Abstract
We present methods for estimating five year birth cohort specific trends in smoking behavior for individuals born 1910–1984.We combine cross-sectional survey data on smoking behavior from the National Health Interview Surveys (NHIS) conducted between 1965 and 2001 into a single data set. The cumulative incidence of smoking by year of age and calendar year is constructed for each birth cohort from this data set and the effect of differential mortality on ever smoking prevalence is adjusted by modeling the ever smoking prevalence of each cohort for each survey year and back extrapolating that effect to age 30. Cumulative incidence is then scaled to match the ever smoking prevalence at age 30. Survival analyses generate the cumulative cessation among ever smokers across year of age and calendar year and are used to estimate current smoking prevalence. Data from Substance Abuse and Mental Health Services Administration (SAMHSA) National Survey on Drug Use and Health is used to divide those initiating smoking into quintiles of number of cigarettes smoked per day (CPD) and the mean CPD for each quintile in each calendar year is estimated from the NHIS data.
For five year birth cohorts of White, African American, Hispanic and all race/ethnicity groupings of males and females born between 1910 and 1984, estimates are provided for prevalence of current and ever-smoking, incidence of cessation, incidence of initiation, and the distribution of smoking duration and cigarettes smoked per day for each calendar year and each single year of age through the year 1999.
We believe that we are the first to provide birth cohort specific estimates of smoking behaviors for the U.S. population that include distributions of duration of smoking and number of cigarettes per day. These additional elements substantively enhance the utility of these estimates for estimating lung cancer risks.
1. Introduction
Tobacco has a long history in the Americas, but cigarettes smoking as the predominant form of tobacco use is largely a phenomena of the past one hundred years(1). During that period, per-capita consumption of cigarettes rose and then fell, smoking shifted from a predominantly male behavior to one shared equally across both genders and dramatic shifts in the distribution of smoking by race and ethnicity occurred(1). Behaviors presented for birth cohorts followed over time can present a more complete picture of these changes in smoking behaviors than cross-sectional survey data which are limited to a single time point.
Several efforts to use multiple cross-sectional survey data to construct birth cohort specific estimates of smoking behaviors over time have been undertaken(1–5) and a number of challenges to converting cross-sectional data into longitudinal estimates are recognized. As the National Cancer Institute (NCI) Cancer Intervention and Surveillance Modeling Network (CISNET) undertook the task of modeling U.S. lung cancer mortality rates, the value of having longitudinal estimates for a range of smoking behaviors going back to early in the last century was evident, and we undertook an effort to update previous estimates and improve the methods by which they were derived.
We develop five-year birth cohort specific smoking-behavior estimates for the United States for birth cohorts born 1910 to 1984 by sex and by race/ethnicity. Estimates are provided for prevalence of current and ever-smoking, incidence of cessation, incidence of initiation, and the distribution of smoking duration and cigarettes smoked per day for each calendar year and each single year of age through the year 1999.
2. Methods
2.1 Smoking BehaviorData
Twenty-five NHIS surveys administered from 1965 to 2001 are used for this analysis (1965, 66, 70, 74, 76, 77, 78, 79, 30 83, 85, 87 (×2), 88, 90, 91, 92, 93, 94, 95, 97, 98, 99, 2000, 2001).The surveys are combined into a single dataset. Not all variables are available in each survey and survey questions have changed over time. Survey questionnaires for each year were obtained, questions describing the variables of interest in our analysis identified, and adjustments for the differences between survey questions to make the variable recording as uniform as possible implemented in order to allow the analysis to be conducted on a single data set. Only self-respondents are used for these analyses. Each respondent is grouped into five-year birth cohorts. While smoking behaviors for earlier birth cohorts have been derived, the data presented in this paper begin with the years 1910 to 1914 and continuing through 1980–84. Since the race-specific analyses are stratified by race/ethnicity, age and gender, we did not use weighted data except when generating estimate for all race and ethnicity cohorts.
