Abstract
Escherichia coli O157:H7 is a predominant foodborne pathogen with severe pathogenicity, leading to increasing attention given to rapid and sensitive detection. Herein, we propose an impedance biosensor using new kinds of screen-printed interdigitated microelectrodes (SPIMs) and wheat germ agglutinin (WGA) for signal amplification to detect E. coli O157:H7 with high sensitivity and time-efficiency. The SPIMs integrate the high sensitivity and short response time of the interdigitated electrodes and the low cost of the screen-printed electrodes. Self-assembling of bi-functional 3-dithiobis-(sulfosuccinimidyl-propionate) (DTSP) on the SPIMs was investigated and was proved to be able to improve adsorption quantity and stability of biomaterials. WGA was further adopted to enhance the signal taking advantage of the abundant lectin-binding sites on the bacteria surface. The immunosensor exhibited a detection limit of 102 cfu·mL−1, with a linear detection range from 102 to 107 cfu·mL−1 (r2 = 0.98). The total detection time was less than 1 h, showing its comparable sensitivity and rapid response. Furthermore, the low cost of one SPIM significantly reduced the detection cost of the biosensor. The biosensor may have great promise in food safety analysis and lead to a portable biosensing system for routine monitoring of foodborne pathogens.
Keywords: Escherichia coli O157:H7, impedance immunosensor, lectin, screen-printed interdigitated microelectrode
1. Introduction
Escherichia coli O157:H7, a common member of a group of pathogenic E. coli strains, has been marked as an enterohaemorrhagic, verocytotoxin-producing, or Shiga-toxin-producing pathogen [1]. In addition to devastating personal losses to the patients, the economic costs can be substantial—the costs of healthcare, social care, and lost productivity come to around $600 million per year in the United States, whereas costs from product recalls and reduced trade can run to tens of millions of dollars [2,3]. E. coli O157:H7 is the predominant serotype associated with human health and food safety [4,5]. Human infections are usually caused by the consumption of contaminated and under-cooked beef, unpasteurized milk, and feces-contaminated vegetables and water [6,7]. Deaths are usually associated with severe extra-renal complications [1]. Considering the serious threats from E. coli O157:H7, it is of great importance to develop rapid and sensitive detection methods to ensure food safety. The early detection at low concentrations still remains a challenge to allow immediate decisions to be made in many important fields.
There are many detection methods for E. coli O157:H7, such as traditional culture methods, immuno-assays, and molecular methods. Although these methods have high sensitivity and reliability, their application to food safety is limited due to time-consuming and complicated operations [8]. Studies on biosensing methods have increased significantly in the recent years, and become one of the most active research areas for pathogen detection [9,10]. Numerous biosensors have been developed for detection and enumeration of E. coli O157:H7, and some of them are promising candidates for rapid screening [11,12,13]. Electrochemical biosensors are widely recognized as powerful analytical tools due to a variety of contributions such as simple instrumentation, easy operation, low cost and short response time [14,15]. Some impedimetric biosensors based on different types of electrodes have recently been reported for pathogen detection, and disposable screen-printed electrodes offer cost-effective methods [16,17]. The increasing commercial availability of low-cost screen-printed electrodes has opened new fields for electrochemical measurements [18]. Interdigitated microelectrodes can maximize the impedance change, reduce the detection time, minimize interfering effects, and work in a two-electrode system (vs. conventional three-electrode system), thus significantly benefiting the fabrication and performance of electrochemical biosensors [19,20,21]. A combination of two electrodes, namely, screen-printed interdigitated microelectrodes (SPIMs), may integrate their merits to develop highly sensitive, rapid-responding, cost-effective biosensors for the detection of E. coli O157:H7. However, related research is rarely reported.
Self-assembled monolayers (SAMs) are broadly used to functionalize the electrode surface, improve adsorption capacity and stability, and retain activity of the biomaterials [22,23,24]. Covalent binding (amide bond formation) results from the reaction between amino group of protein and reactive succinimidyl group of the 3-dithiobis-(sulfosuccinimidylpropionate) (DTSP) on the SAM surface. DTSP can be easily used without strict reaction conditions and additional activation step, compared with other SAMs, such as 4,4-Dithiodibutyric acid (DTBA) and 3-Mercaptopropionic acid (MPA). DTBA and MPA SAMs need to activate with N-Hydroxysuccinimide (NHS)/carbodiimide hydrochloride (EDC) before use under strict reaction conditions which increase the complexity [25,26,27]. The short self-assembling time and mild reaction conditions improve the accuracy and practicality of the microelectrode surface functionalization, which is useful for modification of biomaterials.
More recently, lectin binding sites at the surface of a high percentage of microorganisms can be used for bacteria absorption, which has proven to be greatly promising and effective. Lectins are much smaller than antibodies, thus they allow higher absorption capacity leading to higher sensitivity and lower non-specific adsorption. Furthermore, lectins are readily available and inexpensive recognition agents [28,29,30]. As far as we know, the application of wheat germ agglutinin (WGA) as signal amplification for bacteria detection has never been reported.
