Abstract
Traditionally, patients treated with chemoradiotherapy for node-positive oropharyngeal squamous cell carcinoma (N+ OPSCC) have undergone a planned neck dissection (ND) after treatment. Recently, negative post-treatment positron-emission tomography (PET)/computed tomography (CT) imaging has been found to have a high negative predictive value for the presence of residual disease in the neck. Here we present the first comprehensive analysis of a large, uniform cohort of N+ OPSCC patients achieving a PET/CT-based complete response (CR) after chemoradiotherapy, and undergoing observation, rather than ND. From 2002 to 2009, 302 patients with N+ OPSCC treated with 70 Gy intensity-modulated radiation therapy and concurrent chemotherapy underwent post-treatment clinical assessment including PET/CT. CR was defined as no evidence of disease on clinical examination and post-treatment PET/CT. ND was reserved for patients with <CR on either PET/CT, clinical examination, or other imaging. 260 patients (86.1%) had clinical and radiographic CRs, and underwent neck observation (rate of regional control, 97.7%; 5-year overall survival, 79.8%). The four observed patients experiencing neck recurrence had initial staging of N1 (n=2), N2b (n=1), and N2c (n=1). Three of four were successfully surgically salvaged. There was no association between N stage and rate of neck recurrence (P = 0.74). 52% and 25% of patients undergoing ND had viable tumor in the neck after positive and negative PET/CT, respectively. We conclude that patients achieving CRs after chemoradiation, based on clinical and PET/CT assessment, have a high probability of regional control, with a 2.3% regional failure rate, and may be safely observed without planned ND.
Keywords: Oropharyngeal Squamous Cell Carcinoma, Neck Dissection, Observation, PET
The management of the node-positive (N+) neck in patients with head and neck cancer has evolved in recent years. While definitive chemoradiation is an effective treatment, the optimal management of the N+ neck following chemoradiation has not been well established. Traditionally, many patients with limited N1 disease were observed without planned neck dissection (ND), provided that a complete response (CR) was achieved at the primary site and neck. However, most patients with N2 or N3 disease have generally undergone planned post-treatment NDs. These early experiences demonstrated that the combination of surgery and radiation significantly improved control rates in the neck compared with either modality alone.1–7 However, in recent years, some centers have advocated observation of the neck in patients with initial N2–3 disease, provided a CR is achieved after chemoradiation.8–10 Contemporary CR rates are higher than those in the past. This reflects both a greater proportion of human papillomavirus (HPV)-positive patients in modern series as well as improvements in chemotherapy regimens and radiotherapy techniques.11–13 At the same time, newer diagnostic modalities have also offered higher accuracy for the detection of persistent disease.14 Fused positron emission tomography/computed tomography (PET/CT) imaging, in particular, offers a negative predictive value for persistent neck disease as high as 97%.8, 9 These preliminary data have been used to support observation of the neck in node-positive head and neck cancer patients, provided a clinical and radiographic CR is achieved.8–10 However, the long-term outcomes of patients who experience a clinical and PET/CT-based CR and undergo observation of the neck, in lieu of post-treatment neck dissection, have not been described.
Ten years ago, we began to incorporate PET/CT with clinical examination into our decision making for head and neck cancer patients undergoing chemoradiotherapy. When a clinical and radiographic CR is achieved in the node-positive neck, we observe the neck, omitting planned post-treatment neck dissection. We now report data on long-term regional control in a cohort of patients with oropharyngeal squamous cell carcinoma (OPSCC) who underwent observation of the N+ neck, after achieving a clinical and radiographic CR to chemoradiotherapy.
Materials and Methods
Between January 2002 and April 2009, 352 patients presenting with N+ OPSCC completed definitive chemoradiation therapy at Memorial Sloan-Kettering Cancer Center (MSKCC), and were evaluated with post-treatment clinical examination and PET/CT imaging. Although we currently obtain a post-treatment PET/CT at 12 weeks from the completion of radiation therapy to assess treatment response,8 there was variability in practice during the early years of this study. In this analysis, to reflect actual practice, we included patients whose post-treatment PET/CT was obtained between 6 and 24 weeks following completion of treatment. This identified a final cohort of 302 patients. Fifty patients were excluded on the basis of not having received a post-treatment PET/CT (n=36), having a PET/CT performed outside of the defined 6–24 week time window (n=12), or undergoing a planned neck dissection (n=2). These patients were analyzed separately to ensure that we had not inadvertently excluded a cohort of high-risk patients and thereby biased our results.
The primary outcome was the incidence of neck recurrence among patients experiencing a CR and undergoing neck observation, rather than ND. We categorized based upon response to therapy: either CR or suspected incomplete response (<CR). Our analysis focused on patients achieving a clinical and radiographic CR, defined as no evidence of disease on post-treatment clinical examination, PET/CT, and other imaging (CT or magnetic resonance imaging), if performed. Patients in whom the treating clinicians had any suspicion of residual disease, whether based on PET/CT, other imaging, or clinical examination, were included in the <CR group.
