SUMMARY
Seasonal changes in disease activity have been observed in multiple sclerosis, an autoimmune disorder that affects the central nervous system. These epidemiological observations suggest that environmental factors influence the disease course. Here we report that melatonin levels, whose production is modulated by seasonal variations in night length, negatively correlate with multiple sclerosis activity in humans. Treatment with melatonin ameliorates disease in an experimental model of multiple sclerosis and directly interferes with the differentiation of human and mouse T cells. Melatonin induces the expression of the repressor transcription factor Nfil3, blocking the differentiation of pathogenic Th17 cells as well as boosts the generation of protective Tr1 cells via Erk1/2 and the transactivation of the IL-10 promoter by ROR-α. These results suggest that melatonin is another example of how environmental-driven cues can impact on T cell differentiation and have implications for autoimmune disorders such as multiple sclerosis.
Graphical Abstract
INTRODUCTION
Multiple Sclerosis (MS) is an immune-mediated disease of the central nervous system (CNS) that is thought to result from the destruction of myelin by autoreactive T cells. CD4+ T cells characterized by the production of IFN-γ (Th1 cells) or IL-17 (Th17 cells) are considered important contributors to MS immunopathogenesis (Miossec et al., 2009; Sospedra and Martin, 2005; Steinman, 2014). FoxP3+ regulatory T cells (Tregs) and IL-10 secreting type 1 regulatory T cells (Tr1) regulate the activity of effector T cells, accordingly deficits in Tregs and Tr1 cells have been described in MS (Astier et al., 2006; Sakaguchi et al., 2010; Viglietta et al., 2004). Thus, the balance between effector and regulatory T cells controls MS disease activity (Miossec et al., 2009; Sospedra and Martin, 2005; Steinman, 2014).
Genetic polymorphisms have been associated with MS risk and/or pathogenesis (Beecham et al., 2013; Sawcer et al., 2011). However, environmental factors such as infections (Ascherio et al., 2001; Correale and Farez, 2007; Correale et al., 2006), sodium intake (Farez et al., 2014), smoking (Hernan, 2005) and vitamin D levels (Ascherio et al., 2014) are also known to affect MS development and course. Lower levels of vitamin D, for example, are associated with higher relapse rates (Runia et al., 2012; Simpson et al., 2010). As a result of the regulation of its synthesis by sun exposure, a significant seasonal fluctuation on vitamin D levels is observed in most locations, with a peak in spring-summer and a nadir in autumn and winter (Rosecrans and Dohnal, 2014). Thus, based on the reported anti-inflammatory effects of vitamin D (Correale et al., 2009) (Ascherio et al., 2010), MS relapse occurrence is predicted to peak during autumn and winter. However, several studies, including a meta-analysis (Jin et al., 2000) and a recent multicentric study (Spelman et al., 2014) found that MS disease activity is higher in spring and summer, suggesting that additional factors play a role in MS relapse seasonality.
Here we report that melatonin levels, which peak in autumn-winter, show an inverse correlation with clinical disease activity in MS patients. Moreover, melatonin limits the development of EAE and controls Th17 and Tr1 cell differentiation. Thus, seasonal changes in melatonin levels may contribute to the decreased disease activity observed in autumn and winter through a mechanism mediated, at least partially, by the regulation of effector and regulatory T cells.
RESULTS
Melatonin levels are negatively correlated with MS clinical relapses
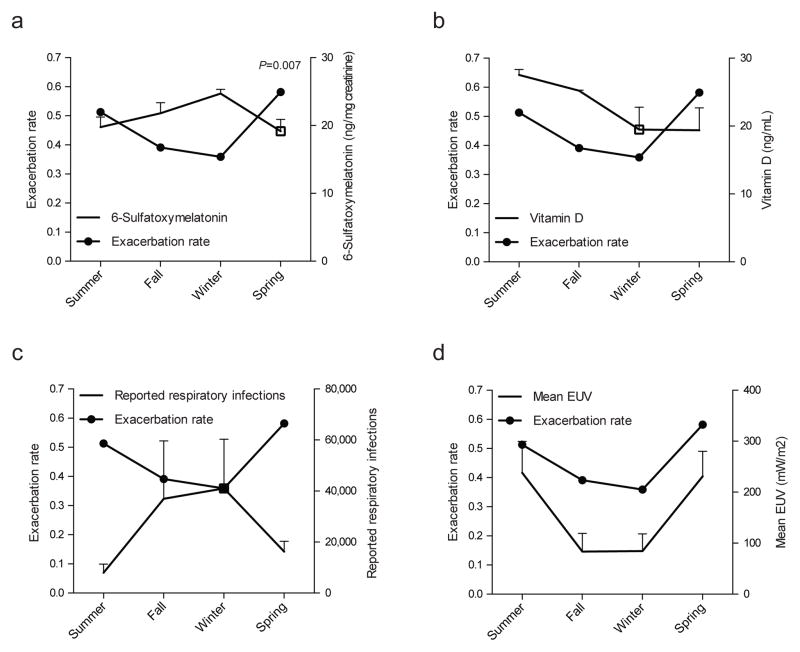
We first established the seasonality of MS relapses in our cohort of 139 relapsing remitting MS patients (Table 1). Using a Poisson regression model, we detected a 32% reduction in the number of relapses occurring during fall and winter (incidence rate-ratio, IRR 0.682, 95% CI 0.49–0.95, P=0.02). Hence, the MS patient cohort used in this study shows the seasonality of MS relapses previously described for other cohorts (Jin et al., 2000; Spelman et al., 2014).
Table 1.
Baseline and clinical characteristics of the study population
| All participants (n=139) | |
|---|---|
|
| |
| Age (years, mean ± SD) | 38.6 ± 10.9 |
|
| |
| F:M (n) | 87:52 |
|
| |
| Disease duration (years, median, range) | 6 (1–20) |
|
| |
| EDSS (median, range) | 1 (0–4) |
|
| |
| Treatment (n) | |
| None | 2 |
| Interferon | 64 |
| Glatiramer Acetate | 34 |
| Natalizumab | 2 |
| Fingolimod | 26 |
| Other | 11 |
|
| |
| 6-SM levels (ng/mg creatinine, mean ± SEM) | |
| Summer | 19.8 ± 1.5 |
| Fall | 21.8 ± 1.6 |
| Winter | 24.7 ± 0.6 |
| Spring | 19.2 ± 1.7 |
|
| |
| Vitamin D levels (ng/mL) | |
| Summer | 27.8 ± 0.8 |
| Fall | 25.2 ± 0.1 |
| Winter | 21.7 ± 3.2 |
| Spring | 21.7 ± 3.3 |
Melatonin production is stimulated by darkness and follows a seasonal pattern with higher levels during fall and winter (Brzezinski, 1997). Melatonin impacts several biological processes, including the circadian clock and the immune response (Brzezinski, 1997). Thus, we investigated the relationship between melatonin and MS disease activity by measuring 6-sulfatoxymelatonin (6-SM) levels in relapsing-remitting MS patients. Since 6-SM is the main melatonin metabolite, its levels in first morning urine are strongly correlated with nighttime melatonin secretion, supporting its use in epidemiological studies (Graham et al., 1998; McMullan et al., 2013). In agreement with previous reports (Morera and Abreu, 2007; Ueno-Towatari et al., 2007), we detected increased melatonin secretion during fall and winter, with lower levels during spring and summer (Fig. 1a and Table 1). Moreover, we found a significant negative correlation between 6-SM levels and MS exacerbation rates (P<0.01 Spearman’s correlation). This was further confirmed in an age and gender-adjusted Poisson regression model, with a 3% reduction in the number of relapses for each 6-SM unit increase (IRR 0.97, 95% CI 0.95–0.99, P=0.007). Finally, to test whether the relationship between melatonin levels and exacerbation rate was synchronous, we lagged the occurrence of relapses for 1 (IRR 1.01, 95% CI 0.97–1.05; P=0.7), 2 (IRR 1.03, 95%CI 0.99–1.07; P=0.1), and 3 months (IRR 1.03, 95% CI 0.99–1.07; P=0.7), with no evidence of a lagged effect in relapse occurrence.
Figure 1. Melatonin levels show an inverse correlation with MS clinical relapses.
(a) Exacerbation rate for each season was estimated for the duration of the follow-up and depicted in the primary axis. 6-sulfatoxymelatonin levels measured in first morning urine in each season is depicted as mean ± s.e.m. in secondary axis. P value corresponds to Poisson regression model. Lack of correlation between exacerbation rate and Vitamin D (b), reported respiratory infections (c), and UV radiation in Buenos Aires city (d). See also Table 1.
