Abstract
Gap junctions, which mediate intercellular communication, are key players in digestive homeostasis. They are also frequently involved in gastrointestinal and liver pathology. This equally holds true for connexin hemichannels, the structural precursors of gap junctions, and pannexin channels, connexin-like proteins assembled in a hemichannel configuration. Both connexin hemichannels and pannexin channels facilitate extracellular communication and drive a number of deteriorative processes, such as cell death and inflammation. Connexins, pannexins and their channels underlie a wide spectrum of gastrointestinal and liver diseases, including gastritis and peptic ulcer disease, inflammatory intestinal conditions, acute liver failure, cholestasis, hepatitis and steatosis, liver fibrosis and cirrhosis, infectious gastrointestinal pathologies, and gastrointestinal and liver cancer. This could open promising perspectives for the characterization of new targets and biomarkers for therapeutic and diagnostic clinical purposes in the area of gastroenterology and hepatology.
Keywords: stomach, intestine, liver, connexin, pannexin, disease
1. Introduction
Gap junctional intercellular communication (GJIC) relies on the exchange of small and hydrophilic substances between adjacent cells, including adenosine triphosphate (ATP), cyclic adenosine monophosphate and inositol triphosphate as well as ions (1-3). Hence, GJIC is considered a key mechanism in the maintenance of tissue functioning. In the gastrointestinal system, gap junctions indeed drive processes such as gastroduodenal (4) and gut motility (5, 6), gastric acid secretion (7), gastric cytoprotection (8, 9) and intestinal innate immune defense (10). Similarly, GJIC underlies critical hepatic functions, including xenobiotic biotransformation (11-13) and plasma protein synthesis (14).
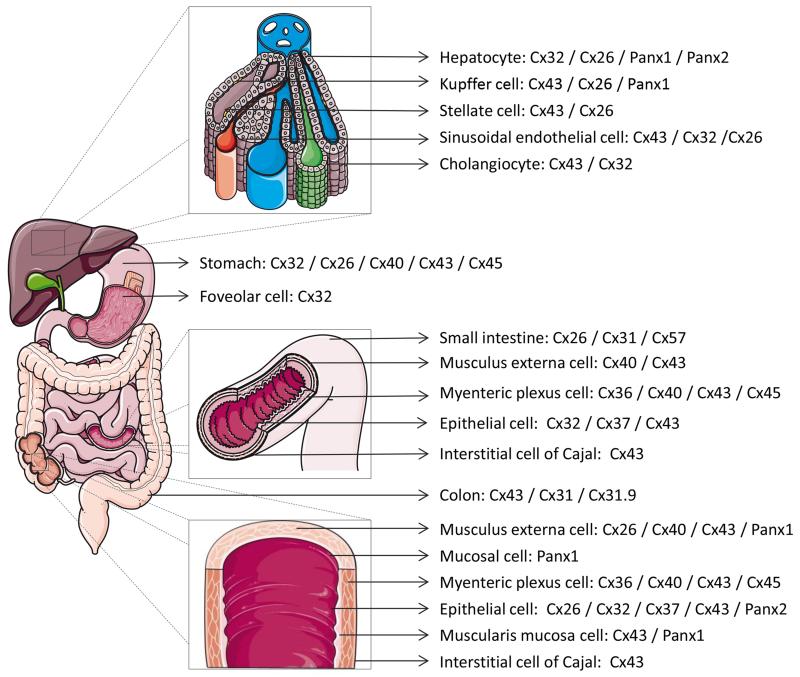
Gap junctions are composed of 2 hemichannels of neighboring cells, which in turn are composed of 6 connexin (Cx) proteins. Today, more than 20 different connexin species have been identified, all which are named after their molecular weight (15). Connexins share a structure consisting of 4 membrane-spanning domains, 2 extracellular loops, a cytoplasmic loop, a cytosolic N-terminal area and a C-terminal region (2, 3). Connexins are expressed in a tissue-specific way. Thus, gastric tissue produces Cx26, Cx32 and Cx43 (4, 8, 9, 16-20). At least 10 different connexin variants have been characterized in the intestinal system, namely Cx26, Cx31, Cx32, Cx36, Cx37, Cx40, Cx43, Cx45 and Cx57 in the small intestine (6, 18, 20-23), and Cx26, Cx31, Cx31.1, Cx32, Cx36, Cx40, Cx43 and Cx45 in the colon (20, 24-32). As much as 5 connexin family members are detectable in liver, among which Cx26 and Cx32 are predominantly expressed by hepatocytes, while non-parenchymal liver cells harbor Cx37, Cx40 and Cx43 (19, 33-35) (Figure 1).
Figure 1. Connexin and pannexin expression in liver, stomach, gallbladder, colon and small intestine.
In the last decade, it has been well documented that connexin hemichannels, in addition to acting as structural precursors of gap junctions, also provide a pathway for communication, albeit between the cytosol and extracellular environment (36, 37). The messengers that permeate connexin hemichannels and pannexin channels show great overlap with those involved in GJIC. Nevertheless, although some physiological roles have been attributed to connexin hemichannels, in particular in the intestine (28), they primarily become active during disease. Furthermore, a novel class of connexin-like proteins was discovered in 2000, the pannexins, which gather in a configuration reminiscent of connexin hemichannels and that also facilitate extracellular communication (38). Only 3 pannexins (Panx) have yet been identified. Of those, Panx1 and Panx2 are expressed in gastric tissue (39, 40), the intestine (40, 41) and the liver (40, 42-47) (Figure 1). Recently, it has become clear that pannexin channels, like connexin hemichannels, are also essentially involved in pathological processes (41, 48-52). In this paper, the role of connexins, pannexins and their channels in gastrointestinal (Table 1) and liver (Table 2) disease is discussed.
Table 1. Effects of gastrointestinal disease on human connexins and pannexins.
| Disease | Effect | References |
|---|---|---|
| Gastric cancer | Cytoplasmic Cx26† protein localization | (16) |
| Upregulated Cx30 protein expression | (77) | |
| Cytoplasmic Cx32 protein localization | (72, 73) | |
| Downregulated Cx32 protein expression | (17, 54, 72-74) | |
| C1019T Cx37 gene polymorphism | (150) | |
| Cytoplasmic Cx43 protein localization | (73) | |
| Downregulated Cx43 mRNA and protein expression | (18, 73-75) | |
|
| ||
| Gastric ulcer | Downregulated Cx32 protein expression | (17, 54) |
|
| ||
| Spontaneous neonatal gastric perforation | Downregulated Cx43 mRNA and protein expression | (70) |
| Gastric Helicobacter pylori infection | Downregulated Cx32 mRNA and protein expression | (62, 64) |
| Hypermethylated Cx32 gene promoter | (65) | |
| C1019T Cx37 gene polymorphism | (150) | |
| Downregulated Cx43 mRNA and protein expression | (62, 64) | |
| Hypermethylated Cx43 gene promoter | (65) | |
|
| ||
| Colon cancer | Cytoplasmic Cx26 protein localization associated with Bax and Bcl-xL | (26) |
| Cytoplasmic Cx26 protein localization associated with insulin-like growth factor-I receptor | (85) | |
| Downregulated Cx31.9 mRNA expression | (89) | |
| Cytoplasmic Cx32 protein localization | (87) | |
| Cytoplasmic Cx43 protein localization | (87) | |
| Downregulated Cx43 protein expression | (25) | |
| Upregulated Cx43 protein expression | (32) | |
| Mutated Cx43 protein expression | (24) | |
| Altered Cx43 protein phosphorylation | (31) | |
| Downregulated Cx45 mRNA expression | (30) | |
| Hypermethylated Cx45 gene promoter | (30) | |
|
| ||
| Small intestine stromal cancer | Cytoplasmic Cx43 protein localization | (18, 86) |
|
| ||
| Inflammatory bowel disease | Downregulated Cx43 protein expression | (22) |
|
| ||
| Crohn’s disease | Downregulated Panx1‡ mRNA and protein expression | (41, 78) |
|
| ||
| Ulcerative disease | Downregulated Panxl mRNA expression | (41) |
|
| ||
| Coeliac disease | Upregulated Cx37 mRNA and protein expression | (21) |
|
| ||
| Diverticular disease | Downregulated Cx26 protein expression | (27) |
| Downregulated Cx43 protein expression | (27) | |
|
| ||
| Hirschsprung’s disease | Downregulated Cx43 protein expression | (6) |
Cx, connexin;
Panx, pannexin
Table 2. Effects of liver disease on human connexins.
| Disease | Effect | References |
|---|---|---|
| Chronic hepatitis | Cytoplasmic Cx32† protein localization | (110) |
| Downregulated Cx32 protein expression | (110, 111) | |
|
| ||
| Cirrhosis | Cytoplasmic Cx32 protein localization | (110) |
| Downregulated Cx32 protein expression | (110, 111, 115) | |
|
| ||
| HCC§ | Downregulated Cx26 protein and mRNA expression | (128) |
| Downregulated Cx32 protein and mRNA expression | (14, 110, 121) | |
| Cytoplasmic Cx32 protein localization | (110, 128, 132) | |
| Upregulated Cx43 protein and mRNA expression | (134, 135, 138) | |
Cx, connexin;
HCC, hepatocellular carcinoma
2. Connexin and pannexin channels in gastrointestinal and hepatic pathology
2.1. Involvement of connexin signaling in gastric disease
2.1.1. Gastritis and peptic ulcer disease
Loss of GJIC has been associated with gastric ulcer formation. Electron microscopic studies of human gastric ulcers indeed showed a marked reduction of gap junction numbers. In areas of intestinal metaplasia, gap junctions have been occasionally seen between absorptive cells of the villi, but not in the lateral membranes of goblet cells (53). On the border of human gastric ulcers, Cx32 spots in the surface mucous cells are significantly fewer than in the surface mucous cells of the body and the antrum distant from the ulcer area. The majority of the foveolar cells adjacent to gastric erosions displays decreased or even absent Cx32 staining (54). Gastric expression of Cx32 is also reduced in experimentally induced atrophic gastritis in rat (55, 56).