To assess the efficacy of actual clinical practice, post-treatment PET/CTs were categorized according to interpretations provided to the treating physicians. Post-treatment PET/CTs were categorized as <CR if the study was interpreted as consistent with persistent disease in the neck. PET/CTs were categorized as CR if the study revealed complete resolution of suspicious metabolic activity in the neck. The determination of CR on PET/CT did not require standardized uptake values of 0, or radiographic absence of residual lymphadenopathy, but did require that any residual uptake or adenopathy was interpreted by the radiologist to be consistent with post-treatment effect and not residual/persistent cancer. If the post-treatment PET/CT was not consistent with CR or <CR, the study was deemed indeterminate. Patients with an indeterminate PET/CT, demonstrating residual, or decreased, metabolic activity in the neck, where persistent disease could not be ruled out, were classified as experiencing a CR if a subsequent PET/CT demonstrated a CR. If a CR was not demonstrated on subsequent PET/CT, or if the patient was triaged to ND without a subsequent PET/CT, the patient was classified as <CR. This reflects our clinical practice at the time, as patients suspected to have experienced <CR underwent post-treatment ND. Such dichotomous categorization is intended to be conservative, by underestimating the rate of CR, and has been used by others in prospective trials.9 To best evaluate the efficacy of decision making in actual clinical practice, rather than in an idealized setting, the categorization of PET/CT response for this analysis was based on the reports available to clinicians at the time, and not on contemporary re-review of imaging studies by expert readers blinded to clinical outcome.
Chemotherapy given during intensity-modulated radiotherapy was recorded as cisplatin based, cetuximab based, or carboplatin based. If chemotherapy was changed mid-treatment, the chemotherapy regimen delivered for the majority of the treatment was recorded. Radiation was given using intensity-modulated radiotherapy to 70 Gy. HPV status was not routinely determined during this time period. Instead, we classified patients as former, current, or never smokers. Patients who quit smoking within 1 year of radiation therapy were considered current smokers.
Regional failure was defined as either radiographic suspicious or biopsy-proven recurrent nodal metastasis. Durations to all endpoints were measured from the first day of chemoradiation. Event time probabilities were estimated using the Kaplan-Meier method and groups compared using the log-rank test. All tests were two-sided and were considered statistically significant at P < 0.05. Analyses were conducted using SPSS Version 19.
Results
Between 2002 and 2009, 302 N+ OPSCC patients were treated with definitive chemoradiation and had treatment response assessed by both clinical exam and PET/CT within 6–24 weeks of completion of treatment. Median follow-up among living patients was 34 months (range, 6–102).
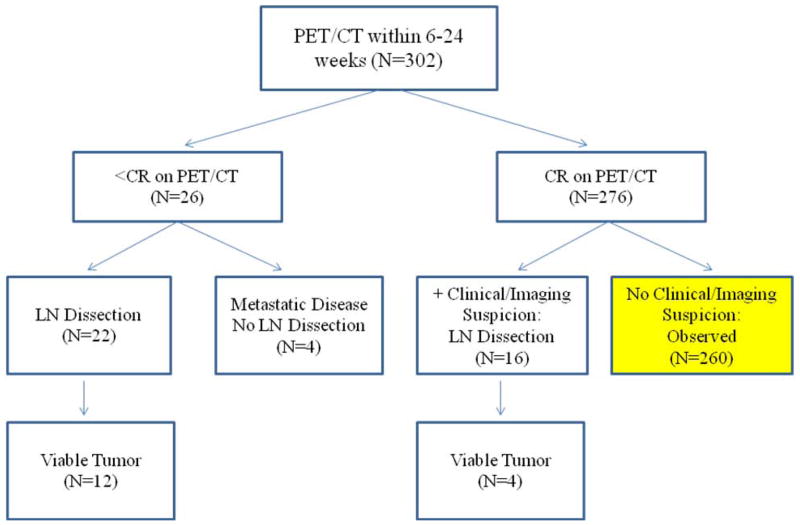
Of these patients, 294 (97.4%) experienced a local CR (at the primary site). Two hundred sixty patients (86.1%) experienced a locoregional CR (at both the primary site and in the neck), based on both clinical and radiographic criteria, and did not undergo a post-treatment ND. These 260 patients remained under active observation. Patient and treatment characteristics for patients who obtained a locoregional CR are listed in Tables 1 and 2. Twenty-six patients (8.6%) had radiographic suspicion of persistent disease: 19 had persistent metabolic activity on PET/CT, and 7 had indeterminate PET/CTs. Sixteen patients (5.3%) had clinical suspicion of residual disease, despite a negative PET/CT (Fig. 1).
Table 1.
