Abstract
Background & Aims
Nonalcoholic fatty liver disease (NAFLD) is a common consequence of human and rodent obesity. Disruptions in lipid metabolism lead to accumulation of triglycerides and fatty acids, which can promote inflammation and fibrosis and lead to nonalcoholic steatohepatitis (NASH). Circulating levels of fibroblast growth factor (FGF)21 increase in patients with NAFLD or NASH, so we assessed the role of FGF21 in the progression of murine fatty liver disease, independent of obesity, caused by methionine and choline deficiency.
Methods
C57BL/6 wild-type and FGF21-knockout (FGF21-KO) mice were placed on methionine- and choline-deficient (MCD), high-fat, or control diets for 8–16 weeks. Mice were weighed; serum and liver tissues were collected and analyzed for histology, levels of malondialdehyde and liver enzymes, gene expression, and lipid content.
Results
The MCD diet increased hepatic levels of FGF21 mRNA more than 50-fold and serum levels 16-fold, compared with the control diet. FGF21-KO mice had more severe steatosis, fibrosis, inflammation, and peroxidative damage than wild-type C57BL/6 mice. FGF21-KO mice had reduced hepatic fatty acid activation and β oxidation, resulting in increased levels of free fatty acid. FGF21-KO mice given continuous subcutaneous infusions of FGF21 for 4 weeks while on MCD diets had reduced steatosis and peroxidative damage, compared with mice not receiving FGF21. The expression of genes that regulate inflammation and fibrosis were reduced in FGF21-KO mice given FGF21, similar to those of wild-type mice.
Conclusions
FGF21 regulates fatty acid activation and oxidation in livers of mice. In the absence of FGF21, accumulation of inactivated fatty acids results in lipotoxic damage and increased steatosis.
Keywords: fatty acid metabolism, long chain fatty acid, acyl-CoA
Background & Aims
In humans nonalcoholic fatty liver disease (NAFLD), characterized by excess hepatic accumulation of triglycerides, is a common complication of obesity and is linked to insulin resistance and the metabolic syndrome. Diagnosis of NAFLD requires exclusion of other causes of liver pathology, including alcohol abuse, viral infections and biliary or autoimmune disease. 10–20% of individuals with NAFLD progress to nonalcoholic steatohepatitis (NASH) which is characterized by hepatocyte lipoapoptosis, inflammation and fibrosis. While fatty liver has a relatively benign prognosis1, NASH poses a high risk for further progression to cirrhosis and hepatocellular carcinoma. As a result of the increasing prevalence of obesity, NAFLD is now the most common cause of chronic liver disease in developed as well as developing countries. In the USA NAFLD affects 30% of the obese population and 53% of obese children2, 3. Additionally, risk increases with weight and prevalence increases to 90% in morbidly obese populations4, 5. Thus, understanding the molecular mechanisms underlying the progression from hepatic steatosis to frank steatohepatitis is of critical importance for clinical prognostication and for pharmacological treatment.
In humans, circulating levels of the metabolic regulator, fibroblast growth factor 21 (FGF21) are increased in individuals with both NAFLD and NASH6–9, suggesting that FGF21 may play a role in the pathogenesis of fatty liver disease. FGF21 has multiple metabolic roles, and in rodents is known to enhance hepatic fatty acid oxidation during fasting or consumption of a ketogenic diets10, 11. Furthermore, mice lacking FGF21 (FGF21-KO) show an atypical metabolic response to a ketogenic diet, including impaired fatty acid oxidation, and weight gain rather than the expected weight loss12. Increased expression of hepatic FGF21 and high circulating levels are seen with genetic and diet induced rodent obesity13.
Mice rendered obese by consuming a high fat diet develop a mild phenotype of inflammation and steatosis which is relatively late onset and is also exacerbated in mice lacking FGF21. Thus supporting accumulating data suggest that FGF21 plays a protective role in steatohepatitis. Understanding the molecular mechanisms of this process is complicated by the fact that studies thus far focused on NAFLD in the context of obesity, which is associated with resistance to insulin, leptin and FGF21 itself. Additionally, pharmacologic doses of FGF21 induce rapid weight loss making it difficult to identify primary effects of FGF21 on liver metabolism as opposed to secondary effects related to resolution of NAFLD as a consequence of weight loss. To define the distinct role of FGF21 on the liver, independent of adiposity, we used a lean model of fatty liver disease induced by consumption of a methionine- and choline-deficient (MCD) diet. Unlike the mild phenotype observed with diet induced obesity, this model recapitulates many of the pathologic processes observed in fatty liver disease including hepatocyte lipoapoptosis, progression to NASH, development of severe inflammation, hepatocyte ballooning, and fibrosis. However, this diet does not cause weight gain or insulin resistance so that the contributions of FGF21 can be isolated independent of adiposity.
Wild-type mice consuming the MCD diet showed elevations in hepatic FGF21 mRNA expression and circulating FGF21 levels. Furthermore, in FGF21-KO mice hepatic steatosis and fibrosis were exacerbated, consistent with a protective role for FGF21 in the pathogenesis of liver disease independent of obesity. Importantly, when hepatic lipid content was analyzed, livers of mice lacking FGF21 showed elevated free fatty acid levels. Strikingly, there was a concomitant and profound reduction in all species of long chain fatty acyl-CoAs, suggesting that FGF21 regulates the activation of free fatty acids, a process necessary for long chain fatty acid oxidation. Consistent with this finding, FGF21-KO mice exhibited significantly decreased hepatic acyl CoA synthetase activity and long chain fatty acid β-oxidation. Treatment with exogenous FGF21 ameliorated MCD-induced steatohepatitis and increased acyl CoA synthetase activity and β-oxidation. Additionally, treatment of FGF21 to WT mice with established NASH reversed many of the associated complications including hepatic steatosis and inflammation. Taken together, these results demonstrate a novel and critical protective role for FGF21 that limits the progression of steatosis to steatohepatitis and associated lipotoxic damage. This results from the action of FGF21 to increase long chain fatty acid activation, β-oxidation, and fatty acid disposal and thus, limit hepatic fatty acid accumulation.