2.1.2. Infectious gastric disease
Helicobacter pylori colonizes the human stomach and confers an increased risk for the development of peptic ulceration, gastric adenocarcinoma and lymphoma. Among the various virulence factors secreted by Helicobacter pylori is cytotoxin-associated gene A (CagA), which is associated with gastric cancer (57). Indeed, CagA-positive Helicobacter pylori, especially the East Asian type, as well as CagA-negative strains abolish GJIC in cultured human gastric epithelial cells, which is accompanied by the inhibition of cell proliferation (58, 59). Upon administration of water extracts of CagA-positive Helicobacter pylori to rats, in which gastric ulcers were induced by acetic acid, healing and reappearance of Cx32 protein expression in gastric mucosa are significantly delayed (60). CagA-positive Helicobacter pylori also downregulates Cx43 production in cultured human gastric carcinoma cells (61). Likewise, in precancerous gastric lesions of patients with Helicobacter pylori infection, especially with the CagA-positive variant, Cx32 and Cx43 levels are reduced compared to noninfected patients (62-64). This is paralleled by hypermethylation of their corresponding gene promoters (65). Eradication of Helicobacter pylori usually results in the restoration of connexin expression in human gastric cells both in vitro (61) and in vivo (62). Another toxin produced by Helicobacter pylori is vacuolating toxin A (VacA), which can cause multiple alterations in gastric epithelial cells, including cell death. In fact, it has been reported that Cx43 is a host cell constituent that contributes to VacA-induced cell death. Furthermore, variation among cell types in the susceptibility to VacA-induced cell death is attributable, at least in part, to cell type-specific differences in Cx43 production (66).
2.1.3. Miscellaneous gastric disease
Gastroparesis or delayed gastric emptying is a condition frequently seen in people with diabetes mellitus. Streptozotocin-induced diabetes mellitus in rats causes functional impairment of neuromuscular transmission, reduces the maximum activity of the electrogenic pump, increases the sensitivity of muscarinic receptors, negatively affects the sensitivity of adrenoceptors and decreases the myogenic activity in gastric smooth muscles (67). This has been linked to a reduced amount of gap junctions in interstitial cells of Cajal in the antrum (68). The number of gap junctions of muscle cells and interstitial cells of Cajal is also decreased in infantile hypertrophic pyloric stenosis, a functional gastric outlet obstruction as a result of hypertrophy and hyperplasia of the muscular layers of the pylorus (69). In addition, Cx43 protein production declines in spontaneous neonatal gastric perforation (70).
2.1.4. Gastric cancer
Cx26 becomes located in the cytoplasm in human gastric carcinoma and is associated with a biologically less aggressive phenotype and pathologic early stage of gastric carcinoma. For this reason, Cx26 has been proposed to act as a gastric tumor suppressor (16). In human and murine gastric tumors, Cx32 protein is strongly downregulated and is located in the cytosol (71, 72) or may even be absent (17, 54, 56). Overexpression of Cx32 in cultured human gastric cancer cells inhibits cell proliferation by upregulating the cell cycle inhibitors p21Cip1 and p27Kip1. This suggests that Cx32, like Cx26, may be a gastric tumor suppressor (72). Cx43 protein quantities are downregulated in human gastric tumors and correlate with the occurrence, development and metastatic potential of stomach cancers (73, 74). It has been suggested that Cx43-based GJIC between gastric cancer cells and mesothelial cells could represent an important regulatory step during metastasis (75). Cx43 mRNA and protein expression is also negatively affected in human gastrointestinal stromal tumors of gastric origin (18), resulting in significantly reduced gap junction numbers (76). Interestingly, Cx30 is not expressed in normal human stomach, but becomes detectable in gastric cancer (77).
2.2. Involvement of connexin and pannexin signaling in intestinal disease
2.2.1. Inflammatory intestinal disease
Inflammatory bowel diseases are characterized by relapsing-remitting epithelial barrier dysfunction that is restricted to the colon, such as in ulcerative colitis, or that may affect any part of the gastrointestinal tract, like in Crohn’s disease. Cellular channels composed of Panx1 are assumed to play a major role in inflammatory bowel disease (41). Using mouse models of experimental colitis, it has been found that inflammation causes enteric neuron death by activating Panx1-based channels, which in turn leads to abnormal gut motility (78). Furthermore, Panx1 mRNA quantities are downregulated in the colonic mucosa and the muscularis externa of patients with Crohn’s disease as well as in the colonic muscularis externa of ulcerative colitis patients. However, this transcriptional deterioration is only reflected at the protein level in the muscularis externa of Crohn’s disease patients (41, 78). Cx43 is completely lost in colonic epithelium in experimental mouse models of acute ulceration and intestinal inflammation (29). In vitro studies pointed out that cells bearing an ulcerative colitis-associated mutant form of Toll-like receptor 2 target Cx43 for increased proteasomal degradation, thus impairing GJIC necessary for intestinal mucosal healing (10). Similarly, Cx43 expression in enterocytes is negatively affected in a mouse model of necrotizing enterocolitis, which is the leading cause of death from gastrointestinal disease in premature infants. Specifically, release of interferon gamma induces Cx43 dephosphorylation and internalization, eventually resulting in suppression of GJIC. This impedes migration of enterocytes, which is crucial for healing (22). Other inflammatory mediators released in necrotizing enterocolitis, in particular nitric oxide, also compromise gap junction integrity (79).
2.2.2. Infectious intestinal disease
Shigella flexneri causes bacillary dysentery by invading colonic mucosa, where it triggers inflammation and destruction of epithelial cells. Upon invasion, Shigella flexneri induces the opening of Cx26-based hemichannels in an actin-dependent and phospholipase C-dependent way, which allows extracellular release of ATP. The latter then favors bacterial dissemination and spreading (49, 50). It has been further demonstrated that ATP release is an early alert response to infection that promotes inflammation of the gut. Of note, Shigella flexneri evolved to escape this inflammatory reaction by secreting imidazoleglycerol-phosphate dehydratase, which blocks ATP release through hemichannels composed of Cx26 via the lipid mediator phosphatidylinositol 5-phosphate (80). An epidemiologic study recently showed a lower frequency of diarrhea in human carriers of genetic Cx26 variants, which might be related to an increased resistance to gastrointestinal infections (81). Another role for connexin hemichannels in infectious disease has been described for Citrobacter rodentium, which causes diarrhea. Upon infection of mouse colon, Citrobacter rodentium induces the expression of Cx43, which gathers in a hemichannel configuration at the apical and lateral membrane areas of colonocytes. Using Cx43-deficient mice, it has been found that subsequent hemichannel opening results in water release and hence diarrhea (48).
2.2.3. Miscellaneous intestinal disease
Coeliac disease is an autoimmune disorder caused by gluten ingestion in genetically susceptible individuals. Enterocytes in the duodenum of patients with coeliac disease show upregulated Cx37 expression and protein staining predominantly in the cytoplasm of epithelial cells. It has been suggested that increased numbers of gap junctions in coeliac disease could promote the passage of immunostimulatory gluten peptides between cells along the epithelial boundary (21). Diverticular disease is one of the most common pathologic conditions affecting the gastrointestinal tract in Western countries. It results from the interplay between genetic factors, dietary habits and coexistence of other bowel abnormalities, including changes in colonic pressure, motility and wall structure. It is associated with reduced Cx26 and Cx43 expression in human colonic circular and longitudinal muscle (27). Hirschsprung’s disease is a gastrointestinal disorder that occurs when the colon is devoid of ganglion cells, resulting in the lack of muscular activity. In line with the latter, patients with Hirschsprung’s disease show reduced Cx43 presence between interstitial cells of Cajal and smooth muscle cells in the colon (6).
2.2.4. Intestinal cancer
Cx32-deficient mice display an increased number of tumors in the small intestine upon X-ray radiation, suggesting that Cx32 behaves as an intestinal tumor suppressor (82, 83). A similar role has been attributed to Cx43 in colorectal cancer, since ectopic expression of Cx43 in human colon cancer cells reduces cell growth in soft agar cultures and in tumor xenografts (31). Expression of connexins, including Cx43, Cx32 and Cx26, is closely related to production of the adherens junction building stones E-cadherin and beta-catenin in human colorectal cancer (84). Connexin expression and localization are altered during carcinogenesis. Thus, Cx26 preferably resides in the cytoplasm of human colorectal epithelial cancer cells (26), where it is colocalized with the insulin-like growth factor-I receptor (85) as well as with proapoptotic Bax and antiapoptotic Bcl-xL (26). The relevance of this finding is unclear, but could suggest a gap junction-independent role for Cx26 in tumor cell turnover and colorectal cancer progression (85). Dot-like cytoplasmic and perinuclear Cx43 staining have also been noticed in human small intestinal stromal tumors (18, 86). Likewise, Cx43, but especially Cx32, is located in the cytoplasm rather than at the membrane surface of human colon cancer cells (87). This is associated with a shift from the phosphorylated to the nonphosphorylated Cx43 isoform (31).