Clinical characteristics of patients receiving positron emission tomography/computed tomography (PET/CT) within 6–24 weeks following completion of conformal radiotherapy
| CR on PET/CT | <CR on PET/CT | Total | P Value | |
|---|---|---|---|---|
| T stage | ||||
| T1–2 | 190 (92%) | 16 (8%) | 206 | 0.45 |
| T3–T4 | 86 (90%) | 10 (10%) | 96 | |
| Nodal status | ||||
| N1 | 68 (97%) | 2 (3%) | 70 | <0.01 |
| N2a | 26 (93%) | 2 (7%) | 28 | |
| N2b | 115 (91%) | 12 (9%) | 127 | |
| N2c | 64 (90%) | 7 (10%) | 71 | |
| N3 | 3 (50%) | 3 (50%) | 6 | |
| Age | ||||
| 40–59 | 170 (93%) | 13 (7%) | 183 | 0.25 |
| ≥60 | 106 (89%) | 13 (11%) | 119 | |
| Gender | ||||
| Male | 244 (91%) | 25 (9%) | 269 | 0.23 |
| Female | 32 (97%) | 1 (3%) | 33 | |
| Primary cancer | ||||
| Base of tongue | 131 (92%) | 11 (8%) | 142 | 0.64 |
| Pharyngeal wall | 7 (100%) | 0 (0%) | 7 | |
| Soft palate | 5 (100%) | 0 (0%) | 5 | |
| Tonsil | 133 (90%) | 15 (10%) | 148 | |
| Concurrent systemic treatment | ||||
| Cisplatin-based | 207 (94%) | 14 (6%) | 221 | 0.06 |
| Cetuximab-based | 35 (83%) | 7 (17%) | 42 | |
| Carboplatin-based | 34 (87%) | 5 (13%) | 39 | |
| Smoking history | 0.38 | |||
| Never smoker | 60 (91%) | 6 (9%) | 66 | |
| Former smoker | 138 (91%) | 14 (9%) | 152 | |
| Current smoker | 77 (93%) | 6 (7%) | 83 | |
| Unknown | 1 (100%) | 0 (0%) | 1 | |
CR = locoregional complete response; <CR = incomplete response.
Table 2.
Details on chemotherapy regimens used concurrently with radiation therapy
| CR on PET/CT | <CR on PET/CT | |
|---|---|---|
| Cisplatin based | ||
| Cisplatin alone | 169 (92%) | 14 (8%) |
| Cisplatin + bevacizumab | 35(97%) | 1 (3%) |
| Cisplatin + paclitaxel | 3 (100%) | 0 (0%) |
| Carboplatin based | ||
| Carboplatin + 5-FU | 26 (90%) | 3 (10%) |
| Carboplatin + paclitaxel | 8 (80%) | 2 (20%) |
| Cetuximab based | ||
| Cetuximab alone | 30 (83%) | 6 (17%) |
| Cetuximab + paclitaxel | 3 (75%) | 1 (25%) |
| Cetuximab + paclitaxel-NAB | 2 (100%) | 0 (0%) |
CR = complete response; <CR = incomplete response; CT = computed tomography; PET = positron emission tomography.
Figure 1.
Characteristics of 302 patients with node-positive (N+) oropharyngeal squamous cell carcinoma who underwent positron emission tomography/computed tomography (PET/CT) within 6–24 weeks following completion of conformal radiotherapy.
Complete Response
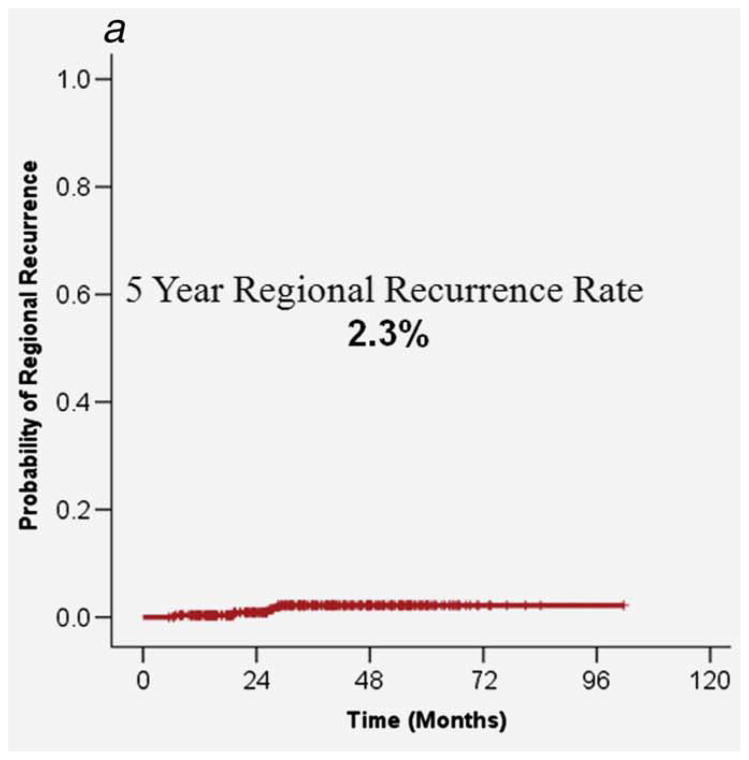
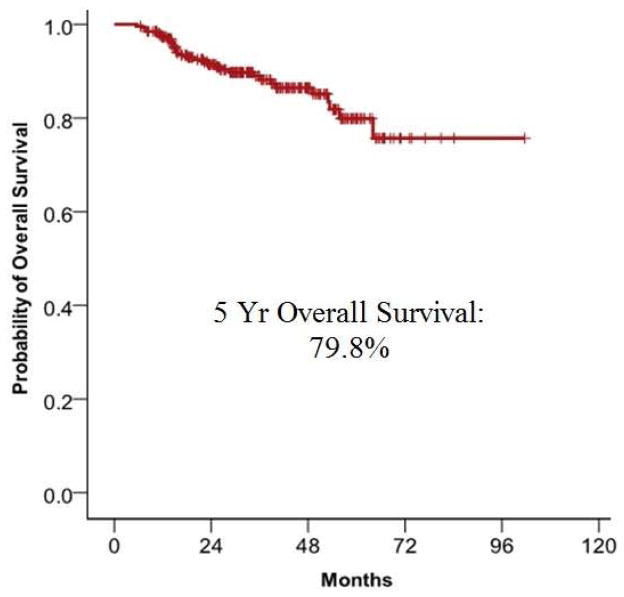
A clinical and radiographic locoregional CR was experienced by 260 patients with initial nodal status as follows: N1, 65 (97.0%); N2a, 25 (92.6%); N2b, 106 (91.4%); N2c, 61 (89.7%); N3, 3 (50.0%) These patients underwent active observation, rather than ND. Of these patients, 5 experienced local recurrence, between 4 and 17 months after treatment, for a cumulative rate of 2.1%. Four patients experienced regional recurrence, between 6 and 28 months after treatment, for a cumulative incidence rate of 2.3%. There were no patients experiencing both local and regional recurrence. Five year overall survival was 79.8% (Fig. 2).