These analyses use definitions of ever smoking as a positive response to the survey question “Have you ever smoked at least 100 cigarettes in your lifetime?”, and current smoking is defined as a positive response to the question “Do you smoke now?” or “Do you currently smoke some days, every day or not at all?”. We defined former smokers as ever smokers who currently report not smoking at all, but because smokers who have quit have a very high rate of relapse in the first two years following cessation we censored the cessation rate data two years prior to the date of each survey as described below in the section on cessation. This censoring results in only those who have achieved long term abstinence being counted as having quit in the development of the birth cohort estimates and avoids an overestimation of long term cessation in the population by inclusion of smokers who are quit at the time of the survey but who will relapse back to smoking in the subsequent months.
2.2 Estimating Ever-Smoking Prevalence
We estimate birth cohort smoking behaviors for five-year birth cohorts of White, African American, Hispanic and all race/ethnicity groupings of males and females born between 1910 and 1984. Estimates are provided for prevalence of current and ever-smoking, incidence of cessation, incidence of initiation, and the distribution of smoking duration and cigarettes smoked per day for each calendar year and each single year of age through the year 1999.
Estimating ever smoking prevalence by birth cohort requires resolution of two problems. First, ever smokers have higher mortality than never smokers resulting in a progressive fall in the residual fraction of the birth cohort who are ever smoker as the cohort ages. Second, the first survey data are for 1965 and it is not possible to directly estimate the prevalence of ever smoking prior to this date. We estimate ever smoking prevalence by five year birth cohorts for each survey year in which smoking data were collected based on a positive response to the question “Have you ever smoked at least 100 cigarettes in your lifetime?”.This gives a reflection of the actual proportion of ever-smokers at each calendar year, but the small numbers of observations for some cohorts leads to substantial variability and broad confidence intervals for the estimates. An example of these estimates is presented for the White female birth cohort born 1920–1924 as individual data points in Figure 1. Because estimates are only available for years for which there are surveys, the earliest of which was conducted in 1965, there is an underestimate of ever smoking prevalence for the calendar years prior to the first survey in many cohorts.
Figure 1.
Development of Ever-Smoking Rates White Females Born 1920 – 1924
An estimate of the actual prevalence of ever smoking at age 30, an age when initiation is largely complete for most cohorts, can be generated by modeling the observed cross-sectional ever-smoking prevalence rates derived from each survey for each birth cohort and back extrapolating to age 30 for the cohort. In order to smooth the rates and obtain rates prior to the first NHIS survey, we use the equation:
The model is fit using the cross-sectional ever-smoking prevalence from each survey and the ever smoking prevalence estimate for each survey is weighted by its denominator. An example of this approach for a single birth cohort of white females is used to describe these methods, and the cross-sectional ever-smoking prevalence is the line labeled number 1 in Figure 1.
The prevalence of ever smoking can also be estimated by constructing the cumulative incidence of smoking for a given birth cohort using the question on age of initiation. The time to initiation of ever-smoking (or age of initiation) is modeled using survival analysis. One minus the survival function for time to initiation of smoking produces the cumulative incidence of ever-smoking. Never smokers are censored at their age when the survey was administered.
An example of this approach is presented as line 2 in Figure 1.
The cumulative incidence of ever-smoking generated using this approach also underestimates the actual prevalence of ever-smoking. This underestimation results from the differential mortality of ever-smokers compared to never-smokers. Ever-smokers die at a disproportionally higher rate than never-smokers at the same age leading to a progressively lower estimated, and actual, prevalence of ever smoking as the cohort ages, particularly for ages past 50 years. Differential mortality also reduces the age specific incidence rates used to construct the cumulative ever smoking prevalence. This underestimate can be corrected if the actual ever smoking prevalence is known for the birth cohort at the age when initiation is complete.
Since initiation is essentially complete by age 30 for most of the cohorts, the cumulative incidence rates (line2 in Figure 1) can be scaled upward for most cohorts to force the cumulative incidence (line 2 in Figure 1) to match the back extrapolated ever fitted model of cross-sectional smoking prevalence for that cohort at age 30 (line 1 in Figure 1.) to generate the final fitted model of ever smoking prevalence presented as lines 3 and 4 in Figure 1. However, a small amount of initiation occurs after the age of 30 in some cohorts and must be accounted for, particularly for older cohorts of females. To correct for this initiation after age 30, the fitted model of cross-sectional ever smoking prevalence (line 1 in Figure 1) is adjusted downward at each age after age 30 by the proportion of eventual initiation that has not already occurred by that age before the cumulative incidence rates (line 2 in Figure 1) is scaled upward to generate the final fitted model of ever smoking prevalence by age adjusted for continuing initiation presented as lines 3. 4and the subsequent portion of line 1 in Figure 1. The same process was conducted to generate estimates by calendar year.