Methods
Mouse maintenance and experiments
All procedures were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee. Mice were housed in groups of two to four mice at 24°C under a 12-hour light-dark cycle (0600–1800 h) with ad libitum access to food and water. Mice were fed either a methionine-choline deficient (MCD) diet (Harlan Teklad TD.90262), the matched control diet (Harlan Teklad TD.94149), or a high fat diet (Research Diets, D12451) for either four or eight or 16 weeks. Mice were euthanized with vaporized isoflurane, exsanguinated via cardiac puncture, serum was collected and frozen immediately. Dissected tissues were weighed and flash frozen in liquid nitrogen. Specific methodic details are contained within the supplemental information.
Statistics
Data are presented as mean ± SEM. Data sets were analyzed for statistical significance on Microsoft Excel using unpaired two-tailed t tests. Statistical significance was assumed at P < 0.05.
Results
Hepatic FGF21 expression is increased during MCD induced steatohepatitis
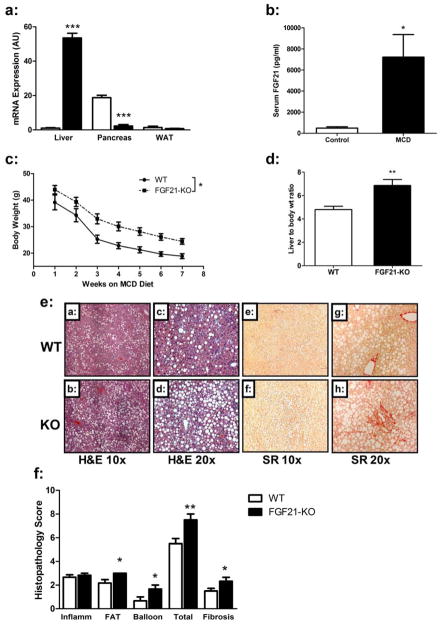
FGF21-KO mice fed a high fat diet for 16 weeks showed evidence of exacerbated fibrosis and inflammation (Sup. Figure 1), however, the phenotype was mild in both the WT and FGF21-KO mice. As we were interested in the role of FGF21 in attenuating the more severe pathologies associated with NASH such as lipotoxycity and inflammation we fed mice an MCD diet (Figure 1). Consumption of the MCD diet led to the development of fatty liver independent of obesity and was associated with a >50 fold increase in hepatic FGF21 mRNA expression (Figure 1a) and a >15 fold increase in serum FGF21 levels (Figure 1b). These changes were sustained through 8 weeks on the MCD diet (data not shown). FGF21 is also expressed in white adipose tissue14 and pancreas15 however there was no change in FGF21 expression in adipose tissue, while expression in the pancreas decreased (Figure 1a) indicating that the increase in serum levels is a consequence of hepatic expression.
Figure 1. FGF21 is up regulated in a mouse model of steatohepatitis a condition which is severely exacerbated in Fgf21-deficient mice.
(a) Fgf21 mRNA expression is markedly increased in livers of WT mice on the MCD diet for 2 weeks, decreased in the pancreas, and unchanged in white adipose tissue. (b) Fgf21 serum levels are elevated in WT mice on the MCD diet for 2 weeks. (c) When consuming an MCD diet for the duration of 8 weeks mice lacking FGF21 (FGF21-KO) remain heavier than WT littermates after 8 weeks on the MCD diet. (d) FGF21-KO mice have increased liver to body weight ratio. Histological analysis of steatohepatitis in FGF21KO mice. (e) FGF21-KO mice have increased lipid accumulation compared to WT mice (panels a–d, stained by H&E, at 10x and 20x magnification), and increased perisinusoidal, perivenular, and periportal fibrosis as assessed by Sirius Red (SR) staining (panels e–h, at 20x and 40x magnification). (f) Histopathology scores assigned to the FGF21-KO livers showed higher scores for fatty change (FAT), hepatocyte ballooning (BALLOON), total NAFLD Activity Score (TOTAL), and fibrosis. Data are expressed as mean ± SEM, N = 6 per group. (*, P < 0.05; **, P < 0.01).
FGF21 deficiency exacerbates hepatic steatosis, inflammation, fibrosis, and lipid peroxidative damage caused by the MCD diet
After 8 weeks of MCD diet both WT and mice lacking FGF21 (FGF21-KO) demonstrated similar rates of weight loss16 (Figure 1c). FGF21-KO mice had heavier livers (0.92g ± 0.063 vs. 1.6g ± 0.14, P=0.0008) and, increased liver to body weight ratios as compared to WT mice (Figure 1d). The differences in liver weight appear to be secondary to increased hepatic triglyceride levels in FGF21-KO mice (Figure 2a). Serum ALT levels were also higher in the FGF21-KO mice (Table 1), indicating increased hepatocyte damage. While there was a small increase in serum cholesterol in FGF21-KO mice, there were no significant differences in circulating triglycerides or non-esterified fatty acid concentrations (Table 1).
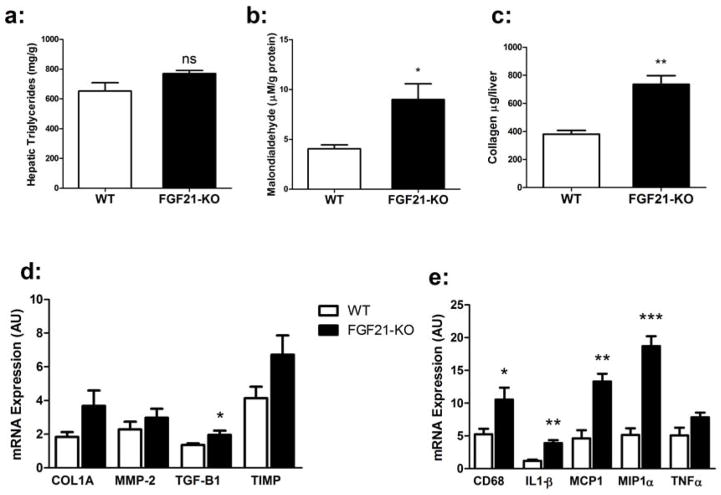
Figure 2. FGF21-KO mice consuming an MCD diet display evidence of progressive steatohepatitis.