Figure 2.
A. Regional control in the 260 patients who were observed following an initial negative PET/CT and with no evidence of residual disease on clinical exam. B. 5-year overall survival in the 260 patients who were observed following an initial negative PET/CT with no evidence of residual disease on clinical exam.
Four patients who experienced a CR and were observed subsequently developed a neck recurrence. In all cases, this occurred in the absence of local or distant recurrence. Initial nodal status of these patients was N1 in 2, N2b in 1, and N2c in 1. All four patients underwent salvage ND, of whom three were successfully salvaged and have not experienced further recurrence with follow-up times of 6, 17, and 27 months. One patient not successfully salvaged had N1 disease on presentation and failed in the neck 30 months following salvage ND in the absence of distant failure.
Initial nodal status of N2 or N3 was not associated with an increased risk of neck recurrence (N1, 4.4%; N2–3, 1.3%; P = 0.64). Regional control rates by initial nodal status were as follows: N1, 95.6%; N2a, 100%; N2b, 98.6%; N2c, 98.4%; N3, 98.4% (P = 0.74).
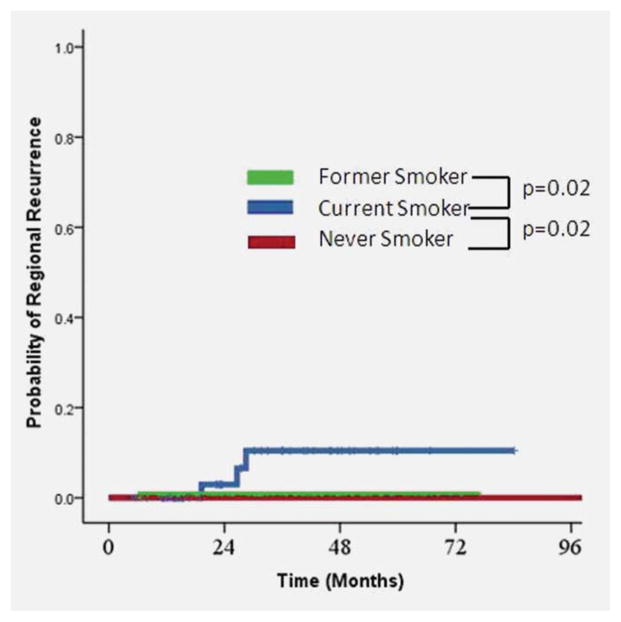
Current smokers experiencing a CR had an increased risk of neck recurrence (n=4, 10.8%) compared with former smokers (n=1, 0.8%, P = 0.024) or never smokers (n=0, 0%, P = 0.02, Fig. 3). Local recurrence was associated with significantly poorer 5-year survival (40.0% vs. 80.3%, p<.0001). Regional recurrence was associated with marginally poorer 5-year survival (75.0% vs. 79.5%, p=.11), although this difference did not reach statistical significance.
Figure 3.
Risk of recurrence by smoking status at time of diagnosis.
Incomplete Response: PET/CT Based
Twenty-six patients did not experience a CR, based on PET/CT imaging (positive in 19, indeterminate in 7). There was an escalating risk of radiographically suspected persistent disease with advancing nodal status (N1, 2.9%, N2a, 7.1%, N2b, 9.5%, N2c, 9.9%, N3, 50.0%; P < 0.01, Table 1). We observed an association between the systemic agent prescribed and the rate of PET/CT-determined CR (cisplatin based, 93.7%; carboplatin based, 87.2%; cetuximab based, 83.3%) although this value was of borderline statistical significance (P = 0.06) (Tables 1, 2).