We also assessed vitamin D levels and, as previously reported for healthy controls and MS patients in our region (Correale et al., 2009; Fassi et al., 2003), overall levels were low throughout the year with higher levels during summer but no significant correlation with MS relapses (Fig. 1b). Finally, we did not detect a correlation between MS relapses and additional environmental factors such as reported upper respiratory tract infections and UV incidence, as determined by national registries and NASA satellites, respectively (Figs. 1c,d). Thus, higher melatonin levels during fall and winter are associated with a reduction in clinical relapses.
Melatonin ameliorates experimental autoimmune encephalitis
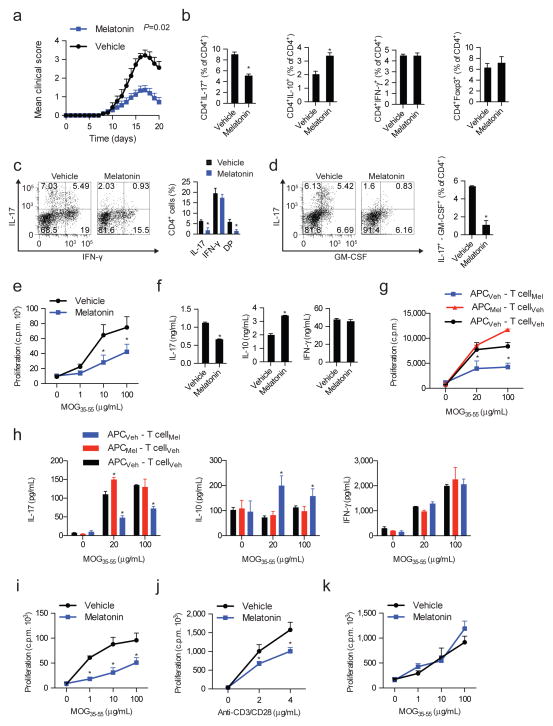
Based on our epidemiological findings, we studied the effects of melatonin on CNS inflammation using the Experimental Autoimmune Encephalitis (EAE) model of MS. Naïve C57BL/6 wild-type mice were immunized with MOG35-55 and treated daily with melatonin (5mg/kg, intraperitoneally) or vehicle. Melatonin administration ameliorated EAE clinical symptoms (Fig. 2a, Table S1 and Fig. S1a). The amelioration of EAE was associated with a decreased number and frequency of Th17 cells in spleen, lymph nodes and CNS; this decrease was also detected in IL-17+ IFNγ+ and IL-17+ GM-CSF+ CD4+ T cells that have been associated to the pathogenesis of EAE (Codarri et al., 2011; El-Behi et al., 2011; Lee et al., 2012a) (Figs. 2c,d). We also detected a concomitant increase in IL-10 secreting CD4+ T cells; no significant changes were detected in the number or frequency of other T cell subsets, B cells, γδ T cells or innate lymphoid cells (ILCs) (Figs. 2b and Fig. S1b–d).
Figure 2. Melatonin administration ameliorates EAE.
(a) EAE development in C57/B6 treated with vehicle (0.01% DMSO) or melatonin (5mg/kg). Data are representative of three independent experiments (means and s.e.m.) (n ≥ 20 mice/group). P value corresponds for the effect of treatment in a repeated measures mixed effect model. (b) Flow cytometry analysis of IL-17+, IL10+, IFN-γ+ and FoxP3+ CD4+ cells from the spleen of vehicle or melatonin treated mice at day 7 after disease induction. At least 4 mice were analyzed per group and data is presented as mean ± SEM. * P<0.05 of unpaired T-test. (c-d) Flow cytometry analysis of IL-17+, IFN-γ+, IL-17+-IFN-γ+ (DP) and IL-17+-GM-CSF+ CD4+ T cells from the CNS of control- or melatonin-treated mice at the clinical peak of EAE * P<0.05 of unpaired T-test. (e) Proliferative responses of CD4+ T cells to MOG35-55 of vehicle or melatonin treated mice. At least 3 mice were analyzed per group and data is presented as mean ± s.e.m. * P<0.05 of one-way ANOVA. (f) Cytokine secretion by proliferating CD4+ T cells from vehicle and melatonin treated. Data are representative of three independent experiments (means and s.e.m.)* P<0.05 of unpaired T-test. (g) Proliferative responses and cytokine profile (h) of CD4+ T cells in co-culture with dendritic cells derived from melatonin-treated or untreated mice. Data are representative of three independent experiments (means and s.e.m.). * P<0.05 of one-way ANOVA. (i) Proliferative responses of melatonin treated 2D2 CD4+ T cells to MOG35-55 in the presence of dendritic cells. Data are representative of three independent experiments (means and s.e.m.)* P<0.05 of one-way ANOVA. (j) Proliferative responses of melatonin treated 2D2 CD4+ T cells to MOG35-55 stimulated only with anti-CD3 and anti-CD28. Data are representative of three independent experiments (means and s.e.m.)* P<0.05 of one-way ANOVA. (k) Proliferative responses of treated 2D2 CD4+ T cells to MOG35-55 stimulated melatonin-treated DCs. Data are representative of three independent experiments (means and s.e.m.). See also Fig. S1a–e
To further characterize the effects of melatonin on the encephalitogenic T-cell response, we analyzed the recall response to MOG35-55. Splenocytes from melatonin-treated mice showed adiminished proliferative response to MOG35-55, reduced IL-17 concomitant with increased IL-10 production, however no significant effects were detected on IFN-γ production (Figs. 2e,f). Thus, melatonin arrests the encephalitogenic Th17 cell response.
To investigate if melatonin acts directly on T cells or whether it controls the T-cell response indirectly through its effects on antigen presenting cells, we co-incubated sorted CD4+ T cells from melatonin-treated or control mice with treatment-switched dendritic cells (DCs). When compared to controls isolated from vehicle-treated mice, CD4+ T cells from melatonin-treated mice co-incubated with splenic DCs isolated from control mice showed decreased proliferation and IL-17 secretion, concomitant with increased IL-10 production, (Figs. 2g,h). Conversely, we did not detect significant differences when we used DCs isolated from melatonin or vehicle treated mice to activate CD4+ T cells from control-treated mice.
In support for a direct effect of melatonin on T cells, melatonin suppressed the in vitro activation of naive 2D2+ transgenic T cells with MOG35-55 and DCs (Fig. 2i, Fig. S1e) or with antibodies to CD3 and CD28 in the absence of DCs (Fig. 2j). Pretreatment of DCs with melatonin did not affect their ability to activate 2D2+ T cells in the presence of MOG35-55 (Fig. 2k). Melatonin did not increase apoptosis in CD4+ T cells stimulated with antibodies against CD3 and CD28, as indicated by the analysis of annexin V and propidium iodide staining by flow cytometry or the expression of Bcl-xl levels (Fig. S1f,g). IL-10 blockade, however, abrogated the suppressive effects of melatonin on T-cell proliferation (Fig. S1h).
Melatonin affects human T-cell differentiation
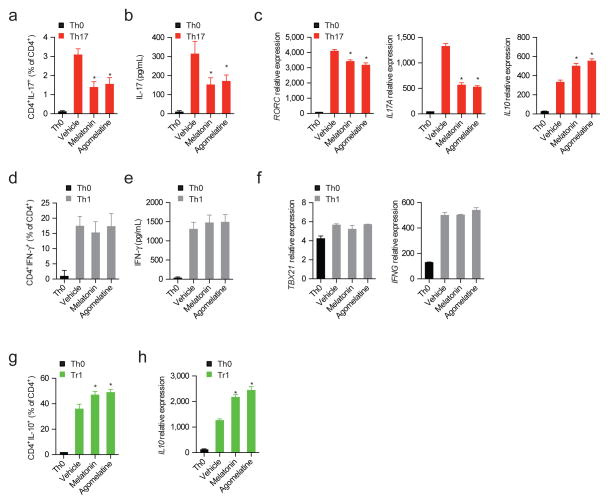
We then studied the effects of melatonin on human CD4+ T cells. In addition, we also analyzed the effects of agomelatine, which activates melatonin-dependent signaling (Hickie and Rogers, 2011). Based on the effects of melatonin administration on T cells during EAE, we focused our studies on human Th17 and Tr1 cells. Melatonin and agomelatine reduced the production of IL-17, RORC and IL17A expression by human CD4+ T cells activated under Th17 polarizing conditions (Figs. 3a–c and Fig. S2), no effect was detected on the differentiation of human Th1 cells (Figs. 3d–f). Concomitantly, melatonin and agomelatine increased IL10 expression. Indeed, melatonin and agomelatine also increased IL-10 production by human CD4+ T cells activated under Tr1 polarizing conditions (Figs. 3g,h).