(a) Hepatic triglyceride content (mg triglyceride/gram of liver) is increased in the FGF21-KO mice compared to WT on the MCD diet. (b) The lipid peroxidation product malondialdehyde is increased in the FGF21-KO mice on the MCD diet. (c) Collagen levels were higher in the liver of FGF21-KO mice (d) Hepatic mRNA expression of the pro-fibrotic genes are elevated in FGF21-KO mice fed MCD. (e) FGF21-KO mice have higher mRNA levels of the pro-inflammatory genes Cd68, Il1β, Mcp1, and Mip1a compared to WT mice. Data are expressed as mean ± SEM, with N = 6 mice per group. (*, P < 0.05; **, P < 0.01; *** P< 0.001).
Table 1.
| 8 Weeks MCD | 4 Weeks MCD | ||||
|---|---|---|---|---|---|
| Metabolite | WT | FGF21-KO | WT | FGF21-KO | FGF21-KO (FGF21 rx) |
| ALT (IU/L) | 66.4±36.5 | 186.2±30.9* | 246.3±16.1 | 253.6±29.9 | 131.5±14.4#& |
| Cholesterol (mg/dL) | 50.6±8.08 | 63.2±7.23* | 65.3±2.09 | 68.4±3.10 | 68.8±2.22 |
| Triglycerides (mg/dL) | 109.1±1.81 | 116.7±3.73 | 104.0±3.65 | 109.4±3.28 | 106.9±2.03 |
| NEFAs (meq/L) | 0.57±0.073 | 0.45±0.044 | 0.63±0.055 | 0.56±0.070 | 0.44±0.048# |
8 week WT v KO
4 v week WT
4 v week KO
We used histological analysis to compare the progression of steatohepatitis in WT and FGF21-KO mice. When scored for degree of steatosis, inflammation, and hepatocyte ballooning according to criteria set forth by a well-validated grading system17, FGF21-KO mice were found to have increased hepatic steatosis and ballooning, with a significantly higher NAFLD Activity Score (Figure 1e and f). Notably, FGF21-KO mice had pronounced perisinusoidal fibrosis (Figure 1e), a pattern characteristic of fibrosis with a metabolic etiology such as NASH18.
To analyze the progression of steatohepatitis in mice lacking FGF21 we measured malondialdehyde levels, a product of lipid oxidation, and found a substantial increase in the livers of FGF21-KO mice (Figure 2b) confirming enhanced oxidative stress in FGF21KO mice3. FGF21-KO mice also exhibited more hepatic fibrosis as assessed by hepatic collagen content (Figure 2c). FGF21-KO mice did not show impaired expression of genes involved in antioxidant pathways, including Nrf2, superoxide dismutase, catalase, or enzymes in the glutathione pathway (data not shown); nor were there differences in glutathione levels between WT and FGF21-KO mice (data not shown). Expression of genes involved in extracellular matrix deposition and remodeling in fibrosis were increased FGF21-KO mice, however only Tgfβ1 was significant (Figure 2d). Strikingly, there was a substantial induction of genes mediating inflammation (IL1β, MCP1, and MIP1a) in the FGF21-KO livers, including the macrophage marker Cd68, (Figure 2e) suggesting enhanced inflammation and innate immune cell infiltration. Taken together, these results demonstrate that FGF21 deficiency leads to increased hepatic steatosis and exacerbated oxidative damage, inflammation and fibrosis and thus enhanced progression to NASH.
Fgf21 alters hepatic long chain acyl CoA content
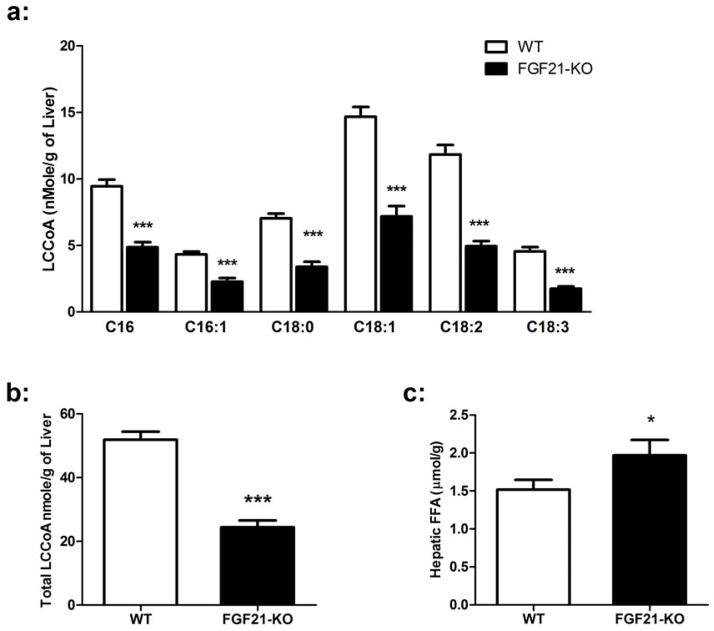
We next sought to identify biochemical pathways targeted by FGF21 that might affect the course of steatohepatitis. Excess free fatty acids have lipotoxic effects which may contribute to progression from simple steatosis to steatohepatitis19. As the initial step in hepatic long chain fatty acid metabolism is activation of fatty acids to long chain acyl CoAs, we profiled the long chain acyl CoA content in the livers of FGF21-KO mice on the MCD diet. As FGF21-KO mice have increased hepatic triglyceride content, we anticipated an accumulation of long chain acyl CoAs. Surprisingly, there was a profound reduction in the levels of all species of long chain acyl CoAs examined (Figure 3a), with a >50% reduction in total long chain acyl CoA content (Figure 3b). These data suggest a global defect in the activation of all species of long chain fatty acids to their respective acyl CoA in mice lacking FGF21 on the MCD diet. As a consequence, FGF21-KO mice have significantly elevated hepatic free fatty acids (Figure 3c), consistent with an abrogated conversion to acyl CoAs for subsequent utilization in downstream metabolic pathways.