Herein, an electrochemical impedance immunosensor based on self-assembled monolayers was proposed for rapid detection of E. coli O157:H7 with signal amplification using WGA (Scheme 1). With the immobilization of the biomaterials onto the surface of the SPIMs, the impedance signal can be changed due to the blocking of electron transfer, which can be utilized as the sensing mechanism of impedance immunosensor. Compared with the expensive non-screen printed microelectrodes, the cost of one SPIM may be less than $1 which is much better for the practical application and can be further reduced by the good reusability. The performance of the electrochemical impedance biosensor showed that this biosensor was sensitive, reliable and effective for detection of E. coli O157:H7, which hold great promise in food safety analysis.
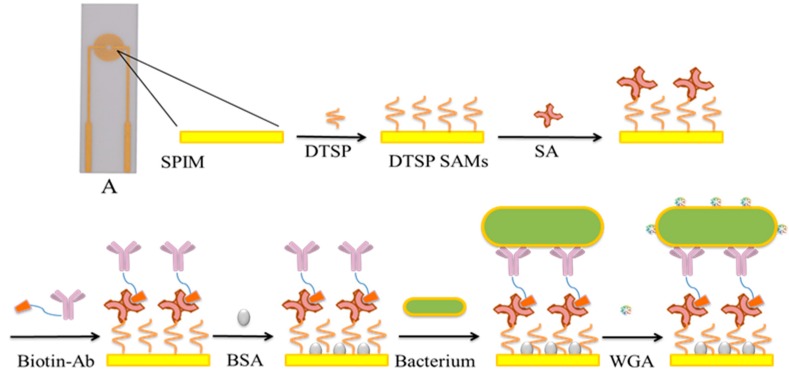
Scheme 1.
A scheme of the proposed immunosensor for bacteria detection. A was a photo of SPIM and the width of a finger and the gap between two fingers of this microelectrode were 200 μm, respectively.
2. Experimental Section
2.1. Apparatus and Reagents
Electrochemical impedance measurements were performed using the ZAHNER chemical station (Kronach, Germany). SPIMs were purchased from AIBIT Biotech Instrument (Jiangyin, China). The width of a finger and the gap between two fingers for the gold interdigitated microelectrode were 200 μm, respectively. Gold interdigitated microelectrodes with 25 μm width and gap were obtained from Institute of Semiconductors of Chinese Academy of Sciences (Beijing, China). Scanning electron microscopy (Hitachi, Japan) was used for the characterization of electrodes surface.
DTSP, MPA, WGA, N-Ethyl-N'-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), N-Hydroxysuccinimide (NHS) and phosphate buffer solutions (PBS, pH 7.4) were purchased from Sigma (St. Louis, MO, USA). E. coli K12 (ATCC29425), E. coli O157:H7 (ATCC43889), Listeria monocytogenes (ATCC19151) and Salmonella Typhimurium (ATCC14028) were purchased from ATCC (Manassas, MD). Streptavidin (SA), bovine serum albumin (BSA) and protein A were purchased from Sangon Biotech (Shanghai, China). MacConkey agar, brain heart infusion (BHI) culture medium (Becton, Dickinson and Company, Sparks, NV), and nutrient agar (Huankai Microbial Sci. & Tech. Co. LTD, Guangzhou, China) were used for the bacteria culture. Anti-E. coli biotin-antibodies (biotin-Ab) were obtained from Meridian Life Science (Saco, ME) and dissolved in PBS (0.4 mg·mL−1). DTSP was dissolved in acetone (2 mM). PBS solution containing 10 mM K3Fe(CN)6/K4Fe(CN)6 (Sangon Biotech., Shanghai, China) was used for electrochemical impedance spectroscopy (EIS) measurements. Ultrapure water (18.2 MΩ·cm) was obtained from a Millipore Milli-Q purification system (Merck Millipore, MA). All chemicals were of analytical grade. All the bacteria dilutions used in this research were inactivated (10 min in boiling water).
2.2. Preparation of Bacterial Samples
E. coli O157:H7 was grown in BHI culture medium at 37 °C for 20 h to the stationary phase. Serial 10-fold dilutions of the culture were made with PBS and 100 μL of the diluted solutions were transferred to MacConkey agar plates and incubated for 24 h at 37 °C for enumeration of colonies. At the same time, the stationary-phase cultures were diluted to 101–107 cfu·mL−1 in PBS (pH 7.4) for use in the tests. S. Typhimurium and E. coli K12 were also incubated at 37 °C for 24 h and L. monocytogenes was incubated for 48 h in BHI culture medium. Incubated microorganisms were serially diluted in PBS and bacterial numbers were determined using a conventional plate counting method. The dilutions containing approximately 105 cfu·mL−1 of each microorganism were prepared for evaluation of the specificity of the proposed impedance immunosensor.
2.3. Immunosensor Fabrication
Two methods were adopted to clean the bare SPIMs. For the first one, NaOH solution (50 μL, 1 M) was dropped on the electrode area, kept for 5 min, and washed with water, followed by similar treatment of HCl solution (50 μL, 1 M) for 2 min. For the second method, piranha solution (H2SO4:H2O2 (v/v) = 7:3, 50 μL) was dropped onto the electrode surface and kept for 15 min, followed by washing with water thoroughly. After the cleaning procedure, the microelectrodes with different sizes (25 µm and 200 µm width) were characterized using cyclic voltammetry (CV) and EIS.
