Abstract
Arboviruses are medically important pathogens that cause human disease ranging from a mild fever to encephalitis. Laboratory diagnosis is essential to differentiate arbovirus infections from other pathogens with similar clinical manifestations. The Arboviral Diseases Branch (ADB) reference laboratory at the CDC Division of Vector-Borne Diseases (DVBD) produces reference antigens used in serological assays such as the virus-specific immunoglobulin M antibody-capture enzyme-linked immunosorbent assay (MAC-ELISA). Antigen production in cell culture has largely replaced the use of suckling mice; however, the methods are not directly transferable. The development of a cell culture antigen production algorithm for nine arboviruses from the three main arbovirus families, Flaviviridae, Togaviridae, and Bunyaviridae, is described here. Virus cell culture growth and harvest conditions were optimized, inactivation methods were evaluated, and concentration procedures were compared for each virus. Antigen performance was evaluated by the MAC-ELISA at each step of the procedure. The antigen production algorithm is a framework for standardization of methodology and quality control; however, a single antigen production protocol was not applicable to all arboviruses and needed to be optimized for each virus.
Keywords: Arbovirus, Antigen, Immunoglobulin M antibody-capture enzyme-linked immunosorbent assay (MAC-ELISA), Beta-propiolactone, Gamma-irradiation
1. Introduction
There are over 500 arthropod-borne viruses, or arboviruses, geographically distributed throughout the world, over 150 of which cause disease in human and/or animal populations (Burke, Monath, 2001; Centers for Disease Control and Prevention, 2014; Cleton et al., 2012; Gubler, 2002; Monath, Heinz, 1996; Rosenberg et al., 2013; Weaver, 2005). Some arboviruses, such as dengue, Japanese encephalitis, and most recently chikungunya (CHIK) viruses, have wide geographical distribution and cause large seasonal epidemics (Powers et al., 2000; Staples et al., 2009). Others, such as West Nile virus, Zika virus and again, CHIK virus, are emerging or reemerging, and may cause sporadic outbreaks in regions in which they were not previously detected (Lanciotti et al., 1999; Lanciotti et al., 2007; Lanciotti et al., 2008; Solomon, Winter, 2004). Other arboviruses, such as Powassan (POW), have low or unknown incidence, and may be detected due to emergence or increased surveillance (Ei Khoury et al., 2013).
Arbovirus infections may present with clinical symptoms similar to those of other bacterial or virological infections, such as an influenza-like illness, encephalitis, or polio-like myelitis (Burke, Monath, 2001). In addition, arboviruses within a serocomplex may cause similar disease syndromes and may be clinically indistinguishable from one another. Laboratory diagnosis is necessary to identify arbovirus infections and differentiate between other bacterial or viral pathogens, particularly if there is an effective treatment or vaccine available. Although detection of viral RNA or virus isolation is the gold standard for diagnosis and identification of a viral infection, these methods are not sensitive in many arbovirus infections due to the brief, transient, low-level viremia (≤100 infectious particles/ml) that may be cleared by the onset of illness. The virus-specific immunoglobulin M (IgM) antibody-capture enzyme-linked immunosorbent assay (MAC-ELISA) can be used for rapid detection of acute arbovirus infections, as IgM antibody is produced early in infection, rises rapidly to detectable levels, and is less cross-reactive than IgG antibodies (Johnson et al., 2000; Johnson et al., 2005; Martin et al., 2000; WHO, 2003; Wong et al., 2003; Wong et al., 2004). The CDC Division of Vector-Borne Diseases (DVBD) Arboviral Diseases Branch (ADB) reference laboratory produces antigens for the MAC-ELISA for a wide array of arboviruses, most of which are not available commercially.
Viral antigen used in serological assays was previously generated from sucrose-acetone extracted suckling mouse brain (SMB) preparations. In order to reduce the use of animals, viral antigen production has shifted towards cell culture. This has necessitated modification and optimization of the methods previously used during SMB antigen production to cell culture, such as virus inactivation and concentration. The development of a cell culture antigen production algorithm for nine arboviruses from the three main arbovirus families, Flaviviridae, Togaviridae, and Bunyaviridae, is reported here. Cell culture conditions and inactivation and concentration procedures were optimized for each virus, using the MAC-ELISA as the performance indicator.
2. Materials and Methods
2.1. Viruses
Yellow Fever virus (YFV) strain 17D; St. Louis Encephalitis virus (SLEV) strain TBH-28; Powassan virus (POWV) strain LB; Chikungunya virus (CHIKV) strains 181/25 and S27; Mayaro virus (MAYV) strain TR15537; Sindbis virus (SINV) strains EgAr 339, 16260, 80–2449, AUS C 263, AUS C 377, AUS MRM 39, INDA 1036, MAL AMM 2215, Michalovce, Reed Warbler, SAAR 86 and UGMP 684; La Crosse virus (LACV) strain Original; Jamestown Canyon virus (JCV) strains 61V-2235 and MN256–260; and Tahyna virus (TAHV) strain Bardos 92 were obtained from the Arbovirus Reference Collection at the Centers for Disease Control and Prevention (CDC), Division of Vector-Borne Diseases (DVBD) in Fort Collins, Colorado.
2.2. Tissue culture
Cell lines used in the growth curves were obtained at CDC DVBD. African green monkey kidney (Vero) cells, baby hamster kidney (BHK-21) clones 13 and 15 cells, rhesus monkey kidney (LLC-MK2) cells, and Vero clone E6 cells were maintained at 37°C in Dulbecco’s Modified Eagle Medium (DMEM, Life Technologies, Grand Island, NY) with 8% fetal bovine serum (FBS, Atlas Biologicals, Fort Collins, CO), 1mM sodium pyruvate (Life Technologies), 27mM sodium bicarbonate (Life Technologies), 0.1mM gentamicin (Lonza, Walkersville, MD), and 1uM amphotericin B (Sigma-Aldrich, St. Louis, MO). Aedes albopictus mosquito C6/36 cells were maintained at 28°C in DMEM (Life Technologies) with 10% FBS (Atlas Biologicals), 0.1mM non-essential amino acids (Life Technologies), 1mM sodium pyruvate (Life Technologies), 9mM sodium bicarbonate (Life Technologies), and 0.1mM gentamicin (Lonza).
2.3. Growth curves
Growth curves were performed in T150 cm2 cell culture flasks (Corning Inc. Life Sciences, Tewksbury, MA) at a multiplicity of infection (MOI) ranging from 0.0005 to 0.1 plaque-forming units (PFU)/cell. Following adsorption of virus in 10 ml of media at 37°C for 1 h, cells were maintained in 60 ml of their respective media as described above, albeit with 2% FBS (Atlas Biologicals). At 24 h intervals, 0.5–1.0 ml of supernatant was removed and frozen at −70°C until tested. Growth curves were carried out for 3 to 16 days until cytopathic effect (CPE) reached ∼90–100%, or until the cells became overgrown in the negative control flask.
2.4. Virus titration
Virus titers were determined by 1% agarose double-overlay plaque titration assay in Vero cells, as previously described (Beaty et al., 1995). Plaques were visualized with second overlays applied with 0.005% neutral red (Sigma-Aldrich) following incubation for 2 days for CHIKV, MAYV, SINV, LACV, and TAHV; 3 days for JCV; 4 days for YFV; and 6 days for SLEV and POWV. Virus titers were recorded as log10 PFU/ml.
2.5. IgM antibody-capture enzyme-linked immunosorbent assay (MAC-ELISA)
Viral antigen activity was evaluated by the CDC MAC-ELISA, as previously described (Martin et al., 2000). Live virus or inactivated antigen was serially diluted two-fold and reacted against both constant IgM positive and normal control sera, obtained from the DVBD diagnostic laboratory, except the SINV IgM positive control, for which no human sera was available. An alphavirus-group reactive mouse/human chimeric monoclonal antibody (cMAb) served as the SINV IgM positive control (Thibodeaux et al., 2011). Virus-specific antigen activity (VSAA) was defined as the optical density (OD) of viral antigen reacted against a constant positive control serum; acceptable VSAA had an OD of >0.8. Nonspecific background reactivity (NBR) was defined as the OD of viral antigen reacted against a constant normal control serum; acceptable NBR had an OD of <0.2. A satisfactory antigen was defined as that which had acceptable MAC-ELISA results, in which both the VSAA and NBR were within acceptable OD ranges; the highest antigen dilution with acceptable VSAA and NBR OD ranges was considered the working antigen dilution, and was a measure of functional antigen concentration.
2.6. Virus production for inactivation and concentration analyses
The optimal virus strain, cell type, and day of harvest were determined by the growth curves from one T150 cm2 flask, after which a second batch was made in additional T150 cm2 flask(s) under the optimized conditions. Supernatant was harvested, with volumes ranging from 60 to 500 ml, clarified at 2400 x g for 10 min at 4°C, and stored at −70°C with 20% FBS (Atlas Biologicals) until further analysis. Flaviviruses grow relatively slowly and it was possible to collect and replenish supernatant on multiple days from one flask. The harvests were then combined to make one batch.
2.7. Virus inactivation methods
2.7.1. Beta-propiolactone (BPL)
Cell culture supernatants were thawed in a 44°C water bath with intermittent shaking, treated with BPL (CTC Organics, Atlanta, GA) at final concentrations ranging from 0.01% to 0.3%, and incubated for 24 h at 4°C with moderate shaking on a refrigerated shaker plate. Mock-treated control supernatants (no addition of BPL) were incubated under the same conditions as the BPL-treated samples. Due to acidic BPL by-products, 7.5% sodium bicarbonate (Life Technologies) was added intermittently to adjust the pH (French, McKinney, 1964). Following BPL treatment the samples were stored at −70°C until further analysis. For hydrolysis analysis, samples were treated with 0.05 or 0.3% BPL and incubated for 48 h at 4°C with moderate shaking. Following BPL treatment, samples that underwent hydrolysis were incubated at 37°C for 2 h, then placed at −70°C until further analysis.
2.7.2. Gamma-irradiation
Gamma-irradiation using a cobalt-60 source was carried out at the CDC irradiation facility in Atlanta, GA. Small volume aliquots of virus were irradiated with doses ranging from1–6 Mrad in a “kill curve.” All material was maintained frozen on dry ice throughout the treatment process. Untreated control supernatants remained frozen without any exposure to gamma-irradiation.
2.7.3. Gamma-irradiation + BPL
Virus supernatant was treated with 5.5 to 6 Mrad, thawed, concentrated 5X to 6X with Centricon Plus-70 100kDa Centrifugal Filter Devices (Millipore), and then treated with BPL at final concentrations ranging from 0.01% to 0.1%.
2.7.4. 37% Formaldehyde (Formalin)
Cell culture supernatants were treated with 0.05% to 0.3% final concentrations of 37% formalin (Fisher Scientific, Waltham, MA) and incubated for 4.5 days at 4°C, room temperature, or 37°C with moderate shaking or stirring (Sabin, 1943). Aliquots were taken at 6, 12, 24, 36, 48, 72, 96, and 108 h and tested for antigen activity.
2.7.4. Hydrogen peroxide
Cell culture supernatants were treated with a 3% final concentration of hydrogen peroxide (Fisher Scientific) and incubated for 2 h at room temperature with moderate shaking, as described (Amanna et al., 2012). To adjust for pH changes, 7.5% sodium bicarbonate (Life Technologies) was added intermittently during incubation.