Figure 3. Hepatic long chain acyl CoA and free fatty acid content.
(a) Livers from FGF21-KO mice on MCD for 8 weeks showed reduced levels of all the species of long chain acyl CoAs measured. (b) Reduced total LCCoA in FGF21-KO. (c) Compared to WT mice, FGF21KO mice have higher hepatic levels of free fatty acids. Data are expressed as mean ± SEM, with N = 6 per group. (*, P < 0.05; **, P < 0.01; *** P< 0.001).
Fgf21 deficiency leads to reduced ACSL activity and mitochondrial β-oxidation
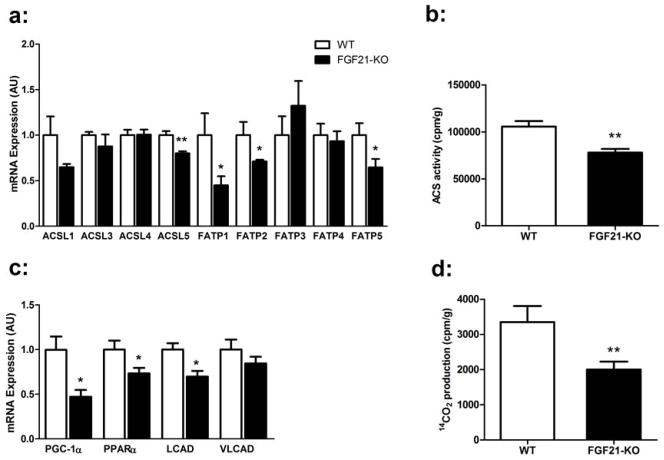
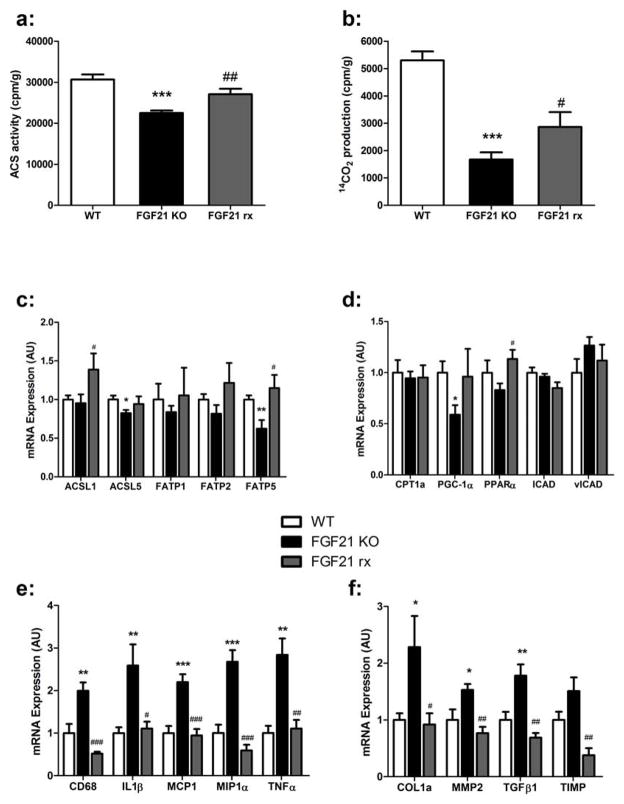
Long chain fatty acids are converted to acyl CoAs by a family of five acyl CoA synthetases (ACSLs) as well as several fatty acid transport proteins (FATPs), many of which are expressed in the liver20, 21. We hypothesized that differences in the expression of ACSLs or FATPs could account for the reduction in long chain acyl CoA content in the livers of FGF21-KO mice. Indeed, substantial decreases in the mRNA expression of ACSL1, ACSL5, FATP1, FATP2 and FATP5 were observed in the livers of FGF21-KO mice on the MCD diet (Figure 4a). This was accompanied by a 25% reduction in total ACSL activity (Figure 4b).
Figure 4. Mice deficient in FGF21 have decreased hepatic acyl CoA synthetase activity and reduced β-oxidation.
(a) FGF21-KO mice on MCD for 8 weeks have decreased hepatic mRNA expression of Acsl1, Acsl5, Fatp1, Fatp2, and Fatp5 compared to WT. (b) FGF21-KO mice have decreased total hepatic acyl CoA synthetase activity, using [1-14C] palmitic acid as the substrate. (c) Reduced hepatic mRNA expression of the fatty acid oxidation genes Cpt1a, Pgc1α, and Pparα in the FGF21-KO mice. (d) Liver mitochondrial β-oxidation of [1-14C] palmitic acid is reduced in FGF21KO mice. Data are expressed as mean ± SEM, with N = 5 per group. (*, P < 0.05; **, P < 0.01)
A consequence of decreased levels of long chain acyl CoAs is reduced mitochondrial β-oxidation. Livers from the FGF21-KO mice demonstrated a 40% reduction in [1-14C] palmitic acid oxidation to CO2 (Figure 4d). We also found significant decreases in mRNA expression of several genes regulating β-oxidation, including, Pgc-1α, Pparα and lCAD (Figure 4c) that may contribute to decreased oxidation. However, given the relatively small differences in oxidative gene expression between WT and FGF21-KO mice, this considerable reduction in palmitate β-oxidation may be more attributable to the decreased levels of reactive substrates as less activated long chain acyl CoAs are available in the hepatocyte.