Although contradicting results have been reported (32), overall Cx43 expression is downregulated in colon cancer cells (25), which is partly due to altered Wnt signaling. Mutations in the tumor suppressor gene adenomatous polyposis coli, a key player in the Wnt cascade, are early and critical events in colon cancer seen in the vast majority of human sporadic adenomas and carcinomas. In the absence of functional adenomatous polyposis coli, beta-catenin moves to the cell nucleus to influence the transcription of its target genes, including Cx43. The resulting reduction in Cx43 levels is accompanied by suppression of GJIC in the colonic mucosa (88). Cancer-related production of truncated adenomatous polyposis coli also reduces Cx32 content in Paneth cells of the murine small intestine (23). In addition, Cx31.9 (89) and Cx45 (30) amounts are negatively affected in colon cancer. Although documented (24), mutations in connexin genes associated with cancer are rare. Rather, their downregulation results from epigenetic modifications. This has been well exemplified for Cx45, of which its gene promoter is hypermethylated in human colon cancer cells (30). Interestingly, while Cx36 is not expressed in colonic tissue, its gene promoter is aberrantly methylated in a mouse model of colorectal cancer (90). Other connexins are upregulated in intestinal cancer. This is the case for Cx26, which shows increased expression in metastatic colon cancer cells, but not in nonmetastatic counterparts. Therefore, Cx26 might facilitate metastasis of colorectal tumors (91). In human and murine colon cancer cells, liver X receptor beta activation leads to pyroptosis by directly interacting with Panx1, thereby inducing extracellular ATP release and ultimately triggering inflammation and cell death (92).
2.3. Involvement of connexin and pannexin signaling in liver disease
2.3.1. Acute liver failure
Short-term administration of high doses of liver toxicants, including acetaminophen, thioacetamide, D-galactosamine and carbon tetrachloride, to Cx32-deficient mice or Cx32-dominant negative transgenic rats results in decreased alanine and aspartate amiotransferase serum levels as well as in less liver damage in comparison with wild-type animals (93-95). Similarly, ceramide synthase 2-null mice show Cx32 mislocalization in the hepatocyte cytosol and gap junction dysfunction, and are resistant to acute liver damage induced by acetaminophen (96). Likewise, cultured hepatocyte doublets isolated from Cx32 knockout mice display reduced synchronized cell death after exposure to acetaminophen (97). These findings suggest a role for Cx32 signaling in the dissemination of damage signals activated by these chemicals or in the removal of defunct hepatocytes in order to restore homeostasis. However, a recent study demonstrated that Cx32 protects against acetaminophen-induced hepatic centrilobular necrosis in mice, which may be related to the exchange of glutathione between hepatocytes mediated by gap junctions (98). This complies with the many reports describing deterioration in Cx32 production and channel activity upon exposure of hepatocytes to liver toxicants in vitro and in vivo (99). During the early stages of centrilobular necrosis induced by single administration of thioacetamide to rats, liver gap junctions are still present, but they disappear in the course of the subsequent restorative proliferative response. Thereafter, gap junctions reappear, first in the perinecrotic region and eventually in all areas (100). Interestingly, hepatocytes of rats overdosed with acetaminophen show de novo expression of Cx43 that is colocalized with caspase 3, which could point to its involvement in cell death (94). This is supported by the observation that, in comparison with wild-type animals, hepatocyte damage and apoptosis are strongly reduced in Cx43-deficient mice that received carbon tetrachloride (101). In a study using an acute-on-chronic liver failure rat model, strongly downregulated Cx32 expression and negligible Cx26 immunoreactivity were found in liver tissue. At the same time, increased Cx43-positive puncta were observed, in particular in the vicinity of inflamed and necrotic areas (102).
2.3.2. Cholestasis
While liver gap junctions seem unaffected in clinical patients suffering from extrahepatic cholestasis (103, 104), they seem to be compromised in cholelithiasis (105). Nonetheless, hepatic gap junction numbers decrease upon bile duct ligation in rodents and this is accompanied by a rapid drop in Cx32 amounts (102, 106-108), a process mediated by the p38 mitogen-activated protein kinase (108). Hepatic Cx26 immunoreactivity also decreases, yet Cx43 production increases following bile duct ligation (102, 106). In addition, bile duct ligated Cx43 heterozygous knockout mice display less hepatic vein angiogenesis, while other parameters, such as biliary duct hyperplasia, remain unchanged (109).
2.3.3. Hepatitis, inflammation and lipotoxic liver injury
Decreased liver Cx32 protein levels have been measured both in hepatitis patients (110, 111) and in laboratory rodents treated with lipopolysaccharide (107, 112, 113). This results from increased Cx32 mRNA degradation by shortening of its poly(A) tail (114). Reduction of Cx32 in primary hepatocyte cultures by proinflammatory cytokines is mediated by mitogen-activated protein kinase and nuclear factor kappa beta signaling leading to suppression of GJIC (115). By contrast, liver Cx26 becomes upregulated under inflammatory conditions both in vitro and in vivo (113, 116). This also holds true for Cx43 in primary stellate cultures and primary Kupffer cell cultures, whereby GJIC becomes more intensified (117, 118). In fact, upon inflammatory challenge, Cx43 in Kupffer cells shuttles from the cytoplasm to the plasma membrane surface and forms functional gap junctions. Increases in Cx43 abundance have been equally observed during liver inflammation in vivo (107, 117). This is believed to reflect the activation of the macrophage activity of Kupffer cells in order to take care of debris clearance and apoptosis of damaged hepatocytes following inflammation (117). Furthermore, administration of lipopolysaccharide (43) as well as ischemia-reperfusion (44) elevates hepatic Panx1 levels in mice. Panx1 is instrumental for activating the inflammasome, a multiprotein complex involved in innate immunity and caspase 1 activation, and subsequent processing and release of the proinflammatory cytokines interleukin 1 beta and interleukin 18 (119, 120). In addition, Panx1 supports ATP release during lipoapoptosis induced by saturated free fatty acids, a key morphologic and pathological feature of human nonalcoholic steatohepatitis. By doing so, Panx1-based channels play an important role in hepatic inflammation associated with lipotoxic liver injury (47).
2.3.4. Fibrosis and cirrhosis
Deterioration of the liver parenchyma in cirrhosis patients is paralleled by a decline in Cx32 protein levels and its relocalization in the cytoplasm of hepatocytes (110, 111, 121) as well as an increase in hepatic Cx43 expression (122). This has been experimentally reproduced in rodents treated for extended periods of time with carbon tetrachloride or thioacetamide (123), yet contradicting results have been obtained, especially with respect to alterations in Cx43 production, depending on the model used (122). The deleterious effects of carbon tetrachloride on gap junctions become manifested at doses that cause an increase in alanine aminotransferase serum levels (101). As is the case for Cx32 in hepatocytes, carbon tetrachloride induces a shift in the cellular localization of Cx26 and Cx43 from the cell plasma membrane surface to the cytoplasm and nuclei of sinusoidal endothelial cells. Similar observations have been made in cultures of spontaneously activated primary stellate cells, whereby both Cx26 and Cx43 reside in the perinuclear region (118). This could explain why stellate cells establish heterologous communication with hepatocytes under these conditions (124), whereas Cx32-based GJIC in primary hepatocyte cultures is suppressed by carbon tetrachloride (125). In turn, these findings indicate active roles for cell type-specific connexins and associated channels in fibrogenesis. In this regard, increased Cx43 production, elevated proliferative activity and collagen content are seen in mouse liver upon induction of hepatic granulomas and concomitant fibrogenesis by Schistosoma mansoni. In a similar way, repetitive administration of carbon tetrachloride to Cx43-lacking mice results in the appearance of tick irregular collagen fibers, less necroinflammatory lesions, lower alanine aspartate aminotransferase serum levels and reduced hepatocyte proliferation compared to wild-type animals. It has been suggested that modified liver cell architecture in Cx43-deficient mice could jeopardize the exchange of growth and toxic signals between hepatocytes, which could explain lower proliferation and injury. Collectively, these observations show an active role for Cx43 in liver fibrogenesis (126).
2.3.5. Liver cancer
Hepatocellular carcinoma (HCC) accounts for as many as 90% of primary liver cancers and typically occurs within an established background of chronic liver disease. It has been well documented that HCC cells display reduced GJIC activity (14, 127, 128). Reduction of Cx26 expression in HCC is due to epigenetic alterations, in particular DNA hypermethylation of its gene promoter (129, 130). In addition, Cx32 tends to accumulate in the cytoplasm of HCC cells. This promotes the motility and metastatic potential (131), a process that was shown to involve expansion of the cancer stem cell population through Cx32-mediated enhancement of cellular self-renewal (132). Another feature of HCC cells includes the appearance of Cx43 both in the cytoplasm and plasma membrane (133-135). The biological relevance of induced Cx43 expression in HCC, reminiscent of liver development, is not fully understood. It has been suggested that the extent of intracellular Cx43 localization is related to the malignant potential of the liver tumor (136). Furthermore, Cx43 production in HCC corresponds well with in vitro migration and invasion capacity, and in vivo metastatic ability in mice (137), though it can delay early recurrence, metastasis and poor prognosis after radical hepatectomy in human patients with hepatitis B-related HCC (135). Knockdown of Cx43 expression in HCC cells triggers cell cycle arrests and boosts the differentiated status in vitro, whereas inverse observations have been made in their Cx43-overexpressing counterparts. Moreover, both Cx32 expression levels and GJIC negatively correlate with Cx43 production in HCC cells. Cx43 might therefore be responsible for the malignancy of liver cancer cells and may thus act as a hepatic oncogene (138). By contrast, Cx32 knockout rodents display increased susceptibility to chemically induced hepatocarcinogenesis and hence Cx32 is considered as a liver tumor suppressor (139, 140).