2.7.5. N-Lauroylsarcosine sodium salt (Sarkosyl)
Cell culture supernatants were treated with 0.1% to 0.3% final concentrations of sarkosyl (Sigma-Aldrich) and incubated for 1 h at room temperature, as previously described (Piret et al., 2002a, 2002b). Prior to treatment, the sarkosyl stock solution was filtered through a Millex GV PVDF 0.22-µm membrane (Millipore, Billerica, MA) for sterilization purposes.
2.8. Antigen Concentration Methods
2.8.1. Ultracentrifugation
Inactivated cell culture supernatants were concentrated by ultracentrifugation at 54,000 x g at 4°C for ∼16 h. Supernatant was completely removed and pelleted antigen was resuspended in 0.1M trizma/BS buffer: 1.0M trizma pH 9.0 (Sigma-Aldrich) + borate saline solution pH 9.0 [1.5M sodium chloride (Daigger, Vernon Hills, IL), 0.5M boric acid (Fisher Scientific), 1.0N sodium hydroxide (Daigger)], or 1X phosphate buffered saline (PBS, Life Technologies) to achieve the desired concentration factor.
2.8.2. Centrifugal filters
Inactivated cell culture supernatants were concentrated in Amicon Ultra-15 100-kDa Centrifugal Filter Devices (Millipore) or Centricon Plus-70 100-kDa Centrifugal Filter Devices at 3500 x g for 10–45 min at 4°C. Any material that was inadvertently concentrated beyond the desired concentration factor was diluted to the correct volume with 0.1M trizma/BS buffer or 1X PBS.
2.9. Infectivity assays
Two methods were used to evaluate infectivity of inactivated antigen as described previously with modifications (Monath et al., 2010). Briefly, plaque titration of the treated antigen material was performed in duplicate in 6-well plates on Vero cells, beginning at neat concentration. In addition, 100 µl of treated antigen was inoculated into duplicate T25 cm2 cell culture flasks containing Vero or BHK-21c.15 cells and passaged once a week for 3 weeks. Virus was considered inactivated if there was no detectable titer by plaque titration and if there was no detectable CPE in any of the three cell culture passages.
3. Results
3.1. Growth curves
Virus growth curves were performed first to determine the optimal virus strain and cell culture type to use for subsequent antigen production. Viruses were inoculated into T150 cm2 flasks at MOIs ranging from 0.0005 to 0.1 and incubated for 3 to 16 days, depending on CPE and the condition of the cell monolayer. Supernatant, 0.5–1 ml, was removed at 24 h time points and tested by plaque titration and/or MAC-ELISA. The growth and harvest conditions resulting in the highest titer and/or greatest acceptable antigen activity were chosen to make a second batch, which was used in the inactivation and concentration analyses. If multiple conditions generated the highest titer and/or greatest acceptable antigen activity equally (e.g. POWV produced from Vero and BHK-21 cells both generated the highest VSAA), the cell culture that yielded more product (as in performing multiple collections), was chosen. Second-batch product was evaluated by MAC-ELISA to confirm acceptable VSAA and NBR ranges prior to inactivation experiments; the results are shown in Table 1 (untreated).
Table 1.
Antigen reactivity in MAC ELISA before (untreated) and after inactivation
| A. YFV/17D/ Vero* | ||||||||
|---|---|---|---|---|---|---|---|---|
| Antigen Dilution |
VSAA | NBR | VSAA | NBR | VSAA | NBR | VSAA | NBR |
| 0.01% BPL | 0.05% BPL | 0.1% BPL | Mock-treated | |||||
| 1:10 | 2.840 | 0.064 | 3.091 | 0.188 | 3.205 | 1.900 | 2.706 | 0.047 |
| 1:20 | 2.162 | 0.047 | 2.367 | 0.112 | 2.009 | 1.058 | 2.050 | 0.042 |
| 1:40 | 1.569 | 0.045 | 1.631 | 0.068 | 1.415 | 0.668 | 1.399 | 0.039 |
| 1:80 | 1.024 | 0.038 | 1.003 | 0.064 | 0.762 | 0.402 | 0.882** | 0.039 |
| 1:160 | 0.587 | 0.038 | 0.550 | 0.056 | 0.431 | 0.254 | 0.519 | 0.034 |
| 1:320 | 0.339 | 0.043 | 0.281 | 0.079 | 0.235 | 0.159 | 0.301 | 0.036 |
| 1mRAD | 3mRAD | 5mRAD | Untreated | |||||
| Neat | 2.497 | 0.019 | 2.536 | 0.029 | 2.167 | 0.034 | 2.636 | 0.099 |
| 1:2 | 2.290 | 0.021 | 2.310 | 0.057 | 1.835 | 0.037 | 2.533 | 0.087 |
| 1:4 | 1.857 | 0.020 | 1.877 | 0.049 | 1.432 | 0.030 | 2.174 | 0.021 |
| 1:8 | 1.453 | 0.015 | 1.290 | 0.037 | 1.041† | 0.025 | 1.759 | 0.018 |
| 1:16 | 0.866‡ | 0.017 | 0.819 | 0.049 | 0.688 | 0.031 | 1.194 | 0.018 |
| 1:32 | 0.701 | 0.015 | 0.540 | 0.051 | 0.429 | 0.030 | 0.859 | 0.025 |
| B. SLEV/TBH-28/Vero | ||||||||
|---|---|---|---|---|---|---|---|---|
| Antigex Dilution |
VSAA | NBR | VSAA | NBR | VSAA | NBR | VSAA | NBR |
| 0.01% BPL | 0.05% BPL | 0.1% BPL | Mock-treated | |||||
| 1:10 | 0.819 | 0.037 | 0.812 | 0.059 | 0.844 | 0.051 | 0.757 | 0.033 |
| 1:20 | 0.492 | 0.032 | 0.472 | 0.035 | 0.490 | 0.038 | 0.455 | 0.030 |
| 1:40 | 0.272 | 0.031 | 0.276 | 0.028 | 0.273 | 0.034 | 0.259 | 0.028 |
| 1:80 | 0.156 | 0.027 | 0.157 | 0.026 | 0.162 | 0.026 | 0.150 | 0.026 |
| 1:160 | 0.097 | 0.028 | 0.097 | 0.024 | 0.102 | 0.028 | 0.089 | 0.026 |
| 1:320 | 0.063 | 0.031 | 0.059 | 0.024 | 0.072 | 0.028 | 0.060 | 0.027 |
| 1mRAD | 3mRAD | 5mRAD | Untreated | |||||
| Neat | 2.454 | 0.068 | 1.823 | 0.078 | 1.563 | 0.066 | 2.267 | 0.034 |
| 1:2 | 2.432 | 0.045 | 1.880 | 0.073 | 1.600 | 0.085 | 2.108 | 0.018 |
| 1:4 | 2.022 | 0.046 | 1.531 | 0.054 | 1.346 | 0.063 | 1.691 | 0.015 |
| 1:8 | 1.335 | 0.038 | 1.026 | 0.034 | 0.868 | 0.038 | 1.201 | 0.016 |
| 1:16 | 0.722 | 0.031 | 0.568 | 0.023 | 0.443 | 0.021 | 0.634 | 0.013 |
| 1:32 | 0.387 | 0.029 | 0.283 | 0.018 | 0.221 | 0.017 | 0.292 | 0.012 |
| C. POWV/LB/Vero | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antigen Dilution |
VSAA | NBR | VSAA | NBR | VSAA | NBR | VSAA | NBR | VSAA | NBR |
| 0.01% BPL | 0.05% BPL | 0.1% BPL | Mock-Treated | Untreated | ||||||
| Neat | 2.113 | 0.025 | 1.949 | 0.031 | 2.366 | 0.116 | 2.325 | 0.022 | 2.824 | 0.027 |
| 1:2 | 1.907 | 0.021 | 2.045 | 0.027 | 2.112 | 0.053 | 2.070 | 0.017 | 2.606 | 0.023 |
| 1:4 | 1.660 | 0.018 | 1.801 | 0.018 | 1.863 | 0.029 | 1.684 | 0.015 | 2.252 | 0.023 |
| 1:8 | 1.317 | 0.016 | 1.575 | 0.018 | 1.573 | 0.023 | 1.366 | 0.015 | 1.614 | 0.025 |
| 1:16 | 1.143 | 0.017 | 1.286 | 0.016 | 1.285 | 0.021 | 1.006 | 0.014 | 1.165 | 0.022 |
| 1:32 | 0.762 | 0.015 | 0.763 | 0.017 | 0.778 | 0.020 | 0.748 | 0.016 | 0.873 | 0.023 |
| D. CHIKV/181-25/C6/36 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Antigen Dilution |
VSAA | NBR | VSAA | NBR | VSAA | NBR | VSAA | NBR |
| 0.1% BPL | 0.2% BPL | 0.3% BPL | Mock-treated | |||||
| 1:10 | 1.572 | 0.129 | 1.413 | 0.350 | 1.151 | 0.358 | 0.575 | 0.050 |
| 1:20 | 1.307 | 0.120 | 0.890 | 0.200 | 0.656 | 0.175 | 0.350 | 0.043 |
| 1:40 | 1.383 | 0.135 | 0.770 | 0.133 | 0.477 | 0.153 | 0.214 | 0.040 |
| 1:80 | 1.077 | 0.118 | 0.682 | 0.120 | 0.428 | 0.088 | 0.116 | 0.038 |
| 1:160 | 0.744 | 0.126 | 0.476 | 0.098 | 0.304 | 0.104 | 0.059 | 0.035 |
| 1:320 | 0.475 | 0.107 | 0.315 | 0.079 | 0.225 | 0.077 | 0.058 | 0.052 |
| 1mRAD | 3mRAD | 6mRAD | Untreated | |||||
| Neat | 2.007 | 0.047 | 2.255 | 0.057 | 2.447 | 0.141 | 2.154 | 0.049 |
