.29 | .04 | −0.44 | .001 | −0.42 | .002 |
| Morning serum cortisol | −0.30 | .03 | −0.31 | .02 | −0.27 | .04 |
| Late-night salivary cortisol | −0.26 | .10 | −0.24 | .12 | −0.17 | .28 |
| Morning salivary cortisol | −0.29 | .13 | −0.48 | .002 | −0.42 | .005 |
Bold values indicate P < .05.
BMD
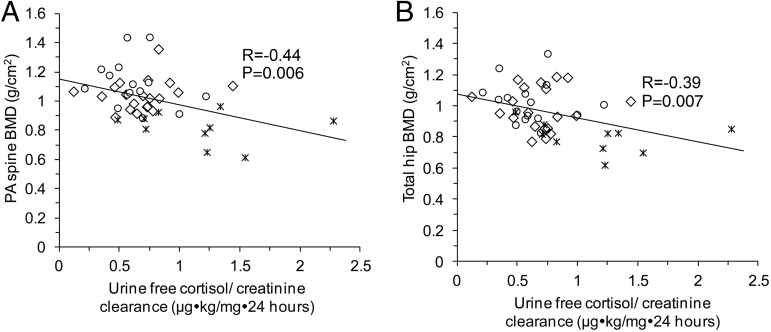
There was a negative linear relationship between most cortisol measures and BMD at the postero-anterior (PA) spine and total hip (Table 3 and Figure 3). After controlling for BMI, the association between UFC/CrCl and PA spine BMD (R = −0.25; P = .09) and total hip BMD (R = −0.27; P = .07) trended toward significance, as did the association between mean overnight serum cortisol and total hip BMD (R = −0.24; P = .09). There were no associations between cortisol measures and BMD within BMI groups (AN, HC, OB). There was a positive linear relationship between BMI and BMD at the PA spine (R = 0.62; P < .0001) and total hip (R = 0.50; P < .0001), which remained significant after controlling for each cortisol measure in separate models.
Figure 3.
A, Significant negative linear relationship between UFC/CrCl and PA spine BMD. B, Significant negative linear relationship between UFC/CrCl and total hip. °, OB group; ⨯⃒, AN group; ⬦, HC group.
Discussion
We demonstrate that cortisol measures are lowest in overweight and class I obese women and increase with more significant obesity, but not to the levels observed in very low BMI states, such as AN. Of note, categorical BMI testing alone, in which mean cortisol levels are compared between the groups, would have missed this critical U-shaped relationship. We also show that there is a U-shaped association between cortisol measures and adiposity (visceral and total fat mass). This is in contrast to the weak negative linear relationship between some cortisol measures and lean body mass. These data suggest that extreme underweight and overweight states are associated with HPA axis activation and raise the question of whether hypercortisolemia may contribute to increased adiposity in the setting of caloric excess, as well as muscle wasting, in the setting of caloric restriction and excess. We observe that cortisol measures are negatively associated with BMD across the weight spectrum, which suggests that relative hypercortisolemia may contribute to bone loss in the setting of caloric restriction and excess. HPA axis activation may not only be a marker of metabolic dysregulation, but also a target for future therapies.
We demonstrate a U-shaped relationship between cortisol measures and BMI, such that cortisol measures are lowest in overweight-class I obese women and increase toward the extremes of BMI, with the highest values in very low BMI states. These data are consistent across multiple parameters of the HPA axis, including UFC, mean overnight serum cortisol, LNSC, and morning salivary and serum cortisol. Most cortisol nadirs in the U-shaped relationship between BMI and cortisol measures are in the class I obese range. Likewise, most cortisol nadirs in the U-shaped relationship between FMI and cortisol measures are in the excess fat range, which is the first FMI category above the normal range. Given that most cortisol measures increase as FMI increases above the normal range, this may further support the hypothesis that it is adiposity that is associated with cortisol measures rather than BMI itself. In contrast, there is either no relationship between cortisol measures and lean body mass, again suggesting that it is adiposity that is associated with relative hypercortisolemia, or a weak negative linear relationship between cortisol measures and lean body mass, possibly because higher cortisol levels contribute to muscle wasting across the weight spectrum.