Exogenous administration of Fgf21 attenuates the development of steatohepatitis
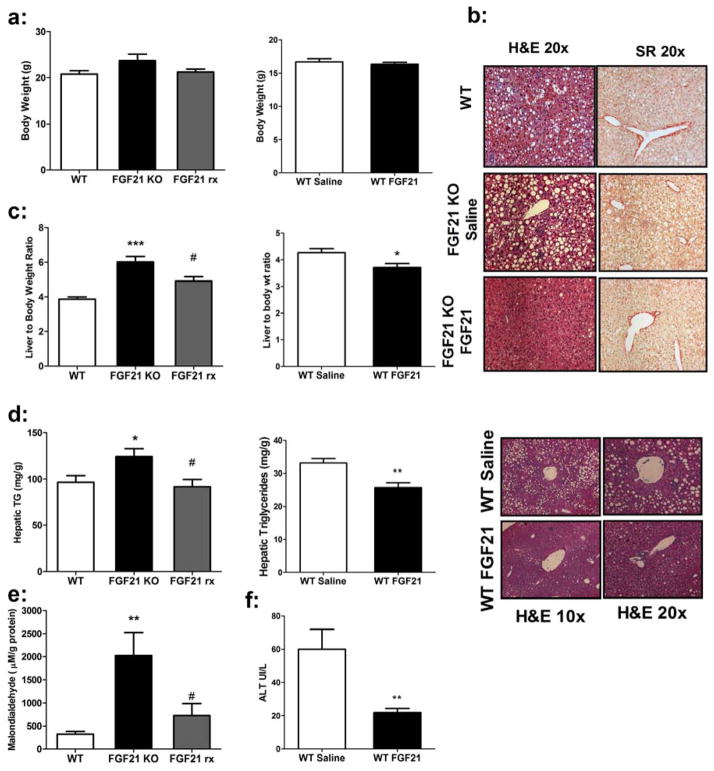
FGF21-KO mice were treated with continuous subcutaneous infusion of either saline or human recombinant FGF21 for 4 weeks while on the MCD diet, and were compared to WT mice on the MCD diet for the same period of time. FGF21 serum levels achieved with treatment were 34.6 ± 8.1 ng/mL compared to endogenously up-regulated serum levels in WT mice consuming the MCD diet of 7.8 ± 2.1 ng/mL (Figure 1b). There were no significant differences in weight between the three cohorts after 4 weeks (Figure 5a). However, a number of beneficial effects of FGF21 administration were noted. FGF21 treated KO mice exhibited lower liver weights and liver to body weight ratio (Figure 5c) and substantially reduced serum ALT levels compared to both WT and KO counterparts (Table 1). There was a small but significant decrease in circulating free fatty acid levels with no change in serum triglyceride or cholesterol concentrations (Table 1). Notably, FGF21 significantly attenuated the development of hepatic steatosis in the FGF21-KO mice, leading to hepatic triglyceride levels comparable to WT animals (Figure 5d). Histologic analysis revealed profoundly reduced macrovesicular steatosis (Figure 5b). In addition, FGF21 treatment decreased peroxidative damage, reflected by malondialdehyde levels (Figure 5e) and was associated with normalization of all pro-inflammatory and pro-fibrotic genes to levels seen in WT mice (Figure 6e and 6f). Consistent with reduced inflammation and fibrosis there were fewer inflammatory infiltrates and reduced Sirius Red staining (Figure 5b). Collagen levels were reduced but did not reach significance (Sup. Figure 2a), however, significant improvements were observed with all components of the histological scoring (Sup. Figure 2b). Additionally, FGF21 treatment to wild type mice with established NASH led to a significant improvement in hepatic steatosis and inflammation (Figure 5a–d). This was also associated with increased acyl CoA synthatase expression and reduced fibrotic and inflammatory gene expression (Sup. Figure 6).
Figure 5. Exogenous administration of FGF21 improves hepatic steatosis in FGF21-KO mice and in wild type mice with established NASH.
(a) WT, saline-treated FGF21-KO, and FGF21-treated FGF21-KO mice (FGF21 rx) showed no significant differences in body weight after 4 weeks on the MCD diet nor when treated to WT mice. (b) Representative liver sections showing that the severe hepatic steatosis in the saline-treated FGF21-KO mice is significantly improved with FGF21 treatment, as assessed by hematoxylin and eosin staining (10x and 20x magnification). Sirius red staining demonstrates improvement in perisinusoidal fibrosis in the FGF21-treated mice. A significant improvement is also observed in WT treated mice. (c) While the KO mice had heavier livers and a higher liver to body weight ratio than WT mice, FGF21-treated mice showed a significant decrease in liver to body weight ratio. This was also seen in WT treated mice. (d) Hepatic triglycerides are reduced to WT levels in FGF21-treated KO mice and are further reduced in treated WT animals. (e) Hepatic malondialdehyde levels are decreased to near that of WT levels in FGF21-treated mice. (f) Dramatic reductions in ALT are observed in mice treated with FGF21 for 10 days. Data are shown as mean ± SEM, N = 5 per group. ((WT v FGF21KO; *, P < 0.05; **, P < 0.01; *** P< 0.001) (FGF21KO v FGF21rx; #, P<0.05)).
Figure 6. Treatment with FGF21 increases hepatic acyl CoA synthetase and fatty acid β-oxidation.
(a) FGF21 treatment increases total hepatic acyl CoA synthetase activity to WT levels, with [1-14C] palmitic acid as the substrate. (b) Hepatic β-oxidation of [1-14C] palmitic acid to CO2 is increased by FGF21 treatment. (c) Chronic FGF21 treatment increases mRNA expression of ACSL1, FATP1 and FATP5 compared to saline treated FGF21KO mice on the MCD diet for 4 weeks. (d) mRNA expression of fatty acid oxidation genes in WT, KO, and FGF21-treated mice on the MCD diet for 4 weeks. (e) Expression of pro-fibrotic genes is reduced to WT levels in FGF21-treated mice. Of note, Timp mRNA expression in the FGF21-treated mice was lower than the level in WT mice. (f) FGF21-treated mice had decreased mRNA expression of pro-inflammatory genes to WT levels. Data is expressed as mean ± SEM, with N = 5 mice per group. ((WT v FGF21KO; *, P < 0.05; **, P < 0.01; *** P< 0.001) (FGF21KO v FGF21rx; #, P<0.05; ##, P<0.01; P<0.001)).