| 1:2 | 1.498 | 0.036 | 1.872 | 0.044 | 2.574 | 0.096 | 1.944 | 0.045 |
| 1:4 | 0.977 | 0.036 | 1.352 | 0.044 | 2.521 | 0.088 | 1.427 | 0.040 |
| 1:8 | 0.782 | 0.037 | 1.081 | 0.044 | 2.259 | 0.086 | 1.151 | 0.040 |
| 1:16 | 0.705 | 0.042 | 0.994 | 0.053 | 1.916 | 0.135 | 1.145 | 0.045 |
| 1:32 | 0.735 | 0.049 | 0.904 | 0.066 | 1.538 | 0.119 | 1.020 | 0.052 |
| CHIKV/S27/BHK-21c.15 | ||||||||
| 1mRAD | 3mRAD | 5mRAD | Untreated | |||||
| Neat | 2.262 | 0.036 | 3.293 | 0.097 | 3.527 | 0.141 | 1.055 | 0.030 |
| 1:2 | 2.094 | 0.036 | 3.247 | 0.054 | 3.311 | 0.070 | 1.078 | 0.030 |
| 1:4 | 1.753 | 0.034 | 2.982 | 0.039 | 3.014 | 0.044 | 0.813 | 0.028 |
| 1:8 | 1.142 | 0.032 | 2.394 | 0.039 | 2.564 | 0.054 | 0.451 | 0.025 |
| 1:16 | 0.669 | 0.036 | 1.763 | 0.035 | 1.876 | 0.058 | 0.273 | 0.027 |
| 1:32 | 0.471 | 0.030 | 1.131 | 0.030 | 1.184 | 0.036 | 0.183 | 0.028 |
|
5.5mRAD + 0.01% BPL |
5.5mRAD + 0.05% BPL |
5.5mRAD + 0.1% BPL |
5.5mRAD only |
|||||
| 1:10 | 3.324 | 0.061 | 3.647 | 0.833 | 3.591 | 1.892 | 3.007 | 0.038 |
| 1:20 | 3.072 | 0.042 | 3.445 | 0.503 | 3.577 | 0.970 | 2.740 | 0.034 |
| 1:40 | 2.718 | 0.040 | 3.225 | 0.433 | 3.208 | 0.803 | 2.409 | 0.032 |
| 1:80 | 2.072 | 0.039 | 2.777 | 0.358 | 2.709 | 0.732 | 1.789 | 0.030 |
| 1:160 | 1.354 | 0.037 | 1.953 | 0.264 | 1.671 | 0.506 | 1.119 | 0.022 |
| 1:320 | 0.827 | 0.033 | 1.211 | 0.154 | 1.225 | 0.356 | 0.652 | 0.020 |
| E. MAYV/TR15537/BHK-21c.15 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Antigen Dilution |
VSAA | NBR | VSAA | NBR | VSAA | NBR | VSAA | NBR |
| 0.1% BPL | 0.2% BPL | 0.3% BPL | Mock-treated | |||||
| 1:4 | 1.868 | 0.035 | 1.245 | 0.079 | 0.670 | 0.151 | 1.619 | 0.028 |
| 1:8 | 1.265 | 0.031 | 0.924 | 0.046 | 0.466 | 0.057 | 0.906 | 0.027 |
| 1:16 | 0.804 | 0.032 | 0.716 | 0.037 | 0.338 | 0.048 | 0.511 | 0.030 |
| 1mRAD | 3mRAD | 6mRAD | Untreated | |||||
| 1:4 | 2.256 | 0.037 | 1.600 | 0.033 | 2.697 | 0.052 | 0.854 | 0.039 |
| 1:8 | 1.552 | 0.044 | 2.200 | 0.038 | 2.411 | 0.065 | 0.783 | 0.036 |
| 1:16 | 1.023 | 0.045 | 1.482 | 0.036 | 1.891 | 0.049 | 0.448 | 0.034 |
|
6mRAD + 0.01% BPL |
6mRAD + 0.05% BPL |
6mRAD + 0.1% BPL |
6mRAD only | |||||
| 1:10 | 2.865 | 0.188 | 3.195 | 0.591 | 3.276 | 0.918 | 2.865 | 0.085 |
| 1:20 | 2.370 | 0.146 | 2.926 | 0.294 | 3.019 | 0.485 | 2.278 | 0.063 |
| 1:40 | 1.962 | 0.129 | 2.475 | 0.269 | 2.467 | 0.305 | 1.681 | 0.055 |
| 1:80 | 1.400 | 0.106 | 1.835 | 0.207 | 1.767 | 0.261 | 1.214 | 0.050 |
| 1:160 | 0.921 | 0.083 | 1.186 | 0.161 | 1.121 | 0.201 | 0.782 | 0.044 |
| 1:320 | 0.561 | 0.074 | 0.698 | 0.120 | 0.648 | 0.164 | 0.492 | 0.040 |
| F. SINV/AUS MRM 39/Vero | ||||||||
|---|---|---|---|---|---|---|---|---|
| Antigen Dilution |
VSAA | NBR | VSAA | NBR | VSAA | NBR | VSAA | NBR |
| 0.1% BPL | 0.25% BPL | 0.3% BPL | Mock-treated | |||||
| 1:8 | 1.013 | 0.043 | 0.961 | 0.047 | 0.874 | 0.047 | 0.916 | 0.035 |
| 1:16 | 0.480 | 0.041 | 0.426 | 0.041 | 0.389 | 0.044 | 0.489 | 0.036 |
| 1:32 | 0.263 | 0.043 | 0.228 | 0.037 | 0.224 | 0.041 | 0.266 | 0.040 |
| 1mRAD | 3mRAD | 6mRAD | Untreated | |||||
| 1:4 | 2.235 | 0.053 | 2.551 | 0.050 | 2.870 | 0.056 | 1.682 | 0.044 |
| 1:8 | 1.183 | 0.052 | 1.572 | 0.050 | 1.873 | 0.058 | 0.922 | 0.043 |
| 1:16 | 0.690 | 0.052 | 0.761 | 0.049 | 0.925 | 0.055 | 0.487 | 0.037 |
| G. LACV/Original/BHK-21c.13 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antigen Dilution |
VSAA | NBR | VSAA | NBR | VSAA | NBR | VSAA | NBR | VSAA | NBR |
| 0.01% BPL | 0.05% BPL | 0.1% BPL | Mock-Treated | Untreated | ||||||
| 1:10 | 3.102 | 0.121 | 3.324 | 0.209 | 3.133 | 0.107 | 3.142 | 0.124 | 3.217 | 0.100 |
| 1:20 | 2.837 | 0.116 | 3.092 | 0.143 | 3.014 | 0.101 | 2.523 | 0.104 | 2.488 | 0.097 |
| 1:40 | 1.912 | 0.100 | 2.365 | 0.111 | 2.433 | 0.094 | 1.543 | 0.102 | 1.594 | 0.092 |
| 1:80 | 1.149 | 0.115 | 1.452 | 0.092 | 1.639 | 0.106 | 0.944 | 0.099 | 0.971 | 0.088 |
| 1:160 | 0.659 | 0.084 | 0.777 | 0.105 | 0.863 | 0.086 | 0.568 | 0.103 | 0.569 | 0.055 |
| 1:320 | 0.390 | 0.105 | 0.464 | 0.109 | 0.508 | 0.105 | 0.360 | 0.106 | 0.346 | 0.077 |
| H. JCV/MN256–260/BHK-21c.13 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antigen Dilution |
VSAA | NBR | VSAA | NBR | VSAA | NBR | VSAA | NBR | VSAA | NBR |
| 0.01% BPL | 0.05% BPL | 0.1% BPL | Mock-Treated | Untreated | ||||||
| Neat | 3.038 | 0.100 | 2.481 | 0.078 | 1.829 | 0.064 | 3.218 | 0.112 | 3.210 | 0.098 |
| 1:2 | 3.308 | 0.104 | 2.833 | 0.076 | 2.064 | 0.066 | 3.319 | 0.100 | 3.140 | 0.112 |
| 1:4 | 3.035 | 0.111 | 2.572 | 0.079 | 1.938 | 0.079 | 3.134 | 0.106 | 2.820 | 0.102 |
| 1:8 | 2.605 | 0.097 | 2.155 | 0.079 | 1.709 | 0.080 | 2.687 | 0.095 | 2.219 | 0.089 |
| 1:16 | 2.097 | 0.095 | 1.709 | 0.080 | 1.347 | 0.081 | 2.138 | 0.095 | 1.487 | 0.082 |
| 1:32 | 1.521 | 0.087 | 1.155 | 0.073 | 0.945 | 0.073 | 1.557 | 0.097 | 1.004 | 0.055 |
| I. TAHV/Bardos 92/BHK-21c.13 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antigen Dilution |
VSAA | NBR | VSAA | NBR | VSAA | NBR | VSAA | NBR | VSAA | NBR |
| 0.01% BPL | 0.05% BPL | 0.1% BPL | Mock-Treated | Untreated | ||||||
| 1:10 | 2.256 | 0.087 | 1.111 | 0.061 | 0.355 | 0.068 | 2.504 | 0.095 | 2.595 | 0.084 |
| 1:20 | 1.511 | 0.074 | 0.797 | 0.057 | 0.269 | 0.063 | 1.811 | 0.083 | 1.872 | 0.074 |
| 1:40 | 1.132 | 0.081 | 0.553 | 0.059 | 0.245 | 0.063 | 1.202 | 0.071 | 1.238 | 0.067 |
| 1:80 | 0.685 | 0.075 | 0.391 | 0.059 | 0.194 | 0.075 | 0.712 | 0.064 | 0.767 | 0.061 |
| 1:160 | 0.414 | 0.076 | 0.260 | 0.066 | 0.126 | 0.069 | 0.446 | 0.069 | 0.452 | 0.055 |
| 1:320 | 0.260 | 0.072 | 0.170 | 0.072 | 0.127 | 0.074 | 0.268 | 0.070 | 0.270 | 0.051 |
Virus name/strain/cell culture type.
Underlined OD readings indicate the working antigen dilution of untreated or mock-treated, infectious antigen.
OD readings in bold indicate the dilution of the unconcentrated antigen that yielded acceptable MAC ELISA results; upon concentration, the antigen yielded acceptable MAC ELISA and infectivity assay results. Shaded boxes indicate the inactivation condition(s) used for final antigen production.
OD readings in italics indicate the dilution of the unconcentrated antigen that yielded acceptable MAC ELISA results; however, the preparation had unacceptable NBR and/or was infectious before or after concentration.
Abbreviations: VSAA – virus-specific antigen activity; NBR – nonspecific background reactivity.
3.1.1. Flaviviruses
Yellow Fever Virus
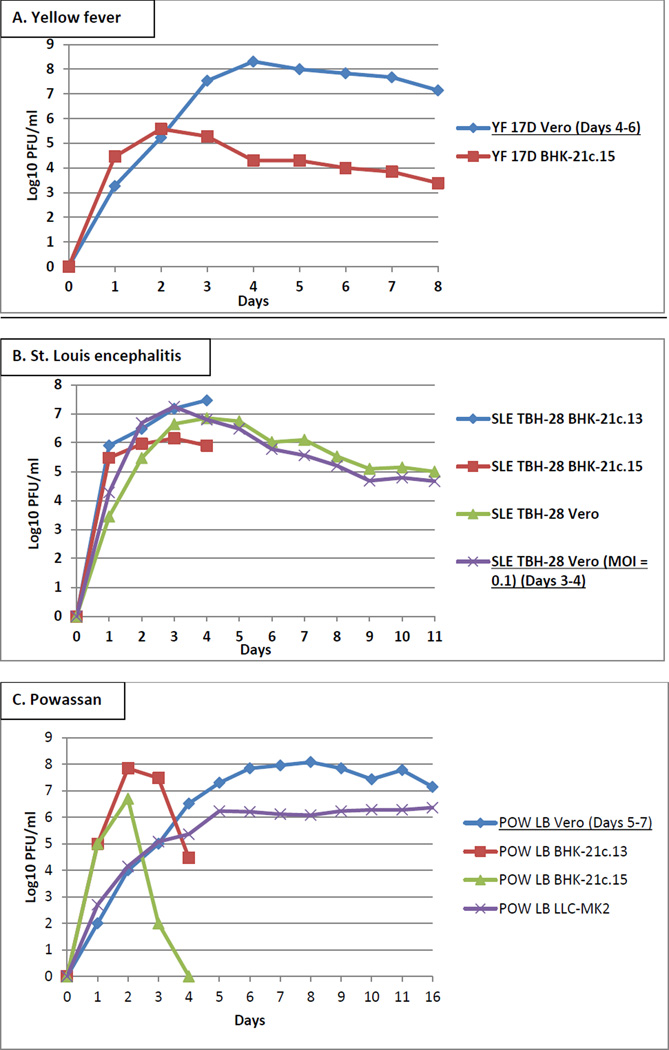
YFV strain 17D was inoculated into Vero and BHK-21c.15 cells at an MOI of 0.01 and incubated for 8 days. YFV grown in Vero cells had the highest titer of 8.3 log10 PFU/ml at day 4 (Figure 1A). The second batch of YFV was grown in Vero cells and supernatant was replaced and harvested on days 4, 5, and 6 and the three collections were combined into one batch. The VSAA and NBR of YFV were acceptable and the working antigen dilution was 1:32 (Table 1A, untreated).
