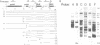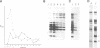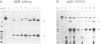Abstract
Chloroplast genes are typically organized into polycistronic transcription units that give rise to complex sets of overlapping RNAs through a series of processing steps. The functional significance of this complicated mode of expression is unknown. To determine whether processing of the primary transcript is required to create translatable mRNAs, the translational properties of the RNAs derived from the maize psbB gene cluster (containing the psbB, psbH, petB and petD genes) were examined. Almost all of the approximately 20 RNAs derived from this region co-sediment with polysomes in sucrose gradients, suggesting that at least one coding region on most transcripts is translated. To determine which sequences are translated on each polycistronic RNA, antibodies to psbB, petB or petD proteins were used to immunoselect polysomes engaged in the synthesis of each protein. Northern and S1 nuclease analyses of the immunoselected RNAs revealed that (i) potential start codons within the petB and petD introns are not functional in translation; (ii) all transcripts containing spliced petB or petD sequences are translated to give these proteins, regardless of upstream or downstream sequences; (iii) psbB is translated from all transcripts encoding it. It is concluded that intercistronic processing is not required for translation of these RNAs, although certain processing steps may enhance translational efficiency.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barkan A., Miles D., Taylor W. C. Chloroplast gene expression in nuclear, photosynthetic mutants of maize. EMBO J. 1986 Jul;5(7):1421–1427. doi: 10.1002/j.1460-2075.1986.tb04378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman G., Nomura M. Localization of the target site for translational regulation of the L11 operon and direct evidence for translational coupling in Escherichia coli. Cell. 1983 Oct;34(3):979–988. doi: 10.1016/0092-8674(83)90555-x. [DOI] [PubMed] [Google Scholar]
- Berends T., Gamble P. E., Mullet J. E. Characterization of the barley chloroplast transcription units containing psaA-psaB and psbD-psbC. Nucleic Acids Res. 1987 Jul 10;15(13):5217–5240. doi: 10.1093/nar/15.13.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J. O., Nikolau B. J., Carr J. P., Klessig D. F. Translational regulation of light-induced ribulose 1,5-bisphosphate carboxylase gene expression in amaranth. Mol Cell Biol. 1986 Jul;6(7):2347–2353. doi: 10.1128/mcb.6.7.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y., Beatty J. T., Cohen S. N., Belasco J. G. An intercistronic stem-loop structure functions as an mRNA decay terminator necessary but insufficient for puf mRNA stability. Cell. 1988 Feb 26;52(4):609–619. doi: 10.1016/0092-8674(88)90473-4. [DOI] [PubMed] [Google Scholar]
- Crossland L. D., Rodermel S. R., Bogorad L. Single gene for the large subunit of ribulosebisphosphate carboxylase in maize yields two differentially regulated mRNAs. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4060–4064. doi: 10.1073/pnas.81.13.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Fromm H., Devic M., Fluhr R., Edelman M. Control of psbA gene expression: in mature Spirodela chloroplasts light regulation of 32-kd protein synthesis is independent of transcript level. EMBO J. 1985 Feb;4(2):291–295. doi: 10.1002/j.1460-2075.1985.tb03628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble P. E., Sexton T. B., Mullet J. E. Light-dependent changes in psbD and psbC transcripts of barley chloroplasts: accumulation of two transcripts maintains psbD and psbC translation capability in mature chloroplasts. EMBO J. 1988 May;7(5):1289–1297. doi: 10.1002/j.1460-2075.1988.tb02943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley-Bowdoin L., Orozco E. M., Jr, Chua N. H. In vitro synthesis and processing of a maize chloroplast transcript encoded by the ribulose 1,5-bisphosphate carboxylase large subunit gene. Mol Cell Biol. 1985 Oct;5(10):2733–2745. doi: 10.1128/mcb.5.10.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson G. S., Mason J. G., Holton T. A., Koller B., Cox G. B., Whitfeld P. R., Bottomley W. A gene cluster in the spinach and pea chloroplast genomes encoding one CF1 and three CF0 subunits of the H+-ATP synthase complex and the ribosomal protein S2. J Mol Biol. 1987 Jul 20;196(2):283–298. doi: 10.1016/0022-2836(87)90690-5. [DOI] [PubMed] [Google Scholar]