3. Conclusion and perspectives
Because of their critical roles in tissue functionality, it is not surprising that connexins, pannexins and their channels are also frequently involved in gastrointestinal (Table 1) and liver (Table 2) disease. Indeed, connexin hemichannels have been found to facilitate bacterial infection in the intestine (48-50, 80) and cell death in the liver (51, 52). Likewise, pannexin channels act as goalkeepers of physiological ATP signaling, yet they also drive inflammatory processes (41-43, 47, 78). This might have important clinical implications. Thus, inhibition of connexin hemichannels and pannexin channels could represent a novel strategy for the clinical management of a plethora of gastrointestinal and hepatic diseases. This will be cordially welcomed, as the latter currently constitute the fifth most common cause of death worldwide with an increasing economic burden on society (141, 142). A prerequisite in this context is the development of pharmacological inhibitors of these specific channel types. Most, if not all, of the presently used inhibitors of connexin hemichannels and pannexin channels also suppress gap junctions (143), which typically maintain normal tissue functioning. Great promise now lies with peptides that reproduce sequences in the cytosolic loop regions of connexins, as they suppress connexin hemichannel activity without affecting GJIC (144, 145). These compounds have been found to reduce experimentally induced cell death in mouse models of ischemia-reperfusion (146). Furthermore, a specific inhibitory Panx1 peptide has been demonstrated to counteract inflammation and to protect against cell death (147, 148). It remains to be established whether these compounds or derivatives thereof can also be used for the treatment of gastrointestinal and liver diseases.
In addition to serving as drug targets, connexin and pannexin proteins as such should be further scrutinized as diagnostic biomarkers that could expedite personalized medicine. In this context, gradually downregulated colonic expression of Cx43 (25, 31) and Cx26 (149) even as cytoplasmic presence of Cx32 (87) have been associated with reduced colon cancer patient survival. Similarly, cytoplasmic occurrence of Cx26 in human gastric carcinoma represents a less aggressive phenotype (16). As regards genetic profiling, C1019T Cx37 gene polymorphism is indicative for human gastric cancer and Helicobacter pylori infection (150). Furthermore, colon cancer seems to have its own epigenetic signature, including altered methylation of the Cx45 (30) and Cx36 gene promoters (90), which also could serve as clinical readouts. This equally holds for human gastric cancers caused by Helicobacter pylori, whereby hypermethylated Cx32 and Cx43 gene promoters are observed (65). In liver disease, Cx43 becomes gradually upregulated in nonparenchymal liver cells and is even de novo expressed in hepatocytes at the expense of Cx32 (133-135). Nevertheless, it has been demonstrated that Cx43-positive expression in hepatitis B-HCC tissue is a predictor of lower early recurrence rates and better prognosis in patients with low alpha-fetoprotein serum levels (135). Although in its infancy, it seems that Panx1 could represent another biomarker of intestinal (41, 78) and hepatic (43) pathology, in particular by reflecting inflammatory conditions. It should be mentioned, however, that connexins and pannexins only have been studied thus far as intestinal and hepatic tissue biomarkers, thus necessitating biopsy. Future research in this direction should focus on their potential use as noninvasive serum biomarkers. Further exploration of connexins and pannexins as biomarkers is anticipated to open new avenues for the early and accurate diagnosis of gastrointestinal and liver disease in the upcoming years.
Acknowledgements
This work was financially supported by the grants of Agency for Innovation by Science and Technology in Flanders (IWT), the University Hospital of the Vrije Universiteit Brussel-Belgium (Willy Gepts Fonds UZ-VUB), the Fund for Scientific Research-Flanders (FWO grants G009514N and G010214N), the European Research Council (ERC Starting Grant 335476), the University of São Paulo-Brazil and the Foundation for Research Support of the State of São Paulo (FAPESP SPEC grant 2013/50420-6).
Abbreviations
- ATP
adenosine triphosphate
- CagA
cytotoxin-associated gene A
- Cx
connexin
- GJIC
gap junctional intercellular communication
- HCC
hepatocellular carcinoma
- Panx
pannexin
- VacA
vacuolating toxin A
Footnotes
All authors have read the journal’s policy on conflicts of interest and have no conflicts of interest to declare. All authors have read the journal’s authorship agreement.
References
- 1.Alexander DB, Goldberg GS. Transfer of biologically important molecules between cells through gap junction channels. Curr Med Chem. 2003;10(19):2045–58. doi: 10.2174/0929867033456927. [DOI] [PubMed] [Google Scholar]
- 2.Dbouk HA, Mroue RM, El-Sabban ME, Talhouk RS. Connexins: a myriad of functions extending beyond assembly of gap junction channels. Cell Commun Signal. 2009;7:4. doi: 10.1186/1478-811X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Decrock E, Vinken M, De Vuyst E, Krysko DV, D’Herde K, Vanhaecke T, et al. Connexin-related signaling in cell death: to live or let die? Cell Death Differ. 2009;16(4):524–36. doi: 10.1038/cdd.2008.196. [DOI] [PubMed] [Google Scholar]
- 4.Iino S, Asamoto K, Nojyo Y. Heterogeneous distribution of a gap junction protein, connexin43, in the gastroduodenal junction of the guinea pig. Auton Neurosci. 2001;93(1-2):8–13. doi: 10.1016/S1566-0702(01)00320-4. [DOI] [PubMed] [Google Scholar]
- 5.Daniel EE, Wang YF. Gap junctions in intestinal smooth muscle and interstitial cells of Cajal. Microsc Res Tech. 1999;47(5):309–20. doi: 10.1002/(SICI)1097-0029(19991201)47:5<309::AID-JEMT2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 6.Nemeth L, Maddur S, Puri P. Immunolocalization of the gap junction protein Connexin43 in the interstitial cells of Cajal in the normal and Hirschsprung’s disease bowel. J Pediatr Surg. 2000;35(6):823–8. doi: 10.1053/jpsu.2000.6851. [DOI] [PubMed] [Google Scholar]
- 7.Radebold K, Horakova E, Gloeckner J, Ortega G, Spray DC, Vieweger H, et al. Gap junctional channels regulate acid secretion in the mammalian gastric gland. J Membr Biol. 2001;183(3):147–53. doi: 10.1007/s00232-001-0062-9. [DOI] [PubMed] [Google Scholar]
- 8.Iwata F, Joh T, Ueda F, Yokoyama Y, Itoh M. Role of gap junctions in inhibiting ischemia-reperfusion injury of rat gastric mucosa. Am J Physiol. 1998;275(5 Pt 1):G883–8. doi: 10.1152/ajpgi.1998.275.5.G883. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi N, Joh T, Yokoyama Y, Seno K, Nomura T, Ohara H, et al. Importance of gap junction in gastric mucosal restitution from acid-induced injury. J Lab Clin Med. 2000;136(2):93–9. doi: 10.1067/mlc.2000.108158. [DOI] [PubMed] [Google Scholar]
- 10.Ey B, Eyking A, Gerken G, Podolsky DK, Cario E. TLR2 mediates gap junctional intercellular communication through connexin-43 in intestinal epithelial barrier injury. J Biol Chem. 2009;284(33):22332–43. doi: 10.1074/jbc.M901619200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neveu MJ, Babcock KL, Hertzberg EL, Paul DL, Nicholson BJ, Pitot HC. Colocalized alterations in connexin32 and cytochrome P450IIB1/2 by phenobarbital and related liver tumor promoters. Cancer Res. 1994;54(12):3145–52. [PubMed] [Google Scholar]
- 12.Shoda T, Mitsumori K, Onodera H, Toyoda K, Uneyama C, Takada K, et al. Liver tumor-promoting effect of beta-naphthoflavone, a strong CYP 1A1/2 inducer, and the relationship between CYP 1A1/2 induction and Cx32 decrease in its hepatocarcinogenesis in the rat. Toxicol Pathol. 2000;28(4):540–7. doi: 10.1177/019262330002800406. [DOI] [PubMed] [Google Scholar]
- 13.Shoda T, Mitsumori K, Onodera H, Toyoda K, Uneyama C, Imazawa T, et al. The relationship between decrease in Cx32 and induction of P450 isozymes in the early phase of clofibrate hepatocarcinogenesis in the rat. Arch Toxicol. 1999;73(7):373–80. doi: 10.1007/s002040050676. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Ichikawa A, Tsuchiya T. A novel function of connexin 32: marked enhancement of liver function in a hepatoma cell line. Biochem Biophys Res Commun. 2003;307(1):80–5. doi: 10.1016/s0006-291x(03)01117-3. [DOI] [PubMed] [Google Scholar]
- 15.Bai D, Wang AH. Extracellular domains play different roles in gap junction formation and docking compatibility. Biochem J. 2014;458(1):1–10. doi: 10.1042/BJ20131162. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Furuya T, Li D, Xu J, Cao X, Li Q, et al. Connexin 26 expression correlates with less aggressive phenotype of intestinal type-gastric carcinomas. Int J Mol Med. 2010;25(5):709–16. doi: 10.3892/ijmm_00000395. [DOI] [PubMed] [Google Scholar]
