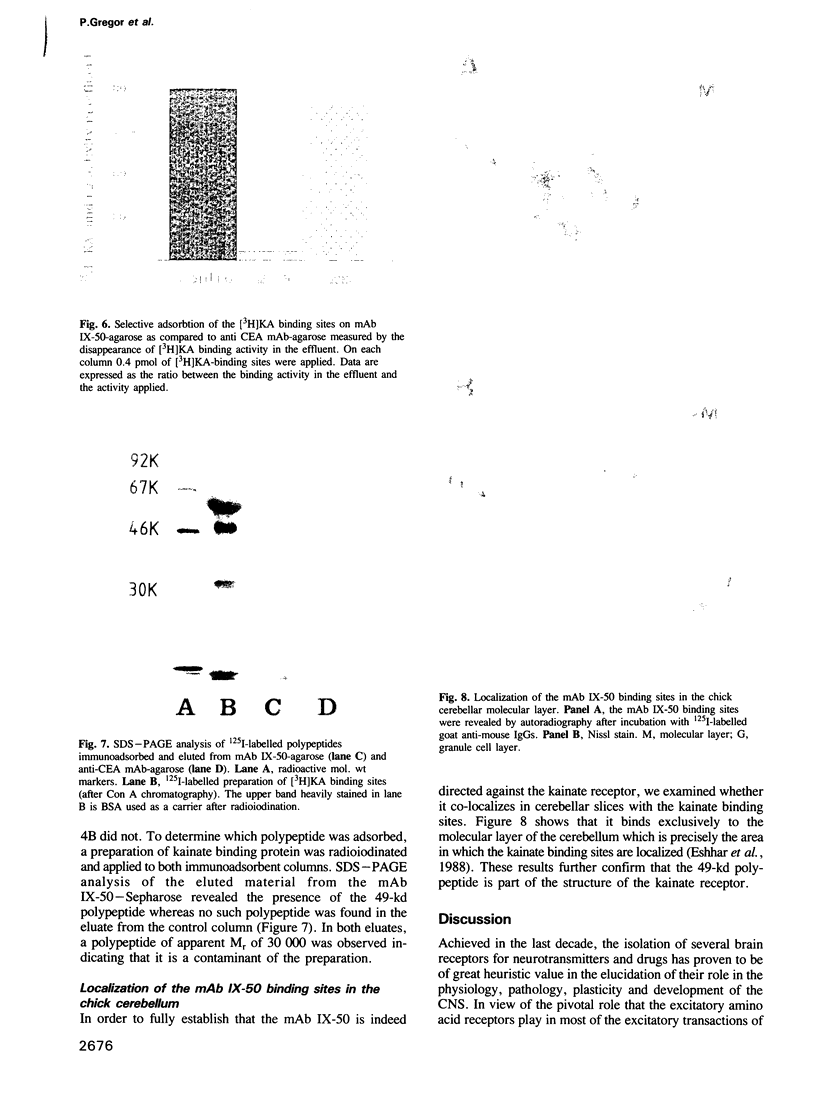
Abstract
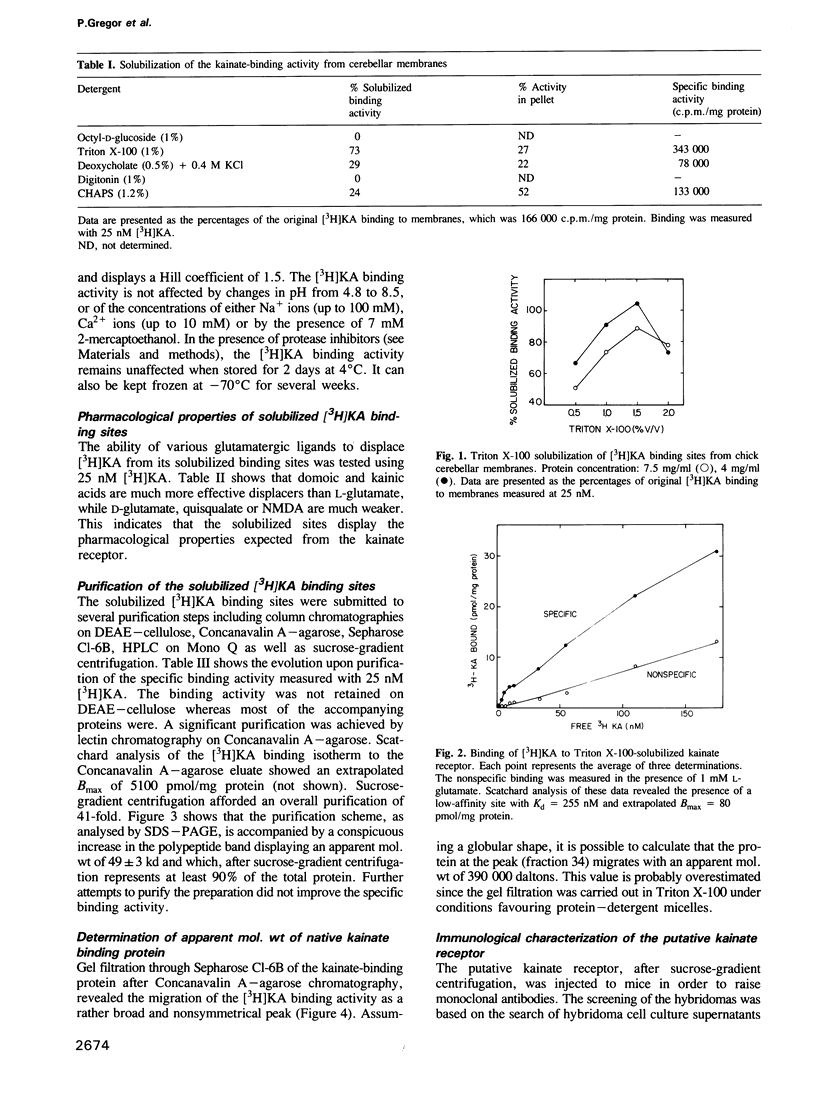
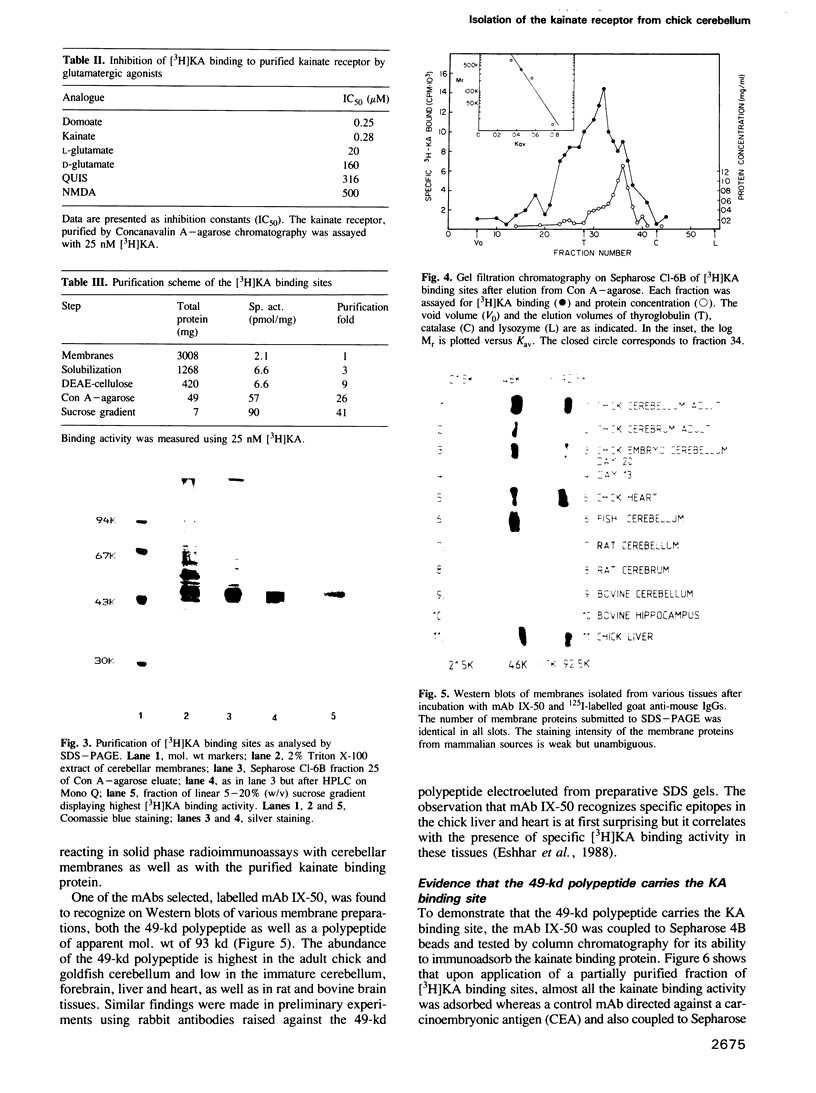
The low-affinity kainate binding sites, present at high density in chick cerebellar membranes, were solubilized with Triton X-100 and purified 41-fold. The purified kainate binding sites, therein referred to as the kainate receptor, displayed the expected pharmacological specificity: domoate = kainate much greater than L-glutamate much greater than D-glutamate, quisqualate, N-methyl-D-aspartate. Analysed by SDS-PAGE under reducing conditions, a single polypeptide with a Mr = 49,000 was observed. Western blots of membranes prepared from different brain areas and animal species were analysed using a monoclonal antibody, named mAb IX-50, raised against the purified kainate receptor. The mAb IX-50 stained the 49,000 polypeptide in chick, goldfish and mammalian brain tissues indicating its conservation during evolution. The staining intensity correlated with the density of kainate binding sites. The mAb IX-50 stained also