Figure 1.
Growth of (A) YFV, (B) SLEV, (C) POWV, (D) CHIKV, (E) MAYV, (F) SINV, (G) LACV, (H) JCV, and (I) TAHV in Vero, BHK-21, LLC-MK2, and C6/36 cells. T-150 cm2 flasks were inoculated with MOIs ranging from 0.0005–0.1 and incubated until CPE was visible, for up to 16 days. Underlined conditions indicate the optimal virus strain, cell type, and harvest day based on virus titer and/or performance in the MAC-ELISA.
St. Louis Encephalitis virus
SLEV strain TBH-28 was inoculated at an MOI of 0.01 into Vero, BHK-21c.13, and BHK-21c.15 cells, and at an MOI of 0.1 into Vero cells. BHK-21c.13 and BHK-21.15 cells were incubated for 4 days and Vero cells were incubated for 11 days. Titers of approximately 7 log10 PFU/ml were similar in Vero (MOI = 0.1) and BHK-21c.13 cells at day 3 (Figure 1B). Vero cells (MOI = 0.1) were selected for second batch production, and supernatant was replaced and harvested on days 3 and 4 and combined into one batch. The VSAA and NBR of SLEV were acceptable and the working antigen dilution was 1:8 (Table 1B, untreated).
Powassan virus
POWV strain LB was inoculated at an MOI of 0.01 and incubated for 16 days in Vero and LLC-MK2 cells and for 4 days in BHK-21c.13 and BHK-21c.15 cells. Peak titers of approximately 8 log10 PFU/ml were similar in Vero cells at days 6–9 and in BHK-21c.13 cells at day 2 (Figure 1C). Time point aliquots were also tested by the POWV MAC-ELISA to determine peak VSAA. POWV produced from Vero cells and collected on day 6 yielded higher VSAA compared to those obtained from BHK-21c.13, BHK-21c.15, and LLC-MK2 cells (working antigen dilutions of 1:32, unusable, unusable, and 1:8, respectively), and was therefore selected for second batch production, with supernatant replaced and harvested on days 5, 6, and 7 and combined into one batch. The VSAA and NBR were acceptable and the working antigen dilution was 1:16 (Table 1C, untreated).
3.1.2. Alphaviruses
Chikungunya virus
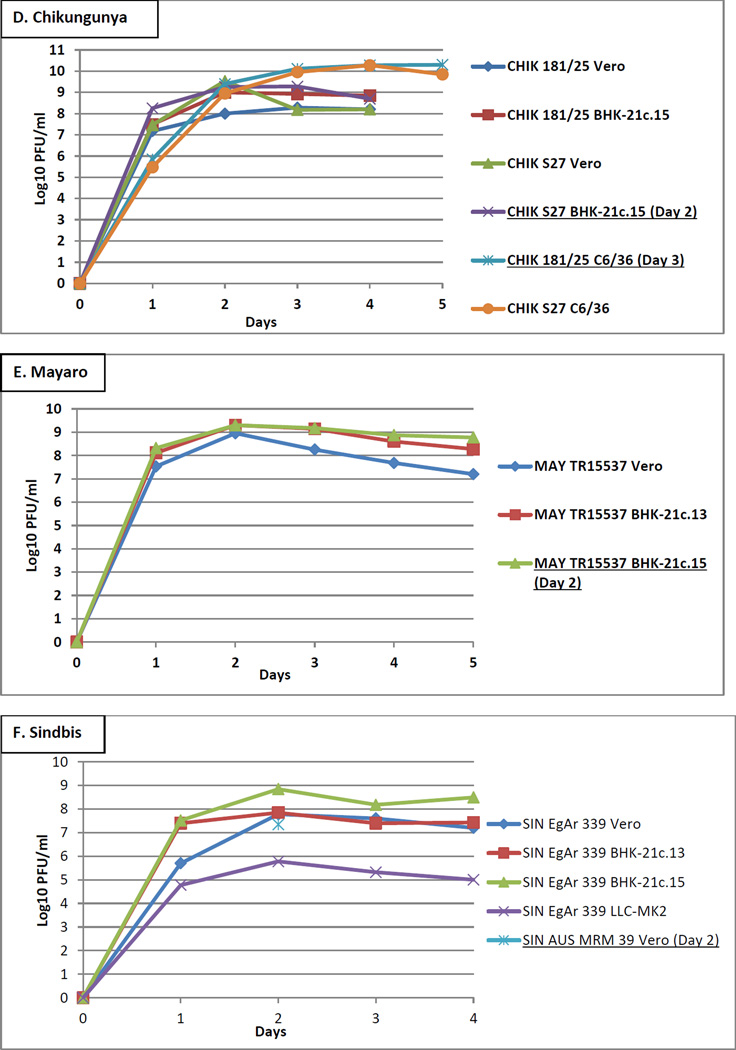
CHIKV strains 181/25 and S27 were inoculated into Vero and BHK-21c.15 cells at an MOI of 0.001 and incubated for 4 days, and into C6/36 cells at an inadvertent MOI of 0.0005 and incubated for 5 days. Peak titers were between 8 and 10 log10 PFU/ml in all cell types (Figure 1D). Time point aliquots tested by the CHIKV MAC-ELISA showed variation in the VSAAs, which did not correspond to the titers. Representative CHIKV titer and MAC-ELISA results are shown in Table 2. CHIKV strain 181/25 grown in C6/36 cells and CHIKV strain S27 grown in BHK-21c.15 cells were the only two preparations to yield both acceptable VSAA and NBR; other CHIKV strain/cell type combinations yielded VSAA below the acceptable range, despite the high titers (Figure 1D). CHIKV strains 181/25 and S27 were both grown in C6/36 cells and had equivalent titers of 10.3 log10 PFU/ml, but only strain 181/25 had acceptable VSAA. CHIKV strain S27 grown in Vero and BHK-21c.15 cells had nearly equivalent titers of 9.4 and 9.3 log10 PFU/ml, respectively; however, only strain S27 grown in BHK-21c.15 cells had acceptable VSAA. Second batches were produced from CHIKV strain 181/25 grown in C6/36 cells and harvested on day 3 and CHIKV strain S27 grown in BHK-21c.15 cells and harvested on day 2: the VSAAs and NBRs were acceptable and the working antigen dilutions were 1:32 and 1:4, respectively (Table 1D, untreated).
Table 2.
Titer and antigen reactivity in MAC ELISA of CHIKV Strains 181/25 and S27 grown in C6/36, Vero, and BHK-21c.15 cells
|
Antigen Dilution |
CHIKV - C6/36 cells | CHIKV - strain S27 | ||||||
|---|---|---|---|---|---|---|---|---|
| Strain 181/25 10.3 log10 PFU/ml |
Strain S27 10.3 log10 PFU/ml |
Vero cells 9.4 log10 PFU/ml |
BHK-21c.15 cells9.3 log10 PFU/ml |
|||||
| VSAA | NBR | VSAA | NBR | VSAA | NBR | VSAA | NBR | |
| Neat | 1.144 | 0.032 | 0.263 | 0.010 | 0.125 | 0.020 | 1.116 | 0.017 |
| 1:2 | 0.956 | 0.010 | 0.220 | 0.007 | 0.071 | 0.014 | 1.220 | 0.014 |
| 1:4 | 0.818* | 0.011 | 0.149 | 0.009 | 0.038 | 0.012 | 0.878 | 0.013 |
| 1:8 | 0.646 | 0.015 | 0.102 | 0.027 | 0.026 | 0.010 | 0.512 | 0.009 |
| 1:16 | 0.613 | 0.043 | 0.092 | 0.036 | 0.022 | 0.013 | 0.303 | 0.012 |
| 1:32 | 0.528 | 0.025 | 0.054 | 0.033 | 0.020 | 0.012 | 0.150 | 0.009 |
Underlined OD readings indicate the working antigen dilution of untreated, infectious antigen.
Abbreviations: VSAA – virus-specific antigen activity; NBR – nonspecific background reactivity.
Mayaro virus
MAYV strain TR15537 was inoculated into Vero, BHK-21.c13, and BHK-21c.15 cells at an MOI of 0.001 and incubated for 5 days. Peak titers were approximately equivalent at 9 log10 PFU/ml in all cell types at day 2 (Figure 1E). Aliquots were also tested by the MAYV MAC-ELISA. MAYV grown in BHK-21c.15 cells and collected on day 2 yielded higher VSAA over those obtained from Vero and BHK-21c.13 cells (working antigen dilutions of 1:8, neat, and 1:2, respectively), and was therefore selected for production of the second batch. MAYV harvested on day 2 from BHK-21c.15 cells had acceptable VSAA and NBR and yielded a working antigen dilution of 1:4 (Table 1E, untreated).
Sindbis virus
SINV strain EgAr 339 was inoculated into Vero, BHK-21c.13, BHK-21c.15, and LLC-MK2 cells at an MOI of 0.001 and incubated for 4 days. Peak titers were >7 log10 PFU/ml by day 2 in all cell types except LLC-MK2 (Figure 1F). However, none of the aliquots tested by the SINV MAC-ELISA yielded acceptable VSAAs (data not shown).
Therefore, additional SINV strains (16260, 80–2449, AUS C 263, AUS C 377, AUS MRM 39, INDA 1036, MAL AMM 2215, Michalovce, Reed Warbler, SAAR 86, and UGMP 684) were inoculated into Vero, BHK-21c.13, and BHK-21c.15 cells at an MOI of 0.001 and supernatant was collected only on day 2. In this instance, the day 2 aliquots were first tested by the SINV MAC-ELISA to determine if any strain yielded acceptable VSAA. SINV strain AUS MRM 39 produced from Vero and BHK-21c.13 cells were the only two preparations to yield acceptable VSAAs (working antigen dilutions of 1:10 for both). Subsequently, titer was determined only for the day 2 aliquot of SINV strain AUS MRM 39 produced from Vero cells (Figure 1F), and SINV strain AUS MRM 39 was selected for second batch production. SINV strain AUS MRM 39 harvested on day 2 from Vero cells had acceptable VSAA and NBR and yielded a working antigen dilution of 1:8 (Table 1F, untreated).