- 17.Uchida Y, Matsuda K, Sasahara K, Kawabata H, Nishioka M. Immunohistochemistry of gap junctions in normal and diseased gastric mucosa of humans. Gastroenterology. 1995;109(5):1492–6. doi: 10.1016/0016-5085(95)90635-5. [DOI] [PubMed] [Google Scholar]
- 18.Nishitani A, Hirota S, Nishida T, Isozaki K, Hashimoto K, Nakagomi N, et al. Differential expression of connexin 43 in gastrointestinal stromal tumours of gastric and small intestinal origin. J Pathol. 2005;206(4):377–82. doi: 10.1002/path.1799. [DOI] [PubMed] [Google Scholar]
- 19.Zhang JT, Nicholson BJ. Sequence and tissue distribution of a second protein of hepatic gap junctions, Cx26, as deduced from its cDNA. J Cell Biol. 1989;109(6 Pt 2):3391–401. doi: 10.1083/jcb.109.6.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang YF, Daniel EE. Gap junctions in gastrointestinal muscle contain multiple connexins. Am J Physiol Gastrointest Liver Physiol. 2001;281(2):G533–43. doi: 10.1152/ajpgi.2001.281.2.G533. [DOI] [PubMed] [Google Scholar]
- 21.Bracken S, Byrne G, Kelly J, Jackson J, Feighery C. Altered gene expression in highly purified enterocytes from patients with active coeliac disease. BMC Genomics. 2008;9:377. doi: 10.1186/1471-2164-9-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leaphart CL, Qureshi F, Cetin S, Li J, Dubowski T, Baty C, et al. Interferon-gamma inhibits intestinal restitution by preventing gap junction communication between enterocytes. Gastroenterology. 2007;132(7):2395–411. doi: 10.1053/j.gastro.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 23.Husøy T, Ølstørn HB, Knutsen HK, Løberg EM, Cruciani V, Mikalsen SO, et al. Truncated mouse adenomatous polyposis coli reduces connexin32 content and increases matrilysin secretion from Paneth cells. Eur J Cancer. 2004;40(10):1599–603. doi: 10.1016/j.ejca.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Dubina MV, Iatckii NA, Popov DE, Vasil’ev SV, Krutovskikh VA. Connexin 43, but not connexin 32, is mutated at advanced stages of human sporadic colon cancer. Oncogene. 2002;21(32):4992–6. doi: 10.1038/sj.onc.1205630. [DOI] [PubMed] [Google Scholar]
- 25.Ismail R, Rashid R, Andrabi K, Parray FQ, Besina S, Shah MA, et al. Pathological implications of Cx43 down-regulation in human colon cancer. Asian Pac J Cancer Prev. 2014;15(7):2987–91. doi: 10.7314/apjcp.2014.15.7.2987. [DOI] [PubMed] [Google Scholar]
- 26.Kanczuga-Koda L, Sulkowski S, Koda M, Skrzydlewska E, Sulkowska M. Connexin 26 correlates with Bcl-xL and Bax proteins expression in colorectal cancer. World J Gastroenterol. 2005;11(10):1544–8. doi: 10.3748/wjg.v11.i10.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattii L, Ippolito C, Segnani C, Battolla B, Colucci R, Dolfi A, et al. Altered expression pattern of molecular factors involved in colonic smooth muscle functions: an immunohistochemical study in patients with diverticular disease. PLoS One. 2013;8(2):e57023. doi: 10.1371/journal.pone.0057023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClain JL, Grubišić V, Fried D, Gomez-Suarez RA, Leinninger GM, Sévigny J, et al. Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology. 2014;146(2):497–507.e1. doi: 10.1053/j.gastro.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sedhom MA, Pichery M, Murdoch JR, Foligné B, Ortega N, Normand S, et al. Neutralisation of the interleukin-33/ST2 pathway ameliorates experimental colitis through enhancement of mucosal healing in mice. Gut. 2013;62(12):1714–23. doi: 10.1136/gutjnl-2011-301785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sirnes S, Honne H, Ahmed D, Danielsen SA, Rognum TO, Meling GI, et al. DNA methylation analyses of the connexin gene family reveal silencing of GJC1 (Connexin45) by promoter hypermethylation in colorectal cancer. Epigenetics. 2011;6(5):602–9. doi: 10.4161/epi.6.5.15237. [DOI] [PubMed] [Google Scholar]
- 31.Sirnes S, Bruun J, Kolberg M, Kjenseth A, Lind GE, Svindland A, et al. Connexin43 acts as a colorectal cancer tumor suppressor and predicts disease outcome. Int J Cancer. 2012;131(3):570–81. doi: 10.1002/ijc.26392. [DOI] [PubMed] [Google Scholar]
- 32.Han Y, Zhang PJ, Chen T, Yum SW, Pasha T, Furth EE. Connexin43 Expression Increases in the Epithelium and Stroma along the Colonic Neoplastic Progression Pathway: Implications for Its Oncogenic Role. Gastroenterol Res Pract. 2011;2011:561719. doi: 10.1155/2011/561719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar NM, Gilula NB. Cloning and characterization of human and rat liver cDNAs coding for a gap junction protein. J Cell Biol. 1986;103(3):767–76. doi: 10.1083/jcb.103.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bode HP, Wang L, Cassio D, Leite MF, St-Pierre MV, Hirata K, et al. Expression and regulation of gap junctions in rat cholangiocytes. Hepatology. 2002;36(3):631–40. doi: 10.1053/jhep.2002.35274. [DOI] [PubMed] [Google Scholar]
- 35.Shiojiri N, Niwa T, Sugiyama Y, Koike T. Preferential expression of connexin37 and connexin40 in the endothelium of the portal veins during mouse liver development. Cell Tissue Res. 2006;324(3):547–52. doi: 10.1007/s00441-006-0165-9. [DOI] [PubMed] [Google Scholar]
- 36.Chandrasekhar A, Bera AK. Hemichannels: permeants and their effect on development, physiology and death. Cell Biochem Funct. 2012;30(2):89–100. doi: 10.1002/cbf.2794. [DOI] [PubMed] [Google Scholar]
- 37.Spray DC, Ye ZC, Ransom BR. Functional connexin “hemichannels”: a critical appraisal. Glia. 2006;54(7):758–73. doi: 10.1002/glia.20429. [DOI] [PubMed] [Google Scholar]
- 38.Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. A ubiquitous family of putative gap junction molecules. Curr Biol. 2000;10(13):R473–4. doi: 10.1016/s0960-9822(00)00576-5. [DOI] [PubMed] [Google Scholar]
- 39.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003;100(23):13644–9. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Vasseur M, Lelowski J, Bechberger JF, Sin WC, Naus CC. Pannexin 2 protein expression is not restricted to the CNS. Front Cell Neurosci. 2014;8:392. doi: 10.3389/fncel.2014.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diezmos EF, Sandow SL, Markus I, Shevy Perera D, Lubowski DZ, King DW, et al. Expression and localization of pannexin-1 hemichannels in human colon in health and disease. Neurogastroenterol Motil. 2013;25(6):e395–405. doi: 10.1111/nmo.12130. [DOI] [PubMed] [Google Scholar]
- 42.Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011;54(1):133–44. doi: 10.1002/hep.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ganz M, Csak T, Nath B, Szabo G. Lipopolysaccharide induces and activates the Nalp3 inflammasome in the liver. World Journal of Gastroenterology. 2011;17(43):4772–8. doi: 10.3748/wjg.v17.i43.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim HY, Kim SJ, Lee SM. Activation of NLRP3 and AIM2 inflammasomes in Kupffer cells in hepatic ischemia/reperfusion. Febs j. 2015;282(2):259–70. doi: 10.1111/febs.13123. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Cao J, Jin Q, Xie C, He Q, Cao R, et al. A proteomic study reveals the diversified distribution of plasma membrane-associated proteins in rat hepatocytes. J Cell Biochem. 2008;104(3):965–84. doi: 10.1002/jcb.21680. [DOI] [PubMed] [Google Scholar]
- 46.Sáez PJ, Shoji KF, Aguirre A, Sáez JC. Regulation of hemichannels and gap junction channels by cytokines in antigen-presenting cells. Mediators Inflamm. 2014;2014:742734. doi: 10.1155/2014/742734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao F, Waldrop SL, Khimji AK, Kilic G. Pannexin1 contributes to pathophysiological ATP release in lipoapoptosis induced by saturated free fatty acids in liver cells. American Journal of Physiology-Cell Physiology. 2012;303(10):C1034–C44. doi: 10.1152/ajpcell.00175.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guttman JA, Lin AE, Li Y, Bechberger J, Naus CC, Vogl AW, et al. Gap junction hemichannels contribute to the generation of diarrhoea during infectious enteric disease. Gut. 2010;59(2):218–26. doi: 10.1136/gut.2008.170464. [DOI] [PubMed] [Google Scholar]
- 49.Simpson C, Kelsell DP, Marchès O. Connexin 26 facilitates gastrointestinal bacterial infection in vitro. Cell Tissue Res. 2013;351(1):107–16. doi: 10.1007/s00441-012-1502-9. [DOI] [PubMed] [Google Scholar]
- 50.Tran Van Nhieu G, Clair C, Bruzzone R, Mesnil M, Sansonetti P, Combettes L. Connexin-dependent inter-cellular communication increases invasion and dissemination of Shigella in epithelial cells. Nat Cell Biol. 2003;5(8):720–6. doi: 10.1038/ncb1021. [DOI] [PubMed] [Google Scholar]
- 51.Vinken M, Decrock E, De Vuyst E, De Bock M, Vandenbroucke RE, De Geest BG, et al. Connexin32 hemichannels contribute to the apoptotic-to-necrotic transition during Fas-mediated hepatocyte cell death. Cell Mol Life Sci. 2010;67(6):907–18. doi: 10.1007/s00018-009-0220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vinken M, Decrock E, Vanhaecke T, Leybaert L, Rogiers V. Connexin43 signaling contributes to spontaneous apoptosis in cultures of primary hepatocytes. Toxicol Sci. 2012;125(1):175–86. doi: 10.1093/toxsci/kfr277. [DOI] [PubMed] [Google Scholar]