The 5-year overall survival in these patients was 57.0%. Of these 26 patients, none had unresectable nodal metastases, but three had synchronous distant metastases, and one had unresectable local progression. The remaining 22 patients, all of whom had suspected isolated lymph node recurrences, underwent post-treatment ND, of whom 12 (54.5%) had residual disease on pathology. Of 15 patients with persistent activity on PET/CT, 11 (73.3%) had pathologically confirmed disease in the neck. Of 7 patients with indeterminate PET/CT receiving immediate ND, 1 (14.3%) had pathologically confirmed residual disease.
Incomplete Response: Clinical or Radiographic (Not PET/CT based)
There were 16 patients who underwent a post-treatment ND due to clinical suspicion of <CR, despite a negative PET/CT. In 3 patients, residual disease was suspected on clinical examination of the neck, of whom 1 (33%) had pathologic confirmation of residual disease after neck dissection. In 11 patients, residual disease was suspected based on both clinical examination and either CT or magnetic resonance imaging. Of these 11, 3 (27%) had pathologically confirmed residual disease. Two patients underwent ND due to other clinical suspicion, of whom neither had pathologically confirmed residual disease. In total, 4 patients (25%) had pathologically confirmed residual disease following ND. Nodal staging of these patients was as follows: N1, 3; N2a, 1; N2b, 9; N2c, 3. These patients have all experienced regional control in the dissected neck with a median follow-up of 51 months.
Excluded Patients
Fifty patients with N+ OPSCC were not included in the final cohort of 302 patients due to practice patterns that differed from the standard management of the neck at MSKCC (Table 3).
Table 3.
Long-term regional control in the 50 patients with N+ oropharyngeal squamous cell carcinoma treated with definitive conformal radiotherapy (CRT) who did not receive positron emission tomography/computed tomography (PET/CT) per standard clinical practice
| PET/CT | PET/CT Timing | N | Post-CRT Neck Dissection | Residual/Recurrent |
|---|---|---|---|---|
| Regional Disease | ||||
| No | NA | 10 | 10 | 3 |
| No | NA | 28 | 0 | 0 |
| Yes | >24 weeks | 5 | 0 | 1 |
| Yes | < 6 weeks | 7 | 2 | 0 |
Ten patients underwent ND after chemoradiation, without post-treatment PET/CT. In 8 cases, this was due to clinical concern for disease persistence or progression (based on clinical examination in 3, clinical and CT findings in 4, and failure at the primary site in 1). Pathologic analysis confirmed residual neck disease in 3 of these 8 patients. In two cases, planned neck dissection was performed due to patient or physician preference, both with pathologically negative necks. All 10 patients have experienced long-term regional control in the dissected neck.
Of the remaining 40 patients excluded from the study cohort, 22 did not receive post-treatment PET/CT imaging at all, 6 had PET imaging without CT, 7 had PET/CT performed earlier than 6 weeks, and 5 had PET/CT performed later than 24 weeks. Of the 22 patients observed without PET/CT imaging, there were no regional failures. Of the 6 patients observed after PET (not PET/CT) imaging, there were no regional failures. Of the 7 patients who had PET/CT performed <6 weeks after treatment, 2 had suspicion of residual neck disease on PET/CT and underwent ND (none had pathologic evidence of residual disease); 5 patients were PET/CT negative, observed, and experienced long-term regional control. Of the 5 patients with PET/CT performed >24 weeks following treatment, 4 were observed and experienced long-term regional control; 1 patient had local, regional, and distant progression of disease, and salvage surgery was not performed. In total, 38 patients underwent neck observation, without neck dissection, after CRT, and were not evaluated by PET/CT in the 6–24 week window. One of these 38 patients experienced failure in the neck. Therefore, the outcomes of the excluded cohort did not differ significantly from the outcomes of the study cohort.
Discussion
We present the first long-term results of neck observation in a large cohort of N+ OPSCC patients who experienced a clinical and radiographic CR, including PET/CT, after chemoradiation. While OPSCC patients with initial N1 nodal disease have commonly undergone neck observation following chemoradiation, there remains controversy regarding the management of the N2 and N3 neck. Initial experiences suggested that there may be improved regional control with a planned post-treatment ND.1–5 However, the addition of PET/CT to post-treatment clinical and radiographic assessment has significantly improved the negative predictive value of post-treatment assessing, allowing clinicians to more accurately rule out persistent nodal disease.8,9,15–17 This has led some centers to observe the N+ neck, rather than performing ND, if a CR is achieved after chemoradiation.
Prior to this study, no long-term data on locoregional control in a large cohort of patients undergoing neck observation has been available to support this practice. The data in this analysis confirm that neck recurrence is uncommon in patients experiencing a CR after chemoradiation, and did not appear to be more frequent in patients with N2/N3 necks compared with N1 necks.
Before proceeding, there are multiple caveats which apply to our data. First, our analysis carries the limitations of a retrospective analysis. We excluded patients who did not undergo post-treatment PET/CT within a defined time window, or who underwent a planned neck dissection. Although we did analyze the excluded cohort separately, in order to confirm that outcomes were not substantially different among excluded patients, the potential for subtle selection bias still exists, and prospective study is still needed. Second, our results may not be generalizable to patients presenting with N3 disease, as this population comprised only 6 patients in this study. Third, HPV status was not routinely determined during this time period and accordingly, could not be studied as a covariate.