3.1.3. Bunyaviruses
La Crosse virus
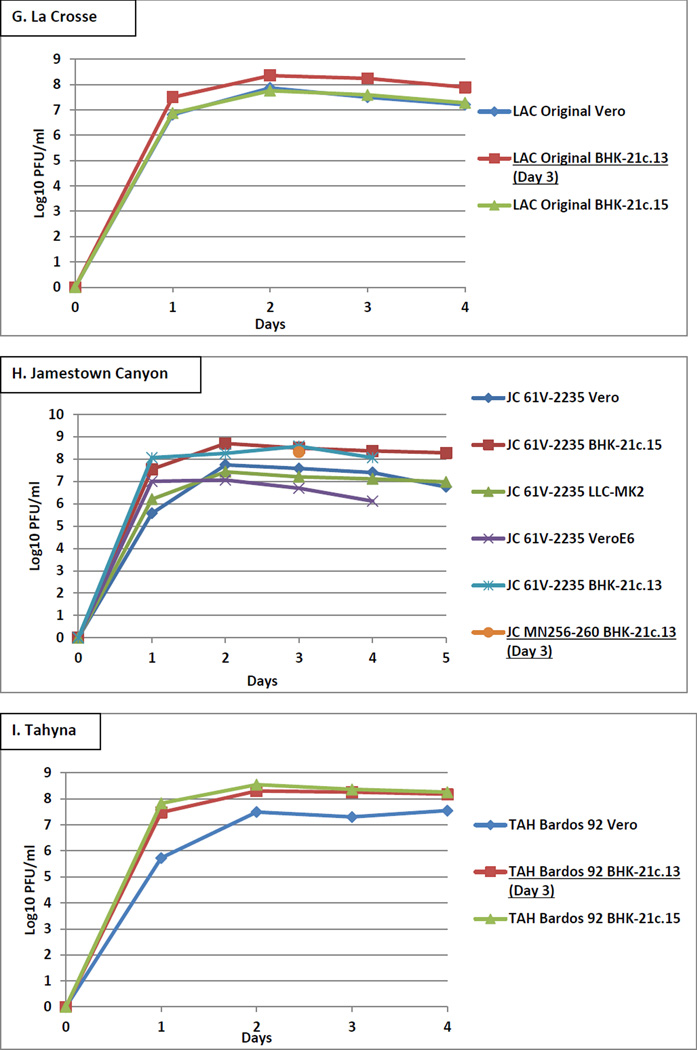
LACV strain Original was inoculated into Vero, BHK-21.c13, and BHK-21c.15 cells at an MOI of 0.01 and incubated for 4 days. Peak titers were approximately equivalent at 8 log10 PFU/ml at day 2 (Figure 1G). All time point aliquots were tested by the LACV MAC-ELISA. LACV grown in BHK-21c.13 cells and collected on day 3 yielded higher VSAA over those obtained from Vero and BHK-21c.15 cells (working antigen dilutions of 1:160, 1:4, and 1:40, respectively), and was therefore selected for production of the second batch. LACV grown in BHK-21c.13 cells and harvested on day 3 had acceptable VSAA and NBR and yielded a working antigen dilution of 1:80 (Table 1G, untreated).
Jamestown Canyon virus
JCV strain 61V-2235 was inoculated at an MOI of 0.01 and incubated for 4 days in BHK-21c.13 and Vero E6 cells and for 5 days in Vero, BHK-21c.15, and LLC-MK2 cells. Additionally, JCV strain MN256–260 was inoculated into BHK-21c.13 cells at an MOI of 0.01 and supernatant was collected only on day 3. Peak titers ranged from 7–9 log10 PFU/ml at days 2–3 (Figure 1H). Time point aliquots were also tested by the JCV MAC-ELISA. JCV strain MN256–260 grown in BHK-21c.13 cells and collected on day 3 yielded higher VSAA over JCV strain 61V-2235 obtained from Vero, Vero E6, BHK-21c.13, BHK-21c.15, and LLC-MK2 cells (working antigen dilutions of 1:32, unusable, unusable, 1:16, 1:8, and unusable, respectively), and was therefore selected for production of the second batch. JCV strain MN256–260 harvested on day 3 from BHK-21c.13 cells had acceptable VSAA and NBR and yielded a working antigen dilution of 1:32 (Table 1H, untreated).
Tahyna virus
TAHV strain Bardos 92 was inoculated into Vero, BHK-21.c13, and BHK-21c.15 cells at an MOI of 0.01 and incubated for 4 days. Peak titers ranged from 7.5–8.5 log10 PFU/ml at day 2 (Figure 1I). Time point aliquots were also tested by the TAHV MAC-ELISA. TAHV grown in BHK-21c.13 cells and collected on day 3 yielded higher VSAA over those obtained from Vero and BHK-21c.15 cells (working antigen dilutions of >1:32, 1:4, and 1:32, respectively), and was therefore selected for production of the second batch. TAHV harvested on day 3 from BHK-21c.13 cells had acceptable VSAA and NBR and yielded a working antigen dilution of 1:40 (Table 1I, untreated).
3.2. Virus inactivation
Five inactivation methods were evaluated. Viruses were successfully inactivated by BPL, gamma-irradiation, or a combination of gamma-irradiation followed by BPL. YFV, SLEV, POWV, LACV, JCV, and TAHV were treated with 0.01, 0.025, 0.05, 0.075, and 0.1% BPL, and CHIKV, MAYV, and SINV were treated with 0.1, 0.15, 0.2, 0.25, and 0.3% BPL. YFV, SLEV, and CHIKV strain S27 were irradiated with 1–5 Mrad, and CHIKV strain 181/25, MAYV, and SINV were irradiated with 1–6 Mrad. For the combination gamma-irradiation/BPL treatments, CHIKV strain S27 and MAYV strain TR15537 were irradiated with 5.5 and 6 Mrad, respectively, concentrated, and then treated with 0.01, 0.025, 0.05, 0.075, and 0.1% BPL. Representative results are shown in Table 1.
Inactivated antigen was evaluated in the MAC-ELISA, and rated as acceptable or unacceptable based on VSAA and NBR. Plaque titration and passage three times in cell culture was used to rule out residual infectivity. The chosen inactivation method was the one that completely inactivated the virus, had VSAA and NBR within the acceptable ranges, and had the highest working antigen dilution compared to the mock-treated or untreated virus. Inactivated virus was then concentrated to a standardized working dilution of ∼1:160 and re-evaluated in the MAC-ELISA and infectivity assays.
3.2.1. Yellow fever virus
Unconcentrated YFV treated with ≤0.05% BPL yielded acceptable MAC-ELISA results (Table 1A), but was infectious at 0.01% BPL. In addition, after concentration, the NBR of antigen treated with 0.025 and 0.05% BPL rose above the acceptable limit. Unconcentrated YFV treated with gamma-irradiation also yielded acceptable MAC-ELISA results, but was infectious at ≤4 Mrad. Concentrated antigen inactivated with 5 Mrad had acceptable MAC-ELISA results, was non-infectious, and was therefore selected as the inactivation method for final, scaled-up YFV antigen production.
3.2.2. St. Louis encephalitis virus
Unconcentrated SLEV treated with ≤0.1% BPL yielded acceptable MAC-ELISA results (Table 1B); however, antigen treated with 0.01% BPL was infectious. Concentrated antigen inactivated with ≥0.05% BPL had acceptable MAC-ELISA results and remained non-infectious. Unconcentrated SLEV treated with gamma-irradiation yielded acceptable MAC-ELISA results, but was infectious at ≤4 Mrad. Concentrated antigen inactivated with 5 Mrad had acceptable MAC-ELISA results and was non-infectious. Due to the ease of use of BPL over gamma-irradiation, inactivation with 0.05% BPL was selected for final, scaled-up SLEV antigen production.
3.2.3. Powassan virus
Unconcentrated POWV treated with ≤0.1% BPL yielded acceptable MAC-ELISA results (Table 1C), but was infectious at 0.01% BPL. Concentrated antigen inactivated with ≥0.05% BPL had acceptable MAC-ELISA results and remained non-infectious. Inactivation with 0.05% BPL was selected for final, scaled-up POWV antigen production.
3.2.4. Chikungunya virus
The VSAA of unconcentrated CHIKV strain 181/25 increased after BPL treatment compared to mock-treated virus, which had a VSAA below the acceptable limit (Table 1D). The greatest increase was after 0.1% BPL treatment, with the VSAA decreasing as the BPL concentration increased to 0.3%. All BPL concentrations completely inactivated the virus; however, after concentration, the NBRs rose above the acceptable limit. The VSAA of unconcentrated CHIKV strain 181/25 treated with ≥4 Mrad also increased compared to the untreated virus and yielded acceptable MAC-ELISA results, but all gamma-irradiated samples were infectious.
VSAAs of unconcentrated CHIKV strain S27 increased as gamma irradiation doses increased (Table 1D), but similar to CHIKV strain 181/25, all samples were infectious. As neither BPL nor gamma-irradiation treatment alone completely inactivated CHIKV, CHIKV strain S27 was treated with a combination of gamma-irradiation followed by BPL. CHIKV strain S27 was irradiated with 5.5 Mrad, concentrated, and then treated with ≤0.1% BPL (Table 1D). The combination treatment of 5.5 Mrad plus ≤0.05% BPL produced acceptable MAC-ELISA results and was non-infectious; therefore, inactivation with 5.5 Mrad + 0.01% BPL (the lowest dose) was selected for final, scaled-up CHIKV antigen production.
3.2.5. Mayaro virus
Unconcentrated MAYV treated with ≤0.2% BPL yielded acceptable MAC-ELISA results (Table 1E) and was non-infectious. Similar to CHIKV, the VSAA of MAYV increased after 0.1% BPL treatment compared to mock-treated MAYV and decreased as the BPL concentration increased to 0.3%. Concentrated antigen treated with ≥ 0.15% BPL produced acceptable MAC-ELISA results and was non-infectious, but at 0.1% BPL remained infectious. Unconcentrated MAYV treated with gamma-irradiation also yielded acceptable MAC-ELISA results, with increased VSAA as Mrads increased, but was infectious at all doses. MAYV was inactivated by a combination of gamma-irradiation followed by BPL. Unconcentrated MAYV was irradiated with 6 Mrad, concentrated, and then treated with ≤0.1% BPL (Table 1E), with 6 Mrad and ≤0.05% BPL yielding acceptable MAC-ELISA and non-infectivity results.
Scale-up production of MAYV antigen with inactivation by 6 Mrad + 0.01% BPL was performed prior to inactivation with BPL. However, due to the ease of use of BPL over the gamma-irradiation/BPL combination, inactivation with 0.15 or 0.2% BPL could be used for future MAYV antigen production.
3.2.6. Sindbis virus
Unconcentrated SINV treated with ≤0.3% BPL yielded acceptable MAC-ELISA results (Table 1F), and was non-infectious. Concentrated antigen treated with ≥0.15% BPL produced acceptable MAC-ELISA results and remained non-infectious, but at 0.1% BPL remained infectious. Unconcentrated SINV treated with gamma-irradiation also yielded acceptable MAC-ELISA results, with VSAA increasing as Mrads increased, but was infectious at ≤5 Mrad. Concentrated antigen inactivated with 6 Mrad produced acceptable MAC-ELISA results and remained non-infectious. Due to the ease of use of BPL over gamma-irradiation, inactivation with 0.25% BPL was selected for final, scaled-up SINV antigen production.