- 53.Ohkusa T, Yamamoto M, Kataoka K, Kyoi T, Ueda F, Fujimoto H, et al. Electron microscopic study of intercellular junctions in human gastric mucosa with special reference to their relationship to gastric ulcer. Gut. 1993;34(1):86–9. doi: 10.1136/gut.34.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohkusa T, Fujiki K, Tamura Y, Yamamoto M, Kyoi T. Freeze-fracture and immunohistochemical studies of gap junctions in human gastric mucosa with special reference to their relationship to gastric ulcer and gastric carcinoma. Microsc Res Tech. 1995;31(3):226–33. doi: 10.1002/jemt.1070310306. [DOI] [PubMed] [Google Scholar]
- 55.Miwa H, Endo K, Wada R, Hirai S, Hirose M, Misawa H, et al. Cellular proliferation and differentiation in rat atrophic gastric mucosa induced by N’-methyl-N’-nitro-N-nitrosoguanidine. J Clin Gastroenterol. 1997;25(Suppl 1):S116–21. doi: 10.1097/00004836-199700001-00020. [DOI] [PubMed] [Google Scholar]
- 56.Nagahara A, Watanabe S, Miwa H, Endo K, Hirose M, Sato N. Reduction of gap junction protein connexin 32 in rat atrophic gastric mucosa as an early event in carcinogenesis. J Gastroenterol. 1996;31(4):491–7. doi: 10.1007/BF02355047. [DOI] [PubMed] [Google Scholar]
- 57.Ceelen L, Haesebrouck F, Vanhaecke T, Rogiers V, Vinken M. Modulation of connexin signaling by bacterial pathogens and their toxins. Cell Mol Life Sci. 2011;68(18):3047–64. doi: 10.1007/s00018-011-0737-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tao R, Hu MF, Lou JT, Lei YL. Effects of H pylori infection on gap-junctional intercellular communication and proliferation of gastric epithelial cells in vitro. World J Gastroenterol. 2007;13(41):5497–500. doi: 10.3748/wjg.v13.i41.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu C, Chen Y, Chen X, Wang F. Effects of different types of Helicobacter pylori on the gap junction intercellular communication in GES-1 cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36(4):294–300. doi: 10.3969/j.issn.1672-7347.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 60.Mine T, Endo C, Kushima R, Kushima W, Kobayashi I, Muraoka H, et al. The effects of water extracts of CagA positive or negative Helicobacter pylori on proliferation, apoptosis and connexin formation in acetic acid-induced gastric ulcer of rats. Aliment Pharmacol Ther. 2000;14(Suppl 1):199–204. doi: 10.1046/j.1365-2036.2000.014s1199.x. [DOI] [PubMed] [Google Scholar]
- 61.Xu CX, Qi YM, Yang WB, Wang F, Zhou JD, Shen SR. Effect of CagA(+) helicobacter pylori strain on the expression of connexin 43 and cell proliferation in BGC-823 cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2007;32(2):288–94. [PubMed] [Google Scholar]
- 62.Jia Y, Xu CX, Yang WB. Expressions of connexin 32 and connexin 43 in patients with gastric precancerous lesion after eradication of Helicobacter pylori. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2008;33(7):628–33. [PubMed] [Google Scholar]
- 63.Xu CX, Jia Y, Yang WB, Zou HF, Wang F, Shen SR. Helicobacter pylori infection and changes of cell gap junction of gastric epithelial cells in patients with gastric cancer and precancerous lesion. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2008;33(4):338–43. [PubMed] [Google Scholar]
- 64.Xu CX, Jia Y, Yang WB, Wang F, Shen SR. Relationship between Helicobacter pylori infection and expression of connexin (Cx) 32 and Cx43 genes in gastric cancer and gastric precancerous lesions. Zhonghua Yi Xue Za Zhi. 2008;88(22):1523–7. [PubMed] [Google Scholar]
- 65.Wang Y, Huang LH, Xu CX, Xiao J, Zhou L, Cao D, et al. Connexin 32 and 43 promoter methylation in Helicobacter pylori-associated gastric tumorigenesis. World J Gastroenterol. 2014;20(33):11770–9. doi: 10.3748/wjg.v20.i33.11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Radin JN, González-Rivera C, Frick-Cheng AE, Sheng J, Gaddy JA, Rubin DH, et al. Role of connexin 43 in Helicobacter pylori VacA-induced cell death. Infect Immun. 2014;82(1):423–32. doi: 10.1128/IAI.00827-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xue L, Suzuki H. Electrical responses of gastric smooth muscles in streptozotocin-induced diabetic rats. Am J Physiol. 1997;272(1 Pt 1):G77–83. doi: 10.1152/ajpgi.1997.272.1.G77. [DOI] [PubMed] [Google Scholar]
- 68.Long QL, Fang DC, Shi HT, Luo YH. Gastro-electric dysrhythm and lack of gastric interstitial cells of cajal. World J Gastroenterol. 2004;10(8):1227–30. doi: 10.3748/wjg.v10.i8.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Langer JC, Berezin I, Daniel EE. Hypertrophic pyloric stenosis: ultrastructural abnormalities of enteric nerves and the interstitial cells of Cajal. J Pediatr Surg. 1995;30(11):1535–43. doi: 10.1016/0022-3468(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 70.Xia LN, Wang ZQ, Wang ZM, Zhang P. Expression of c-kit and Cx43 in neonates with spontaneous gastric perforation. Zhongguo Dang Dai Er Ke Za Zhi. 2011;13(10):787–9. [PubMed] [Google Scholar]
- 71.Jee H, Nam KT, Kwon HJ, Han SU, Kim DY. Altered expression and localization of connexin32 in human and murine gastric carcinogenesis. Dig Dis Sci. 2011;56(5):1323–32. doi: 10.1007/s10620-010-1467-z. [DOI] [PubMed] [Google Scholar]
- 72.Jee H, Lee SH, Park JW, Lee BR, Nam KT, Kim DY. Connexin32 inhibits gastric carcinogenesis through cell cycle arrest and altered expression of p21Cip1 and p27Kip1. BMB Rep. 2013;46(1):25–30. doi: 10.5483/BMBRep.2013.46.1.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang B, Peng ZH, Yu PW, Yu G, Qian F. Expression and significance of Cx43 and E-cadherin in gastric cancer and metastatic lymph nodes. Med Oncol. 2011;28(2):502–8. doi: 10.1007/s12032-010-9492-5. [DOI] [PubMed] [Google Scholar]
- 74.Wu J, Zhou HF, Wang CH, Zhang B, Liu D, Wang W, et al. Decreased expression of Cx32 and Cx43 and their function of gap junction intercellular communication in gastric cancer. Zhonghua Zhong Liu Za Zhi. 2007;29(10):742–7. [PubMed] [Google Scholar]
- 75.Tang B, Peng ZH, Yu PW, Yu G, Qian F, Zeng DZ, et al. Aberrant expression of Cx43 is associated with the peritoneal metastasis of gastric cancer and Cx43-mediated gap junction enhances gastric cancer cell diapedesis from peritoneal mesothelium. PLoS One. 2013;8(9):e74527. doi: 10.1371/journal.pone.0074527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park SH, Kim MK, Kim H, Song BJ, Chi JG. Ultrastructural studies of gastrointestinal stromal tumors. J Korean Med Sci. 2004;19(2):234–44. doi: 10.3346/jkms.2004.19.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sentani K, Oue N, Sakamoto N, Anami K, Naito Y, Aoyagi K, et al. Upregulation of connexin 30 in intestinal phenotype gastric cancer and its reduction during tumor progression. Pathobiology. 2010;77(5):241–8. doi: 10.1159/000314966. [DOI] [PubMed] [Google Scholar]
- 78.Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med. 2012;18(4):600–4. doi: 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chokshi NK, Guner YS, Hunter CJ, Upperman JS, Grishin A, Ford HR. The role of nitric oxide in intestinal epithelial injury and restitution in neonatal necrotizing enterocolitis. Semin Perinatol. 2008;32(2):92–9. doi: 10.1053/j.semperi.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Puhar A, Tronchère H, Payrastre B, Nhieu GT, Sansonetti PJ. A Shigella effector dampens inflammation by regulating epithelial release of danger signal ATP through production of the lipid mediator PtdIns5P. Immunity. 2013;39(6):1121–31. doi: 10.1016/j.immuni.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 81.Vuckovic D, Dallapiccola B, Franzè A, Mauri L, Perrone MD, Gasparini P. Connexin 26 variant carriers have a better gastrointestinal health: is this the heterozygote advantage? Eur J Hum Genet. 2014 [Google Scholar]
- 82.King TJ, Lampe PD. Mice deficient for the gap junction protein Connexin32 exhibit increased radiation-induced tumorigenesis associated with elevated mitogen-activated protein kinase (p44/Erk1, p42/Erk2) activation. Carcinogenesis. 2004;25(5):669–80. doi: 10.1093/carcin/bgh071. [DOI] [PubMed] [Google Scholar]
- 83.King TJ, Gurley KE, Prunty J, Shin JL, Kemp CJ, Lampe PD. Deficiency in the gap junction protein connexin32 alters p27Kip1 tumor suppression and MAPK activation in a tissue-specific manner. Oncogene. 2005;24(10):1718–26. doi: 10.1038/sj.onc.1208355. [DOI] [PubMed] [Google Scholar]
- 84.Kanczuga-Koda L, Wincewicz A, Fudala A, Abrycki T, Famulski W, Baltaziak M, et al. E-cadherin and β-catenin adhesion proteins correlate positively with connexins in colorectal cancer. Oncol Lett. 2014;7(6):1863–70. doi: 10.3892/ol.2014.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sulkowski S, Kanczuga-Koda L, Koda M, Wincewicz A, Sulkowska M. Insulin-like growth factor-I receptor correlates with connexin 26 and Bcl-xL expression in human colorectal cancer. Ann N Y Acad Sci. 2006;1090:265–75. doi: 10.1196/annals.1378.029. [DOI] [PubMed] [Google Scholar]