The risk of regional failure in the neck at 5 years was 2.3%, indicating that the initially N+ neck can be safely observed in the absence of either clinical or radiographic (including PET/CT) suspicion of persistent neck disease. Among patients who developed pathologically confirmed persistent or recurrent disease isolated to the neck (n=17), the majority of patients (n=16, 94.1%) were able to undergo post-treatment neck dissection, of whom 75% (n=12) experienced long-term regional control. While it has been speculated that delayed ND might result in progression of disease, compromising the ability to successfully achieve salvage, we found this scenario to be uncommon.
We caution that, although PET/CT is an accurate tool with high negative predictive values, it does not replace the clinical examination. In our study, there were 16 patients who, despite a negative PET/CT, underwent ND, due to clinical concern of residual disease, most commonly a palpable mass. On pathologic analysis, 4 necks (25%) harbored residual disease.
The results of this study do not necessarily imply superiority of PET/CT over other imaging modalities for neck assessment after chemoradiation. We did not routinely utilize post-treatment contrast-enhanced CT, as others have. Moeller et al performed PET/CT and contrast-enhanced CT on 98 patients at 8 weeks post-treatment. PET/CT was not superior to CT for detection of persistent nodal disease, except in high-risk patients, in whom PET/CT offered slightly higher specificity and positive predictive value.18 These findings may in part be due to the timing of PET/CT; post-treatment PET/CT is more accurate at 10–16 weeks,9, 19–21 which is our current schedule for post-treatment imaging.
Among patients who had PET/CT-based radiographic suspicion of <CR and underwent ND, 52% had persistent disease identified in the ND specimen. This low positive predictive value reflects the heterogeneous group of patients who comprise this group. We included both patients with an indeterminate PET/CT, who were triaged to immediate ND, likely due to clinician suspicion, and patients with findings on PET/CT that were strongly suggestive of residual disease. In patients undergoing ND following a PET/CT consistent with residual disease, 73% harbored persistent disease, in contrast to only 14% of patients with an indeterminate PET/CT report.
To account for the possibility of selection bias, should higher-risk patients have been inadvertently excluded from the study because they were less likely to receive post-treatment PET/CT imaging during the defined time period, we evaluated the outcomes of all excluded patients. Of the 38 patients without concern for persistent disease who were observed, only 1 patient experienced a neck recurrence. Accordingly, we conclude that our exclusion criteria did not introduce significant selection bias into the study cohort.
We note that HPV status was not routinely determined during the period of time in which these patients were treated. However, tobacco history was informative. We found that the probability of neck failure following a complete CR was substantially higher in current smokers. This is consistent with the escalated risk of recurrence and death in current smokers,22–24 and may also be consistent with poorer outcomes in patients with HPV-negative tumors, although this hypothesis requires further investigation.25–27 Therefore we would caution applying the results of this study to an HPV-negative population.
It is also important to note that the interpretation of post-treatment PET/CT requires significant expertise, since metabolically active tumor may be difficult to discern from treatment-related inflammation or infectious processes.28 The high negative predictive value of PET imaging in our cohort may in part be reflective of the expertise of an experienced nuclear medicine department. To accurately evaluate the efficacy of our practice, we based PET/CT data on reports available to clinicians at the time of decision making. We did not perform contemporary re-review of imaging, as the purpose of this study was to evaluate the outcomes of actual practice, rather than to assess the diagnostic accuracy, under ideal conditions, of PET/CT in the post-chemoradiation neck.
We observed a potential association between the chemotherapy regimen used and CR rate (cetuximab based, 83%; carboplatin based, 87%; cisplatin based, 94%; P = 0.06). However, this difference was of borderline statistical significance, and these data should be interpreted with caution. We were unable to adjust for potentially confounding factors such as performance status and medical comorbidity. Nevertheless, these findings may have some clinical significance, and warrant further investigation.29
In conclusion, these data confirm that the addition of PET/CT to our standard post-treatment clinical evaluation of response following chemoradiation has allowed us to observe the neck in N+ OPSCC patients who achieved a CR to treatment. The long-term follow-up in this large uniform cohort showed that the rate of regional failure was low. In patients who do have a regional recurrence, salvage surgical resection appears to be associated with excellent long-term control. This cost-effective approach30 in reserving ND for salvage in this population also decreases the likelihood of surgical morbidity.31 There is now evolving interest in the use of transoral robotic surgery and ND as upfront therapy for N+ OPSCC, with a goal of de-escalating adjuvant therapy. The results of this current analysis will serve as important benchmark outcomes data, as prospective cohort data on robotic surgery begins to be reported.32, 33
Novelty/Impact.
This is the first uniform cohort of node-positive head and neck cancer patients undergoing post-chemoradiotherapy PET/CT-based determination of response, and triaged to observation of the neck, rather than neck dissection. The safety of this practice had been inferred from data revealing the low rate of pathologic residual disease in these patients, but clinical outcome data have been limited. These data now confirm a very low rate of recurrence in these observed patients.