Figure 3. Melatonin interferes with human Th17 cell differentiation and boosts Tr1 generation.
(a) Flow cytometry analysis of IL-17 expression in human Th17 differentiated CD4+ T cells (IL-1β, IL-6 and TGF-β1) in the presence or absence of melatonin (500ng/ml) and agomelatine (500ng/ml). Data are representative of three independent experiments (means and s.e.m.) * P<0.05 of one-way ANOVA. (b). Cytokine quantification by ELISA of IL-17 in human Th17 differentiated CD4+ T cells in the presence or absence of melatonin (500ng/ml) and agomelatine (500ng/ml). Data are representative of three independent experiments (means and s.e.m.) * P<0.05 of one-way ANOVA. (c) RT-PCR analysis of Th17 cells cultured as in a. Data are representative of three independent experiments (means and s.e.m.) * P<0.05 of one-way ANOVA. (d). Cytokine quantification by ELISA of IFN-γ in human Th1 differentiated CD4+ T cells in the presence or absence of melatonin (500ng/ml) and agomelatin (500ng/ml). Data are representative of three independent experiments (means and s.e.m.) * P<0.05 of one-way ANOVA. (e) RT-PCR analysis of Th1 cells cultured as in d. Data are representative of three independent experiments (means and s.e.m.) * P<0.05 of one-way ANOVA. (f) Flow cytometry analysis of IL-10 expression in human Tr1 differentiated CD4+ T cell in the presence or absence of melatonin (500ng/ml)and agomelatin (500ng/ml). Data are representative of three independent experiments (means and s.e.m.) * P<0.05 of one-way ANOVA. (g) Quantitative PCR analysis of Tr1 cells cultured as in f. Data are representative of three independent experiments (means and s.e.m.). * P<0.05 of one-way ANOVA. See also Fig. S2
To further investigate the role of melatonin on the immune response in MS, we analyzed the correlation between serum melatonin levels and IL17 and IL10 expression in peripheral CD4+ T cells of 26 RRMS patients (Table S2). Using an age- and gender-adjusted linear regression model we detected a negative correlation between melatonin in serum and IL17 expression in peripheral CD4+ T cells (P=0.012): higher serum melatonin levels were associated to lower IL17 expression (Table S3). Conversely, linear regression analysis identified a positive correlation between higher IL10 expression in peripheral CD4+ T cells and melatonin in serum (P=0.003). We did not detect a significant correlation between melatonin levels and the expression of RORC, NR1D1 or NFIL3 in CD4+ T cells (Table S3). Thus, melatonin modulates the differentiation of human Th17 and Tr1 cells in vitro, and endogenous melatonin levels are associated to the expression levels of IL17 and IL10 in peripheral CD4+ T cells in RRMS patients.
Melatonin interferes with Th17 generation
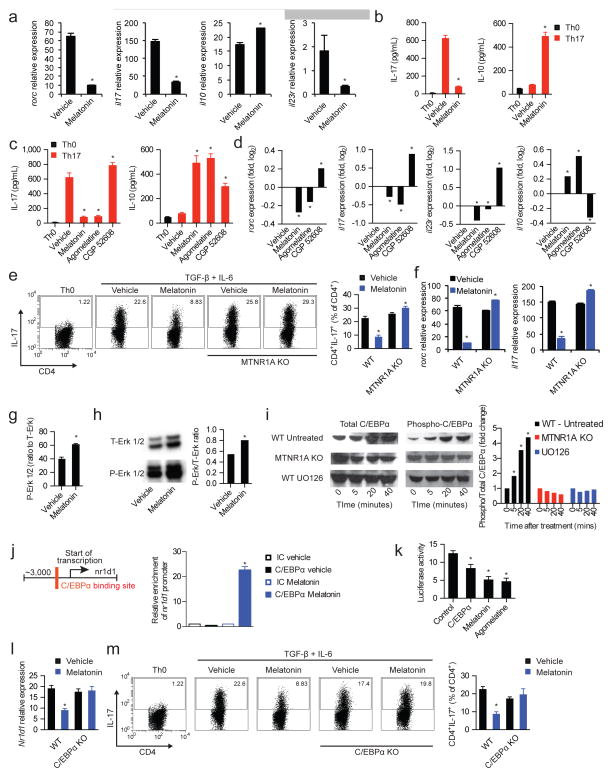
Together with Th1 cells, Th17 cells promote the development of EAE and are thought to contribute to MS pathogenesis (Korn et al., 2009). Based on the suppressive effects of melatonin on EAE and IL-17 production by CD4+ T cells, we studied the effects of melatonin on murine Th17 cell differentiation. Melatonin interfered with the differentiation of Th17 cells in vitro as indicated by the expression of rorc, IL-17, and the IL-23 receptor necessary for the differentiation of Th17 cells into fully pathogenic cells; no effects were detected on the differentiation of FoxP3+ iTregs, Th1 or Th2 cells. (Fig. 4a,b and Fig. S3) (Lee et al., 2012b). Melatonin also increased the expression of IL-10, associated to non-pathogenic Th17 cells (Lee et al., 2012b; McGeachy et al., 2007) (Fig. 4a,b).
Figure 4. Melatonin interferes with Th17 cell differentiation via the Erk1/2-C/EBPα pathway.
(a) CD4+ naïve T cells were differentiated into Th17 cells by the addition of TFG-β, IL-6 (0 h) and IL-23 (48hs) in the presence or absence of melatonin (2ng/ml) and analyzed by RT-PCR after 72hs. Displayed image is representative of five experiments. * P<0.05 of unpaired T-test (b) Cytokine secretion analysis of IL-17 and IL-10 after 72hs of culture as in a. Data are representative of three independent experiments (means and s.e.m.)* P<0.05 of unpaired T-test (c) Cytokine secretion in Th17 differentiated CD4+ T cells in the presence or absence of melatonin (2ng/ml), agomelatine (20ng/ml, MTNR1A ligand) and CGP 52608 (20ng/ml, ROR-α ligand). Data are representative of three independent experiments (means and s.e.m.). * P<0.05 of one-way ANOVA. (d) RT-PCR analysis of Th17 cells cultured as in c. Data are representative of three independent experiments (means and s.e.m.). * P<0.05 of one-way ANOVA. (e) Flow cytometry analysis of IL-17 expression as in a, in wild type mice and MTNR1A-deficient mice. Data are representative of three independent experiments (means and s.e.m.) * P<0.05 of unpaired T-test. (f) Quantitative PCR analysis of wild type and MTNR1A deficient mice cultured as in e. Data are representative of three independent experiments (means and s.e.m.)* P<0.05 of unpaired T-test. (g) Signal transduction profiling using reverse protein arrays. Data are representative of two independent experiments (means and s.e.m.)* P<0.05 of unpaired T-test. (h) Immunoblot analysis of T- and P-Erk1/2. Data are representative of two independent experiments (means and s.e.m.). (i) Immunoblot analysis of T- and P-C/EBPα Data are representative of two independent experiments (means and s.e.m.). (j) Putative binding sites of C/EBPα in nr1d1 (left panel); chromatin immunoprecipitation with anti-C/EBPα (right panel). Data are representative of three independent experiments (means and s.e.m.) * P<0.05 of one-way ANOVA. (k) Luciferase activity of HEK-293 cells transfected with a luciferase reporter construct for the nr1d1 promoter. Data are representative of three independent experiments (means and s.e.m.)* P<0.05 of unpaired T-test. (l) Flow cytometry analysis of IL-17 expression as in a, in wild type mice and C/EBPα-deficient mice. Data are representative of two independent experiments (means and s.e.m.) * P<0.05 of one-way ANOVA. See also Fig. S3–S4
IFNγ and IL-2 have been shown to limit Th17 cell differentiation (Korn et al., 2009). However, in our studies Th17 cells were differentiated in the presence of IFNγ-blocking antibodies, and IL-2 blocking antibodies failed to abrogate the suppression of Th17 differentiation by melatonin (Fig. S4a,b). Thus, melatonin suppresses Th17 cell differentiation through a mechanism independent of IFNγ or IL-2.