Fgf21 treatment increases hepatic acyl CoA synthetase activity and fatty acid β-oxidation
Livers from the FGF21-treated KO mice had a 20% increase in total ACS activity compared to saline-treated KO mice (Figure 6a). FGF21 treatment was associated with increased expression of ACSL1, and FATP5 compared to saline treated KO mice (figure 6c). FGF21-treated mice demonstrated a robust 71% increase in CO2 production from [1-14C] palmitic acid compared to the saline-treated FGF21-KO mice (Figure 6b), although the level of β-oxidation in the FGF21-treated mice was lower than that seen WT cohort. Surprisingly, there were few significant changes in the expression levels of genes mediating mitochondrial β-oxidation (Figure 6C).
Conclusions
FGF21 is a novel metabolic regulator that has potent effects on glucose and lipid homeostasis. Administration of FGF21 reduces circulating triglycerides, NEFAs, and glucose and leads to weight loss in obese animals. This occurs through enhanced insulin sensitivity and increased adipose tissue energy expenditure22 caused, in part, by increased white adipose tissue thermogenesis23. In humans, an FGF21 analog was found to improve serum lipid profiles and reduce body mass24. In addition, FGF21 is essential for appropriate fatty acid oxidation in mice during prolonged fasting25 or while consuming a ketogenic diet12.
While FGF21 has multiple effects on a wide range of tissues, the liver is an important source of systemic FGF21 as well as a key site of action. In humans, FGF21 serum levels correlate with obesity and importantly appear to reflect the degree of fatty infiltration in the liver, suggesting that levels could serve as a marker for NAFLD6, 26. In diet induced obese mice, FGF21 treatment leads to resolution of obesity-associated NAFLD, suggesting that FGF21 could serve as a therapeutic agent for this disease27. However, interpreting the effects of FGF21 on fatty liver is complicated; FGF21 treatment potently induces weight loss which in turn leads to resolution of fatty liver. This makes it difficult to isolate a direct effect on hepatic metabolism from indirect effects related to weight loss27–30. By using the MCD diet to induce NAFLD and NASH in the context of leanness we can isolate the effects of FGF21 independent of weight loss. Consumption of an MCD diet led to substantial increases in hepatic mRNA expression and serum levels of FGF2131. In the absence of changes in expression in other FGF21 expressing tissues, including adipose tissue and pancreas, strongly suggests that the increased serum levels are derived from the liver.
Here we show that FGF21 plays an important role in the pathogenic elements of NASH independent of body weight. In mice consuming an MCD diet, lack of FGF21 was associated with significantly worsened lipid peroxidative damage, apoptosis, inflammation, fibrosis, and thus the progression to severe NASH. Importantly, treatment of FGF21-KO mice with FGF21 attenuated or eliminated the adverse effects of the MCD diet. This included reduced hepatic triglycerides normalized expression of pro-inflammatory and pro-fibrotic genes to levels seen in WT animals. Impressively, FGF21 administration actually reduced serum ALT and hepatic lipid peroxidative damage to below WT levels. These novel findings demonstrate that FGF21 treatment improves hepatic steatosis and most importantly inflammation and fibrosis independent of adiposity suggesting a direct beneficial effect on hepatic metabolism as well as a plausible therapeutic role.
The mechanism by which excess hepatic fat leads to tissue damage is multifactorial and not completely understood. Triglycerides have been considered potentially causal, however more recent work indicates that pathology stems from increased levels of non-esterified fatty acids. For example deletion of diacylglycerol-acyltransferase 2, which esterifies fatty acids, leads to decreased triglyceride accumulation, but also results in increased FFA content and is associated with substantially worse fibrosis19.
Fatty acids are metabolized through activation to long chain acyl CoAs, which then serve as substrates for anabolic and catabolic downstream pathways21. We profiled the hepatic long chain acyl CoA content in our model. FGF21-KO mice demonstrated a marked decrease (>50%) in total hepatic long chain acyl CoA content and a profound reduction in all species of long chain acyl CoAs examined. These differences were only observed in mice challenged with the MCD diet, as livers from FGF21-KO mice on a control diet showed no significant differences in hepatic lipid parameters when compared to WT mice (data not shown). Thus FGF21 becomes physiologically important in states requiring increased hepatic free fatty acid metabolism and removal.
These data define a novel and potentially very important metabolic role of FGF21 in the activation of long chain fatty acids to acyl CoAs. Long chain fatty acids are converted to acyl CoAs by a family of five acyl-CoA synthetases (ACSL) and six fatty acid transport proteins (FATP) possessing acyl-CoA synthetase activity20. Consistent with impaired fatty acid activation, FGF21-KO livers demonstrate reduced expression of Acsl1, Acsl5, Fatp1, Fatp2, and Fatp5, while treatment with FGF21 induces Acsl1 and Fatp5. In addition, mice lacking FGF21 have decreased total hepatic acyl CoA synthetase activity while FGF21 treatment augments acyl CoA synthetase activity. These data indicate that FGF21 serves to activate fatty acids at least in part by up regulating expression of the ACSLs. This pathway appears independent of dietary status as FGF21 is able to induce acyl CoA synthetase gene expression and enhance fatty acid oxidation in lean WT chow fed mice (Sup. Figure 3a+b).
Consistent with a role for FGF21 in fatty acid oxidation, FGF21-KO mice on the MCD diet demonstrate significantly decreased β-oxidation of palmitic acid to CO2. This is accompanied by reduced expression of genes involved in mitochondrial oxidation, including PGC-1α, a transcriptional coactivator involved in numerous mitochondrial pathways that is up regulated by FGF2132. Treatment of mice on MCD diet with FGF21 restores PPARα and PGC-1α levels and increases fatty acid oxidation. Taken together, our data demonstrate that FGF21 promotes hepatocyte fatty acid oxidation by activating two key processes: 1) FGF21 up regulates ACSL/FATP expression and increases acyl CoA synthetase activity in the cytosol to activate fatty acids to acyl CoAs more efficiently, and 2) FGF21 enhances mitochondrial β-oxidation of fatty acids.