Glossary
- <CR
incomplete response
- CR
complete response
- CRT
conformal radiotherapy
- CT
computed tomography
- HPV
human papillomavirus
- MSKCC
Memorial Sloan-Kettering Cancer Center
- N+
node positive
- ND
neck dissection
- OPSCC
oropharyngeal squamous cell carcinoma
- PET
positron emission tomography
Footnotes
The authors have no conflicts of interest or funding sources to declare.
References
- 1.Brizel DM, Prosnitz RG, Hunter S, et al. Necessity for adjuvant neck dissection in setting of concurrent chemoradiation for advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;58:1418–1423. doi: 10.1016/j.ijrobp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Parsons JT, Mendenhall WM, Cassisi NJ, Stringer SP, Million RR. Neck dissection after twice-a-day radiotherapy: morbidity and recurrence rates. Head Neck. 1989;11:400–404. doi: 10.1002/hed.2880110504. [DOI] [PubMed] [Google Scholar]
- 3.Mendenhall WM, Million RR. Elective neck irradiation for squamous cell carcinoma of the head and neck: analysis of time-dose factors and causes of failure. Int J Radiat Oncol Biol Phys. 1986;12:741–746. doi: 10.1016/0360-3016(86)90031-3. [DOI] [PubMed] [Google Scholar]
- 4.Mendenhall WM, Amdur RJ, Stringer SP, Villaret DB, Cassisi NJ. Radiation therapy for squamous cell carcinoma of the tonsillar region: a preferred alternative to surgery? J Clin Oncol. 2000;18:2219–2225. doi: 10.1200/JCO.2000.18.11.2219. [DOI] [PubMed] [Google Scholar]
- 5.Mendenhall WM, Stringer SP, Amdur RJ, Hinerman RW, Moore-Higgs GJ, Cassisi NJ. Is radiation therapy a preferred alternative to surgery for squamous cell carcinoma of the base of tongue? J Clin Oncol. 2000;18:35–42. doi: 10.1200/JCO.2000.18.1.35. [DOI] [PubMed] [Google Scholar]
- 6.Mendenhall WM, Million RR, Cassisi NJ. Squamous cell carcinoma of the head and neck treated with radiation therapy: the role of neck dissection for clinically positive neck nodes. Int J Radiat Oncol Biol Phys. 1986;12:733–740. doi: 10.1016/0360-3016(86)90030-1. [DOI] [PubMed] [Google Scholar]
- 7.Lavertu P, Adelstein DJ, Saxton JP, et al. Management of the neck in a randomized trial comparing concurrent chemotherapy and radiotherapy with radiotherapy alone in resectable stage III and IV squamous cell head and neck cancer. Head Neck. 1997;19:559–566. doi: 10.1002/(sici)1097-0347(199710)19:7<559::aid-hed1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Ong SC, Schoder H, Lee NY, et al. Clinical utility of 18F-FDG PET/CT in assessing the neck after concurrent chemoradiotherapy for Locoregional advanced head and neck cancer. J Nucl Med. 2008;49:532–540. doi: 10.2967/jnumed.107.044792. [DOI] [PubMed] [Google Scholar]
- 9.Porceddu SV, Jarmolowski E, Hicks RJ, et al. Utility of positron emission tomography for the detection of disease in residual neck nodes after (chemo) radiotherapy in head and neck cancer. Head Neck. 2005;27:175–181. doi: 10.1002/hed.20130. [DOI] [PubMed] [Google Scholar]
- 10.Connell CA, Corry J, Milner AD, et al. Clinical impact of, and prognostic stratification by, F-18 FDG PET/CT in head and neck mucosal squamous cell carcinoma. Head Neck. 2007;29:986–995. doi: 10.1002/hed.20629. [DOI] [PubMed] [Google Scholar]
- 11.Yao M, Luo P, Hoffman HT, et al. Pathology and FDG PET correlation of residual lymph nodes in head and neck cancer after radiation treatment. Am J Clin Oncol. 2007;30:264–270. doi: 10.1097/01.coc.0000257611.65290.aa. [DOI] [PubMed] [Google Scholar]
- 12.Lango MN, Myers JN, Garden AS. Controversies in surgical management of the node-positive neck after chemoradiation. Semin Radiat Oncol. 2009;19:24–28. doi: 10.1016/j.semradonc.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Pletcher SD, Kaplan MJ, Eisele DW, Singer MI, Quivey JM, Lee N. Management of cervical metastases in advanced squamous cell carcinoma of the base of tongue. Arch Otolaryngol Head Neck Surg. 2003;129:983–986. doi: 10.1001/archotol.129.9.983. [DOI] [PubMed] [Google Scholar]
- 14.Ferlito A, Corry J, Silver CE, Shaha AR, Thomas Robbins K, Rinaldo A. Planned neck dissection for patients with complete response to chemoradiotherapy: a concept approaching obsolescence. Head Neck. 2010;32:253–261. doi: 10.1002/hed.21173. [DOI] [PubMed] [Google Scholar]
- 15.Goguen LA, Posner MR, Tishler RB, et al. Examining the need for neck dissection in the era of chemoradiation therapy for advanced head and neck cancer. Arch Otolaryngol Head Neck Surg. 2006;132:526–531. doi: 10.1001/archotol.132.5.526. [DOI] [PubMed] [Google Scholar]