Self-assembly was carried out by immersing electrodes in 2 mM DTSP. The effect of different incubation times (0, 2, 4, 8 h) on sensor performance was studied. After incubation, the microelectrodes were washed immediately using acetone to remove free DTSP. The self-assembled monolayers of MPA were obtained and activated with EDC + NHS before use [31]. Briefly, EIS was used to characterize the electrodes modified with different SAMs.
After DTSP immobilization, the electrodes were incubated in SA solution (50 μL, 0.5 mg·mL−1) for 45 min and then washed with ultrapure water. Then electrodes were incubated with biotin-Ab solution for another 45 min and washed with ultrapure water again. Different biotin-Ab concentrations (0.1, 0.2 and 0.4 mg·mL−1) were evaluated for optimization. Finally, BSA solution (10 mg·mL−1) was applied to block non-specific adsorption sites on the surface of the microelectrodes.
2.4. Bacteria Detection
Different dilutions of inactivated bacterial cultures (from 101 to 107 cfu·mL−1) were dropped on the surface of electrodes and incubated for 45 min. Impedance measurements were conducted using a 10 mM K3Fe(CN)6/K4Fe(CN)6 (1:1) mixture in PBS (1 Hz–1 MHz, 10 mV). Each detection was repeated more than three times. Several inactivated non-target bacteria, including E. coli K12, L. monocytogenes and S. Typhimurium were used as non-target bacteria to evaluate the specificity of this immunosensor.
2.5. Lectin Absorption
The WGA solution (50 μL, 0.5 mg·mL−1) was used to amplify the impedance signal. The incubation time was 45 min and then washed with ultrapure water. Impedance measurements were also conducted using a 10 mM K3Fe(CN)6/K4Fe(CN)6 (1:1) mixture in PBS (1 Hz–1 MHz, 10 mV).
2.6. Regeneration of SPIMs
After each test, the used SPIMs were treated with piranha solution to disassociate the bacterial cells and other materials attached on the surface of the SPIMs. Then, impedance data were collected to compare with the initial value. The electrodes were reused if their impedance values were similar to initial values.
3. Results and Discussion
3.1. Characterization of the Microelectrodes
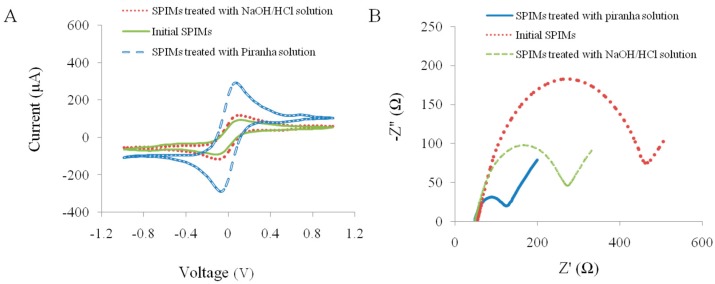
To effectively clean these new kind of SPIMs, different cleaning procedures were conducted and compared. CV and EIS methods using a K3Fe(CN)6/K4Fe(CN)6 probe were adopted to investigate the cleaning efficiency by comparing peak current of electrochemical probes and electron transfer resistance (Ret), respectively (Figure 1) [31,32]. After being treated with conventional NaOH/HCl and piranha solutions, SPIMs showed the increases of peak currents of the electrochemical probe by 25% and 180%, and the decreases of Ret by 45% and 75%, respectively, indicating the cleanliness was improved. Obviously, piranha solution was more efficient in cleaning, which was, therefore, selected for further use. We also found that peak current and Ret kept constant after a 15 min immersion. Accordingly, we could readily obtain a clean SPIM through immerging electrodes into piranha solution for 15 min. This treatment is much more convenient and time-saving compared with treatments commonly used for rod electrodes or plate electrodes, which generally need complicated polishing procedures [33,34,35]. These merits should highlight the convenience of SPIMs and benefit the facile fabrication of biosensors.
Figure 1.
Characterization of the SPIMs by cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) after the washing with piranha solution and NaOH/HCl solution. (A) the current change; and (B) the impedance change.
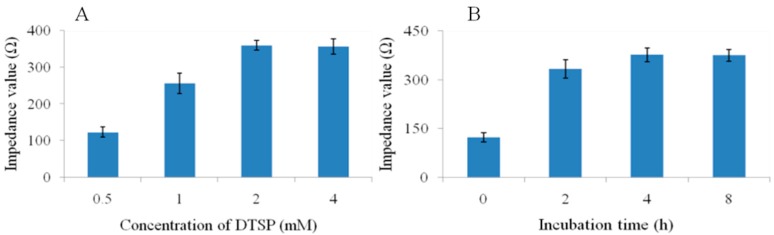
The self-assembling of DTSP was monitored through measuring the impedance value at 100 Hz, as shown in Figure 2. After 2 h incubation, impedance values had minor change (p > 0.05), indicating nearly full self-assembling, though the arrangement of DTSP molecules might be still going to finally form a perfect monolayer. Here, we chose 4 h incubation time considering both the self-assembling efficiency and time efficiency. Similarly, the concentration of DTSP was also investigated and optimized to be 2 mM.