Our data extend the previously described physiologic actions of FGF21 to include induction of long chain fatty acid activation and mitochondrial β-oxidation, promoting fatty acid disposal and reducing the potential for fatty acid-induced lipotoxicity. These results add further support to the emerging view that non triglyceride lipid species, especially hepatic free fatty acids, play a pivotal role in the development of NAFLD and NASH19, 33, 34, and further implicate FGF21 as a key regulator of fatty acid metabolism in the liver. Fatty acids have been shown to induce hepatic TNFα expression35 and cause hepatocyte apoptosis36. In addition, impairments in mitochondrial fatty acid oxidation have been linked to the development of NASH37, as reactive oxygen species generated by peroxisomal β- and microsomal ω-oxidation of accumulated fatty acids can lead to lipid peroxidation, DNA and protein damage, inflammation and fibrosis38. By potentiating the activation of long chain fatty acids to acyl CoAs and partitioning them towards mitochondrial β-oxidation, FGF21 limits the accumulation of free fatty acids. This leads to attenuated hepatic steatosis and diminishes the lipotoxic effects that can lead hepatocyte lipoapoptosis, inflammation and fibrosis. Notably, the latter appears to be a primary effect of FGF21 independent of diet, as we observe the same result in the healthy liver of WT chow fed mice.
The mechanism by which FGF21 targets hepatic metabolism is subject to debate. We have found that FGF21 can activate hepatic signaling in vivo13 and has been found to enhance oxidative gene expression in HepG2 cells treated with resveratrol39. Recent studies found adiponectin important to the insulin sensitizing effects of pharmacologic doses of FGF21 suggesting adiponectin may be an intermediate in the hepatic actions of FGF2140, 41. However, both studies are mainly concerned with the pharmacologic action of FGF21 to improve carbohydrate metabolism and not its physiologic function to regulate oxidative metabolism in the liver. Furthermore, the action to enhance FFA activation is likely direct as it has been shown that FGF21 reduces hepatic FFA levels in mice lacking adipose FGF21 signaling42.
Overall, our data suggest that up-regulation of FGF21 in NAFLD and NASH is a physiologic adaptation to hepatic stress that increases hepatic fatty acid activation, oxidation and disposal, but depending on the metabolic milieu, may be an insufficient compensatory response. Mice overexpressing FGF21 appear to be somewhat protected from the development of lipotoxic damage on the MCD diet (Sup. Figure 4 and 5). Furthermore, exogenous administration of FGF21 to WT mice with established NASH significantly improved liver function and abrogated the progression of steatohepatitis, and future studies will determine whether increasing systemic FGF21 to supra-physiologic levels confers this beneficial effect in patients. Taken together, our work provides major insights into the pathophysiology of NAFLD and NASH, the mechanism for FGF21 action in these disorders, and raises the exciting possibility that FGF21 or other agents mimicking its action may be effective and viable therapies for the treatment of both hepatic steatosis and steatohepatitis.
Supplementary Material
Acknowledgments
We are grateful for the help from Gary Cline at the Yale Mouse Metabolic Phenotyping Core and the technical assistance of Patrick Antonellis, Deanna Sverdlov, and Anisha Sharma. This work was supported by the following grants from the National Institutes of Health: R01 DK069983 and R37 DK28082 (J.S.F.), and 5 T32 DK007760-12 (P.C.C.).
Footnotes
Disclosures: A.K. works for Eli Lilly and Co. E.M.-F. has consulted for Novo/Nordisk and Novartis regarding FGF21. The remaining authors have no conflict of interest to report.
Author Contributions: F.M.F. Wrote manuscript designed and planned study, researched data. P.C.C. Wrote manuscript designed and planned study, researched data. I.N. researched data. Y.P. researched data. J.C. researched data. T.L. Researched data, A.K. reviewed/edited manuscript. D.S. reviewed/edited manuscript. J.S.F. reviewed/edited manuscript and research design. E.M.F. Wrote, reviewed and edited manuscript, research design.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Day CP. Natural history of NAFLD: remarkably benign in the absence of cirrhosis. Gastroenterology. 2005;129:375–8. doi: 10.1053/j.gastro.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 2.Tominaga K, Kurata JH, Chen YK, Fujimoto E, Miyagawa S, Abe I, Kusano Y. Prevalence of fatty liver in Japanese children and relationship to obesity. An epidemiological ultrasonographic survey Digestive diseases and sciences. 1995;40:2002–9. doi: 10.1007/BF02208670. [DOI] [PubMed] [Google Scholar]
- 3.Schattenberg JM, Schuppan D. Nonalcoholic steatohepatitis: the therapeutic challenge of a global epidemic. Curr Opin Lipidol. 2011;22:479–88. doi: 10.1097/MOL.0b013e32834c7cfc. [DOI] [PubMed] [Google Scholar]
- 4.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 5.Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. Journal of hepatology. 2006;45:600–6. doi: 10.1016/j.jhep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM, Badman MK, Martinez-Chantar ML, Maratos-Flier E. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139:456–63. doi: 10.1053/j.gastro.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alisi A, Ceccarelli S, Panera N, Prono F, Petrini S, De Stefanis C, Pezzullo M, Tozzi A, Villani A, Bedogni G, Nobili V. Association between Serum Atypical Fibroblast Growth Factors 21 and 19 and Pediatric Nonalcoholic Fatty Liver Disease. PLoS One. 2013;8:e67160. doi: 10.1371/journal.pone.0067160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H, Fang Q, Gao F, Fan J, Zhou J, Wang X, Zhang H, Pan X, Bao Y, Xiang K, Xu A, Jia W. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. Journal of hepatology. 2010;53:934–40. doi: 10.1016/j.jhep.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Yilmaz Y, Eren F, Yonal O, Kurt R, Aktas B, Celikel CA, Ozdogan O, Imeryuz N, Kalayci C, Avsar E. Increased serum FGF21 levels in patients with nonalcoholic fatty liver disease. European journal of clinical investigation. 2010;40:887–92. doi: 10.1111/j.1365-2362.2010.02338.x. [DOI] [PubMed] [Google Scholar]