Previous literature on HPA dynamics in overweight and obese subjects is inconsistent. For instance, some, but not all, prior studies demonstrated a negative linear relationship between BMI and morning serum or salivary cortisol (4–8), and no relationship between BMI and UFC (35, 36). Some possible explanations for this discrepancy between our and previous results may have been that we included more subjects at the extremes of BMI, or that we analyzed BMI as a continuous rather than categorical variable. Similarly, previous studies have reported inconsistent results regarding the association between body composition parameters and cortisol measures (9–11); only one prior study radiographically measured visceral adipose tissue by computed tomography and found no significant association with UFC (12). Our data demonstrate a U-shaped relationship between visceral adipose tissue and most cortisol measures, and, to our knowledge, we are the first to report a U-shaped relationship between total fat mass and most cortisol measures.
We replicated previous studies demonstrating that very low BMI states, such as AN, are associated with hypercortisolemia. Our data are consistent with the previously reported 40–50% prevalence of elevated UFC in women with AN (13, 37), and with prior reports of elevated LNSC in AN (15). In contrast, the percentage of AN subjects in our study who failed the dex-CRH test was smaller than the 47% previously published by Duclos et al (14); this difference may be due to a higher mean BMI in our AN group (18.2 vs 15.3 kg/m2). Despite the reported lack of specific symptoms and signs of hypercortisolemia, AN has been reported to be associated with a metabolic myopathy similar to steroid myopathy (38), and we found that some measures of hypercortisolemia are associated with decreased lean body mass. A clear relationship between cortisol levels and neurocognitive measures in AN has not been established. Although one study found no correlation between cortisol measures and cognitive function in AN (39), another cross-sectional study demonstrated a positive linear relationship between cortisol measures and anxiety/depression scores (23), indicating that cortisol excess may be associated with some comorbid features of AN.
Only two studies have previously evaluated HPA dynamics across the weight spectrum. Kumari et al (7) evaluated the relationship between BMI or waist circumference and salivary cortisol. Similar to our data, they published a U-shaped relationship between BMI and LNSC; unlike our data, they reported a negative linear relationship between BMI and morning salivary cortisol (7). This difference in results may be due to improper outpatient salivary cortisol collection or due to differences in BMI ranges between the two studies; the latter cannot be determined as Kumari et al did not report their subjects' BMI range. Putignano et al (15) found that AN subjects had higher morning and late-night salivary and serum cortisols, higher UFC, and decreased cortisol suppression after dexamethasone suppression testing compared with controls; severely obese subjects (mean BMI, 42 kg/m2) had lower 0800 h serum cortisols compared with controls. Although our data also show that most cortisol measures are higher in AN subjects compared with HC, the U-shaped relationship across multiple cortisol measures, with a nadir in the overweight-class I obese range, has not been demonstrated previously.
Multiple mechanisms, both central and peripheral, may be mediating the U-shaped relationship between cortisol measures adiposity. In AN, we hypothesize that it is the underweight state itself that activates the HPA axis centrally, which in turn may contribute to muscle wasting, but does not result in visceral adiposity due to lack of caloric substrate. In severe obesity, the relationship between body composition and HPA axis hyperactivity may be bidirectional. Central activation of the HPA axis may contribute to the accumulation of adiposity and/or may result as a response to the metabolic stress of adiposity. Visceral adipose tissue itself is rich in 11β-hydroxysteroid dehydrogenase type I, which converts inactive cortisone to cortisol, although it is unclear whether this regulation at the tissue level also contributes to systemic cortisol regulation.
Our data suggest that relative hypercortisolemia may contribute to bone loss in the setting of caloric restriction and excess, consistent with that seen in Cushing's syndrome, in which UFC negatively correlates with BMD (2). Similarly, in AN, studies have shown a negative association between UFC and BMD (22) and between mean overnight serum cortisol levels and BMD (23). However, no previous studies have evaluated the association between cortisol measures and BMD in overweight/obese individuals. Our data demonstrate that there is a negative linear relationship between most cortisol measures and BMD across the weight spectrum. Therefore, relative hypercortisolemia may contribute to increased fracture risk in both AN and obesity (21, 24). Of note, there are positive linear relationships between BMI and both hip and spine BMD that remain significant after controlling for cortisol measures. These data are consistent with prior literature, which supports a positive association between BMI and BMD (40, 41); however, this may not correlate directly with reduced fracture risk given that recent studies have shown an association between obesity and fracture risk at certain skeletal sites (24, 42).