Figure 2.
The effect of (A) the concentration of DTSP and (B) incubation time of DTSP on the impedance value.
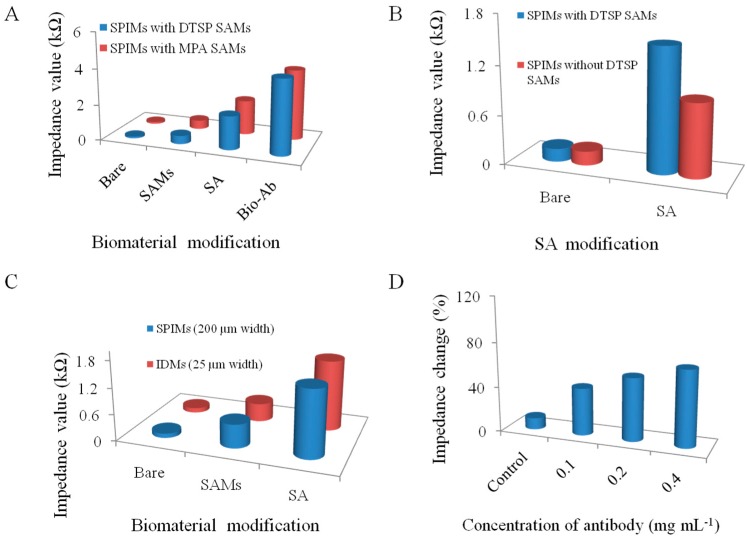
For comparison, a conventional method for the modification of materials on the surface of the SPIMs using MPA SAMs plus EDC/NHS cross-linking was also used to immobilize antibodies (Figure 3A). We found that the immobilization efficiency of the new DTSP-based method performed even better than this classic method. Additionally, the conventional method requires 4 h for self-assembling of MPA plus another 3 h activation of carboxyl group using EDC/NHS. Therefore, DTSP self-assembling should be a better choice with comparable performance but shorter preparation time. Moreover, we evaluated the absorption capacity of SPIMs with or without the DTSP SAMs (Figure 3B) and found that the absorption capacity increased significantly when the SPIM was modified with SAMs. The increase improved detection performance, verifying the necessity of the SAMs.
Figure 3.
(A) The performance of the different SAMs (DTSP SAMs and 3-Mercaptopropionic acid (MPA) SAMs); (B) The absorption capacity of SPIMs with or without SAMs; (C) Comparison of the SPIMs (200 µm width of electrode fingers) and interdigitated microelectrodes (IDMs, 25 µm width of electrode fingers); and (D) The optimization of the antibody concentration. All the experiments were repeated more than three times.
The impedance changes of the microelectrodes with different sizes are shown in Figure 3C. The results indicated that there was no significant change between the 25 µm and 200 µm microelectrodes (p > 0.05) after the biomaterials immobilization. Compared with 25 µm interdigitated microelectrodes, the application of SPIMs is promising due to their low cost.
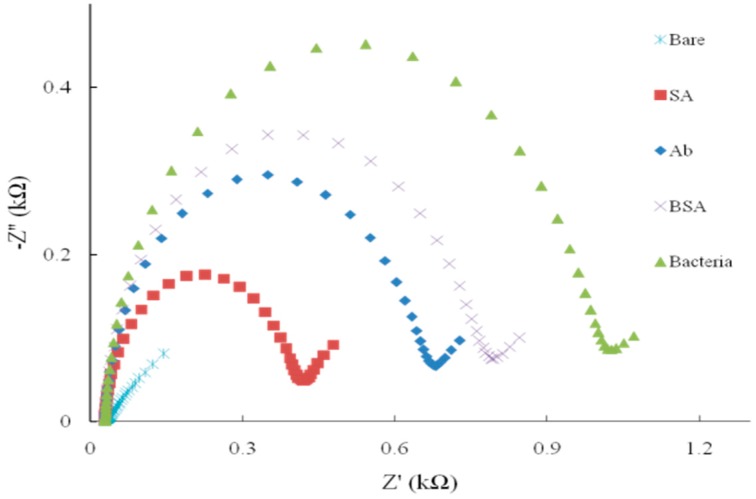
Besides the self-assembling time and the concentration of DTSP, we also optimized the concentration of the antibody (Figure 3D). The impedance change showed that the adsorbing capacity was induced with the different dilutions. Compared with the control, the lower dilution (0.2 mg·mL−1) of antibody was enough for detection of bacteria. There was no significant difference between the concentrations of 0.2 and 0.4 mg·mL−1 (p > 0.05). The disadvantage of the lower concentration of antibodies is that the sensitivity of the method may be affected due to less amount of the antibody available to capture the target pathogen. Each step of the surface modification was measured and the result was shown in Figure 4. The bare microelectrode showed a very small impedance value (curve a) because of the low resistance of the [Fe(CN)6]3−/4− charge transfer process. When SA (curve b), antibody (curve c) and BSA (curve d) were immobilized onto the microelectrode surface, impedance increased, which confirmed the successful surface immobilization.
Figure 4.
The impedance change after the SPIMs modification with biomaterials (SA, Ab and BSA) and the capture of target bacteria E. coli O157:H7 (Nyquist plot).