Physiological concentrations of melatonin result in the activation of signaling pathways controlled by membrane and nuclear receptors (Brzezinski, 1997). The melatonin membrane receptor MTNR1A is expressed by a variety of tissues including cells of the immune system (Jockers et al., 2008; Pozo et al., 1997). In addition, melatonin binds to the nuclear retinoid-related orphan receptor alpha (ROR-α), which is also expressed by immune cells (Pozo et al., 2004) and plays a role in Th17 development (Yang et al., 2008). We detected the expression of both MTNR1A and ROR-α on Th17 cells (Fig. S4c,d). To study the role of MTNR1A signaling on the effects of melatonin on Th17 cells, we used the MTNR1A-specific agonists agomelatine and ramelteon (Karim et al., 2006) (Fig. S4e). Similar to our observations with melatonin, MTNR1A activation by agomelatine or ramelteon suppressed the differentiation of Th17 cells (Figs. 4c,d and Fig. S4f,g). Conversely, melatonin failed to suppress the differentiation of MTNR1A-deficient (MTNR1A KO) Th17 cells used (Figs. 4e,f). Thus, MTNR1A mediates the suppressive effects of melatonin on Th17 cell differentiation.
Melatonin suppresses Th17 cell differentiation via Erk1/2 and C/EBPα activation
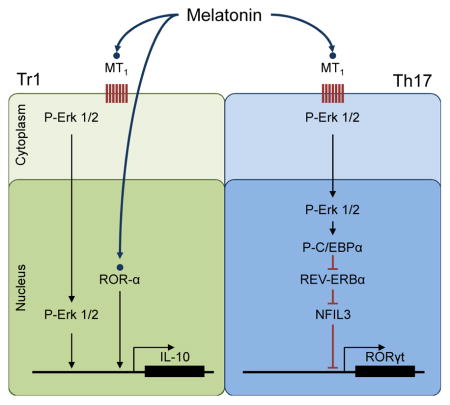
REV-ERBα (encoded by nr1d1) is a component of the circadian clock that promotes Th17 differentiation by limiting the expression of NFIL3, a direct inhibitor of rorc transcription (Yu et al., 2013). Melatonin regulates the activity of both circadian and seasonal clocks (Pévet, 2003). Indeed, melatonin levels show a circadian inverse correlation with nr1d1 expression, suggesting that melatonin affects REV-ERBα expression (Kojetin and Burris, 2014). Thus, we investigated whether melatonin acts on REV-ERBα to suppress Th17 cell differentiation.
Using reverse protein arrays (Farez et al., 2009) we analyzed signaling pathways triggered by melatonin in T cells and detected an MTNR1A-dependent increase in the activation of Erk1/2 (Fig. 4g,h; Fig. S4h,i). Of note, Erk1/2 inhibition has been previously shown to enhance Th17 cell differentiation (Tan and Lam, 2010) and Erk1/2 phosphorylation has been linked to the reduced expression of REV-ERB proteins (Castellano et al., 2014; Kojetin and Burris, 2014), but the mechanism involved and its relevance for T cells has not been characterized yet. Through a bioinformatic analysis of the nr1d1 promoter we identified a binding site for the CAAT/enhancer-binding protein α (C/EBPα), a leucine zipper transcription factor involved in the regulation of cellular differentiation (Lekstrom-Himes and Xanthopoulos, 1998). C/EBPα is a downstream target of Erk1/2 activated by phosphorylation (Johnson, 2005). Thus, we analyzed whether Erk1/2 regulates the transcriptional activity of the nr1d1 promoter in a C/EBPα dependent manner.
Th17 cell differentiation in the presence of melatonin led to C/EBPα phosphorylation and the recruitment of C/EBPα to the nr1d1 promoter (Figs. 4i,j). C/EBPα phosphorylation and recruitment to the nr1d1 promoter were suppressed in MTNR1A KO T cells and in the presence of the Erk1/2 inhibitor UO216 (Figs. 4i,j). Hence, melatonin triggers the recruitment of C/EBPα to the nr1d1 promoter in an MTNR1A- and Erk1/2-dependent manner.
To analyze the effects of C/EBPα on the transcriptional activity of the nr1d1 promoter we used a reporter construct in which the nr1d1 promoter controls luciferase expression. Treatment of nr1d1 reporter-transfected HEK293 cells with melatonin or agomelatine resulted in decreased luciferase activity and similar effects were achieved by C/EBPα overexpression (Fig. 4k). Finally, to investigate the role of C/EBPα on the suppression of Th17 cell differentiation by melatonin we used C/EBPα deficient T cells (Yang et al., 2005). C/EBPα-deficiency abrogated the decrease in nr1d1 expression and the suppression of Th17 differentiation induced by melatonin (Fig 4l,m). Thus, melatonin suppresses the differentiation of Th17 through a mechanism mediated by MTNR1A, Erk1/2 and C/EBPα.
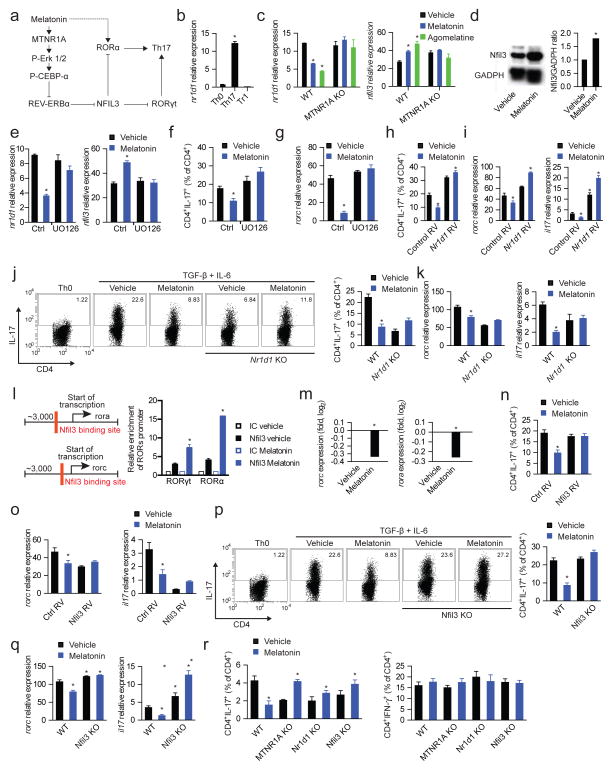
Melatonin inhibits ROR-γt and ROR-α expression in Th17 cells by inducing Nfil3
NFIL3 limits Th17 cell differentiation by suppressing the expression of ROR-γt (Yu et al., 2013). REV-ERBα inhibits nfil3 expression (Yu et al., 2013). Thus, we hypothesized that the decrease in nr1d1 expression induced by melatonin results in the NFIL3-dependent inhibition of rorc expression (Fig. 5a). We detected nr1d1 expression in Th17 cells, but not in Th0 or Tr1 cells (Fig. 5b). Melatonin suppressed nr1d1 expression during Th17 cell differentiation, resulting in a concomitant increase in the expression of the ROR-γt repressor NFIL3 (Figs. 5c,d). In agreement with our results on Th17 cell differentiation, the regulation of REV-ERBα and NFIL3 expression by melatonin was mediated by its membrane receptor MTNR1A and Erk1/2 (Figs. 5c–g). The relevance of the regulation of REV-ERBα expression for the modulation of Th17 cell differentiation by melatonin was confirmed in nr1d1 overexpression experiments and by the use of REV-ERBα deficient T cells. Nr1d1 overexpression and REV-ERBα deficiency abrogated the effects of melatonin on Th17 cell differentiation (Figs. 5h–k). Hence, MTNR1A-dependent signaling triggered by melatonin suppresses Th17 cell differentiation through the regulation of REV-ERBα expression.
Figure 5. Melatonin interferes with Th17 cell differentiation by limiting NFIL3 expression.