2.3 Estimating Incidence of Cessation
Using the smoking histories of each survey respondent, the incidence of cessation by calendar year or year of age is calculated by dividing the number of smokers who report abstinence of 2 or more years duration, and who began that abstinence in the specified calendar year or year of age, by the number of smokers at the start of that calendar year or year of age. To account for the variability of rates at each age and the changing denominators, the rates need to be smoothed with the smoothing weighted by the number of observations in the denominator of the rate. Additionally, the small denominators in the earliest and oldest ages produce “end effects.” Using restricted cubic splines to smooth the rates forces the tails to be linear, ameliorating these “end effects.”(6) The number of knots used in the analysis of each birth cohort is a somewhat subjective judgment since the addition of an additional knot always improves the fit. The selected number of knots is therefore based on an evaluation of whether the residual sum of squares that results from the addition of an additional knot is substantively reduced, an admittedly subjective judgment.. In general, the addition of knots improves the fit; therefore, the point at which adding a knot to the smoothing technique does not greatly reduce the residual sum of squares determines the number of knots used to smooth each particular birth-cohort analysis. Rates with denominators less than one hundred are excluded from the analyses since these estimates are based upon responses by a small number of people, and therefore the reliability of the rates is uncertain.
The incidence of cessation might be affected by the differential mortality between current and former smokers, particularly for long term former smokers, resulting in a slight overestimate of cessation rates. This effect is partially diminished by the application of the cessation incidence to ever smoking prevalence (which has been adjusted for differential mortality) and the use of survival analyses to estimate cumulative cessation in the calculation of current smoking prevalence. The good match between calculated and observed current smoking prevalence suggests that the magnitude of this underestimation of cessation is likely to be small.
Estimating Current-Smoking Prevalence
Current smoking prevalence at any age is the prevalence of ever smoking at that age multiplied by the cumulative proportion of ever smokers at that age who have not quit by that age. We model age at cessation for each cohort using survival analysis to estimate the cumulative proportion of smokers who have not quit by that age. In the cross-sectional survey data many of the smokers who report achieving abstinence recently will relapse back to smoking, overestimating the actual long term successful abstinence achieved by that cohort. In order to minimize the effect of quit attempts that eventually fail, we only considered in the analyses reports of successful cessation that were two or more years old at the survey administration date. Respondents who quit smoking less than two years before survey administration are considered current smokers up to two years prior to survey administration and then are censored in the analyses. Current smokers are also censored at their age two years prior to survey administration.