- 86.Nikolić M, Boban M, Ljubicić N, Bekavac-Beslin M, Tomas D, Pezo-Nikolić B, et al. First report of connexin 43-positive gastrointestinal stromal tumor (GIST) Acta Clin Croat. 2010;49(3):359–63. [PubMed] [Google Scholar]
- 87.Kanczuga-Koda L, Koda M, Sulkowski S, Wincewicz A, Zalewski B, Sulkowska M. Gradual loss of functional gap junction within progression of colorectal cancer -- a shift from membranous CX32 and CX43 expression to cytoplasmic pattern during colorectal carcinogenesis. In Vivo. 2010;24(1):101–7. [PubMed] [Google Scholar]
- 88.Husøy T, Cruciani V, Knutsen HK, Mikalsen SO, Ølstørn HB, Alexander J. Cells heterozygous for the ApcMin mutation have decreased gap junctional intercellular communication and connexin43 level, and reduced microtubule polymerization. Carcinogenesis. 2003;24(4):643–50. doi: 10.1093/carcin/bgg007. [DOI] [PubMed] [Google Scholar]
- 89.Sirnes S, Lind GE, Bruun J, Fykerud TA, Mesnil M, Lothe RA, et al. Connexins in colorectal cancer pathogenesis. Int J Cancer. 2014 doi: 10.1002/ijc.28911. [DOI] [PubMed] [Google Scholar]
- 90.Borinstein SC, Conerly M, Dzieciatkowski S, Biswas S, Washington MK, Trobridge P, et al. Aberrant DNA methylation occurs in colon neoplasms arising in the azoxymethane colon cancer model. Mol Carcinog. 2010;49(1):94–103. doi: 10.1002/mc.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Orian-Rousseau V, Mink S, Mengwasser J, HogenEsch H, Guo F, Thies WG, et al. Genes upregulated in a metastasizing human colon carcinoma cell line. Int J Cancer. 2005;113(5):699–705. doi: 10.1002/ijc.20644. [DOI] [PubMed] [Google Scholar]
- 92.Derangère V, Chevriaux A, Courtaut F, Bruchard M, Berger H, Chalmin F, et al. Liver X receptor β activation induces pyroptosis of human and murine colon cancer cells. Cell Death Differ. 2014;21(12):1914–24. doi: 10.1038/cdd.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Asamoto M, Hokaiwado N, Murasaki T, Shirai T. Connexin 32 dominant-negative mutant transgenic rats are resistant to hepatic damage by chemicals. Hepatology. 2004;40(1):205–10. doi: 10.1002/hep.20256. [DOI] [PubMed] [Google Scholar]
- 94.Naiki-Ito A, Asamoto M, Naiki T, Ogawa K, Takahashi S, Sato S, et al. Gap junction dysfunction reduces acetaminophen hepatotoxicity with impact on apoptotic signaling and connexin 43 protein induction in rat. Toxicol Pathol. 2010;38(2):280–6. doi: 10.1177/0192623309357951. [DOI] [PubMed] [Google Scholar]
- 95.Patel SJ, Milwid JM, King KR, Bohr S, Iracheta-Velle A, Li M, et al. Gap junction inhibition prevents drug-induced liver toxicity and fulminant hepatic failure. Nat Biotechnol. 2012;30(2):179–83. doi: 10.1038/nbt.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park WJ, Park JW, Erez-Roman R, Kogot-Levin A, Bame JR, Tirosh B, et al. Protection of a ceramide synthase 2 null mouse from drug-induced liver injury: role of gap junction dysfunction and connexin 32 mislocalization. J Biol Chem. 2013;288(43):30904–16. doi: 10.1074/jbc.M112.448852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saito C, Shinzawa K, Tsujimoto Y. Synchronized necrotic death of attached hepatocytes mediated via gap junctions. Sci Rep. 2014;4:5169. doi: 10.1038/srep05169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Igarashi I, Maejima T, Kai K, Arakawa S, Teranishi M, Sanbuissho A. Role of connexin 32 in acetaminophen toxicity in a knockout mice model. Exp Toxicol Pathol. 2014;66(2-3):103–10. doi: 10.1016/j.etp.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 99.Vinken M, Doktorova T, Decrock E, Leybaert L, Vanhaecke T, Rogiers V. Gap junctional intercellular communication as a target for liver toxicity and carcinogenicity. Crit Rev Biochem Mol Biol. 2009;44(4):201–22. doi: 10.1080/10409230903061215. [DOI] [PubMed] [Google Scholar]
- 100.Kojima T, Sawada N, Zhong Y, Oyamada M, Mori M. Sequential changes in intercellular junctions between hepatocytes during the course of acute liver injury and restoration after thioacetamide treatment. Virchows Arch. 1994;425(4):407–12. doi: 10.1007/BF00189579. [DOI] [PubMed] [Google Scholar]
- 101.Cogliati B, Da Silva TC, Aloia TP, Chaible LM, Real-Lima MA, Sanches DS, et al. Morphological and molecular pathology of CCL4-induced hepatic fibrosis in connexin43-deficient mice. Microsc Res Tech. 2011;74(5):421–9. doi: 10.1002/jemt.20926. [DOI] [PubMed] [Google Scholar]
- 102.Balasubramaniyan V, Dhar DK, Warner AE, Vivien Li WY, Amiri AF, Bright B, et al. Importance of Connexin-43 based gap junction in cirrhosis and acute-on-chronic liver failure. J Hepatol. 2013;58(6):1194–200. doi: 10.1016/j.jhep.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 103.Robenek H, Herwig J, Themann H. The morphologic characteristics of intercellular junctions between normal human liver cells and cells from patients with extrahepatic cholestasis. Am J Pathol. 1980;100(1):93–114. [PMC free article] [PubMed] [Google Scholar]
- 104.Robenek H, Rassat J, Themann H. A quantitative freeze-fracture analysis of gap and tight junctions in the normal and cholestatic human liver. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;38(1):39–56. doi: 10.1007/BF02892801. [DOI] [PubMed] [Google Scholar]
- 105.Snigerevskaia ES, Veselov VS. Changes in the structure of the plasmalemma of hepatocytes and its specialized portions intercellular junctions in man in complicated forms of cholelithiasis. Arkh Anat Gistol Embriol. 1986;90(1):59–65. [PubMed] [Google Scholar]
- 106.Fallon MB, Nathanson MH, Mennone A, Sáez JC, Burgstahler AD, Anderson JM. Altered expression and function of hepatocyte gap junctions after common bile duct ligation in the rat. Am J Physiol. 1995;268(5 Pt 1):C1186–94. doi: 10.1152/ajpcell.1995.268.5.C1186. [DOI] [PubMed] [Google Scholar]
- 107.Gonzalez HE, Eugenin EA, Garces G, Solis N, Pizarro M, Accatino L, et al. Regulation of hepatic connexins in cholestasis: possible involvement of Kupffer cells and inflammatory mediators. Am J Physiol Gastrointest Liver Physiol. 2002;282(6):G991–G1001. doi: 10.1152/ajpgi.00298.2001. [DOI] [PubMed] [Google Scholar]
- 108.Kojima T, Yamamoto T, Murata M, Lan M, Takano K, Go M, et al. Role of the p38 MAP-kinase signaling pathway for Cx32 and claudin-1 in the rat liver. Cell Commun Adhes. 2003;10(4-6):437–43. doi: 10.1080/cac.10.4-6.437.443. [DOI] [PubMed] [Google Scholar]
- 109.Teixeira TF, da Silva TC, Fukumasu H, de Lima CE, Lúcia Zaidan Dagli M, Guerra JL. Histological alterations in the livers of Cx43-deficient mice submitted to a cholestasis model. Life Sci. 2007;81(5):380–4. doi: 10.1016/j.lfs.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 110.Nakashima Y, Ono T, Yamanoi A, El-Assal ON, Kohno H, Nagasue N. Expression of gap junction protein connexin32 in chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. J Gastroenterol. 2004;39(8):763–8. doi: 10.1007/s00535-003-1386-2. [DOI] [PubMed] [Google Scholar]
- 111.Yamaoka K, Nouchi T, Kohashi T, Marumo F, Sato C. Expression of gap junction protein connexin 32 in chronic liver diseases. Liver. 2000;20(2):104–7. doi: 10.1034/j.1600-0676.2000.020002104.x. [DOI] [PubMed] [Google Scholar]
- 112.Correa PR, Guerra MT, Leite MF, Spray DC, Nathanson MH. Endotoxin unmasks the role of gap junctions in the liver. Biochem Biophys Res Commun. 2004;322(3):718–26. doi: 10.1016/j.bbrc.2004.07.192. [DOI] [PubMed] [Google Scholar]
- 113.Temme A, Ott T, Haberberger T, Traub O, Willecke K. Acute-phase response and circadian expression of connexin26 are not altered in connexin32-deficient mouse liver. Cell Tissue Res. 2000;300(1):111–7. doi: 10.1007/s004410000177. [DOI] [PubMed] [Google Scholar]
- 114.Theodorakis NG, De Maio A. Cx32 mRNA in rat liver: effects of inflammation on poly(A) tail distribution and mRNA degradation. Am J Physiol. 1999;276(5 Pt 2):R1249–57. doi: 10.1152/ajpregu.1999.276.5.R1249. [DOI] [PubMed] [Google Scholar]
- 115.Yamamoto T, Kojima T, Murata M, Takano K, Go M, Chiba H, et al. IL-1beta regulates expression of Cx32, occludin, and claudin-2 of rat hepatocytes via distinct signal transduction pathways. Exp Cell Res. 2004;299(2):427–41. doi: 10.1016/j.yexcr.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 116.Temme A, Traub O, Willecke K. Downregulation of connexin32 protein and gap-junctional intercellular communication by cytokine-mediated acute-phase response in immortalized mouse hepatocytes. Cell Tissue Res. 1998;294(2):345–50. doi: 10.1007/s004410051184. [DOI] [PubMed] [Google Scholar]
- 117.Eugenin EA, Gonzalez HE, Sanchez HA, Branes MC, Saez JC. Inflammatory conditions induce gap junctional communication between rat Kupffer cells both in vivo and in vitro. Cell Immunol. 2007;247(2):103–10. doi: 10.1016/j.cellimm.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fischer R, Reinehr R, Lu TP, Schonicke A, Warskulat U, Dienes HP, et al. Intercellular communication via gap junctions in activated rat hepatic stellate cells. Gastroenterology. 2005;128(2):433–48. doi: 10.1053/j.gastro.2004.11.065. [DOI] [PubMed] [Google Scholar]