- 10.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–37. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–25. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology. 2009;150:4931–40. doi: 10.1210/en.2009-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, Maratos-Flier E. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;59:2781–9. doi: 10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muise ES, Azzolina B, Kuo DW, El-Sherbeini M, Tan Y, Yuan X, Mu J, Thompson JR, Berger JP, Wong KK. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor gamma and altered metabolic states. Molecular pharmacology. 2008;74:403–12. doi: 10.1124/mol.108.044826. [DOI] [PubMed] [Google Scholar]
- 15.Johnson CL, Weston JY, Chadi SA, Fazio EN, Huff MW, Kharitonenkov A, Koester A, Pin CL. Fibroblast growth factor 21 reduces the severity of cerulein-induced pancreatitis in mice. Gastroenterology. 2009;137:1795–804. doi: 10.1053/j.gastro.2009.07.064. [DOI] [PubMed] [Google Scholar]
- 16.Rizki G, Arnaboldi L, Gabrielli B, Yan J, Lee GS, Ng RK, Turner SM, Badger TM, Pitas RE, Maher JJ. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. Journal of lipid research. 2006;47:2280–90. doi: 10.1194/jlr.M600198-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annual review of pathology. 2011;6:425–56. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 19.Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–88. doi: 10.1002/hep.23719. [DOI] [PubMed] [Google Scholar]
- 20.Ellis JM, Frahm JL, Li LO, Coleman RA. Acyl-coenzyme A synthetases in metabolic control. Current opinion in lipidology. 2010;21:212–7. doi: 10.1097/mol.0b013e32833884bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li LO, Klett EL, Coleman RA. Acyl-CoA synthesis, lipid metabolism and lipotoxicity. Biochimica et biophysica acta. 2010;1801:246–51. doi: 10.1016/j.bbalip.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camporez JP, Jornayvaz FR, Petersen MC, Pesta D, Guigni BA, Serr J, Zhang D, Kahn M, Samuel VT, Jurczak MJ, Shulman GI. Cellular mechanisms by which FGF21 improves insulin sensitivity in male mice. Endocrinology. 2013;154:3099–109. doi: 10.1210/en.2013-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–81. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, Kharitonenkov A, Bumol T, Schilske HK, Moller DE. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013;18:333–40. doi: 10.1016/j.cmet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, Burgess SC. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A. 2009;106:10853–8. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yilmaz Y. Review article: fructose in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2012;35:1135–44. doi: 10.1111/j.1365-2036.2012.05080.x. [DOI] [PubMed] [Google Scholar]
- 27.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang Z, Ko HJ, Kim JK, Veniant MM. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–9. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–27. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 29.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. The Journal of clinical investigation. 2005;115:1627–35. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–81. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 31.Ables GP, Perrone CE, Orentreich D, Orentreich N. Methionine-Restricted C57BL/6J Mice Are Resistant to Diet-Induced Obesity and Insulin Resistance but Have Low Bone Density. PLoS One. 2012;7:e51357. doi: 10.1371/journal.pone.0051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, Burgess SC. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:10853–8. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neuschwander-Tetri BA. Nontriglyceride hepatic lipotoxicity: the new paradigm for the pathogenesis of NASH. Curr Gastroenterol Rep. 2010;12:49–56. doi: 10.1007/s11894-009-0083-6. [DOI] [PubMed] [Google Scholar]
- 34.Walther TC, Farese RV., Jr Lipid droplets and cellular lipid metabolism. Annu Rev Biochem. 2012;81:687–714. doi: 10.1146/annurev-biochem-061009-102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ, Gores GJ. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–94. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 36.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. The Journal of biological chemistry. 2006;281:12093–101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 37.Caldwell SH, Swerdlow RH, Khan EM, Iezzoni JC, Hespenheide EE, Parks JK, Parker WD., Jr Mitochondrial abnormalities in non-alcoholic steatohepatitis. Journal of hepatology. 1999;31:430–4. doi: 10.1016/s0168-8278(99)80033-6. [DOI] [PubMed] [Google Scholar]
- 38.Berson A, De Beco V, Letteron P, Robin MA, Moreau C, El Kahwaji J, Verthier N, Feldmann G, Fromenty B, Pessayre D. Steatohepatitis-inducing drugs cause mitochondrial dysfunction and lipid peroxidation in rat hepatocytes. Gastroenterology. 1998;114:764–74. doi: 10.1016/s0016-5085(98)70590-6. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Wong K, Giles A, Jiang J, Lee JW, Adams AC, Kharitonenkov A, Yang Q, Gao B, Guarente L, Zang M. Hepatic SIRT1 attenuates hepatic steatosis and controls energy balance in mice by inducing fibroblast growth factor 21. Gastroenterology. 2014;146:539–49 e7. doi: 10.1053/j.gastro.2013.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin Z, Tian H, Lam KS, Lin S, Hoo RC, Konishi M, Itoh N, Wang Y, Bornstein SR, Xu A, Li X. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17:779–89. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM, Bauer SM, Wade M, Singhal E, Cheng CC, Volk K, Kuo MS, Gordillo R, Kharitonenkov A, Scherer PE. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013;17:790–7. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams AC, Yang C, Coskun T, Cheng CC, Gimeno RE, Luo Y, Kharitonenkov A. The breadth of FGF21’s metabolic actions are governed by FGFR1 in adipose tissue. Mol Metab. 2012;2:31–7. doi: 10.1016/j.molmet.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.