Abstract
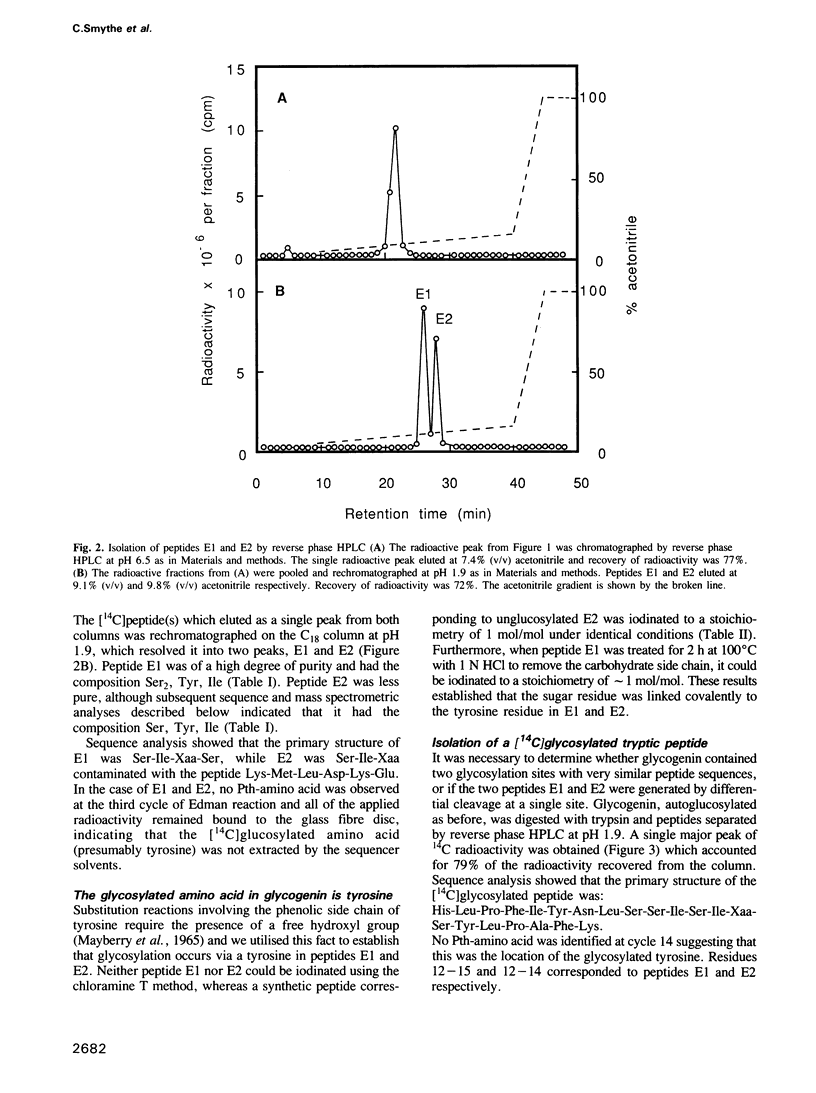
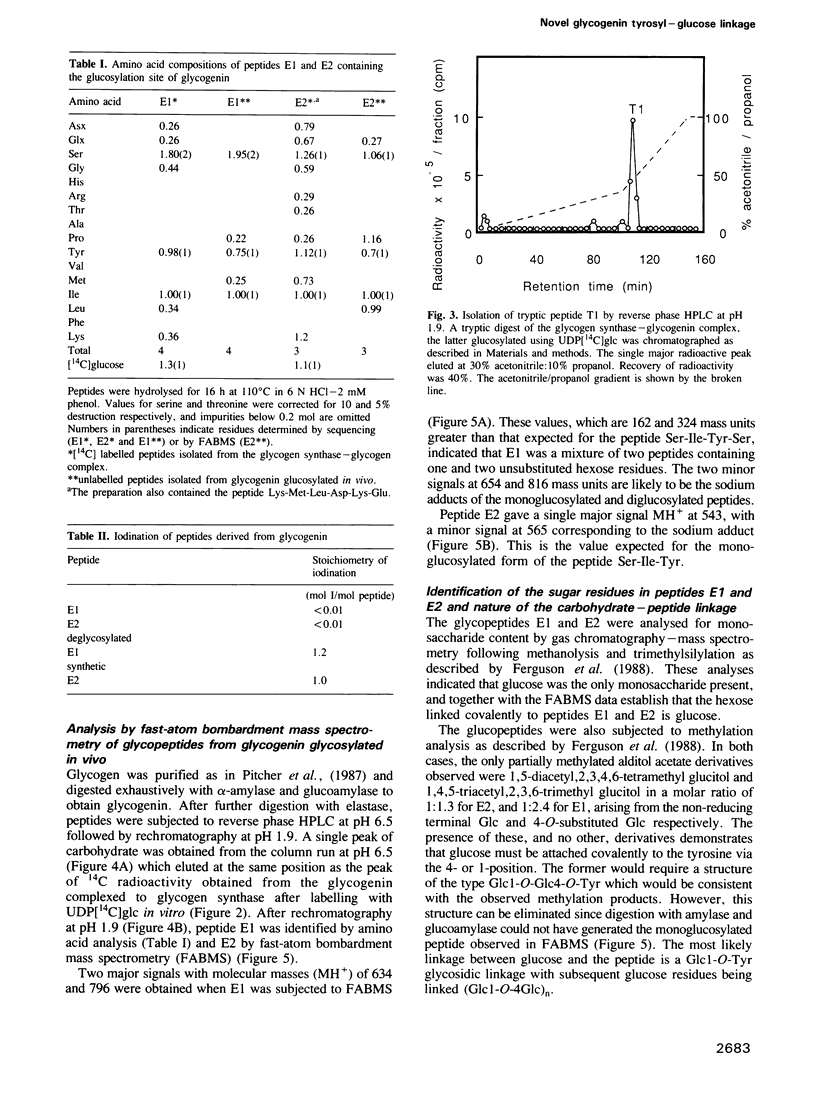
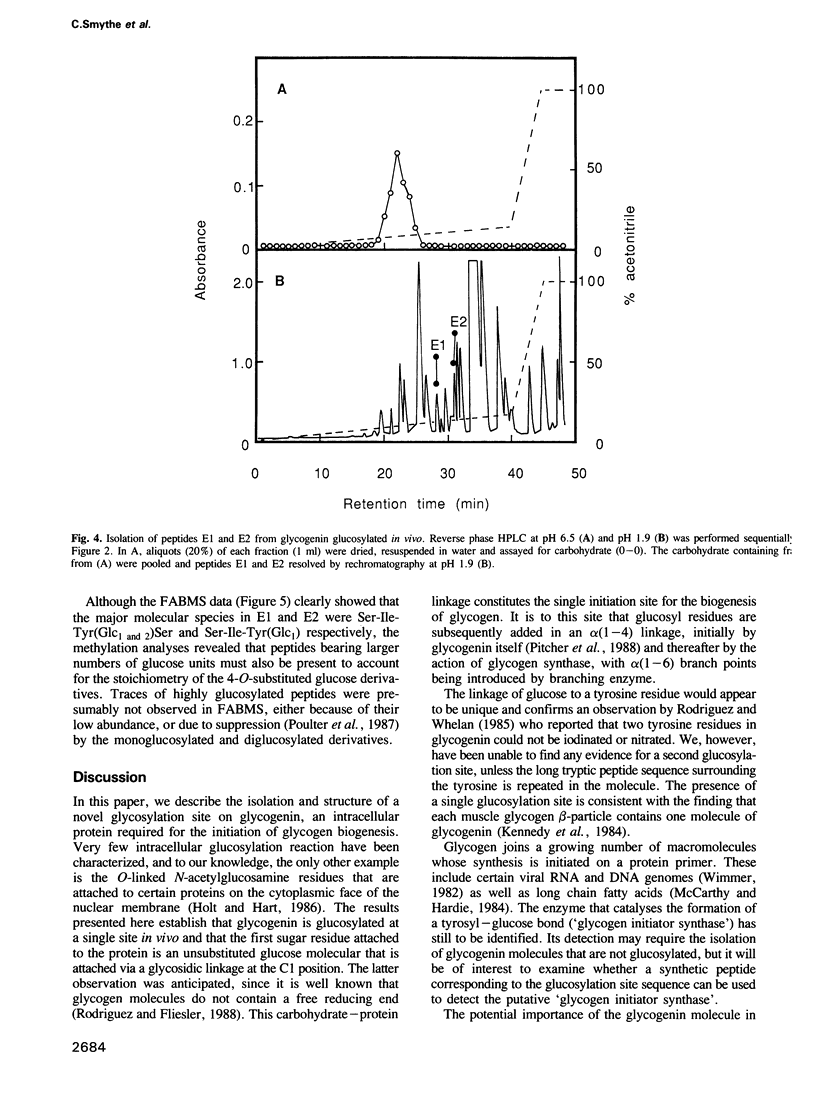
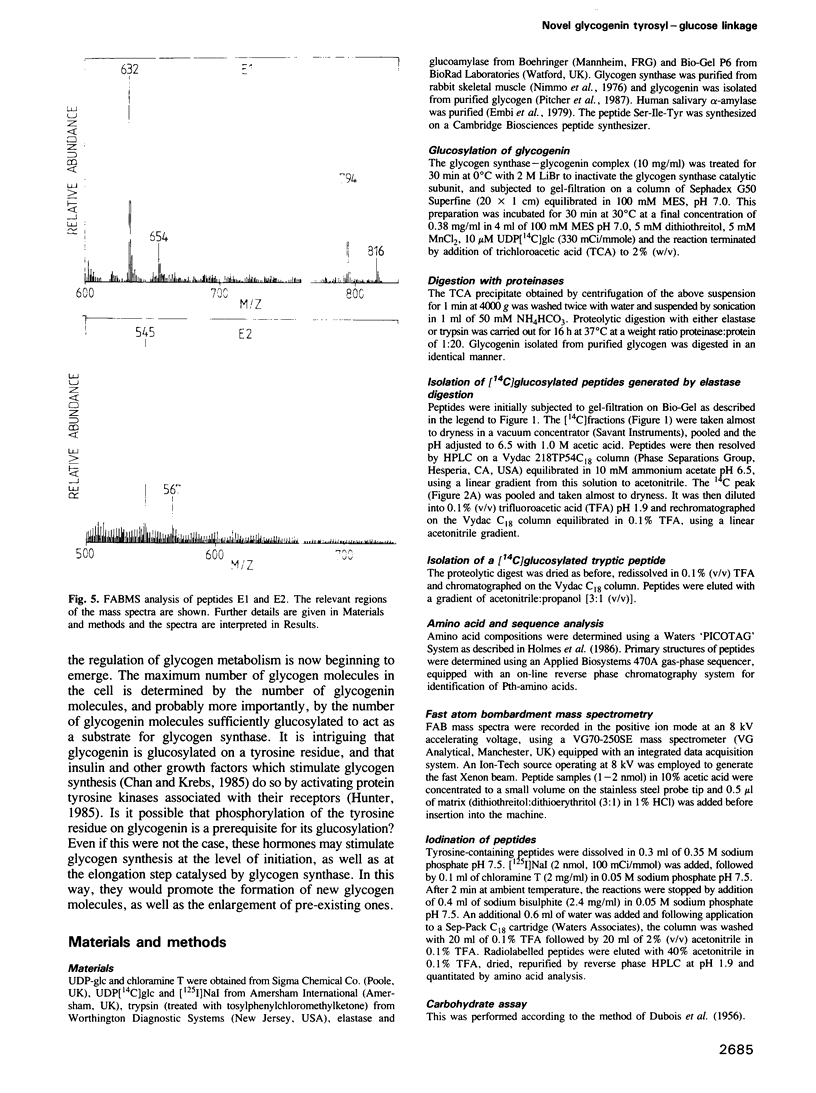
The glucosylation site on glycogenin, the protein primer required for de novo glycogen synthesis, has been identified. The glucose is attached at position C1 in a glycosidic linkage with a unique tyrosine, and the sequence surrounding this residue was found to be: His-Leu-Pro-Phe-Ile-Tyr-Asn-Leu-Ser-Ser-Ile-Ser-Ile-Tyr(Glc)-Ser-Tyr-Leu -Pro- Ala-Phe-Lys. The same tyrosine residue is glycosylated whether glycogenin is isolated as a complex with the catalytic subunit of glycogen synthase, or covalently attached to glycogen. The possibility that insulin and growth factors may enhance glycogen synthesis via stimulation of the priming reaction is discussed.