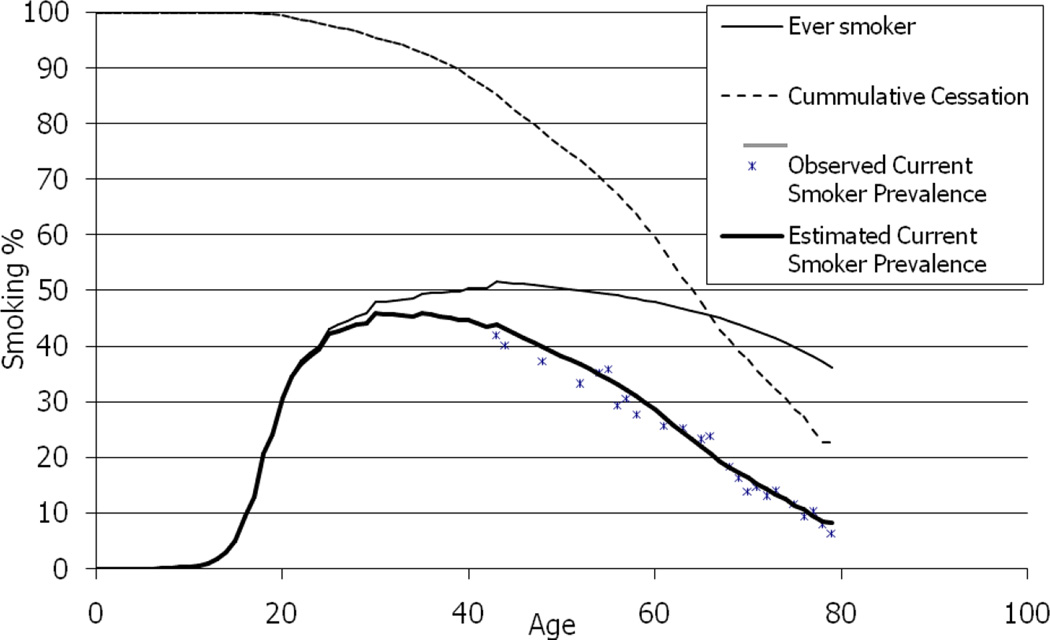
Current-smoking prevalence (dark solid line in Figure 2) is calculated by multiplying the estimated ever-smoking prevalence at each age (light solid line in Figure 2 which is identical to lines 3, 4 and the subsequent portion of line 1 in Figure 1) by the cumulative proportion of smokers who have not quit smoking by that age (dotted line in Figure 2).
Figure 2.
Calculating Current Smoker Prevalence for White Females Born 1920 – 1924
We then validated this approach for calculating current smoking prevalence by comparing the calculated rates to the rates observed for each cohort in each survey. An example of this comparison is provided in Figure 2.
2.4 Estimating Incidence of Initiation
Birth cohort specific initiation of cigarette smoking can be presented either as the cumulative incidence of ever smoking, which has the sum of all current, former and never smokers in the cohort as the denominator, or as the incidence of initiation which is the rate of smoking initiation at any given year of age or calendar year and has only the population at risk (never smokers at the start of that age or year) as the denominator. The age specific incidence rates can be estimated from the cumulative incidence curve by scaling the annual incidence rates upward so that the cumulative incidence rate matches the ever smoking prevalence at age 30 as was done to estimate ever smoking prevalence in Figure 1. The incidence of initiation is the percentage of the birth cohort who began smoking in that calendar year or year of age divided by the proportion of the cohort who remained never smokers at the beginning of that calendar year or year of age (the population at risk for initiation).The rates are smoothed using Loess with a local quadratic fit and a span of 0.25 to obtain estimated incidence of initiation as presented in Figure 3. Unlike the cessation rates, however, it is not necessary to use a restricted cubic spline to moderate the “end effects,” as initiation primarily takes place during a relatively short span of ages.
Figure 3.
Comparison of Calculate and Smoothed Incidence Rates for White Females Born 1920 – 1924
2.5 Estimating the Distribution of Smoking Duration
Duration of smoking is an important determinant of the risk of smoking related disease. The distribution of duration of smoking changes with each advancing year for a birth cohort and is not identical at each given age from one birth cohort to another. We calculate the distribution of duration of smoking for current and former smokers. For current smokers, we use the distribution of age specific initiation and the age of the birth cohort to generate the distribution of duration of smoking in any given year or at any given year for the cohort.
Subtracting the current-smoking prevalence at each age from the ever-smoking prevalence provides the former-smoking prevalence at that age/year. For former smokers, we make the assumption that the distribution of duration for those smokers who become former smokers at each age for each birth cohort is the same as the distribution for current smokers of that age in the same birth cohort. For each birth cohort, at the age at which cessation first occurs, the distribution of duration for those who become former smokers in that year is equal to the distribution of duration for current smokers at that age. At each subsequent age, the distribution of duration is a weighted average of that year’s current-smoking distribution weighted by the proportion of those quitting in that year and the distribution of duration of those who had been former smokers at the start of the year weighted by the proportion who been former smokers at the start of the year. To obtain population estimates, the distribution of former smokers must then be applied to the percentage of former smokers in that year. The distribution of initiation by age can then be advanced forward to each subsequent year to give the distribution of duration of current smokers.