(a) Schematic diagram of the proposed mechanisms mediating the effects of melatonin on Th17 cell differentiation. (b) RT-PCR analysis of nr1d1 expression in CD4+ T cells activated under Th0, Th17 and Tr1 polarizing conditions for 3 days. Data are representative of three independent experiments (means and s.e.m.). * P<0.05 of unpaired T-test. (c) RT-PCR analysis of nr1d1 (left panel) and nfil3 (right panel) expression in CD4+ T cells activated under Th17 polarizing conditions for 3 days treated with vehicle, melatonin (2ng/ml) or agomelatine 20ng/ml). Data are representative of three independent experiments (means and s.e.m.). * P<0.05 of unpaired T-test. NFIL3 expression was further confirmed by western blot (d) Data are representative of two independent experiments (means and s.e.m.). (e) RT-PCR analysis of nfil3 expression in CD4+ T cells activated under Th17 polarizing conditions for 3 days in the presence of melatonin (2ng/ml) and/or UO126. Data are representative of five independent experiments (means and s.e.m.). * P<0.05 of one-way ANOVA. (f,g) Flow cytometry analysis of IL-17 expression (f) and rorc expression (g) in CD4+ T cells activated under Th17 polarizing conditions in the presence of melatonin (2ng/ml) and/or UO126. Data are representative of three independent experiments (means and s.e.m.) * P<0.05 of one-way ANOVA. (h,i) Flow cytometry analysis of IL-17 expression (h) and rorc and il17 expression (i) in CD4+ T cells activated under Th17 polarizing conditions in the presence of melatonin (2ng/ml), following infecting with a control or an nr1d1-encoding retrovirus. Data are representative of three independent experiments (means and s.e.m.) * P<0.05 of one-way ANOVA. (j,k) Flow cytometry analysis of IL-17 expression (j) and rorc and il17 expression (k) ) in wild type and REV-ERBα deficient CD4+ T cells activated under Th17 polarizing conditions in the presence of melatonin (2ng/ml). Data are representative of three independent experiments (means and s.e.m.) * P<0.05 of one-way ANOVA. (l) Putative binding sites of Nfil3 in rorc and rora (left panel); ChIP analysis of the interaction of NFIL3 with its putative binding sites in CD4+ T cells activated under Th17 polarizing conditions (right panel). Data are representative of three independent experiments (means and s.e.m.) * P<0.05 of one-way ANOVA. (m) RT-PCR analysis of rorc and rora expression in CD4+ T cells activated under Th17 polarizing conditions in the presence of melatonin (2ng/ml). Data are representative of three independent experiments (means and s.e.m.) * P<0.05 of unpaired T-test. (n,o) Flow cytometry analysis of IL-17 expression (n) and rorc and il17 expression (o) in CD4+ T cells activated under Th17 polarizing conditions in the presence of melatonin (2ng/ml) and transduced with a control or nfil3-encoding retrovirus. Data are representative of three independent experiments (means and s.e.m.) * P<0.05 of one-way ANOVA. (p,q) Flow cytometry analysis of IL-17 expression (p) and rorc and il17 expression (q) in wild type mice and NFIL3-deficient in CD4+ T cells activated under Th17 polarizing conditions in the presence of melatonin (2ng/ml). Data are representative of three independent experiments (means and s.e.m.) * P<0.05 of one-way ANOVA. (r) Flow cytometry analysis of IL-17 and IFN-γ expression in CD4+ T cells from RAG-1 deficient mice reconstituted with wild type, MTNR1A-REV-ERBα or NFIL3-deficient CD4+ T cells, immunized with MOG35-55 in CFA and treated with vehicle or melatonin (5mg/kg). *P<0.05 of unpaired T-test. See also Fig. S5
ROR-α promotes Th17 cell differentiation (Yang et al., 2008). Accordingly, ROR-α activation by the specific agonist CGP 52608 boosted Th17 cell differentiation (Figs. 4c,d). ROR-α is directly activated by melatonin (Brzezinski, 1997). Indeed, melatonin boosted the differentiation of MTNR1A-deficient Th17 cells (Fig. 4e), suggesting that melatonin-triggered MTNR1A signaling interferes with the promotion of Th17 cell differentiation by ROR-α. Based on the inhibitory effects of NFIL3 on ROR-γt expression and Th17 cell differentiation (Yu et al., 2013), we studied whether NFIL3 also inhibits ROR-α expression.
A bioinformatics analysis identified NFIL3 binding sites in the rora and rorc promoters. Accordingly, we detected the recruitment of NFIL3 to the rora and rorc promoters in CD4+ T cells activated under Th17 polarizing conditions in the presence of melatonin, concomitant with a reduced expression of both ROR-α and ROR-γt (Figs. 5l,m). We then investigated the relevance of the regulation of NFIL3 expression for the modulation of Th17 cell differentiation. Overexpression of NFIL3 (Fig 5n,o) and NFIL3-deficiency (Fig 5p,q) abrogated the suppressive effects of melatonin on Th17 cell differentiation. Thus, the regulation of NFIL3 expression by melatonin mediates its inhibitory effects on the differentiation of Th17 cells in vitro. To evaluate the role of MTNR1A and NFIL3 on the suppression of Th17 cell differentiation by melatonin in vivo we used RAG-1 deficient mice reconstituted with wild type, MTNR1A, REV-ERBα or NFIL3-deficient CD4+ T cells and immunized with MOG35-55 in CFA. In agreement with our in vitro observations, the suppression of Th17 cell differentiation by melatonin in vivo was abrogated by MTNR1A, REV-ERBα and NFIL3-deficiency (Fig. 5r, Fig. S5). Indeed, we detected increased Th17 cell differentiation in response to treatment of mice reconstituted with MTNR1A-, REV-ERBα or NFIL3-deficient T cells, most likely reflecting the unopposed agonistic activity of melatonin on ROR-α and its promoting effects on the differentiation of Th17 cells. Taken together, these data suggest that melatonin interferes with Th17 cell differentiation via the inhibition of ROR-γt and ROR-α expression through an NFIL3-dependent mechanism.
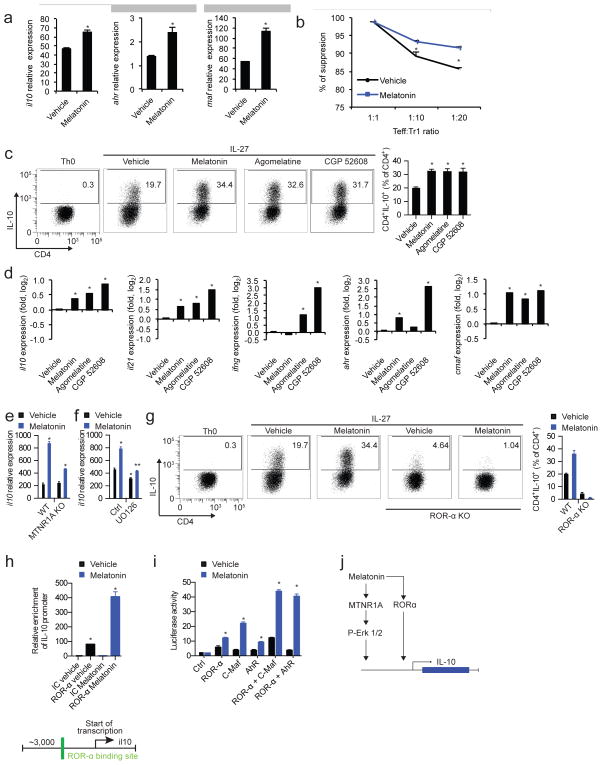
Melatonin boosts Tr1 cell differentiation via Erk1/2 and ROR-α
CD4+ IL-10 producing Tr1 cells play an important role in the regulation of the immune response (Pot et al., 2011; Roncarolo et al., 2006). The amelioration of EAE by melatonin administration was associated with an increase in IL-10 producing T cells (Fig. 2). Thus, we investigated the effects of melatonin on the activation of naïve CD4+ T cells under Tr1 polarizing conditions. We found that melatonin boosted the expression of IL-10 and the Tr1-associated molecules il21, ahr and cmaf (Apetoh et al., 2010) (Fig. 6a). In addition, melatonin boosted the suppressive activity of Tr1 cells in vitro (Fig. 6b).
Figure 6. Melatonin boosts Tr1 cell differentiation.