Limitations of this study include its cross-sectional design, which prevents us from determining whether the associations between cortisol measures and BMI, body composition, and/or BMD are causal. In addition, samples that rely on patient compliance, such as an outpatient UFC collection, may be confounded by improper collection. We cannot determine whether the relatively stronger association between mean overnight serum cortisol and BMI or adiposity is due to the greater precision of this frequent sampling measurement compared with UFC or a relatively stronger relationship between mean overnight serum cortisol and BMI or body composition. The lack of a relationship between cortisol measures and lean body mass or BMD within BMI groups may have been due to the relatively small sample size and lack of range within each BMI group. Free cortisol was calculated from total cortisol and CBG using the Coolens equation rather than measured directly. An available subset of 15 morning serum samples including samples from each group (AN, OB, and HC) was run for free cortisol by LC/MS/equilibrium dialysis, and the Coolens equation correlated strongly (R = 0.70; P = .004) with free cortisol by LC/MS/equilibrium dialysis. Another limitation is that this study focused on systemic hypercortisolemia and did not evaluate tissue-level cortisol dynamics, which may be relevant due to local glucocorticoid regulation by 11β-hydroxysteroid dehydrogenase type I and type II (43).
Our study demonstrates that cortisol measures are lowest in overweight-class I obese women, and that cortisol measures increase with more significant obesity but not to the levels observed in very low BMI states. The HPA axis activation associated with obesity and excess adiposity (both visceral and total fat mass) raises the question of whether hypercortisolemia contributes to increased adiposity in the setting of caloric excess, whether increased adiposity drives HPA activation, or whether the relationship between hypercortisolemia and adiposity is bidirectional. There is no relationship or a weak negative linear relationship between cortisol measures and lean body mass, which may be because it is the adipose component that is associated with cortisol measures or because hypercortisolemia contributes to muscle wasting. We observe that cortisol measures are negatively associated with BMD across the weight spectrum, which suggests that relative hypercortisolemia may contribute to bone loss in the setting of both caloric restriction and excess. Given the fact that obesity has reached epidemic proportions and significantly increases the risk of the metabolic syndrome and cardiovascular disease among other comorbidities, insight into the factors that contribute to obesity, and/or its complications may have important therapeutic implications.
Acknowledgments
This work was supported by National Institutes of Health Grants K24 HL092902, T32 DK007028, R01 DK052625, and UL1 RR025758.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AN
- anorexia nervosa
- BMD
- bone mineral density
- BMI
- body mass index
- CBG
- cortisol-binding globulin
- CrCl
- creatinine clearance
- dex-CRH
- dexamethasone suppression-CRH stimulation
- FMI
- fat mass index
- HC
- healthy control
- HPA
- hypothalamic-pituitary-adrenal
- IBW
- ideal body weight
- LC/MS/equilibrium dialysis
- liquid chromatography tandem mass spectrometry, equilibrium dialysis
- LNSC
- late-night salivary cortisol
- OB
- overweight/obese
- PA
- postero-anterior
- UFC
- urinary free cortisol
- WHO
- World Health Organization.
References
- 1. Wajchenberg BL, Bosco A, Marone MM, et al. Estimation of body fat and lean tissue distribution by dual energy X-ray absorptiometry and abdominal body fat evaluation by computed tomography in Cushing's disease. J Clin Endocrinol Metab. 1995;80(9):2791–2794. [DOI] [PubMed] [Google Scholar]
- 2. Chiodini I, Carnevale V, Torlontano M, et al. Alterations of bone turnover and bone mass at different skeletal sites due to pure glucocorticoid excess: Study in eumenorrheic patients with Cushing's syndrome. J Clin Endocrinol Metab. 1998;83:1863–1867. [DOI] [PubMed] [Google Scholar]
- 3. Ten S, New M, Maclaren N. Clinical review 130: Addison's disease 2001. J Clin Endocrinol Metab. 2001;86:2909–2922. [DOI] [PubMed] [Google Scholar]