- Inamine G., Nash B., Weissbach H., Brot N. Light regulation of the synthesis of the large subunit of ribulose-1,5-bisphosphate carboxylase in peas: Evidence for translational control. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5690–5694. doi: 10.1073/pnas.82.17.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. R., Mullet J. E. Regulation of chloroplast-encoded chlorophyll-binding protein translation during higher plant chloroplast biogenesis. J Biol Chem. 1986 Aug 25;261(24):11138–11145. [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus J. P., Rosenberg L. E. Purification of low-abundance messenger RNAs from rat liver by polysome immunoadsorption. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4015–4019. doi: 10.1073/pnas.79.13.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi T., Wakasugi T., Shinozaki K., Yamaguchi-Shinozaki K., Zaita N., Hidaka T., Meng B. Y., Ohto C., Tanaka M., Kato A. Six chloroplast genes (ndhA-F) homologous to human mitochondrial genes encoding components of the respiratory chain NADH dehydrogenase are actively expressed: determination of the splice sites in ndhA and ndhB pre-mRNAs. Mol Gen Genet. 1987 Dec;210(3):385–393. doi: 10.1007/BF00327187. [DOI] [PubMed] [Google Scholar]
- Rock C. D., Barkan A., Taylor W. C. The maize plastid psbB-psbF-petB-petD gene cluster: spliced and unspliced petB and petD RNAs encode alternative products. Curr Genet. 1987;12(1):69–77. doi: 10.1007/BF00420729. [DOI] [PubMed] [Google Scholar]
- Schuster G., Ohad I., Martineau B., Taylor W. C. Differentiation and development of bundle sheath and mesophyll thylakoids in maize. Thylakoid polypeptide composition, phosphorylation, and organization of photosystem II. J Biol Chem. 1985 Sep 25;260(21):11866–11873. [PubMed] [Google Scholar]
- Stern D. B., Gruissem W. Control of plastid gene expression: 3' inverted repeats act as mRNA processing and stabilizing elements, but do not terminate transcription. Cell. 1987 Dec 24;51(6):1145–1157. doi: 10.1016/0092-8674(87)90600-3. [DOI] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Obokata J, Chunwongse J, Shinozaki K, Sugiura M. Rapid splicing and stepwise processing of a transcript from the psbB operon in tobacco chloroplasts: determination of the intron sites in petB and petD. Mol Gen Genet. 1987 Oct;209(3):427–431. doi: 10.1007/BF00331145. [DOI] [PubMed] [Google Scholar]
- Thomas M. S., Bedwell D. M., Nomura M. Regulation of alpha operon gene expression in Escherichia coli. A novel form of translational coupling. J Mol Biol. 1987 Jul 20;196(2):333–345. doi: 10.1016/0022-2836(87)90694-2. [DOI] [PubMed] [Google Scholar]
- Westhoff P., Farchaus J. W., Herrmann R. G. The gene for the Mr 10,000 phosphoprotein associated with photosystem II is part of the psbB operon of the spinach plastid chromosome. Curr Genet. 1986;11(3):165–169. doi: 10.1007/BF00420602. [DOI] [PubMed] [Google Scholar]
- Westhoff P., Herrmann R. G. Complex RNA maturation in chloroplasts. The psbB operon from spinach. Eur J Biochem. 1988 Feb 1;171(3):551–564. doi: 10.1111/j.1432-1033.1988.tb13824.x. [DOI] [PubMed] [Google Scholar]
- Young E. G., Hanson M. R. A fused mitochondrial gene associated with cytoplasmic male sterility is developmentally regulated. Cell. 1987 Jul 3;50(1):41–49. doi: 10.1016/0092-8674(87)90660-x. [DOI] [PubMed] [Google Scholar]