3.2. Detection and Signal Amplification
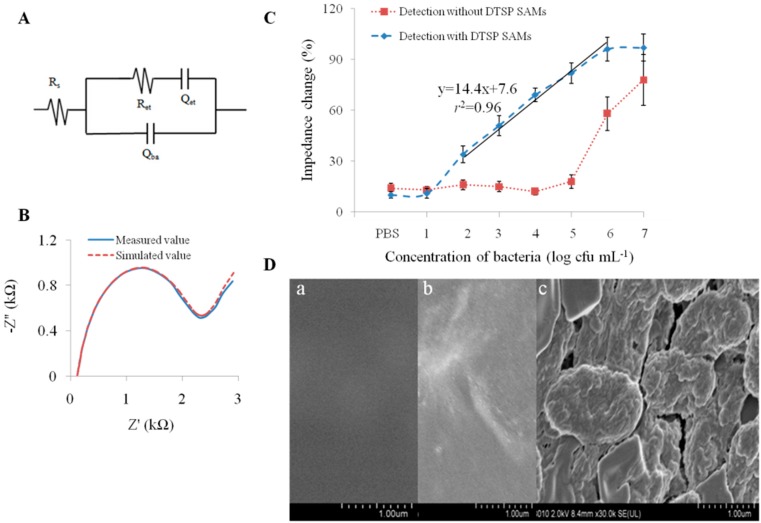
For better understanding of the detection mechanism, a modified Randles’ equivalent circuit obtained using Zpswin 3.10 software was used to adequately fit the measurement data over the whole frequency range (Figure 5). Wherein, Rs stands for the resistance of the electrolyte solution and Ret for charge transfer resistance, Qet is the constant phase elements associated with the capacitance of the double layer and Qba is the capacitance of the biomaterial absorption. All the impedance spectra of different concentrations of bacteria were fitted to the equivalent circuit. During the modification and detection, the loading of non-conductive proteins and bacteria would significantly retard the interfacial electron-transfer kinetics and increase the electron-transfer resistance, leading to the changes of Ret values. The Ret value is the most important electrical parameter in analyzing the impedance signal change for detection of bacteria and can be used to evaluate the detection performance [19,20,21]. When bacterial cells were introduced to the fabricated immunosensor, there was a further increase in Ret, indicating that the microelectrode surface had been attached with a large number of bacterial cells.
Figure 5.
(A) The plot of the measured and simulated data using the immunosensor with DTSP SAMs; (B) A modified Randles’ equivalent circuit; (C) The detection of E. coli O157:H7 at different concentrations using the immunosensor with DTSP SAMs or not; (D) Characterization of the surface of the bare SPIM (a), the surface of the SPIMs before (b) and after (c) the bacteria incubation by scanning electron microscope.
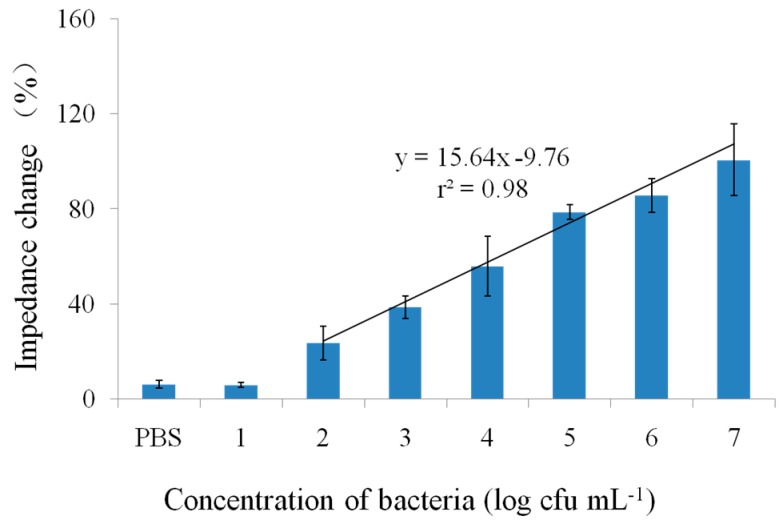
Under optimized conditions, the immunosensor provided a detection limit of 102 cfu·mL−1 and a linear detection range (r2 = 0.96) for E. coli O157:H7 between 102 and 106 cfu·mL−1 (Figure 5C). The total detection time including incubation was less than 1h. The detection performance was comparable to the previous research and the immunosensor was easily fabricated [36,37]. The detection performance of the proposed immunosensor was comparable or better than some of reported biosensors that used interdigitated microelectrodes [19,20,21]. The proposed biosensor presented a lower detection limit and higher specificity, as compared with those of the previously reported biosensors using lectin as recognition element [38,39]. We also evaluated the detection performance of the physical absorption of SA in which only 106 cfu·mL−1 and higher concentrations of bacterial cells could be distinguished (Figure 5C). The reasonable explanation was that DTSP SAMs could be used to improve the stability of the immobilization and increase the absorption capacity. Moreover, the bacteria attached onto the surface of the SPIM was characterized by scanning electron microscopy (Figure 5D) demonstrating the good performance of the constructed immunosensor. The absorption of bacteria cells and WGA has been verified in other research [29,30]. WGA molecules can be successfully absorbed by the fabricated biosensor after the bacteria absorption to improve the impedance change compared with control. Compared to the detection with antibody only (blue curve in Figure 5C), the impedance value was enhanced and the value of the r2 was improved from 0.96 to 0.98 (Figure 6). The results demonstrate proof of this concept that lectins, including WGA, can be utilized for signal amplification. Further work can be done to select one lectin with better specificity for the bacteria from different lectins. We have prepared five lectins, including WGA, canavailiaensiformis (Con A), ulexeuropaeus (UEA), M. amurensis (MAL) and arachishypogaea (PNA) for the optimization. Detection parameters’ optimization is also further research for good performance.