- 4. Travison T, O'Donnell A, Araujo A, Matsumoto A, McKinlay J. Cortisol levels and measures of body composition in middle-aged and older men. Clin. Endocrinol. (Oxf.) 2007;67:71–77. [DOI] [PubMed] [Google Scholar]
- 5. Walker B, Soderberg S, Lindahl B, Olsson T. Independent effects of obesity and cortisol in predicting cardiovascular risk factors in men and women. J. Intern. Med. 2000;247:198–204. [DOI] [PubMed] [Google Scholar]
- 6. Reynolds RM, Labad J, Strachan MW, et al. Elevated fasting plasma cortisol is associated with ischemic heart disease and its risk factors in people with type 2 diabetes: The Edinburgh Type 2 Diabetes Study. J Clin Endocrinol Metab. 2010;95:1602–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kumari M, Chandola T, Brunner E, Kivimaki M. A nonlinear relationship of generalized and central obesity with diurnal cortisol secretion in the Whitehall II Study. J Clin Endocrinol Metab. 2010;95:4415–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Champaneri S, Xu X, Carnethon MR, et al. Diurnal salivary cortisol is associated with body mass index and waist circumference: The Multiethnic Study of Atherosclerosis. Obesity. 2013;21:E56–E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duclos M, Marquez Pereira P, Barat P, Gatta B, Roger P. Increased cortisol bioavailability, abdominal obesity, and the metabolic syndrome in obese women. Obes. Res. 2005;13:1157–1166. [DOI] [PubMed] [Google Scholar]
- 10. Pasquali R, Cantobelli S, Casimirri F, et al. The hypothalamic-pituitary-adrenal axis in obese women with different patterns of body fat distribution. J Clin Endocrinol Metab. 1993;77:341–346. [DOI] [PubMed] [Google Scholar]
- 11. Mårin P, Darin N, Amemiya T, Andersson B, Jern S, Björntorp P. Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism. 1992;41:882–886. [DOI] [PubMed] [Google Scholar]
- 12. Zamboni M, Armellini F, Turcato E, et al. Relationship between visceral fat, steroid hormones, and insulin sensitivity in premenopausal obese women. J Inter Med. 1994;236:521–527. [DOI] [PubMed] [Google Scholar]
- 13. Gold PW, Gwirtsman H, Avgerinos PC, et al. Abnormal hypothalamic-pituitary-adrenal function in anorexia nervosa. N Eng J Med. 1986;314:1335–1342. [DOI] [PubMed] [Google Scholar]
- 14. Duclos M, Corcuff J, Roger P, Tabarin A. The dexamethasone-suppressed corticotrophin-releasing hormone stimulation test in anorexia nervosa. Clin Endocrinol (Oxf.) 1999;51:725–731. [DOI] [PubMed] [Google Scholar]
- 15. Putignano P, Dubini A, Toja P, et al. Salivary cortisol measurement in normal-weight, obese and anorexic women: Comparison with plasma cortisol. Eur J Endocrinol. 2001;145:165–171. [DOI] [PubMed] [Google Scholar]
- 16. Estour B, Pugeat M, Lang F, al. Rapid escape of cortisol from suppression in response to I.V. dexamethasone in anorexia nervosa. Clin Endocrinol Oxf. 1990;(33):45–52. [DOI] [PubMed] [Google Scholar]
- 17. Grinspoon S, Thomas L, Miller K, Pitts S, Herzog D, Klibanski A. Changes in regional fat redistribution and the effects of estrogen during spontaneous weight gain in women with anorexia nervosa. Am J Clin Nutr. 2001;73:865–869. [DOI] [PubMed] [Google Scholar]
- 18. Doerr P, Fichter M, Pirke KM, Lund R. Relationship between weight gain and hypothalamic pituitary adrenal function in patients with anorexia nervosa. J Steroid Biochem. 1980;13:529–537. [DOI] [PubMed] [Google Scholar]
- 19. Vestergaard P, Lindholm J, Jørgensen J, et al. Increased risk of osteoporotic fractures in patients with Cushing's syndrome. Eur J Endocrinol. 146(2002):51–56. [DOI] [PubMed] [Google Scholar]
- 20. Grinspoon S, Baum H, Lee K, Anderson E, Herzog D, Klibanski A. Effects of short-term recombinant human insulin-like growth factor I administration on bone turnover in osteopenic women with anorexia nervosa. J Clin Endocrinol Metab. 1996;81(11):3864–3870. [DOI] [PubMed] [Google Scholar]
- 21. Lucas AR, Melton LJ, 3rd, Crowson CS, O'Fallon WM. Long-term fracture risk among women with anorexia nervosa: A population-based cohort study. Mayo Clin Proc. 1999;74:972–977. [DOI] [PubMed] [Google Scholar]
- 22. Biller BM, Saxe V, Herzog DB, Rosenthal DI, Holzman S, Klibanski A. Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab. 1989;68:548–554. [DOI] [PubMed] [Google Scholar]