2.6 Estimating Cigarettes Smoked Per Day
The NHIS records the number of cigarettes smoked per day for current smokers in the survey year but does not systematically attempt to examine the changes in number of cigarettes smoked per day (CPD) over their lifetime of smoking for the smokers surveyed. In addition the mean number of cigarettes smoked per day by current smokers changes with age(7).
In order to construct estimates of CPD for the birth cohorts over their lifetimes, we divide the process of estimation into the period of smoking uptake (ages under age 30) and the process of maintenance of smoking behavior over the age 30. Current smokers over the age of 30 are divided into five smoking-intensity groups - quintiles 1 through 5 or lightest to heaviest smokers by their reported number of cigarettes smoked at each age. The distribution of CPD at each year of age for the NHIS is used to calculate the mean CPD at each age for each quintile, after the age of 30. While age is the principal determinant of the change in CPD with time in a birth cohort, there is also a modest shift in the mean number of CPD with calendar year(7, 8), a part of which may be due to confounding with age, but this effect of calendar year is not included in the calculation. This approach generates five lines of different intensities for each of the cohorts which define the relationship of CPD with age after age 30.
In order to define CPD at ages under age 30 we modeled the reported CPD at ages under 30 in the Substance Abuse and Mental Health Services Administration (SAMHSA) National Surveys on Drug Use and Health from 1979 to 2003 in order to define the relationships of age, age of initiation and CPD. The resulting models are:
These uptake formulae are then scaled so that the value at age 30 matches the value for each quintile at age 30. The result is a series of uptake curves for each quintile that are different for each age of initiation but all arrive at the same CPD at age 30. The age of initiation is used to weight the probability of being in the different intensity quintiles based on the observed probabilities of being in each quintile as an adult at each age of initiation.
3. Results
The principal purpose of this paper is to describe the methods used in generating the birth cohort smoking estimates and a full presentation of all of the estimates for all of the birth cohorts and all of the race/ethnicity and gender groupings is beyond the space limitations allowed. A full presentation of the actual results in tabular form is available on the CISNET website http://cisnet.cancer.gov/resources/impact_reviewID_A3Z.html. However, a brief presentation of some of the more interesting results is offered for White males and females. The white population was selected as examples because it represented the largest of the race/ethnicity groups and because the all race/ethnicity data are presented elsewhere in this supplement.
3.1 Current Smoking Prevalence
The prevalence of current smoking at any pointis a function of the cumulative initiation, the cumulative cessation and the differential mortality between smokers and never smokers. Initiation occurs predominantly early in life and cessation is the major factor influencing current prevalence after age 30. Birth cohort specific current smoking prevalence by calendar year is presented for White males and females in Figures 4 and 5. Tobacco control outcomes can be assessed by examining both the trend in the peak smoking prevalence across cohorts and by the rate of decline in smoking prevalence within a cohort as the cohort ages.
The difference between this form of presenting smoking behavior and cross-sectional analyses based on age specific rates can be appreciated by examining the rates along a vertical line in any calendar year. For example, if one examines the prevalence of smoking across the range of birth cohorts in figure 4 for 1975, the current smoking prevalence is relatively similar, particularly for those cohorts born after 1925–29. However, those cohorts arrive at the similar prevalence from very different peak smoking prevalence and therefore have very different fractions of the cohort who are former smokers with continuing elevate risks for lung cancer.
Differences in historical patterns of smoking behaviors between males and females is also evident with lower peak prevalence of smoking and later age of achieving peak prevalence for females in the older cohorts. These differences, which are largely responsible for male-female differences in lung cancer rates, disappear for more recent birth cohorts. An additional observation worth noting is the sudden reversal of the trend of declining peak prevalence for male and female birth cohorts born 1975–79. This increased smoking uptake by these cohorts is consistent with data from the Monitoring the Future Study which shows increased and then decreased smoking during the years individuals in these cohorts would have been in high school(9).