3.2.7. La Crosse virus antigen
Unconcentrated and concentrated LACV antigen treated with ≤0.1% BPL yielded acceptable MAC-ELISA results (Table 1G), and was non-infectious. Additionally, VSAA increased after BPL treatment compared to mock-treated virus, and increased as the BPL concentration increased. Treatment with 0.1% BPL was selected for final, scaled-up LACV antigen production.
3.2.8. Jamestown Canyon virus antigen
Unconcentrated and concentrated JCV antigen treated with ≤0.1% BPL yielded acceptable MAC-ELISA results (Table 1H), and was non-infectious. Even though concentrated 0.01% BPL-treated JCV material produced the highest VSAA and remained non-infectious during small-scale production, 0.05% BPL was selected for final, scaled-up JCV antigen production to assure that the scale-up material was completely inactivated, as 0.01% BPL had been shown to be ineffective at inactivating the flaviviruses. Also, the working antigen dilution could be adjusted by the concentration factor.
3.2.9. Tahyna virus antigen
Unconcentrated and concentrated TAHV treated with ≤0.05% BPL yielded acceptable MAC-ELISA results (Table 1I), and was non-infectious. Similar to JCV, concentrated 0.01% BPL-treated TAHV material produced the highest VSAA and remained non-infectious during small-scale production, but 0.05% BPL was selected for final, scaled-up TAHV antigen production to assure complete inactivation.
3.2.10. Virus inactivation with formalin, hydrogen peroxide, and sarkosyl
YFV was treated with formalin at final concentrations ranging from 0.05% to 0.3%. The MAC-ELISA results were acceptable, but YFV was infectious at all formalin concentrations (data not shown).
YFV, SLEV, POWV, CHIKV, LACV, and JCV were treated with hydrogen peroxide at a final concentration of 3%. The VSAA decreased and was unacceptable in the MAC-ELISA compared to untreated virus (data not shown). In addition, infectivity assays of the treated material could not be conducted, as the residual hydrogen peroxide was toxic to the Vero cells used in both assays (data not shown).
YFV, SLEV, MAYV, and LACV treated with sarkosyl at final concentrations of 0.1% to 0.3% resulted in unacceptably low VSAA in the MAC-ELISA, and residual infectivity at all sarkosyl concentrations (data not shown).
3.2.11. Hydrolysis of BPL
Aliquots of SLEV and CHIKV treated with final concentrations of 0.05 and 0.3% BPL were incubated to facilitate the complete hydrolysis of BPL. Hydrolyzed versus non-hydrolyzed SLEV samples had similar VSAA and NBR in the MAC-ELISA, and both conditions generated usable working SLEV antigen dilutions at 1:10 (Table 3). In contrast, the VSAA of CHIKV decreased from 1:32 to 1:16 following hydrolysis, although the NBR decreased as well.
Table 3.
Comparison of antigen reactivity in MAC ELISA of BPL-treated SLEV and CHIKV: with or without hydrolysis treatment
|
Antigen Dilution |
SLEV - 0.05% BPL | |||
|---|---|---|---|---|
| Hydrolysis | No Hydrolysis | |||
| VSAA | NBR | VSAA | NBR | |
| 1:10 | 0.852 | 0.050 | 0.934 | 0.048 |
| 1:20 | 0.613 | 0.048 | 0.744 | 0.044 |
| 1:40 | 0.401 | 0.046 | 0.499 | 0.047 |
| CHIKV - 0.3% BPL | ||||
| 1:8 | 1.472 | 0.156 | 1.942 | 0.512 |
| 1:16 | 0.801 | 0.096 | 1.329 | 0.274 |
| 1:32 | 0.477 | 0.071 | 0.740 | 0.145 |
Abbreviations: VSAA – virus-specific antigen activity; NBR – nonspecific background reactivity.
3.3. Antigen Concentration
In order to evaluate the best method to concentrate antigen to the standardized working dilution of ∼1:160, 15 ml of gamma-irradiated YFV antigen was concentrated 10X and 35 ml of BPL-inactivated SLEV antigen was concentrated 20X by ultracentrifugation or in centrifugal filters. Following ultracentrifugation, all of the supernatant was removed and the antigen pellet was resuspended in 1.5 ml (YFV) or 1.8 ml (SLEV) final volume of either 0.1M trizma/BS buffer or 1X PBS. During the centrifugal filter method, the cell culture supernatant was centrifuged until the supernatant volume remaining in the filter reached approximately the final volume needed to achieve the desired concentration factor. The volume was then adjusted to 1.5 ml (YFV) or 1.8 ml (SLEV) with 0.1M trizma/BS buffer or 1X PBS. The concentration method that resulted in the highest working antigen dilution with both VSAA and NBR in the acceptable ranges was selected for future use (Figure 2).
Figure 2.
Comparison of ultracentrifugation and centrifugal filter concentration methods. (A) Yellow fever virus inactivated by gamma-irradiation was concentrated 10X; (B) St. Louis Encephalitis Virus inactivated with BPL was concentrated 20X.
YFV antigen concentrated 10X by ultracentrifugation and resuspended in 0.1M trizma/BS buffer or 1X PBS yielded working antigen dilutions of 1:40 and 1:20, respectively; in contrast, concentration by centrifugal filters yielded working antigen dilutions of 1:160 and 1:80, respectively. SLEV antigen concentrated 20X by ultracentrifugation and resuspended in 0.1M trizma/BS buffer or 1X PBS yielded working antigen dilutions of 1:40 and 1:10, respectively, whereas centrifugal filters yielded working antigen dilutions of 1:80 in both buffers. All methods and buffers resulted in acceptable NBR. The centrifugal filter method with volume adjustments using 0.1M trizma/BS buffer yielded the highest concentrated product. The remaining antigens were then concentrated 5X to 30X by centrifugal filters to obtain a standard working antigen dilution of ∼1:160.
4. Discussion
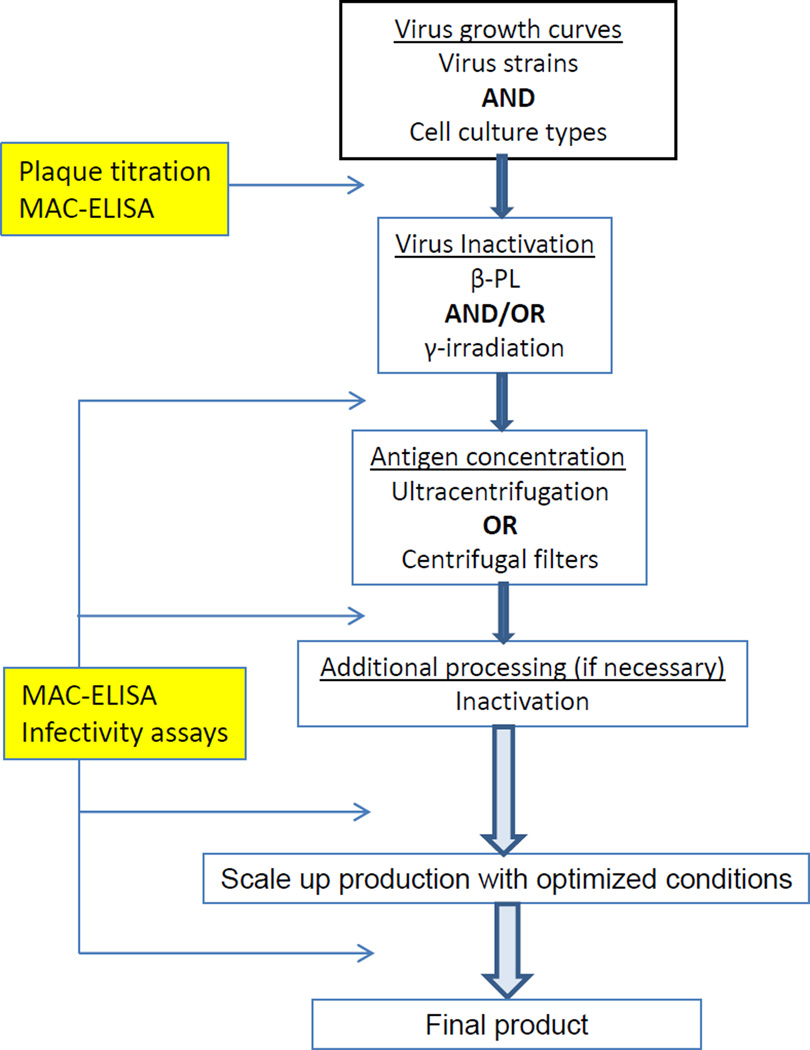
CDC DVBD ADB produces and maintains an inventory of the many arbovirus reference antigens used in serological assays. Cell culture has largely replaced the use of animals, and inactivation and concentration methods needed to be modified and optimized for cell culture. The antigen production process and evaluations of nine arbovirus antigens used in the CDC MAC-ELISA, the end-use assay for this study, were described here. There were three each from Flaviviridae, Togaviridae, and Bunyaviridae, and they were produced based on a prioritized list set by the diagnostic laboratory in the following order: SLEV, YFV, CHIKV, JCV, POWV, LACV, TAHV, MAYV, and SINV. Production of each antigen proceeded one at a time, from growth curves to finished product, according to the algorithm illustrated in Figure 3. During the development of each antigen, modifications were made to the methods and the procedure was optimized based on previous antigen production results and observations, at times necessitating going back and changing methods and repeating evaluations. Although there were trends, and in some cases one method was clearly superior to another for all the antigen preparations, such as concentrating the antigen using centrifugal filters, in general, the production method needed to be optimized for each virus.
Figure 3.
Arbovirus antigen production algorithm.
Additionally, antigens were produced in the most inexpensive and time-efficient manner as possible, so that if BPL treatment produced an acceptable antigen, gamma-irradiation was generally not pursued as a treatment option due to the expense and hands-on time this method required. SLEV, MAYV, and SINV were the exceptions in that both BPL and gamma-irradiation treatments were performed, even though acceptable antigens were produced by BPL. SLEV was one of the first antigens produced, and was included in the batch of YFV and CHIKV samples that were gamma-irradiated out of necessity. MAYV and SINV were preemptively sent for gamma irradiation treatment because these alphaviruses were assumed to behave similarly to CHIKV.
The first step in the production process was to conduct growth curves to determine the best virus strain and cell type to use. A second small batch was then produced under the optimal conditions and used for inactivation and concentration analyses. Once the inactivation and concentration procedure was finalized, a large batch of 2 to 10 T150 cm2 flasks was produced, yielding 100–500 vials of antigen.
Initially, titer was the performance indicator during the growth curve analyses and the conditions that resulted in the highest titer were assumed to also yield the highest VSAA in the MAC-ELISA. However, some inactivated viruses with high titers had low VSAA. In order to determine if the antigen degradation was caused by the inactivation procedure, the VSAA of the live virus was compared to the inactivated antigen by MAC-ELISA. In some cases the VSAA was unacceptably low for both the live and inactivated virus, and the highest titers did not necessarily correspond to the highest VSAA. Notably in CHIKV antigen production, the third antigen in the sequence, strains 181/25 and S27 both had titers of 10.3 log10 PFU/ml in C6/36 cells, but strain 181/25 had acceptable VSAA at a working antigen dilution of 1:4, whereas the VSAA of CHIKV strain S27 was unacceptable at any dilution (Table 2). Thereafter, viral growth curve samples were titrated but also evaluated by MAC-ELISA, and the MAC-ELISA became the primary performance indicator to evaluate antigen activity at every step in the production process, including the live virus.