(a) RT-PCR analysis of il10, ahr and maf expression in Tr1 differentiated CD4+ T cells in the presence or absence of melatonin (2ng/ml). Data are representative of three independent experiments (means and s.e.m.). * P<0.05 of one-way ANOVA (b) In vitro suppression assay, treated or untreated differentiated Tr1 cells as in a, were co-cultured after 72hs with CD4+ T cells previously labeled with CSFE, and proliferation cycles (CSFE dilution) were measured after 48hs by flow cytometry. Data are representative of two independent experiments (means and s.e.m.) * P<0.05 of one-way ANOVA. (c) Flow cytometry analysis of IL-10 expression in Tr1 differentiated CD4+ T cells in the presence or absence of melatonin (2ng/ml), agomelatine (20ng/ml, MTNR1A ligand) and CGP 52608 (20ng/ml, ROR-α ligand). Data are representative of three independent experiments (means and s.e.m.) * P<0.05 of one-way ANOVA. (d) RT-PCR analysis of Tr1 cells cultured as in c. Data are representative of three independent experiments (means and s.e.m.) * P<0.05 of one-way ANOVA. (e) RT-PCR analysis of il10 expression as in c, in wild type mice and MTNR1A deficient mice. Data are representative of three independent experiments (means and s.e.m.) * P<0.05 of one-way ANOVA. (f) RT-PCR expression of il10 in melatonin treated Tr1 cells with or without the addition of UO126. Data are representative of five independent experiments (means and s.e.m.). * P<0.05 of unpaired T-test vs vehicle and signaling inhibitor control condition. ** P<0.05 vs vehicle of UO126-treated condition. (g) Flow cytometry analysis of IL-10 expression as in c, in wild type mice and ROR-α deficient mice. (h) ROR-α putative binding site present in the il10 promoter (lower panel), and chromatin immunoprecipitation with anti-ROR-α (upper panel) Data are representative of three independent experiments (means and s.e.m.). * P<0.05 of unpaired T-test. (i) Luciferase activity of HEK-293 cells transfected with a luciferase reporter construct for the il10 promoter. Data are representative of three independent experiments (means and s.e.m.)* P<0.05 of unpaired T-test. (j) Schematic diagram depicting the effects of melatonin in Tr1 cells.
We then investigated the mechanisms underlying the effects of melatonin on Tr1 regulatory cells. We detected the expression of both MTNR1A and ROR-α by Tr1 cells (Fig. S4c,d). Indeed, both agomelatine and CGP 52608, specific agonist for MTNR1A and ROR-α, respectively, boosted Tr1 cell differentiation (Fig. 6c,d). In agreement with these results, MTNR1A deficiency or inhibition of MTNR1A-activated Erk1/2 by UO126 interfered with the boost in Tr1 differentiation by melatonin (Fig. 6e,f). Of note, Erk1/2 activation is reported to promote cmaf-dependent IL-10 production by CD4+ T cells (Saraiva et al., 2009). In addition, ROR-α deficiency suppressed the differentiation of Tr1 cells induced by IL-27 and its boost by melatonin (Fig 6g).
ROR-α exerts its biological effects by binding to ROR response elements (ROREs) in target genes (Jetten, 2009). A bioinformatic analysis identified ROR-α binding sites in the il10 promoter (Fig. 6h), suggesting that melatonin may increase the recruitment of ROR-α to the il10 promoter and consequently, il10 transcription. In agreement with this hypothesis, we detected increased binding of ROR-α to the il10 promoter following T-cell activation under Tr1 polarizing conditions in the presence of melatonin (Fig. 6h). Moreover, ROR-α transactivated the il10 promoter in reporter assays, and synergized with the aryl hydrocarbon receptor (AhR) and c-Maf to boost their ability to promote il10 expression (Apetoh et al., 2010; Gandhi et al., 2010) (Fig. 6i). Taken together, these data suggest that melatonin boosts Tr1 cell differentiation through its effects on MTNR1A and ROR-α (Fig. 6j).
DISCUSSION
Strong epidemiological evidence supports the role of vitamin D in reducing MS relapses (Ascherio et al., 2012). Strikingly, vitamin D levels are higher during spring and summer, when relapse occurrence in MS patients peaks. Thus, the observation of a lower occurrence of relapses in seasons characterized by lower vitamin D levels represents a “seasonal paradox”: relapses should be less frequent in spring and summer when vitamin D levels are higher, yet the opposite is found in most studies (Jin et al., 2000; Spelman et al., 2014), with a few exceptions (Løken-Amsrud et al., 2012). Our data may solve this paradox by identifying melatonin, whose levels are regulated by seasonal fluctuations in day length, as an additional regulator of the immune response in MS. Note that night shift work, which is associated with lower overall melatonin levels (Schernhammer et al., 2004), increases the risk of developing MS (Hedström et al., 2011). These findings suggest that melatonin may also be an MS risk factor, the relationship between melatonin levels and the risk of developing MS is the focus of ongoing investigations. Finally, the interplay between melatonin and other seasonal environmental factors known to impact MS such as vitamin D in different geographic locations remains to be further elucidated.
The rise in the past 50 years in the incidence of autoimmune disorders has reached an epidemic proportion and can not be accounted by genetic risk only. Thus, increasing attention is being paid to environmental factors and their impact in the immune response and T cell differentiation in particular. For example: several compounds present in household products can activate the aryl hydrocarbon receptor and impact both Th17 and regulatory cell differentiation (Quintana et al., 2008); sodium in westernized diet and processed foods can also enhance Th17 cell differentiation (Wu et al., 2013); the composition of commensal microbiota impacts T cell differentiation and response (Lathrop et al., 2011); and the lack of sun exposure and dietary habits can diminish vitamin D levels and affect regulatory cell function (Correale et al., 2009). Each of these environmental factors trigger different signaling pathways and the characterization of the complex interaction between them can shed light on the impact of the environment on the immune system.
Pro-inflammatory Th17 cells are thought to contribute to the pathogenesis of EAE and MS (Miossec et al., 2009). Th17 cell differentiation is regulated by ROR-α and ROR-γt and therapies targeting Th17 cells are currently being tested in MS and other autoimmune diseases with preliminary encouraging results (Dominique L. P. Baeten and Kuchroo, 2013). Melatonin, despite having the potential to activate ROR-α, suppresses the generation of Th17 cells via its membrane receptor in a NFIL3-dependent fashion. Interestingly, it has been recently shown that the circadian clock suppresses Th17 development during nighttime through a similar NFIL3-dependent mechanism (Yu et al., 2013). Our work suggests that, in addition to Th17 cells, Tr1 cells are also regulated by melatonin during nighttime in an Erk1/2- and ROR-α dependent manner. Based on the high evolutionary conservation of melatonin production by the pineal gland and its regulation by daylight (Macchi and Bruce, 2004), it is likely that the circadian and seasonal effects of melatonin on the immune response play an important role that resulted in its positive selection during evolution.
Tr1 cells are characterized by the production of IL-10 (Pot et al., 2011; Roncarolo et al., 2006). AhR, c-Maf and Erk1/2 have been shown to regulate Tr1 cell development and IL-10 expression (Apetoh et al., 2010; Gandhi et al., 2010). Our work shows that melatonin promotes Tr1 cell differentiation by activating Erk1/2 signaling, which has been previously described to control IL-10 expression in T cells and DCs (Saraiva and O’Garra, 2010). We also identified ROR-α as a mediator of the effects of melatonin in Tr1 cells. Thus, these data suggest that melatonin utilizes multiple pathways to boost Tr1 cell differentiation.
The interplay between pro-inflammatory and regulatory cells controls the development of autoimmune diseases such as MS. Here we report that melatonin, whose levels show seasonal variability, control the balance between pathogenic and regulatory T cells. However, in MS patients melatonin is likely to act on several cell types to affect disease activity. Indeed, NFIL3 has been shown to play a role in human inflammatory bowel disease and autoimmune colitis through its activity on innate immune cells (Kobayashi et al., 2014). Thus, future studies should investigate the effects of melatonin on innate immune cells in MS patients, and also its role in inflammatory bowel disease and other immune-mediated disorders. Finally, although our data identifies melatonin-dependent signaling as a potential target for therapeutic immunomodulation, the pathways involved are complex and likely cross-regulated. Thus, extreme caution should be exercised to evaluate the translational potential of these findings.
EXPERIMENTAL PROCEDURES
Patients
Consecutive patients with relapsing-remitting MS according to McDonald criteria (Polman et al., 2011) were recruited from the MS clinic at the Raúl Carrea Institute for Neurological Research (FLENI) between September of 2011 and November of 2012. Study protocol was approved by the Institutional Ethics Committee, and all subjects signed an informed consent form. See Supplemental Experimental Procedures for detailed information.
Animals and EAE
EAE was induced as follows: mice were immunized with 100 μg MOG35–55 and 500 μg Mycobacterium tuberculosis extract H37Ra (Difco). Mice were also injected intraperitoneally with 200 ng pertussis toxin on days 0 and 2. Melatonin (5mg/kg) or vehicle (0.01% DMSO) was administered daily at 7:00 PM.
Flow cytometry staining and acquisition
For intracellular cytokine staining, cells were stimulated for 4 h at 37 °C with phorbol 12-myristate 13-acetate (50 ng/ml; Sigma), ionomycin (1 μg/ml; Sigma) and monensin (GolgiStop; 1 μg/ml; BD Biosciences). After being stained for surface markers, cells were fixed and made permeable according to the manufacturer’s instructions (BD Biosciences). All antibodies against cytokines were from Biolegend. All experiments were started at the same time (8–9am). Data were collected with a LSR II or FACSAria (BD Biosciences), then were analyzed with FlowJo software (Treestar).