3.2 Incidence of Initiation
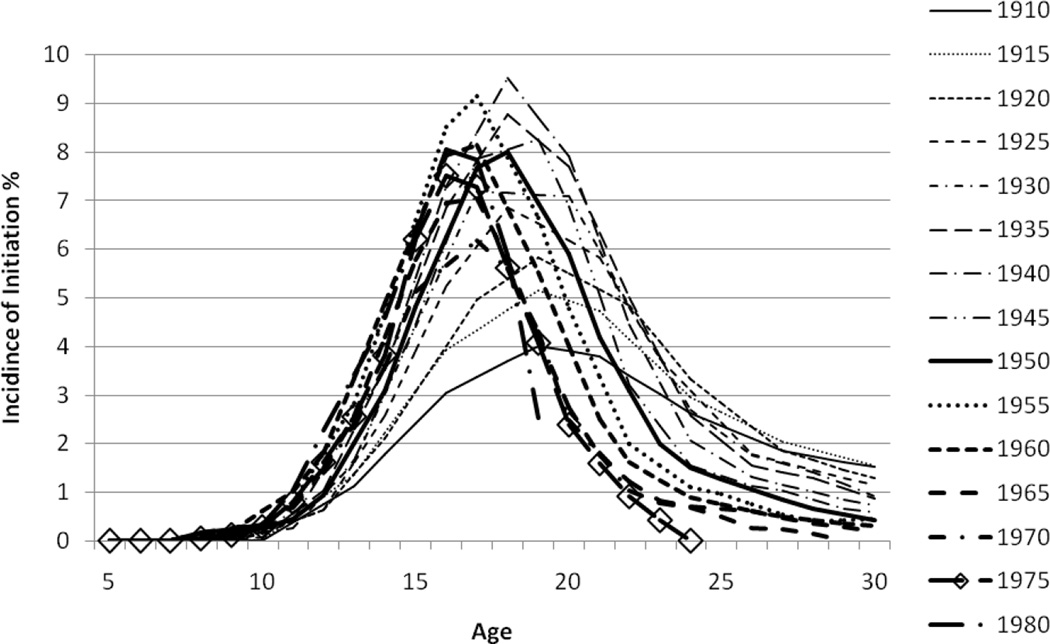
Age-specific incidence of smoking for White males and females by birth cohort is presented in Figures 6 and 7 by year of age. It is evident that smoking initiation peaks in adolescence for most cohorts, but earlier birth cohorts of females demonstrate smoking uptake over a much broader range of ages. Males have higher rates of initiation than females among older cohorts but these differences disappear for more recent cohorts. There is a progressive decline in incidence rates at each age as the cohorts progress from older to more recent cohorts representing progress in tobacco control, but the extent of the decline in incidence rates is greater at older ages suggesting that tobacco control messages and programs may have had larger impacts on older adolescents and young adults than on younger adolescents.
Figure 6.
Incidence of Initiation of Cigarette Smoking By 5-Year Birth Cohorts and Year of Age for White Males
Figure 7.
Incidence of Initiation of Cigarette Smoking By 5-Year Birth Cohorts and Year of Age for White Females
The lines for the 1975–79 cohorts are distinguished with a hollow diamond shaped marker in the figures and the male data show substantially higher rates of initiation at ages 17 and 18 consistent with the increased prevalence demonstrated for that cohort in the prevalence data. A similar increased incidence was not evident for females suggesting that the increased prevalence observed in Figure 5 for that cohort may have resulted from modestly higher incidence rates across a broader range of ages.
Figure 5.
Prevalence of Cigarette Smoking By 5-Year Birth Cohorts and Calendar Year for White Females
3.3 Annual Cessation Rates
Efforts to increase rates of cessation are a major component of tobacco control programs and are the most effective method of reducing tobacco related mortality among those who are regular smokers. Data for the incidence of cessation (annual cessation rates) for birth cohorts of White males and females are presented in figures 8 and 9. In general, cessation rates increase with increasing age, but the pattern is complex and suggests that different birth cohorts may have had different cessation outcomes.
Figure 8.
Annual Cessation Rates of Cigarette Smoking by 5-year Birth Cohorts and Year of Age for White Females
Figure 9.