The differences in antigen activity between the two CHIKV strains described above also highlight the importance of evaluating a variety of virus strains during the initial growth curve evaluation. The prototype strain was generally used during SMB antigen production, and this strain was assumed to make the best, most reactive antigen in cell culture as well. However, as was demonstrated with JCV and SINV antigen production, the prototype strains were not as reactive as some of the non-prototype strains from the reference collection. Only SINV strain AUS MRM 39, a non-prototype Australian isolate, reacted with the SINV IgM positive control. However, a caveat to this evaluation is that DVBD does not have a reference human anti-SINV IgM positive control serum. Instead, an alphavirus-group reactive mouse/human chimeric monoclonal antibody (cMAb) was used, which may have affected the reactivity of the various SINV strains tested (Thibodeaux et al., 2011). A SINV IgM-specific positive control from a natural human infection may produce different results. Additionally, there were limited positive control reference sera with which to evaluate the other antigens as well. Sera from a variety of geographical regions would be needed to determine if the difference in reactivity was due to the virus strains.
The cell type the viruses were cultured in also affected titer and antigen reactivity (Figure 1 and Table 2). Vero cells secrete cell contact inhibition factors which slow cell division and maintain the cell monolayer for 7–10 days. (Earley, Johnson, 1988). Thus Vero cells are the preferred cell type to culture the slow-growing flaviviruses. Arboviruses such as alphaviruses and bunyaviruses, with higher replication rates, are cultured efficiently in BHK-21 cells during the exponential growth phase, before the BHK-21 cells reach confluency, overgrow, and the cell sheet breaks up. Generally, arboviruses grow well in mosquito C6/36 cells, in which they become persistently infected with very little, if any, observable CPE. However, the VSAA from supernatant collected from C6/36 cell culture on multiple days was shown to be inconsistent and the NBR increased over time. Therefore, C6/36 cells were chosen only if the resulting antigen activity was clearly superior to that of the other cell types.
As the growth curves in Figure 1 show, the flaviviruses grew to high titers in Vero cells for a longer period of time than in BHK-21 cells, allowing for 2–3 collections of supernatant, which increased virus yield. Bunyaviruses replicated equally well in Vero and BHK-21 cells, but the VSAAs were higher for bunyaviruses grown in BHK-21c.13 cells. Alphaviruses also grew to high titers in both Vero and BHK-21 cell culture; however there was considerable variability between the VSAAs of the 3 alphaviruses in the different cell types. For example, CHIKV strain S27 cultured in BHK-21c.15 cells produced acceptable VSAA at a working antigen dilution of 1:4, whereas CHIKV S27 grown in Vero cells was unreactive (Table 2). These results illustrate the importance of conducting growth curves in a variety of cells to determine the optimal cell type to use in antigen production.
Chemical compounds and radiation have been used to destroy the ability of viruses to infect cells, including BPL, formalin, hydrogen peroxide, sarkosyl, aziridine compounds, ultraviolet light, and gamma irradiation (Amanna et al., 2012; Brand, Allen, 1970; Brown, 2001; Hearn, Dawson, 1961; Hiatt, 1964; Nims et al., 2011; Piret et al., 2002b). For inactivated viral vaccines and antigens used as reagents in immunoassays, it is also essential that antigenic reactivity is preserved (Sabin, 1943). Because large volumes of over 50 different antigens are produced at DVBD, virus inactivation methods were needed that required little downstream processing such as purification or removal of residue. The methods also needed to be effective to inactivate large volumes of live virus, as antigen in this procedure is concentrated to a standard working dilution after inactivation, and not before, as in other applications.
BPL has been used for inactivation of bacteria, fungi, and viruses, as well as disinfection and sterilization, and has been widely used to inactivate viral vaccines and antigens used in serological assays (French, McKinney, 1964). BPL is an alkylating agent that modifies the structure of nucleic acids and proteins, and can cause DNA-protein cross-linking (Bonnafous et al., 2014; Lawrence, 2000; Uittenbogaard et al., 2011). The efficiency of BPL is dose-specific and specimen-dependent, as accessibility for BPL molecules is related to a diffusion gradient across the viral membrane (French, McKinney, 1964). High BPL concentrations are required to reach the most buried parts of the virus, and differences in BPL diffusion within the virus may in part explain the differences reported in the literature on the BPL concentration required to inactivate a specific virus. Described as the “tailing phenomenon,” the bulk of the virus is rapidly inactivated by a small concentration of BPL, but a disproportionately larger concentration is required to inactivate the residual active virus (French, McKinney, 1964; Logrippo, 1960). Although BPL efficiency depends on the corresponding alterations of the viral structures and higher BLP concentrations will completely inactivate the virus, over-inactivation by BPL can modify viral proteins, resulting in loss or increase of antigenicity (Bonnafous et al., 2014; French, McKinney, 1964; Uittenbogaard et al., 2011).
DVBD ADB has used BPL to inactivate viruses because it is inexpensive and easy to obtain and use. In addition, BPL hydrolyzes in aqueous solution; its activity is self-limiting and there is no residual BPL that needs to be removed. However, considerable batch-to-batch inconsistency following inactivation by BPL was previously observed. Complete batches had been rendered unusable as the antigen appeared to be degraded, resulting in a loss of VSAA, or the NBR rose above the acceptable limit. Because of this, the optimal virus inactivation method was determined empirically with a range of BPL concentrations. Finding the correct concentration of BPL was a balance between using a high enough concentration to completely inactivate the virus with no or minimal reduction in VSAA, and keeping the NBR within the acceptable range, particularly after concentration. The tailing phenomenon was seen during BPL treatment as well (most notably with the alphaviruses), in which the inactivation kinetics did not decrease linearly to zero, but tapered off below the threshold of detection with persistence of low levels of viable virus particles in unconcentrated material; post-concentration, these particles were concentrated above the threshold of detection.
YFV treated with increasing doses of BPL resulted in lowered VSAA and increased NBR, and at the higher BPL concentrations (0.1%), the NBR of the YFV antigen rose above the acceptable limit (Table 1A). In contrast, the NBRs remained low at all BPL concentrations for the remaining flaviviruses, SLEV and POWV (Tables 1B and 1C, respectively). BPL was most effective at inactivating the three bunyaviruses. There was minimal loss of VSAA, and the NBRs were low and stable, even at the highest concentration of 0.1% BPL (Table 1G-I). Interestingly, similar to work described previously by French and McKinney, VSAAs of some of the viruses studied here increased following BPL treatment, with the greatest enhancement seen with CHIKV, MAYV, and LACV (French, McKinney, 1964). Presumably, the BPL causes conformational changes in the envelope protein that exposes or “opens-up” the antigenic epitopes recognized in the MAC-ELISA (Table 1D, Table 1E, and 1G, respectively). However, higher concentrations of BPL tended to generate higher NBR, most notably for CHIKV and MAYV, as it did with YFV, and lower concentrations did not completely inactivate these viruses.
To determine if the NBR was due to residual BPL, the BPL was subjected to complete hydrolysis following treatment of SLEV and CHIKV (Table 3). BPL-treated SLEV did not have high NBR initially, and hydrolysis had little effect on further lowering NBR. The NBR of CHIKV did decrease, but the VSAA also decreased, most likely due to antigen degradation after being held at 37°C for an additional 2 h, as has been shown previously (French, McKinney, 1964). Whereas the NBR of CHIKV decreased to the acceptable range after hydrolysis, this antigen was not concentrated, and the NBR often increases after concentration. BPL hydrolysis will be further investigated in BPL-treated YFV and CHIKV to determine if the NBR remains in the acceptable range following concentration. Therefore, while BPL remained the first-line method of inactivation, additional inactivation methods were needed for CHIKV and YFV.
Inactivation by gamma-irradiation has advantages over BPL, as there are no chemicals added to the supernatant which might interfere with antigen activity by changing pH or causing NBR. In addition, the virus remains frozen throughout the irradiation procedure, preventing the degradation of labile viruses, as can occur when these viruses are held at higher temperatures for extended periods of time. Unfortunately, the process is expensive and a dedicated radiation facility and certified personnel are required, limiting its availability. Consequently, gamma irradiation analysis was generally performed on viral material for which BPL was an inadequate inactivation method. Gamma irradiation modifies nucleic acids by causing base mutations, strand cross-linking, and strand breakage, but also generates free radicals and peroxides that can modify the antigenic protein epitopes (Nims et al., 2011). Consequently, kill curves, or treatment with radiation doses ranging from 1–6 Mrad, were performed to determine the dose of radiation needed to completely inactivate the virus but not compromise the antigen activity.
Because BPL-treated YFV produced an unacceptable antigen, it was the first to be evaluated by gamma irradiation. At the highest dose of 5 Mrad, YFV was completely inactivated and there was very low NBR, although some decrease in VSAA did occur. The alphaviruses were also treated with gamma-irradiation but there was residual infectivity (1–2 log10 PFU/ml) at 5–6 Mrad. Similar to BPL treatment, gamma irradiation increased alphavirus antigen reactivity, possibly by changing antigen conformation to expose the antigenic epitopes by the same mechanism as BPL (Table 1D-F).
For problematic viruses such as CHIKV, in which BPL treatment resulted in unacceptably high NBR and gamma irradiation did not completely inactivate the virus, a combination of the two methods proved effective. CHIKV strain S27 was first treated with 5.5 Mrad which lowered the titer to ≤2 log10 PFU/ml, and then treated with ≤0.05% BPL, which inactivated the virus but did not increase the NBR above the acceptable limit.
None of the other inactivation methods were suitable. Either they did not inactivate the virus or the antigen activity was destroyed. Aziridine compounds such as binary ethylenimine (BEI) were not evaluated, as BEI did not have any advantages over BPL and vaccines inactivated by BEI have been shown to have a short shelf-life of ≤ 2 years (Barteling, 2002).
At DVBD ADB, inactivated antigens are concentrated to a standard working dilution of ∼1:160 and then aliquoted into vials and lyophilized. In any concentration method there is some loss of product, but it should be minimized as much as possible. Centrifugal filters yielded higher VSAA and lower NBR compared to ultracentrifugation, as antigen remained in the retentate in the centrifugal filter method, whereas there was likely some loss of ultracentrifuged pelleted antigen when the supernatant was removed. In addition, the ultracentrifuged antigen pellet did not fully re-solubilize in the resuspension buffer. Subsequently, centrifugal filters are used to concentrate all antigen preparations, even though more hands-on time is required to process large antigen volumes (Figure 2).
Arboviruses are sensitive to acidic conditions, whereas alkaline conditions tend to preserve arbovirus viability (Beaty et al., 1995; Brand, Allen, 1970). To optimize the storage buffer, concentrated YFV and SLEV antigen were resuspended in two different buffers, 0.1M trizma/BS (pH 9.0) and 1X PBS (pH 7.4), and antigen activity was compared. Working antigen dilutions tended to be higher when antigens were resuspended in 0.1M trizma/BS buffer compared to 1X PBS, suggesting that the more alkaline pH of the 0.1M trizma/BS buffer helped contribute to antigen preservation. However, additional experiments would need to be performed to determine whether antigen preservation was primarily due to pH or whether the actual buffer components contributed as well. A future experiment to help discern this may consist of resuspending concentrated antigen in 0.1M trizma/BS with a more acidic pH and 1X PBS with a more alkaline pH.