Measurement of cytokines
Secreted cytokines were measured in tissue culture supernatants after 72–96hs by enzyme-linked immunosorbent assay as previously described (Farez et al., 2009).
Quantitative RT-PCR
RNA was extracted with RNAeasy columns (Qiagen, USA), then cDNA was prepared according to the manufacturer’s instructions (Applied Biosystems) and was used as template for real-time PCR. All primers and probes were provided by Applied Biosystems and were used on the ViiA 7 Real-Time PCR System (Applied Biosystems). Expression was normalized to the expression of the housekeeping gene Gapdh.
Immunoblot analysis
For immunoblot analysis, cells were lysed with radio-immunoprecipitation buffer supplemented with protease inhibitor ‘cocktail’ (Sigma-Aldrich). Total lysates of the different T-cell subsets (40 μg) were resolved by electrophoresis through 4–12% Bis-Tris Nupage gels (Invitrogen, USA) and were transferred onto PVDF membranes (Millipore). The following primary antibodies were used: anti-ROR-α (Abcam); anti-MTNR1A (Santa Cruz), anti total and phospho-Erk1/2 (Cell Signalling), anti-total C/EBPα (Cell Signaling), anti-phospho C/EBPα (Cell Signaling), anti-Nfil3 (Santa Cruz), and anti-GADPH (Abcam). Blots were developed with SuperSignal West Femto Maximum Sensitivity Substrate as suggested by the manufacturer (Pierce).
Statistical analysis
A Poisson regression model was used to assess the impact of season, 6-SM levels and the number of clinical relapses, generating an incidence rate ratio (IRR) and corresponding 95% confidence intervals (CI). A repeated measures mixed model was used to asses the effect of treatment and its interaction with time in EAE experiments. A linear regression model was used to analyze the relationship between serum melatonin levels and IL-17 or IL-10 gene expression. Differences between two or more conditions were analyzed with Student’s t test, Mann-Whitney test, One-way ANOVA or Wilcoxon Rank Sum test when appropriate. P values of less than 0.05 were considered significant. Unless otherwise specified, all data is presented as mean ± SEM. All statistical analyses were performed using Stata v12 (Statacorp LP, Texas, USA).
Supplementary Material
Highlights.
Melatonin levels negatively correlate with multiple sclerosis relapses in humans.
Melatonin treatment ameliorates pathology in a mouse model of multiple sclerosis
Melatonin blocks ROR-γt expression and Th17 differentiation.
Melatonin boosts Tr1 development via Erk1/2 and ROR-α.
Acknowledgments
This study was supported by the Allende Foundation and the MSIF Du-Preé grant (to M.F.F.), a grant from Biogen Idec and Novartis Argentina (to M.F.F. and J.C.) and Merck Serono Argentina (to J.C.), and AI093903 and NS087867 from the National Institutes of Health, RG4111A1 and JF2161-A-5 from the National Multiple Sclerosis Society and PA0069 from the International Progressive MS Alliance to F.J.Q. We would like to thank Drs. Lora Hooper, Bart Staels, Vincent Laudent, Mitch Lazar and Daniel Tenen for generously providing reagents used in these studies. We would also like to thank Jessica Kenison-White for technical assistance with mice colonies.
Footnotes
AUTHORS’ CONTRIBUTION
M.F.F. performed epidemiologic analyses, in vitro and in vivo experiments, analyzed data and wrote the manuscript, I.D.M. performed in vitro and in vivo experiments, S.P.M. performed in vitro and in vivo experiments, A.Y. performed in vitro and in vivo experiments, M.E.B. participated in human sample recollection, data analysis and interpretation, L.G. and M.G. performed human in vitro experiments, B.P. performed bioinformatics analysis, M.C.Y. participated in human sample recollection, data analysis and interpretation, C.Z. provided knockout mice, V.K.K. and G.A.R. edited the manuscript and participated in data interpretation, M.F.F., F.J.Q. and J.C. interpreted data, conceived and supervised the study and edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, Burns EJ, Sherr DH, Weiner HL, Kuchroo VK. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascherio A, Munger KL, Lennette ET, Spiegelman D, Hernan MA, Olek MJ, Hankinson SE, Hunter DJ. Epstein-Barr virus antibodies and risk of multiple sclerosis: a prospective study. Jama. 2001;286:3083–3088. doi: 10.1001/jama.286.24.3083. [DOI] [PubMed] [Google Scholar]
- Ascherio A, Munger KL, Lünemann JD. The initiation and prevention of multiple sclerosis. Nature Reviews Neurology. 2012;8:602–612. doi: 10.1038/nrneurol.2012.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascherio A, Munger KL, Simon KC. Vitamin D and multiple sclerosis. The Lancet Neurology. 2010;9:599–612. doi: 10.1016/S1474-4422(10)70086-7. [DOI] [PubMed] [Google Scholar]
- Ascherio A, Munger KL, White R, Köchert K, Simon KC, Polman CH, Freedman MS, Hartung HP, Miller DH, Montalban X, et al. Vitamin D as an Early Predictor of Multiple Sclerosis Activity and Progression. JAMA Neurol. 2014;71:306. doi: 10.1001/jamaneurol.2013.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astier AL, Meiffren G, Freeman S, Hafler DA. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. Journal of Clinical Investigation. 2006;116:3252–3257. doi: 10.1172/JCI29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecham AH, Patsopoulos NA, Xifara DK, Davis MF, Kemppinen A, Cotsapas C, Shah TS, Spencer C, Booth D, Goris A, et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet. 2013;45:1353–1360. doi: 10.1038/ng.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski A. Melatonin in humans. N Engl J Med. 1997;336:186–195. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- Castellano I, Ercolesi E, Palumbo A. Nitric Oxide Affects ERK Signaling through Down-Regulation of MAP Kinase Phosphatase Levels during Larval Development of the Ascidian Ciona intestinalis. PLoS ONE. 2014;9:e102907. doi: 10.1371/journal.pone.0102907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codarri L, Gyülvészi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- Correale J, Ysrraelit MC, Gaitan MI. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain. 2009;132:1146–1160. doi: 10.1093/brain/awp033. [DOI] [PubMed] [Google Scholar]
- Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. 2007;61:97–108. doi: 10.1002/ana.21067. [DOI] [PubMed] [Google Scholar]
- Correale J, Fiol M, Gilmore W. The risk of relapses in multiple sclerosis during systemic infections. Neurology. 2006;67:652–659. doi: 10.1212/01.wnl.0000233834.09743.3b. [DOI] [PubMed] [Google Scholar]
- Baeten Dominique LP, Kuchroo VK. Interleukin-17 and a tale of two autoimmune diseases. Nature Medicine. 2013;19:824–825. doi: 10.1038/nm.3268. [DOI] [PubMed] [Google Scholar]
- El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farez MF, Fiol MP, Gaitan MI, Quintana FJ, Correale J. Sodium intake is associated with increased disease activity in multiple sclerosis. Journal of Neurology, Neurosurgery & Psychiatry. 2014 doi: 10.1136/jnnp-2014-307928. [DOI] [PubMed] [Google Scholar]
- Farez MF, Quintana FJ, Gandhi R, Izquierdo G, Lucas M, Weiner HL. Toll-like receptor 2 and poly(ADP-ribose) polymerase 1 promote central nervous system neuroinflammation in progressive EAE. Nat Immunol. 2009;10:958–964. doi: 10.1038/ni.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassi J, Russo Picasso MF, Furci A, Sorroche P, Jáuregui R, Plantalech L. Seasonal variations in 25-hydroxyvitamin D in young and elderly and populations in Buenos Aires City. Medicina (B Aires) 2003;63:215–220. [PubMed] [Google Scholar]
- Gandhi R, Kumar D, Burns EJ, Nadeau M, Dake B, Laroni A, Kozoriz D, Weiner HL, Quintana FJ. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol. 2010;11:846–853. doi: 10.1038/ni.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham C, Cook MR, Kavet R, Sastre A, Smith DK. Prediction of nocturnal plasma melatonin from morning urinary measures. J Pineal Res. 1998;24:230–238. doi: 10.1111/j.1600-079x.1998.tb00538.x. [DOI] [PubMed] [Google Scholar]