- 119.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25(21):5071–82. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pelegrin P, Surprenant A. Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem. 2007;282(4):2386–94. doi: 10.1074/jbc.M610351200. [DOI] [PubMed] [Google Scholar]
- 121.Yamaoka K, Nouchi T, Tazawa J, Hiranuma S, Marumo F, Sato C. Expression of gap junction protein connexin 32 and E-cadherin in human hepatocellular carcinoma. J Hepatol. 1995;22(5):536–9. doi: 10.1016/0168-8278(95)80447-1. [DOI] [PubMed] [Google Scholar]
- 122.Hernández-Guerra M, González-Méndez Y, de Ganzo ZA, Salido E, García-Pagán JC, Abrante B, et al. Role of gap junctions modulating hepatic vascular tone in cirrhosis. Liver Int. 2014;34(6):859–68. doi: 10.1111/liv.12446. [DOI] [PubMed] [Google Scholar]
- 123.Nakata Y, Iwai M, Kimura S, Shimazu T. Prolonged decrease in hepatic connexin32 in chronic liver injury induced by carbon tetrachloride in rats. J Hepatol. 1996;25(4):529–37. doi: 10.1016/s0168-8278(96)80213-3. [DOI] [PubMed] [Google Scholar]
- 124.Rojkind M, Novikoff PM, Greenwel P, Rubin J, Rojas-Valencia L, de Carvalho AC, et al. Characterization and functional studies on rat liver fat-storing cell line and freshly isolated hepatocyte coculture system. Am J Pathol. 1995;146(6):1508–20. [PMC free article] [PubMed] [Google Scholar]
- 125.Saez JC, Bennett MV, Spray DC. Carbon tetrachloride at hepatotoxic levels blocks reversibly gap junctions between rat hepatocytes. Science. 1987;236(4804):967–9. doi: 10.1126/science.3576214. [DOI] [PubMed] [Google Scholar]
- 126.Oloris SC, Mesnil M, Reis VN, Sakai M, Matsuzaki P, Fonseca Ede S, et al. Hepatic granulomas induced by Schistosoma mansoni in mice deficient for connexin 43 present lower cell proliferation and higher collagen content. Life Sci. 2007;80(13):1228–35. doi: 10.1016/j.lfs.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 127.Mesnil M, Crespin S, Avanzo JL, Zaidan-Dagli ML. Defective gap junctional intercellular communication in the carcinogenic process. Biochim Biophys Acta. 2005;1719(1-2):125–45. doi: 10.1016/j.bbamem.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 128.Yano T, Hernandez-Blazquez FJ, Omori Y, Yamasaki H. Reduction of malignant phenotype of HEPG2 cell is associated with the expression of connexin 26 but not connexin 32. Carcinogenesis. 2001;22(10):1593–600. doi: 10.1093/carcin/22.10.1593. [DOI] [PubMed] [Google Scholar]
- 129.Shimizu K, Onishi M, Sugata E, Sokuza Y, Mori C, Nishikawa T, et al. Disturbance of DNA methylation patterns in the early phase of hepatocarcinogenesis induced by a choline-deficient L-amino acid-defined diet in rats. Cancer Sci. 2007;98(9):1318–22. doi: 10.1111/j.1349-7006.2007.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tsujiuchi T, Shimizu K, Itsuzaki Y, Onishi M, Sugata E, Fujii H, et al. CpG site hypermethylation of E-cadherin and Connexin26 genes in hepatocellular carcinomas induced by a choline-deficient L-Amino Acid-defined diet in rats. Mol Carcinog. 2007;46(4):269–74. doi: 10.1002/mc.20268. [DOI] [PubMed] [Google Scholar]
- 131.Li Q, Omori Y, Nishikawa Y, Yoshioka T, Yamamoto Y, Enomoto K. Cytoplasmic accumulation of connexin32 protein enhances motility and metastatic ability of human hepatoma cells in vitro and in vivo. Int J Cancer. 2007;121(3):536–46. doi: 10.1002/ijc.22696. [DOI] [PubMed] [Google Scholar]
- 132.Kawasaki Y, Omori Y, Li Q, Nishikawa Y, Yoshioka T, Yoshida M, et al. Cytoplasmic accumulation of connexin32 expands cancer stem cell population in human HuH7 hepatoma cells by enhancing its self-renewal. Int J Cancer. 2011;128(1):51–62. doi: 10.1002/ijc.25308. [DOI] [PubMed] [Google Scholar]
- 133.Krutovskikh V, Mazzoleni G, Mironov N, Omori Y, Aguelon AM, Mesnil M, et al. Altered homologous and heterologous gap-junctional intercellular communication in primary human liver tumors associated with aberrant protein localization but not gene mutation of connexin 32. Int J Cancer. 1994;56(1):87–94. doi: 10.1002/ijc.2910560116. [DOI] [PubMed] [Google Scholar]
- 134.Oyamada M, Krutovskikh VA, Mesnil M, Partensky C, Berger F, Yamasaki H. Aberrant expression of gap junction gene in primary human hepatocellular carcinomas: increased expression of cardiac-type gap junction gene connexin 43. Mol Carcinog. 1990;3(5):273–8. doi: 10.1002/mc.2940030507. [DOI] [PubMed] [Google Scholar]
- 135.Wang ZS, Wu LQ, Yi X, Geng C, Li YJ, Yao RY. Connexin-43 can delay early recurrence and metastasis in patients with hepatitis B-related hepatocellular carcinoma and low serum alpha-fetoprotein after radical hepatectomy. BMC Cancer. 2013;13:306. doi: 10.1186/1471-2407-13-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kawasaki Y, Kubomoto A, Yamasaki H. Control of intracellular localization and function of Cx43 by SEMA3F. J Membr Biol. 2007;217(1-3):53–61. doi: 10.1007/s00232-007-9051-y. [DOI] [PubMed] [Google Scholar]
- 137.Ogawa K, Pitchakarn P, Suzuki S, Chewonarin T, Tang M, Takahashi S, et al. Silencing of connexin 43 suppresses invasion, migration and lung metastasis of rat hepatocellular carcinoma cells. Cancer Sci. 2012;103(5):860–7. doi: 10.1111/j.1349-7006.2012.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang D, Kaneda M, Nakahama K, Arii S, Morita I. Connexin 43 expression promotes malignancy of HuH7 hepatocellular carcinoma cells via the inhibition of cell-cell communication. Cancer Lett. 2007;252(2):208–15. doi: 10.1016/j.canlet.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 139.Dagli ML, Yamasaki H, Krutovskikh V, Omori Y. Delayed liver regeneration and increased susceptibility to chemical hepatocarcinogenesis in transgenic mice expressing a dominant-negative mutant of connexin32 only in the liver. Carcinogenesis. 2004;25(4):483–92. doi: 10.1093/carcin/bgh050. [DOI] [PubMed] [Google Scholar]
- 140.Igarashi I, Makino T, Suzuki Y, Kai K, Teranishi M, Takasaki W, et al. Background lesions during a 24-month observation period in connexin 32-deficient mice. J Vet Med Sci. 2013;75(2):207–10. doi: 10.1292/jvms.12-0280. [DOI] [PubMed] [Google Scholar]
- 141.Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58(3):593–608. doi: 10.1016/j.jhep.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 142.Fagiuoli S, Daina E, D’Antiga L, Colledan M, Remuzzi G. Monogenic diseases that can be cured by liver transplantation. J Hepatol. 2013;59(3):595–612. doi: 10.1016/j.jhep.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 143.Bodendiek SB, Raman G. Connexin modulators and their potential targets under the magnifying glass. Curr Med Chem. 2010;17(34):4191–230. doi: 10.2174/092986710793348563. [DOI] [PubMed] [Google Scholar]
- 144.Abudara V, Bechberger J, Freitas-Andrade M, De Bock M, Wang N, Bultynck G, et al. The connexin43 mimetic peptide Gap19 inhibits hemichannels without altering gap junctional communication in astrocytes. Front Cell Neurosci. 2014;8:306. doi: 10.3389/fncel.2014.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Iyyathurai J, D’hondt C, Wang N, De Bock M, Himpens B, Retamal MA, et al. Peptides and peptide-derived molecules targeting the intracellular domains of Cx43: gap junctions versus hemichannels. Neuropharmacology. 2013;75:491–505. doi: 10.1016/j.neuropharm.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 146.Wang N, De Vuyst E, Ponsaerts R, Boengler K, Palacios-Prado N, Wauman J, et al. Selective inhibition of Cx43 hemichannels by Gap19 and its impact on myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2013;108(1):309. doi: 10.1007/s00395-012-0309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Orellana JA, Shoji KF, Abudara V, Ezan P, Amigou E, Saez PJ, et al. Amyloid beta-induced death in neurons involves glial and neuronal hemichannels. The Journal of neuroscience. 2011;31(13):4962–77. doi: 10.1523/JNEUROSCI.6417-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pelegrin P, Barroso-Gutierrez C, Surprenant A. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J Immunol. 2008;180(11):7147–57. doi: 10.4049/jimmunol.180.11.7147. [DOI] [PubMed] [Google Scholar]
- 149.Nomura S, Maeda K, Noda E, Inoue T, Fukunaga S, Nagahara H, et al. Clinical significance of the expression of connexin26 in colorectal cancer. J Exp Clin Cancer Res. 2010;29:79. doi: 10.1186/1756-9966-29-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jing YM, Guo SX, Zhang XP, Sun AJ, Tao F, Qian HX. Association between C1019T polymorphism in the connexin 37 gene and Helicobacter pylori infection in patients with gastric cancer. Asian Pac J Cancer Prev. 2012;13(5):2363–7. doi: 10.7314/apjcp.2012.13.5.2363. [DOI] [PubMed] [Google Scholar]