Most concentrated antigens demonstrated higher working antigen dilutions when resuspended in 0.1M trizma/BS, although SLEV antigen concentrated by centrifugal filters and resuspended in either 0.1M trizma/BS or 1X PBS had equivalent working antigen dilutions of 1:80. This lack of difference may be due to the smaller volume of buffer added to the retentate following filtration, in contrast to the complete removal of cell culture supernatant and buffer exchange that occurred during ultracentrifugation.
Concomitant with recent global arbovirus emergence and re-emergence, there has been an increase in demand for arbovirus diagnostic testing and the availability of reagents, both in terms of the variety of arboviruses tested as well as the amount of reagents needed. Lack of commercial sources for arbovirus antigens has necessitated increased production in the DVBD ADB reference laboratory. The development of the cell culture arbovirus antigen production algorithm and optimization of the methods and processes will enhance the capacity of the reference laboratory to respond to the changing needs of the diagnostic laboratory. In addition, this study illustrates the importance of optimizing the antigen production procedure for each virus and evaluating antigen performance in the end-use assay at every step in the production process.
Acknowledgments
We would like to thank Lyle Petersen, Wendi Kuhnert, Paul Simpson, and Kathi Kellar for their exceptional assistance in coordinating gamma-irradiation services between CDC-Fort Collins, CO and CDC-Atlanta, GA.
References
- Amanna IJ, Raue HP, Slifka MK. Development of a new hydrogen peroxide-based vaccine platform. Nat Med. 2012;18:974–979. doi: 10.1038/nm.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barteling SJ. Development and performance of inactivated vaccines against foot and mouth disease. Rev Sci Tech. 2002;21:577–588. doi: 10.20506/rst.21.3.1361. [DOI] [PubMed] [Google Scholar]
- Beaty B, Calisher C, Shope R. Arboviruses. In: Lennette E, Lennette D, Lennette E, editors. Diagnostic procedures for viral, rickettsial, and chlamydial infections. Washington, DC: American Public Health Association; 1995. pp. 189–212. [Google Scholar]
- Bonnafous P, Nicolai MC, Taveau JC, Chevalier M, Barriere F, Medina J, Le Bihan O, Adam O, Ronzon F, Lambert O. Treatment of influenza virus with Beta-propiolactone alters viral membrane fusion. Biochim Biophys Acta. 2014;1838:355–363. doi: 10.1016/j.bbamem.2013.09.021. [DOI] [PubMed] [Google Scholar]
- Brand OM, Allen WP. Preparation of noninfectious arbovirus antigens. Appl Microbiol. 1970;20:298–302. doi: 10.1128/am.20.3.298-302.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown F. Inactivation of viruses by aziridines. Vaccine. 2001;20:322–327. doi: 10.1016/s0264-410x(01)00342-5. [DOI] [PubMed] [Google Scholar]
- Burke D, Monath TP. Flaviviruses. In: Knipe D, Howley P, editors. Fields Virology. 4th edition. Philadelphia: Lippincott Williams and Wilkins; 2001. pp. 1043–1125. [Google Scholar]
- Centers for Disease Control and Prevention. International Catalog of Arboviruses Including Certain Other Viruses of Vertebrates. 2014 Available at wwwn.cdc.gov/arbocat/index.asp.
- Cleton N, Koopmans M, Reimerink J, Godeke GJ, Reusken C. Come fly with me: review of clinically important arboviruses for global travelers. J Clin Virol. 2012;55:191–203. doi: 10.1016/j.jcv.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Earley E, Johnson K. The lineage of the Vero, Vero 76 and its clone C1008 in the United States. In: Simizu B, Terasima T, editors. Vero Cells: Origin proterties and biomedical application. Tokyo: Chiba University; 1988. [Google Scholar]
- Ei Khoury MY, Camargo JF, Wormser GP. Changing epidemiology of Powassan encephalitis in North America suggests the emergence of the deer tick virus subtype. Expert Rev Anti Infect Ther. 2013;11:983–985. doi: 10.1586/14787210.2013.837805. [DOI] [PubMed] [Google Scholar]
- French GR, McKinney RW. Use of Beta-Propiolactone in Preparation of Inactivated Arbovirus Serologic Test Antigens. J Immunol. 1964;92:772–778. [PubMed] [Google Scholar]
- Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33:330–342. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- Hearn HJ, Jr, Dawson FW. Comparative effects of beta-propiolactone on mice, mouse-derived cell cultures, and Venezuelan equine encephalomyelitis virus. Appl Microbiol. 1961;9:278–282. doi: 10.1128/am.9.4.278-282.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt CW. Kinetics of the Inactivation of Viruses. Bacteriol Rev. 1964;28:150–163. doi: 10.1128/br.28.2.150-163.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AJ, Martin DA, Karabatsos N, Roehrig JT. Detection of anti-arboviral immunoglobulin G by using a monoclonal antibody-based capture enzyme-linked immunosorbent assay. Journal of Clinical Microbiology. 2000;38:1827–1831. doi: 10.1128/jcm.38.5.1827-1831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AJ, Noga AJ, Kosoy O, Lanciotti RS, Johnson AA, Biggerstaff BJ. Duplex microsphere-based immunoassay for detection of anti-West Nile virus and anti-St. Louis encephalitis virus immunoglobulin m antibodies. Clinical and diagnostic laboratory immunology. 2005;12:566–574. doi: 10.1128/CDLI.12.5.566-574.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Kosoy OL, Laven JJ, Panella AJ, Velez JO, Lambert AJ, Campbell GL. Chikungunya virus in US travelers returning from India, 2006. Emerg Infect Dis. 2007;13:764–767. doi: 10.3201/eid1305.070015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe KE, Crabtree MB, Scherret JH, Hall RA, MacKenzie JS, Cropp CB, Panigrahy B, Ostlund E, Schmitt B, Malkinson M, Banet C, Weissman J, Komar N, Savage HM, Stone W, McNamara T, Gubler DJ. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- Lawrence SA. beta-Propiolactone: viral inactivation in vaccines and plasma products. PDA J Pharm Sci Technol. 2000;54:209–217. [PubMed] [Google Scholar]
- Logrippo GA. Investigations of the use of beta-propiolactone in virus inactivation. Ann N Y Acad Sci. 1960;83:578–594. doi: 10.1111/j.1749-6632.1960.tb40931.x. [DOI] [PubMed] [Google Scholar]
- Martin DA, Muth DA, Brown T, Johnson AJ, Karabatsos N, Roehrig JT. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. Journal of Clinical Microbiology. 2000;38:1823–1826. doi: 10.1128/jcm.38.5.1823-1826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monath TP, Heinz F. Flaviviruses. In: Knipe D, Howley P, editors. Fields Virology. Philadelphia: Lippincott Williams and Wilkins; 1996. pp. 1043–1125. [Google Scholar]
- Monath TP, Lee CK, Julander JG, Brown A, Beasley DW, Watts DM, Hayman E, Guertin P, Makowiecki J, Crowell J, Levesque P, Bowick GC, Morin M, Fowler E, Trent DW. Inactivated yellow fever 17D vaccine: development and nonclinical safety, immunogenicity and protective activity. Vaccine. 2010;28:3827–3840. doi: 10.1016/j.vaccine.2010.03.023. [DOI] [PubMed] [Google Scholar]
- Nims RW, Gauvin G, Plavsic M. Gamma irradiation of animal sera for inactivation of viruses and mollicutes--a review. Biologicals. 2011;39:370–377. doi: 10.1016/j.biologicals.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Piret J, Desormeaux A, Bergeron MG. Sodium lauryl sulfate, a microbicide effective against enveloped and nonenveloped viruses. Curr Drug Targets. 2002a;3:17–30. doi: 10.2174/1389450023348037. [DOI] [PubMed] [Google Scholar]
- Piret J, Roy S, Gagnon M, Landry S, Desormeaux A, Omar RF, Bergeron MG. Comparative study of mechanisms of herpes simplex virus inactivation by sodium lauryl sulfate and n-lauroylsarcosine. Antimicrob Agents Chemother. 2002b;46:2933–2942. doi: 10.1128/AAC.46.9.2933-2942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers AM, Brault AC, Tesh RB, Weaver SC. Re-emergence of Chikungunya and O’nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J Gen Virol. 2000;81:471–479. doi: 10.1099/0022-1317-81-2-471. [DOI] [PubMed] [Google Scholar]
- Rosenberg R, Johansson MA, Powers AM, Miller BR. Search strategy has influenced the discovery rate of human viruses. Proc Natl Acad Sci U S A. 2013;110:13961–13964. doi: 10.1073/pnas.1307243110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin A. The St. Louis and Japanese B types of epidemic encephalitis: Development of noninfective vaccines. The Journal of the American Medical Association. 1943;122:10. [Google Scholar]
- Solomon T, Winter PM. Neurovirulence and host factors in flavivirus encephalitis--evidence from clinical epidemiology. Arch Virol. 2004;(Suppl):161–170. doi: 10.1007/978-3-7091-0572-6_14. [DOI] [PubMed] [Google Scholar]
- Staples JE, Breiman RF, Powers AM. Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin Infect Dis. 2009;49:942–948. doi: 10.1086/605496. [DOI] [PubMed] [Google Scholar]
- Thibodeaux BA, Liss NM, Panella AN, Roehrig JT. Development of a human-murine chimeric immunoglobulin M for use in the serological detection of human alphavirus antibodies. Clin Vaccine Immunol. 2011;18:2181–2182. doi: 10.1128/CVI.05269-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uittenbogaard JP, Zomer B, Hoogerhout P, Metz B. Reactions of beta-propiolactone with nucleobase analogues, nucleosides, and peptides: implications for the inactivation of viruses. J Biol Chem. 2011;286:36198–36214. doi: 10.1074/jbc.M111.279232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC. Host range, amplification and arboviral disease emergence. Arch Virol. 2005;(Suppl):33–44. doi: 10.1007/3-211-29981-5_4. [DOI] [PubMed] [Google Scholar]
- WHO. WHO-recommended standards for surveillance of selected vaccine-preventable diseases. 2003 [Google Scholar]
- Wong SJ, Boyle RH, Demarest VL, Woodmansee AN, Kramer LD, Li H, Drebot M, Koski RA, Fikrig E, Martin DA, Shi PY. Immunoassay targeting nonstructural protein 5 to differentiate West Nile virus infection from dengue and St. Louis encephalitis virus infections and from flavivirus vaccination. J Clin Microbiol. 2003;41:4217–4223. doi: 10.1128/JCM.41.9.4217-4223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SJ, Demarest VL, Boyle RH, Wang T, Ledizet M, Kar K, Kramer LD, Fikrig E, Koski RA. Detection of human anti-flavivirus antibodies with a west nile virus recombinant antigen microsphere immunoassay. J Clin Microbiol. 2004;42:65–72. doi: 10.1128/JCM.42.1.65-72.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]