Full text
PDF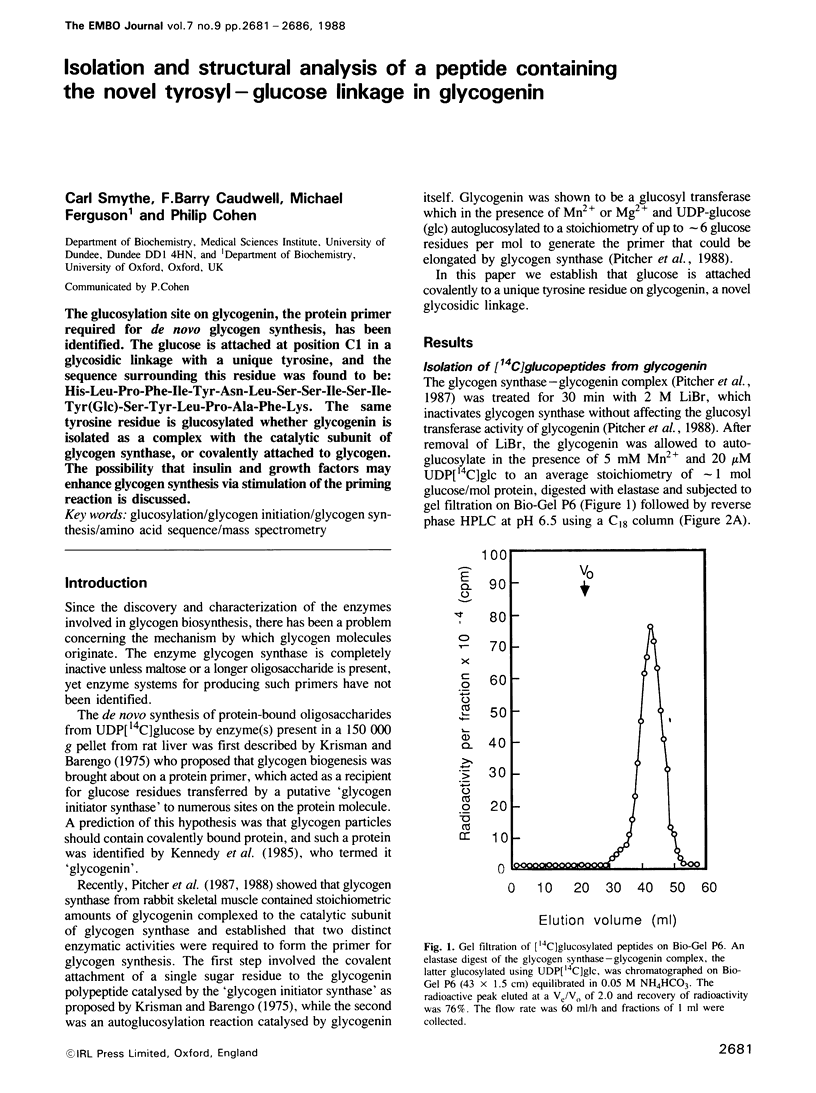

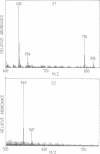
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan C. P., Krebs E. G. Epidermal growth factor stimulates glycogen synthase activity in cultured cells. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4563–4567. doi: 10.1073/pnas.82.14.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embi N., Rylatt D. B., Cohen P. Glycogen synthase kinase-2 and phosphorylase kinase are the same enzyme. Eur J Biochem. 1979 Oct 15;100(2):339–347. doi: 10.1111/j.1432-1033.1979.tb04176.x. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Homans S. W., Dwek R. A., Rademacher T. W. Glycosyl-phosphatidylinositol moiety that anchors Trypanosoma brucei variant surface glycoprotein to the membrane. Science. 1988 Feb 12;239(4841 Pt 1):753–759. doi: 10.1126/science.3340856. [DOI] [PubMed] [Google Scholar]
- Holmes C. F., Campbell D. G., Caudwell F. B., Aitken A., Cohen P. The protein phosphatases involved in cellular regulation. Primary structure of inhibitor-2 from rabbit skeletal muscle. Eur J Biochem. 1986 Feb 17;155(1):173–182. doi: 10.1111/j.1432-1033.1986.tb09473.x. [DOI] [PubMed] [Google Scholar]
- Holt G. D., Hart G. W. The subcellular distribution of terminal N-acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc. J Biol Chem. 1986 Jun 15;261(17):8049–8057. [PubMed] [Google Scholar]
- Krisman C. R., Barengo R. A precursor of glycogen biosynthesis: alpha-1,4-glucan-protein. Eur J Biochem. 1975 Mar 3;52(1):117–123. doi: 10.1111/j.1432-1033.1975.tb03979.x. [DOI] [PubMed] [Google Scholar]