a 93,000 polypeptide but the latter did not copurify with the 49,000 polypeptide. The kainate binding activity was selectively immunoadsorbed on mAb IX-50 coupled to Sepharose which, upon elution, released a 49,000 polypeptide. The immunohistochemical localization of mAb IX-50 binding sites in the chick cerebellar molecular layer coincided with that of the kainate receptor. We conclude that the 49,000 polypeptide is part of the kainate receptor and carries the kainate recognition site.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen J. W., Cunningham M. D., Galton N., Michaelis E. K. Immune labeling and purification of a 71-kDa glutamate-binding protein from brain synaptic membranes. Possible relationship of this protein to physiologic glutamate receptors. J Biol Chem. 1988 Jan 5;263(1):417–426. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian G. G., Moss R. L., Greaser M. Improved methodology for analysis and quantitation of proteins on one-dimensional silver-stained slab gels. Anal Biochem. 1983 Mar;129(2):277–287. doi: 10.1016/0003-2697(83)90551-1. [DOI] [PubMed] [Google Scholar]
- Hampson D. R., Wenthold R. J. A kainic acid receptor from frog brain purified using domoic acid affinity chromatography. J Biol Chem. 1988 Feb 15;263(5):2500–2505. [PubMed] [Google Scholar]
- Honoré T., Drejer J., Nielsen M. Calcium discriminates two [3H]kainate binding sites with different molecular target sizes in rat cortex. Neurosci Lett. 1986 Mar 28;65(1):47–52. doi: 10.1016/0304-3940(86)90118-7. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- London E. D., Klemm N., Coyle J. T. Phylogenetic distribution of [3H]kainic acid receptor binding sites in neuronal tissue. Brain Res. 1980 Jun 23;192(2):463–476. doi: 10.1016/0006-8993(80)90897-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voukelatou G., Angelatoy F., Kouvelas E. D. The binding properties and regional ontogeny for [3H]glutamic acid Na+-independent and [3H]kainic acid binding sites in chick brain. Int J Dev Neurosci. 1986;4(4):339–352. doi: 10.1016/0736-5748(86)90051-1. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]