Annual Cessation Rates of Cigarette Smoking by 5-year Birth Cohorts and Year of Age for White Males
Figure 8 shows the cessation rates for sequential cohorts of white females. The data for older cohorts show the results hoped for in tobacco control initiatives. As one moves from the oldest cohort to younger cohorts there is a trend toward higher age specific rates of cessation at every age suggesting that cessation messages are being increasingly heard and successfully acted upon. This is what would be expected and hoped for as knowledge about smoking related risks is disseminated and more effective tobacco control programs implemented.
However, beginning with the cohorts born in the late 1930s and continuing through the cohort born in the 1960s, more recent cohorts often have lower rates of cessation at given ages than the cohorts which preceded them. It is unclear whether this represents a temporal effect of decreased effectiveness of tobacco control initiatives for these cohorts, an effect of lower rates of initiation for these cohorts resulting in more intensely addicted smokers among those who did smoke, or a phenomena of hardening of the remaining smoking population once those who can easily quit have done so. Plotting the data by calendar year (data not shown) show similar trends but no consistent association with any specific time periods. While this observation is of great concern, it is reassuring to note that the most recent cohorts, those born after 1965, appear to have reversed the decline in cessation and are once again demonstrating increasing age specific cessation rates as progressively more recent cohorts are examined. A similar pattern is observed for white males in Figure 9.
4. Discussion
We present a set of methods for converting multiple cross-sectional surveys conducted at different points in time into a longitudinal presentation of smoking behaviors by birth cohort, including the estimation of behaviors for years prior to the first survey data. This work builds on the approaches presented by Harris(2, 3) as well as subsequent work by ourselves and others(1, 4, 5). New elements of this approach include the use of cohort specific back extrapolation to adjust for the effects of differential mortality on ever smoking prevalence, the development of single year of age and single calendar year estimates of the distribution of the duration of smoking for current and former smokers and the inclusion of estimates of changes in cigarettes smoked per day over time for each birth cohort.
The difference in mortality rates between smokers and never smokers results in a progressive fall in the prevalence of ever smoking as a cohort ages and this fall is progressively steeper after age 50. Harris(3) used the differences in death rates observed in two prospective mortality studies to adjust for this effect, but that approach limits the mortality assessment to the years in which the utilized mortality studies were conducted and applies the same adjustment to all of the cohorts. We utilize the fall in ever smoking prevalence observed across multiple NHIS over 35 years to model the actual change in ever smoking rate in each cohort and to then back extrapolate the change to age 30. Since the cohorts have different compositions of current and former smokers and different durations of smoking, it would be expected that they may have different differential mortality effects on ever smoking prevalence. Adjusting each cohort individually has the advantage of allowing different adjustments for different cohorts and also allows incorporation of whatever self redefinition of former smokers to never smokers may occur in the cohort. One limitation of this approach is that back extrapolation of the ever smoking prevalence is less accurate if substantial differential mortality has already occurred prior to the first survey measurement. For this reason we have limited our presentation of birth cohort data to cohorts born after 1910 who would be in their early fifties or less at the time of the first NHIS (1965).
The potential for differential mortality to alter the distribution of ages of initiation due to those who had earlier initiation having longer durations of smoking and consequently higher risks was examined by comparing the cumulative initiation curves for surveys which obtained age of initiation data in different decades. There is no statistically significant difference in the initiation curves from different surveys and therefore we do not attempt any adjustment for the effect of differential mortality on initiation rates.
One of the principal reasons for conducting these analyses is to allow modeling of lung cancer rates in the U.S. population. Development of detailed distributions of duration of smoking for current and former smokers and distributions of CPD are needed to enhance that modeling effort since they are the major determinants of lung cancer risk(10). The calculation of distribution of duration is straightforward, but we assume that age of initiation does not substantively alter the likelihood of subsequent cessation and distribute ages of initiation uniformly across all of the smokers who quit in a given year. There is considerable evidence that this assumption may not be correct(11–13), but we are not able to identify a simple method for adjusting for this effect, particularly for older adults, and therefore we leave this question open for further investigation.