- Mayberry W. E., Rall J. E., Bertoli D. Kinetics of iodination. IV. A comparison of the kinetics of iodination of L-tyrosine and some derivatives. Biochemistry. 1965 Dec;4(12):2606–2611. doi: 10.1021/bi00888a009. [DOI] [PubMed] [Google Scholar]
- Nimmo H. G., Proud C. G., Cohen P. The purification and properties of rabbit skeletal muscle glycogen synthase. Eur J Biochem. 1976 Sep;68(1):21–30. doi: 10.1111/j.1432-1033.1976.tb10761.x. [DOI] [PubMed] [Google Scholar]
- Pitcher J., Smythe C., Campbell D. G., Cohen P. Identification of the 38-kDa subunit of rabbit skeletal muscle glycogen synthase as glycogenin. Eur J Biochem. 1987 Dec 15;169(3):497–502. doi: 10.1111/j.1432-1033.1987.tb13637.x. [DOI] [PubMed] [Google Scholar]
- Poulter L., Ang S. G., Williams D. H., Cohen P. Observations on the quantitation of the phosphate content of peptides by fast-atom bombardment mass spectrometry. Biochim Biophys Acta. 1987 Jul 29;929(3):296–301. doi: 10.1016/0167-4889(87)90256-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez I. R., Fliesler S. J. A 42,000-Da protein in rabbit tissues and in a glycogen synthase preparation cross-reacts with antibodies to glycogenin. Arch Biochem Biophys. 1988 Feb 1;260(2):628–637. doi: 10.1016/0003-9861(88)90491-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez I. R., Whelan W. J. A novel glycosyl-amino acid linkage: rabbit-muscle glycogen is covalently linked to a protein via tyrosine. Biochem Biophys Res Commun. 1985 Oct 30;132(2):829–836. doi: 10.1016/0006-291x(85)91206-9. [DOI] [PubMed] [Google Scholar]
- Wimmer E. Genome-linked proteins of viruses. Cell. 1982 Feb;28(2):199–201. doi: 10.1016/0092-8674(82)90335-x. [DOI] [PubMed] [Google Scholar]