- Hedström AK, Åkerstedt T, Hillert J, Olsson T, Alfredsson L. Shift work at young age is associated with increased risk for multiple sclerosis. Ann Neurol. 2011;70:733–741. doi: 10.1002/ana.22597. [DOI] [PubMed] [Google Scholar]
- Hernan MA. Cigarette smoking and the progression of multiple sclerosis. Brain. 2005;128:1461–1465. doi: 10.1093/brain/awh471. [DOI] [PubMed] [Google Scholar]
- Hickie IB, Rogers NL. Novel melatonin-based therapies: potential advances in the treatment of major depression. Lancet. 2011;378:621–631. doi: 10.1016/S0140-6736(11)60095-0. [DOI] [PubMed] [Google Scholar]
- Jetten AM. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal. 2009;7:e003. doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, de Pedro-Cuesta J, Söderström M, Stawiarz L, Link H. Seasonal patterns in optic neuritis and multiple sclerosis: a meta-analysis. Journal of the Neurological Sciences. 2000;181:56–64. doi: 10.1016/s0022-510x(00)00408-1. [DOI] [PubMed] [Google Scholar]
- Jockers R, Maurice P, Boutin JA, Delagrange P. Melatonin receptors, heterodimerization, signal transduction and binding sites: what’s new? British Journal of Pharmacology. 2008;154:1182–1195. doi: 10.1038/bjp.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PF. Molecular stop signs: regulation of cell-cycle arrest by C/EBP transcription factors. J Cell Sci. 2005;118:2545–2555. doi: 10.1242/jcs.02459. [DOI] [PubMed] [Google Scholar]
- Karim A, Tolbert D, Cao C. Disposition kinetics and tolerance of escalating single doses of ramelteon, a high-affinity MT1 and MT2 melatonin receptor agonist indicated for treatment of insomnia. J Clin Pharmacol. 2006;46:140–148. doi: 10.1177/0091270005283461. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Steinbach EC, Russo SM, Matsuoka K, Nochi T, Maharshak N, Borst LB, Hostager B, Garcia-Martinez JV, Rothman PB, et al. NFIL3-deficient mice develop microbiota-dependent, IL-12/23-driven spontaneous colitis. The Journal of Immunology. 2014;192:1918–1927. doi: 10.4049/jimmunol.1301819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojetin DJ, Burris TP. REV-ERB and ROR nuclear receptors as drug targets. Nat Rev Drug Discov. 2014;13:197–216. doi: 10.1038/nrd4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012a:1–11. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012b;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekstrom-Himes J, Xanthopoulos KG. Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J Biol Chem. 1998;273:28545–28548. doi: 10.1074/jbc.273.44.28545. [DOI] [PubMed] [Google Scholar]
- Løken-Amsrud KI, Holmøy T, Bakke SJ, Beiske AG, Bjerve KS, Bjørnarå BT, Hovdal H, Lilleås F, Midgard R, Pedersen T, et al. Vitamin D and disease activity in multiple sclerosis before and during interferon-β treatment. Neurology. 2012;79:267–273. doi: 10.1212/WNL.0b013e31825fdf01. [DOI] [PubMed] [Google Scholar]
- Macchi MM, Bruce JN. Human pineal physiology and functional significance of melatonin. Front Neuroendocrinol. 2004;25:177–195. doi: 10.1016/j.yfrne.2004.08.001. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell–mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- McMullan CJ, Schernhammer ES, Rimm EB, Hu FB, Forman JP. Melatonin secretion and the incidence of type 2 diabetes. Jama. 2013;309:1388–1396. doi: 10.1001/jama.2013.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- Morera AL, Abreu P. Daytime/night-time and summer/winter melatonin and malondialdehyde rhythms: an inverse relationship. J Pineal Res. 2007;43:313–314. doi: 10.1111/j.1600-079X.2007.00467.x. [DOI] [PubMed] [Google Scholar]
- Pévet P. Melatonin: from seasonal to circadian signal. J Neuroendocrinol. 2003;15:422–426. doi: 10.1046/j.1365-2826.2003.01017.x. [DOI] [PubMed] [Google Scholar]
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pot C, Apetoh L, Awasthi A, Kuchroo VK. Induction of regulatory Tr1 cells and inhibition of TH17 cells by IL-27. Seminars in Immunology. 2011;23:438–445. doi: 10.1016/j.smim.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo D, Delgado M, Fernandez-Santos JM, Calvo JR, Gomariz RP, Martin-Lacave I, Ortiz GG, Guerrero JM. Expression of the Mel1a-melatonin receptor mRNA in T and B subsets of lymphocytes from rat thymus and spleen. Faseb J. 1997;11:466–473. doi: 10.1096/fasebj.11.6.9194527. [DOI] [PubMed] [Google Scholar]
- Pozo D, García-Mauriño S, Guerrero JM, Calvo JR. mRNA expression of nuclear receptor RZR/RORalpha, melatonin membrane receptor MT1, and hydroxindole-O-methyltransferase in different populations of human immune cells. J Pineal Res. 2004;37:48–54. doi: 10.1111/j.1600-079X.2004.00135.x. [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- Rosecrans R, Dohnal JC. Clinical Biochemistry. Clinical Biochemistry. 2014;47:670–672. doi: 10.1016/j.clinbiochem.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Runia TF, Hop WCJ, de Rijke YB, Buljevac D, Hintzen RQ. Lower serum vitamin D levels are associated with a higher relapse risk in multiple sclerosis. Neurology. 2012;79:261–266. doi: 10.1212/WNL.0b013e31825fdec7. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nature Reviews Immunology. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nature Reviews Immunology. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- Saraiva M, Christensen JR, Veldhoen M, Murphy TL, Murphy KM, O’Garra A. Interleukin-10 Production by Th1 Cells Requires Interleukin-12-Induced STAT4 Transcription Factor and ERK MAP Kinase Activation by High Antigen Dose. Immunity. 2009;31:209–219. doi: 10.1016/j.immuni.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawcer S, Hellenthal G, Pirinen M, Spencer CCA, Patsopoulos NA, Moutsianas L, Dilthey A, Su Z, Freeman C, Hunt SE, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schernhammer ES, Rosner B, Willett WC, Laden F, Colditz GA, Hankinson SE. Epidemiology of urinary melatonin in women and its relation to other hormones and night work. Cancer Epidemiol Biomarkers Prev. 2004;13:936–943. [PubMed] [Google Scholar]
- Simpson S, Taylor B, Blizzard L, Ponsonby AL, Pittas F, Tremlett H, Dwyer T, Gies P, van der Mei I. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol. 2010;68:193–203. doi: 10.1002/ana.22043. [DOI] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Spelman T, Gray O, Trojano M, Petersen T, Izquierdo G, Lugaresi A, Hupperts R, Bergamaschi R, Duquette P, Grammond P, et al. Seasonal variation of relapse rate in multiple sclerosis is latitude dependent. Ann Neurol. 2014 doi: 10.1002/ana.24287. [DOI] [PubMed] [Google Scholar]
- Steinman L. Immunology of Relapse and Remission in Multiple Sclerosis. Annu Rev Immunol. 2014;32:257–281. doi: 10.1146/annurev-immunol-032713-120227. [DOI] [PubMed] [Google Scholar]
- Tan AHM, Lam KP. Pharmacologic Inhibition of MEK-ERK Signaling Enhances Th17 Differentiation. The Journal of Immunology. 2010;184:1849–1857. doi: 10.4049/jimmunol.0901509. [DOI] [PubMed] [Google Scholar]
- Ueno-Towatari T, Norimatsu K, Blazejczyk K, Tokura H, Morita T. Seasonal Variations of Melatonin Secretion in Young Females under Natural and Artificial Light Conditions in Fukuoka, Japan. J Physiol Anthropol. 2007;26:209–215. doi: 10.2114/jpa2.26.209. [DOI] [PubMed] [Google Scholar]
- Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013:1–5. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Croniger CM, Lekstrom-Himes J, Zhang P, Fenyus M, Tenen DG, Darlington GJ, Hanson RW. Metabolic response of mice to a postnatal ablation of CCAAT/enhancer-binding protein alpha. J Biol Chem. 2005;280:38689–38699. doi: 10.1074/jbc.M503486200. [DOI] [PubMed] [Google Scholar]
- Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, et al. T Helper 17 Lineage Differentiation Is Programmed by Orphan Nuclear Receptors RORα and RORγ. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Rollins D, Ruhn KA, Stubblefield JJ, Green CB, Kashiwada M, Rothman PB, Takahashi JS, Hooper LV. TH17 cell differentiation is regulated by the circadian clock. Science. 2013;342:727–730. doi: 10.1126/science.1243884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.