- 16.Zundel MT, Michel MA, Schultz CJ, et al. Comparison of Physical Examination and Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography 4–6 Months After Radiotherapy to Assess Residual Head-and-Neck Cancer. Int J Radiat Oncol Biol Phys. 2011 doi: 10.1016/j.ijrobp.2010.11.072. [DOI] [PubMed] [Google Scholar]
- 17.Kim SY, Kim JS, Yi JS, et al. Evaluation of (18)F-FDG PET/CT and CT/MRI with Histopathologic Correlation in Patients Undergoing Salvage Surgery for Head and Neck Squamous Cell Carcinoma. Ann Surg Oncol. 2011 doi: 10.1245/s10434-011-1655-x. [DOI] [PubMed] [Google Scholar]
- 18.Moeller BJ, Rana V, Cannon BA, et al. Prospective risk-adjusted [18F]Fluorodeoxyglucose positron emission tomography and computed tomography assessment of radiation response in head and neck cancer. J Clin Oncol. 2009;27:2509–2515. doi: 10.1200/JCO.2008.19.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gourin CG, Williams HT, Seabolt WN, Herdman AV, Howington JW, Terris DJ. Utility of positron emission tomography-computed tomography in identification of residual nodal disease after chemoradiation for advanced head and neck cancer. Laryngoscope. 2006;116:705–710. doi: 10.1097/01.MLG.0000215176.98582.A9. [DOI] [PubMed] [Google Scholar]
- 20.Greven KM, Williams DW, 3rd, McGuirt WF, Sr, et al. Serial positron emission tomography scans following radiation therapy of patients with head and neck cancer. Head Neck. 2001;23:942–946. doi: 10.1002/hed.1136. [DOI] [PubMed] [Google Scholar]
- 21.Yao M, Smith RB, Graham MM, et al. The role of FDG PET in management of neck metastasis from head-and-neck cancer after definitive radiation treatment. Int J Radiat Oncol Biol Phys. 2005;63:991–999. doi: 10.1016/j.ijrobp.2005.03.066. [DOI] [PubMed] [Google Scholar]
- 22.Chen AM, Chen LM, Vaughan A, et al. Tobacco smoking during radiation therapy for head-and-neck cancer is associated with unfavorable outcome. Int J Radiat Oncol Biol Phys. 2011;79:414–419. doi: 10.1016/j.ijrobp.2009.10.050. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz LH, Ozsahin M, Zhang GN, et al. Synchronous and metachronous head and neck carcinomas. Cancer. 1994;74:1933–1938. doi: 10.1002/1097-0142(19941001)74:7<1933::aid-cncr2820740718>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 24.Khuri FR, Kim ES, Lee JJ, et al. The impact of smoking status, disease stage, and index tumor site on second primary tumor incidence and tumor recurrence in the head and neck retinoid chemoprevention trial. Cancer Epidemiol Biomarkers Prev. 2001;10:823–829. [PubMed] [Google Scholar]
- 25.Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus--associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 26.Sturgis EM, Ang KK. The epidemic of HPV-associated oropharyngeal cancer is here: is it time to change our treatment paradigms? J Natl Compr Canc Netw. 2011;9:665–673. doi: 10.6004/jnccn.2011.0055. [DOI] [PubMed] [Google Scholar]
- 27.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ojiri H, Mendenhall WM, Stringer SP, Johnson PL, Mancuso AA. Post-RT CT results as a predictive model for the necessity of planned post-RT neck dissection in patients with cervical metastatic disease from squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2002;52:420–428. doi: 10.1016/s0360-3016(01)02603-7. [DOI] [PubMed] [Google Scholar]
- 29.Koutcher L, Sherman E, Fury M, et al. Concurrent cisplatin and radiation versus cetuximab and radiation for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;81:915–922. doi: 10.1016/j.ijrobp.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Sher DJ, Tishler RB, Annino D, Punglia RS. Cost-effectiveness of CT and PET-CT for determining the need for adjuvant neck dissection in locally advanced head and neck cancer. Ann Oncol. 2010;21:1072–1077. doi: 10.1093/annonc/mdp405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davidson BJ, Newkirk KA, Harter KW, Picken CA, Cullen KJ, Sessions RB. Complications from planned, posttreatment neck dissections. Arch Otolaryngol Head Neck Surg. 1999;125:401–405. doi: 10.1001/archotol.125.4.401. [DOI] [PubMed] [Google Scholar]
- 32.Quon H, O’Malley BW, Jr, Weinstein GS. Postoperative adjuvant therapy after transoral robotic resection for oropharyngeal carcinomas: rationale and current treatment approach. ORL J Otorhinolaryngol Relat Spec. 2011;73:121–130. doi: 10.1159/000319890. [DOI] [PubMed] [Google Scholar]
- 33.Weinstein GS, Quon H, O’Malley BW, Jr, Kim GG, Cohen MA. Selective neck dissection and deintensified postoperative radiation and chemotherapy for oropharyngeal cancer: a subset analysis of the University of Pennsylvania transoral robotic surgery trial. Laryngoscope. 2010;120:1749–1755. doi: 10.1002/lary.21021. [DOI] [PubMed] [Google Scholar]