Figure 6.
The signal amplification of the immunosensor with WGA solution.
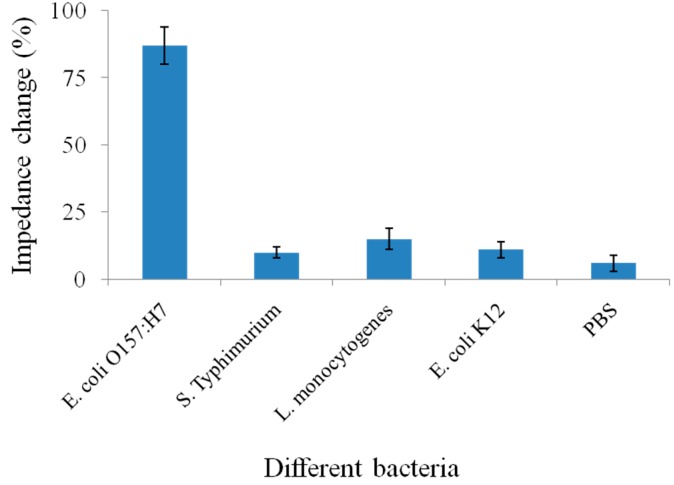
The specificity of the proposed biosensor was evaluated using E. coli K12, L. monocytogenes and S. Typhimurium as non-target bacteria. There was no significant attachment when these bacteria were incubated, which clearly evidenced the sensing specificity to E. coli O157:H7 (Figure 7). Moreover, the cost of one SPIM may be less than $1 which is much better for the practical application compared with the expensive non-screen printed microelectrodes. Moreover, the cost can be further reduced by good reusability. The proposed biosensor has the potential in the development of a simple, low cost and portable biosensing system for rapid and sensitive detection of pathogens other than E. coli O157:H7.
Figure 7.
The specificity of the immunosensor for detection of E. coli O157:H7.
4. Conclusions
An electrochemical impedance immunosensor based on self-assembled monolayers was proposed for rapid detection of E. coli O157:H7 with signal amplification using WGA. The application of WGA as signal amplification for bacteria detection was utilized to amplify the signal and the results demonstrated proof of this concept that lectins, including WGA, can be utilized for signal amplification. With the optimized parameters, the proposed impedance immunosensor could detect E. coli O157:H7 at concentrations as low as 102 cfu·mL−1with a linear detection range between 102 and 107 cfu·mL−1 (r2 = 0.98) and a total detection time of less than 1 h. The cost of one SPIM can be further reduced by good reusability. Considering the ease of the electrodes and assay integration, the method we proposed presents an opportunity to develop a portable biosensing system for routine monitoring of foodborne pathogens.
Acknowledgments
This research was supported by the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (Grant No. 2013BAD19B02). The authors thank Bryan Pierce at the University of Florida for his reviewing this manuscript.
Author Contributions
Zhanming Li conceived the main ideas, conducted the experiments and wrote the manuscript. Yingchun Fu proposed the idea of the monolayers and reviewed the manuscript. Weihuan Fang reviewed the manuscript and modified several paragraphs of the text. Yanbin Li oversaw this research and reviewed the manuscript with his corrections and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Pennington H. Escherichia coli O157. Lancet. 2010;376:1428–1435. doi: 10.1016/S0140-6736(10)60963-4. [DOI] [PubMed] [Google Scholar]
- 2.Matthews L., Reeve R., Gally D.L., Low J.C., Woolhouse M.E., McAteer S.P., Locking M.E., Chase-Topping M.E., Haydon D.T., Allison L.J. Predicting the public health benefit of vaccinating cattle against Escherichia coli O157. Proc. Natl. Acad. Sci. USA. 2013;110:16265–16270. doi: 10.1073/pnas.1304978110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scharff R.L. Economic burden from health losses due to foodborne illness in the United States. J. Food Protect. 2012;75:123–131. doi: 10.4315/0362-028X.JFP-11-058. [DOI] [PubMed] [Google Scholar]
- 4.Griffin P.M., Tauxe R.V. The epidemiology of infections caused by Escherichia coli O157: H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 5.Tokarskyy O., Marshall D.L. Immunosensors for rapid detection of Escherichia coli O157: H7—Perspectives for use in the meat processing industry. Food Microbiol. 2008;25:1–12. doi: 10.1016/j.fm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Cassin M.H., Lammerding A.M., Todd E.C., Ross W., McColl R.S. Quantitative risk assessment for Escherichia coli O157: H7 in ground beef hamburgers. Int. J. Food Microbiol. 1998;41:21–44. doi: 10.1016/S0168-1605(98)00028-2. [DOI] [PubMed] [Google Scholar]
- 7.Beuchat L.R. Survival of enterohemorrhagic Escherichia coli O157: H7 in bovine feces applied to lettuce and the effectiveness of chlorinated water as a disinfectant. J. Food Protect. 1999;62:845–849. doi: 10.4315/0362-028x-62.8.845. [DOI] [PubMed] [Google Scholar]