We also do not alter the likelihood of successful cessation for the different intensity quintiles to adjust for the known effect of intensity of smoking on cessation success. The absence of this adjustment is mitigated by the adjustment of mean number of cigarettes (and the range of CPD) smoked by each quintile as the cohort ages. Since the data is intended to offer those modeling lung cancer risk the distribution of CPD in the population of current smokers in that year, it is not necessary to be able to track individual smokers through their cessation, it is only necessary to know the distribution of CPD for the current smokers in that year.
A final substantive limitation of this approach is the reality that the population of a birth cohort from which the sample was drawn in the 1965 survey is different from the population of that same cohort in the year 2000. In migration, out migration and mortality from factors other than smoking are some of the forces that have led to changes in the composition of the birth cohort population over time. The estimates we provide are based on a weighted mix of the population of that birth cohort as these changes are sampled in the multiple surveys.
We believe that we are the first to provide birth cohort specific estimates of smoking behaviors for the U.S. population that include distributions of duration of smoking and number of cigarettes per day. These additional elements substantively enhance the utility of these estimates for estimating lung cancer risks.
Figure 4.
Prevalence of Cigarette Smoking By 5-Year Birth Cohorts and Calendar Year for White Males
References
- 1.Burns DM, Lee LL, Gilpin B, Tolley HD, Vaughn J, Shanks T. Cigarette Smoking Behavior in the United States. In: Burns DM, Garfinkel L, Samet JM, editors. Changes in Cigarette Related Disease Risks and Their Implications for Prevention and Control. Bethesda: National Cancer Institute; 1997. pp. 13–112. [Google Scholar]
- 2.United States. Public Health Service. The health consequences of smoking for women : a report of the Surgeon General. Prepublication copy. ed. Rockville, Md: ept. of Health and Human Services, Public Health Service, Office of the Assistant Secretary for Health, Office on Smoking and Health; 1980. Office of the Surgeon General., United States. Office on Smoking and Health. [Google Scholar]
- 3.Harris JE. Cigarette smoking among successive birth cohorts of men and women in the United States during 1900–80. J Natl Cancer Inst. 1983;71(3):473–479. [PubMed] [Google Scholar]
- 4.United States. Public Health Service. The Health consequences of smoking : cancer and chronic lung disease in the workplace, a report of the Surgeon General. Rockville, MD. Washington, D.C: The Office; For sale by the Supt. of Doc., U.S. G.P.O.; 1985. Office of the Surgeon General, United States. Office on Smoking and Health. [Google Scholar]
- 5.National Cancer Institute (U.S.) Rockville, Md: U.S. Dept. of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute; 1991. Strategies to control tobacco use in the United States a blueprint for public health action in the 1990's. Available from: http://purl.access.gpo.gov/GPO/LPS117865. [Google Scholar]
- 6.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989 May;8(5):551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 7.National Institutes of Health (U.S.), National Cancer Institute (U.S.) Those who continue to smoke : is achieving abstinence harder and do we need to change our interventions? Bethesda, MD: National Cancer Institute; 2003. [Google Scholar]
- 8.Warner KE, Burns DM. Hardening and the hard-core smoker: concepts, evidence, and implications. Nicotine Tob Res. 2003;5(1):37–48. doi: 10.1080/1462220021000060428. [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Institute (U.S.) Changing adolescent smoking prevalence : where it is and why. Bethesda, MD: U.S. Dept. of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute; 2001. [Google Scholar]
- 10.Doll R, Peto R. Cigarette smoking and bronchial carcinoma: dose and time relationships among regular smokers and lifelong non-smokers. J Epidemiol Community Health. 1978;32(4):303–313. doi: 10.1136/jech.32.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breslau N, Peterson EL. Smoking cessation in young adults: age at initiation of cigarette smoking and other suspected influences. Am J Public Health. 1996;86(2):214–220. doi: 10.2105/ajph.86.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hymowitz N, Cummings KM, Hyland A, Lynn WR, Pechacek TF, Hartwell TD. Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tob Control. 1997;6(Suppl 2):S57–S62. doi: 10.1136/tc.6.suppl_2.s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khuder SA, Dayal HH, Mutgi AB. Age at smoking onset and its effect on smoking cessation. Addict Behav. 1999;24(5):673–677. doi: 10.1016/s0306-4603(98)00113-0. [DOI] [PubMed] [Google Scholar]