- 23. Lawson EA, Donoho D, Miller KK, et al. Hypercortisolemia is associated with severity of bone loss and depression in hypothalamic amenorrhea and anorexia nervosa. J Clin Endocrinol Metab. 2009;94:4710–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johansson H, Kanis J, Odén A, et al. A meta-analysis of the association of fracture risk and body mass index in women. J Bone Min. Res. 2014;29(1):223–233. [DOI] [PubMed] [Google Scholar]
- 25. Lawson EA, Eddy KT, Donoho D, et al. Appetite-regulating hormones cortisol and peptide YY are associated with disordered eating psychopathology, independent of body mass index. Eur J Endocrinol. 2011;164:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lawson EA, Miller KK, Blum JI, et al. Leptin levels are associated with decreased depressive symptoms in women across the weight spectrum, independent of body fat. Clin Endocrinol (Oxf). 2012;76:520–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lawson EA, Donoho DA, Blum JI, et al. Changes in regional fat redistribution and the effects of estrogen during spontaneous weight gain in women with anorexia nervosa. J Clin Psychiatry. 2011;72:1546–1551.21903023 [Google Scholar]
- 28. World Health Organization. Physical status: The use and interpretation of anthropometry. Geneva, Switzerland: World Health Organization 1995; WHO Technical Report Series. [PubMed] [Google Scholar]
- 29. Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51:1106–1112. [DOI] [PubMed] [Google Scholar]
- 30. Kelly T, Wilson K, Heymsfield S. Dual energy x-ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4:e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walsh BT, Katz JL, Levin J, et al. Adrenal activity in anorexia nervosa. Psychosom Med. 1978;40:499–506. [DOI] [PubMed] [Google Scholar]
- 32. Yanovski JA, Cutler GB, Jr, Chrousos GP, Nieman LK. Corticotropin-releasing hormone stimulation following low-dose dexamethasone administration. A new test to distinguish Cushing's syndrome from pseudo-Cushing's states. JAMA. 1993;269:2232–2238. [PubMed] [Google Scholar]
- 33. Coolens JL, Van Baelen H, Heyns W. Clinical use of unbound plasma cortisol as calculated from total cortisol and corticosteroid-binding globulin. J Steroid Biochem. 1987;26:197–202. [DOI] [PubMed] [Google Scholar]
- 34. Meier U. A note on the power of Fisher's least significant difference procedure. Pharm Stat. 2006;5:253–263. [DOI] [PubMed] [Google Scholar]
- 35. Stewart PM, Boulton A, Kumar S, Clark PM, Shackleton CH. Cortisol metabolism in human obesity: Impaired cortisone⇒cortisol conversion in subjects with central adiposity. J Clin Endocrinol Metab. 1999;84:1022–1027. [DOI] [PubMed] [Google Scholar]
- 36. Abraham SB, Rubino D, Sinaii N, Ramsey S, Nieman L. Cortisol, obesity and the metabolic syndrome: A cross-sectional study of obese subjects and review of the literature. Obes (Silver Spring). 2013;21:E105–E117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boyar RM, Hellman LD, Roffwarg H, et al. Cortisol secretion and metabolism in anorexia nervosa. N Engl J Med. 1977;296:190–193. [DOI] [PubMed] [Google Scholar]
- 38. McLoughlin DM, Wassif WS, Morton J, Spargo E, Peters TJ, Russell GF. Metabolic abnormalities associated with skeletal myopathy in severe anorexia nervosa. Nutrition. 2000;16:192–196. [DOI] [PubMed] [Google Scholar]
- 39. Seed JA, Dixon RA, McCluskey SE, Young AH. Basal activity of the hypothalamic-pituitary-adrenal axis and cognitive function in anorexia nervosa. Eur Arch Psychiatry Clin Neurosci. 2000;250(1):11–15. [DOI] [PubMed] [Google Scholar]
- 40. Ravn P, Cizza G, Bjarnason NH, et al. Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. J Bone Min Res. 1999;14:1622–1627. [DOI] [PubMed] [Google Scholar]
- 41. Marcus R, Greendale G, Blunt BA, et al. Correlates of bone mineral density in the postmenopausal estrogen/progestin intervention trials. J Bone Min Res. 1994;9(9):1467–1476. [DOI] [PubMed] [Google Scholar]
- 42. Compston JE, Watts NB, Chapurlat R, et al. Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med. 2011;124(11):1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rask E, Olsson T, Söderberg S, et al. Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metab. 2001;86:1418–1421. [DOI] [PubMed] [Google Scholar]