- 8.Blanco M., Blanco J., Mora A., Dahbi G., Alonso M., González E., Bernárdez M., Blanco J. Serotypes, virulence genes, and intimin types of Shiga toxin (verotoxin)-producing Escherichia coli isolates from cattle in Spain and identification of a new intimin variant gene (eae-ξ) J. Clin. Microbiol. 2004;42:645–651. doi: 10.1128/JCM.42.2.645-651.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Tan C., Fei R., Liu X., Zhou Y., Chen J., Chen H., Zhou R., Hu Y. Sensitive chemiluminescence immunoassay for E. coli O157:H7 detection with signal dual-amplification using glucose oxidase and laccase. Anal. Chem. 2014;86:1115–1122. doi: 10.1021/ac4028774. [DOI] [PubMed] [Google Scholar]
- 10.Liu J., Wagan S., Dávila M.M., Taylor J., White R.J. Achieving reproducible performance of electrochemical, folding aptamer-based sensors on microelectrodes: challenges and prospects. Anal. Chem. 2014;86:11417–11424. doi: 10.1021/ac503407e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Q., Gan Z., Zhuang Q. Electrochemical sensors based on carbon nanotubes. Electroanalysis. 2002;14:1609–1613. doi: 10.1002/elan.200290000. [DOI] [Google Scholar]
- 12.Chen L., Sheng Z., Zhang A., Guo X., Li J., Han H., Jin M. Quantum-dots-based fluoroimmunoassay for the rapid and sensitive detection of avian influenza virus subtype H5N1. Luminescence. 2010;25:419–423. doi: 10.1002/bio.1167. [DOI] [PubMed] [Google Scholar]
- 13.Shao Y., Wang J., Wu H., Liu J., Aksay I.A., Lin Y. Graphene based electrochemical sensors and biosensors: A review. Electroanalysis. 2010;22:1027–1036. doi: 10.1002/elan.200900571. [DOI] [Google Scholar]
- 14.Yang X., Qian J., Jiang L., Yan Y., Wang K., Liu Q., Wang K. Ultrasensitive electrochemical aptasensor for ochratoxin A based on two-level cascaded signal amplification strategy. Bioelectrochemistry. 2014;96:7–13. doi: 10.1016/j.bioelechem.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Vidal J.C., Bonel L., Ezquerra A., Duato P., Castillo J.R. An electrochemical immunosensor for ochratoxin A determination in wines based on a monoclonal antibody and paramagnetic microbeads. Anal. Bioanal. Chem. 2012;403:1585–1593. doi: 10.1007/s00216-012-5951-5. [DOI] [PubMed] [Google Scholar]
- 16.Escamilla-Gómez V., Campuzano S., Pedrero M., Pingarrón J.M. Gold screen-printed-based impedimetric immunobiosensors for direct and sensitive Escherichia coli quantisation. Biosens. Bioelectron. 2009;24:3365–3371. doi: 10.1016/j.bios.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y., Ye Z., Ying Y. New trends in impedimetric biosensors for the detection of foodborne pathogenic bacteria. Sensors. 2012;12:3449–3471. doi: 10.3390/s120303449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernalte E., Sánchez C.M., Gil E.P. Determination of mercury in ambient water samples by anodic stripping voltammetry on screen-printed gold electrodes. Anal. Chim. Acta. 2011;689:60–64. doi: 10.1016/j.aca.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 19.Barreiros D.S.M., Agusil J., Prieto-Simón B., Sporer C., Teixeira V., Samitier J. Highly sensitive detection of pathogen Escherichia coli O157:H7 by electrochemical impedance spectroscopy. Biosens. Bioelectron. 2013;45:174–180. doi: 10.1016/j.bios.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Joung C.K., Kim H.N., Lim M.C., Jeon T.J., Kim H.Y., Kim Y.R. A nanoporous membrane-based impedimetric immunosensor for label-free detection of pathogenic bacteria in whole milk. Biosens. Bioelectron. 2013;44:210–215. doi: 10.1016/j.bios.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 21.Dastider S.G., Barizuddin S., Dweik M., Almasri M. A micromachined impedance biosensor for accurate and rapid detection of E. coli O157:H7. RSC Adv. 2013;3:26297–26306. [Google Scholar]
- 22.Subramanian A., Irudayaraj J., Ryan T. A mixed self-assembled monolayer-based surface plasmon immunosensor for detection of E. coli O157: H7. Biosens. Bioelectron. 2006;21:998–1006. doi: 10.1016/j.bios.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Su X., Li Y. A self-assembled monolayer-based piezoelectric immunosensor for rapid detection of Escherichia coli O157:H7. Biosens. Bioelectron. 2004;19:563–574. doi: 10.1016/S0956-5663(03)00254-9. [DOI] [PubMed] [Google Scholar]
- 24.Samanta D., Sarkar A. Immobilization of bio-macromolecules on self-assembled monolayers: methods and sensor applications. Chem. Soc. Rev. 2011;40:2567–2592. doi: 10.1039/c0cs00056f. [DOI] [PubMed] [Google Scholar]
- 25.Varshney M., Li Y. Interdigitated array microelectrodes based impedance biosensors for detection of bacterial cells. Biosens. Bioelectron. 2009;24:2951–2960. doi: 10.1016/j.bios.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Dou W., Tang W., Zhao G. A disposable electrochemical immunosensor arrays using 4-channel screen-printed carbon electrode for simultaneous detection of Escherichia coli O157:H7 and Enterobacter sakazakii. Electrochim. Acta. 2013;97:79–85. doi: 10.1016/j.electacta.2013.02.136. [DOI] [Google Scholar]
- 27.Jang L.S., Keng H.K. Modified fabrication process of protein chips using a short-chain self-assembled monolayer. Biomed. Microdevices. 2008;10:203–211. doi: 10.1007/s10544-007-9126-7. [DOI] [PubMed] [Google Scholar]
- 28.Kaji H., Yamauchi Y., Takahashi N., Isobe T. Mass spectrometric identification of N-linked glycopeptides using lectin-mediated affinity capture and glycosylation site-specific stable isotope tagging. Nat. Protocols. 2007;1:3019–3027. doi: 10.1038/nprot.2006.444. [DOI] [PubMed] [Google Scholar]
- 29.Shen Z., Huang M., Xiao C., Zhang Y., Zeng X., Wang P.G. Nonlabeled quartz crystal microbalance biosensor for bacterial detection using carbohydrate and lectin recognitions. Anal. Chem. 2007;79:2312–2319. doi: 10.1021/ac061986j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y., Ping J., Ye Z., Wu J., Ying Y. Impedimetric immunosensor based on gold nanoparticles modified graphene paper for label-free detection of Escherichia coli O157:H7. Biosens. Bioelectron. 2013;49:492–498. doi: 10.1016/j.bios.2013.05.061. [DOI] [PubMed] [Google Scholar]
- 31.Lucarelli F., Marrazza G., Mascini M. Enzyme-based impedimetric detection of PCR products using oligonucleotide-modified screen-printed gold electrodes. Biosens. Bioelectron. 2005;20:2001–2009. doi: 10.1016/j.bios.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 32.Rowe A.A., Chuh K.N., Lubin A.A., Miller E.A., Cook B., Hollis D., Plaxco K.W. Electrochemical biosensors employing an internal electrode attachment site and achieving reversible, high gain detection of specific nucleic acid sequences. Anal. Chem. 2011;83:9462–9466. doi: 10.1021/ac202171x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ling T.R., Li C.S., Jow J.J., Lee J.F. Mesoporous nickel electrodes plated with gold for the detection of glucose. Electrochim. Acta. 2011;56:1043–1050. doi: 10.1016/j.electacta.2010.10.041. [DOI] [Google Scholar]
- 34.Kim Y.S., Jung H.S., Matsuura T., Lee H.Y., Kawai T., Gu M.B. Electrochemical detection of 17β-estradiol using DNA aptamer immobilized gold electrode chip. Biosens. Bioelectron. 2007;22:2525–2531. doi: 10.1016/j.bios.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z., Duan N., Hun X., Wu S. Electrochemiluminescent aptamer biosensor for the determination of ochratoxin A at a gold-nanoparticles-modified gold electrode using N-(aminobutyl)-N-ethylisoluminol as a luminescent label. Anal. Bioanal. Chem. 2010;398:2125–2132. doi: 10.1007/s00216-010-4146-1. [DOI] [PubMed] [Google Scholar]
- 36.Geng P., Zhang X., Meng W., Wang Q., Zhang W., Jin L., Feng Z., Wu Z. Self-assembled monolayers-based immunosensor for detection of Escherichia coli using electrochemical impedance spectroscopy. Electrochim. Acta. 2008;53:4663–4668. doi: 10.1016/j.electacta.2008.01.037. [DOI] [Google Scholar]
- 37.Wang R., Ruan C., Kanayeva D., Lassiter K., Li Y. TiO2 nanowire bundle microelectrode based impedance immunosensor for rapid and sensitive detection of Listeria monocytogenes. Nano Lett. 2008;8:2625–2631. doi: 10.1021/nl080366q. [DOI] [PubMed] [Google Scholar]
- 38.Gamella M., Campuzano S., Parrado C., Reviejo A.J., Pingarron J.M. Microorganismsrecognition and quantification by lectin adsorptive affinity impedance. Talanta. 2009;78:1303–1309. doi: 10.1016/j.talanta.2009.01.059. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y., Ye Z., Si C., Ying Y. Monitoring of Escherichia coli O157:H7 in food samples using lectin based surface plasmon resonance biosensor. Food Chem. 2013;136:1303–1308. doi: 10.1016/j.foodchem.2012.09.069. [DOI] [PubMed] [Google Scholar]