Abstract
Although vitamin C (ascorbate) is present in whole blood, measurements in red blood cells (RBCs) are problematic because of interference, instability, limited sensitivity, and sample volume requirements. We describe a new technique using HPLC with coulometric electrochemical detection for ascorbate measurement in RBCs of humans, wild-type mice, and mice unable to synthesize ascorbate. Exogenously added ascorbate was fully recovered even when endogenous RBC ascorbate was below the detection threshold (25 nM). Twenty microliters of whole blood or 10 μl of packed RBCs was sufficient for assay. RBC ascorbate was stable for 24 h from whole-blood samples at 4 °C. Processed, stored samples were stable for >1 month at −80 °C. Unlike other tissues, ascorbate concentrations in human and mouse RBCs were linear in relation to plasma concentrations (R = 0.8 and 0.9, respectively). In healthy humans, RBC ascorbate concentrations were 9–57 μM, corresponding to ascorbate plasma concentrations of 15–90 μM. Mouse data were similar. In human blood stored as if for transfusion, initial RBC ascorbate concentrations varied approximately sevenfold and decreased 50% after 6 weeks of storage under clinical conditions. With this assay, it becomes possible for the first time to characterize ascorbate function in relation to endogenous concentrations in RBCs.
Keywords: Ascorbic acid, Vitamin C, Red blood cells, Electrochemical detection, High-pressure liquid chromatography
Ascorbate (ascorbic acid, vitamin C) was first described to be present in whole blood more than 70 years ago [1,2]. However, spectrophotometric assays for ascorbate in red blood cells (RBCs)2 were subject to multiple types of interference [3–5]. As a consequence of inconsistency and lack of reliability, ascorbate concentrations in RBCs were controversial and considered inaccurate [3,6,7]. Despite the uncertainties, based on estimates from several investigators a fixed value for RBC ascorbate was taken as either as 19.9 [2,8,9] or 28.9 μM [10]. Although the former value appeared in older hematology textbooks [11], the knowledge that ascorbate is found in RBCs, with potential functional consequences, seems to be disappearing from modern hematology [12,13].
We addressed ascorbate measurements in RBCs for two major reasons. First, a concentration–function approach to nutrient recommendations [14] can be applied to ascorbate in RBCs. Although RBCs are easily obtained for function studies, it is unknown how physiologic RBC ascorbate concentrations are related to RBC function. Several functions of ascorbate within RBCs have been postulated, including actions as an antioxidant or in maintaining plasma ascorbate concentrations [15–17]. However, firm evidence for ascorbate function in RBCs is elusive. Concentration–function relationships for ascorbate in RBCs may be difficult to address in humans alone without strict inpatient diet control. This is because ascorbate plasma and tissue values reflect ingested amounts, and there is a steep relationship between concentrations and ingested amounts over the lower end of the physiologic dose range [18,19]. Humans depend on dietary ascorbate because we, as well as other primates, cannot synthesize it [20]. Most rodents synthesize ascorbate, which complicated vitamin C studies in animal models until gulonolactone oxidase knockout (gulo−/−) mice were introduced [21]. Similar to primates, gulo−/− mice lack the terminal enzyme in the ascorbate biosynthesis pathway and do not make ascorbate [20,21]. Although they appear normal if ascorbate-replete, gulo−/− mice must be supplemented with ascorbate to survive. Gulo−/− mice would be ideal for investigating concentration–function relationships for RBCs, if a suitable ascorbate assay were available.
The second reason for addressing ascorbate in RBCs is due to emerging data linking transfusion of packed RBCs to increased mortality under some circumstances, for unknown reasons [22–24]. To address the storage lesion, or why stored blood might deteriorate after collection, multiple RBC parameters have been measured as a function of storage time [25–27]. However, ascorbate measurement is absent from investigations. Because ascorbate concentrations in plasma, leukocytes, and platelets vary as a function of intake [18,19], variations in ascorbate concentration might also occur in RBCs. If such variations occurred, due to either initial concentration in donors or storage, it is possible that low ascorbate concentrations might be linked to the RBC storage lesion.
To address both concentration–function relationships and the storage lesion, an essential prerequisite was to develop a new ascorbate assay for RBCs. Key goals were to solve measurement problems of sensitivity, specificity, interference, instability, and inadvertent ascorbate oxidation and the need for comparatively high sample volumes (>300 μl whole blood) [4,5,10,28–30]. Poor sensitivity, lack of specificity, and interfering substances were key problems for prior assays. Ascorbate instability and inadvertent ascorbate oxidation were of particular concern for the RBC. Ascorbate is inherently unstable because it oxidizes in aqueous media. Ascorbate instability during analysis is expected to be heightened in RBC samples with their abundant iron, because iron accelerates ascorbate oxidation. We sought to use minimal sample volume so that multiple experiments could easily be performed using the same mice or humans. Development of an assay that addressed the many concerns could jump start the investigation of ascorbate in RBCs as a function of plasma concentration and measurements of ascorbate in stored human blood over time. Assay of ascorbic acid using HPLC coupled to coulometric electrochemical detection has resolved other concerns inherent to ascorbate measurement [31]. We predicted that this method could be modified to address problems of ascorbate detection in RBCs. This paper describes such an assay and its use.
Methods
Reagents and materials
l-Ascorbic acid, sodium acetate anhydrous, tetraoctylammonium bromide, dodecyltrimethylammonium chloride, orthophosphoric acid, bromine solution, and EDTA were supplied by Sigma–Aldrich (St. Louis, MO, USA). Sodium phosphate monobasic was supplied by Mallinckrodt Chemicals (Paris, KY, USA). Methanol was supplied by Omnisolv (Charlotte, NC, USA). Centrifugal filter units (Ultracel-10K) were purchased from Millipore (Billerica, MA, USA). Tubes with lithium heparin were purchased from Becton–Dickinson (Franklin Lakes, NJ, USA). Heparinized microhematocrit capillary tubes were purchased from Fisher Scientific (Pittsburgh, PA, USA). Tris(2-carboxyethyl)phosphine was purchased from Thermo Scientific (Rockford, IL, USA). Dehydroascorbic acid was prepared by oxidizing 10 mM ascorbic acid solution with bromine solution [32].
HPLC assay
Ascorbic acid was analyzed by HPLC with coulometric electrochemical detection [31] with modifications. The following instruments were used: HPLC autosampler and pump from Waters Chromatography (Milford, MA, USA) and Coulochem III detector from ESA-Dionex (Chelmsford, MA, USA). Detector settings were electrode 2, 250 mV; electrode 1, 0 mV. Mobile phase contained 0.05 M sodium phosphate monobasic, 0.05 M sodium acetate anhydrous, 189 μM dodecyltrimethylammonium bromide, and 36.6 μM tetraoctylammonium bromide. Tetraoctylammonium bromide was dissolved in 100% methanol. Other reagents were dissolved in HPLC-grade water (Milli-Q; Millipore) and methanol percentage was adjusted to 30% of final volume and pH adjusted to 4.8 with orthophosphoric acid. All concentrations are final concentrations. Standards and samples were analyzed with mobile phase at 1 ml/min. Injection volume was 10 μl. The column was 5 μm, 4.6 mm × 25 cm ODS-DABS C18 (Ultrasphere 240002; Beckman Coulter, Brea, CA, USA). The column was conditioned with mobile phase at 1 ml/min for 24–36 h prior to running standards and samples. The column was washed once monthly with 30% methanol/water for 24 h, 1 ml/min. Guard Cartridges Bioadvantage Basic C18 5 μm were essential for optimum performance (Thomson Instruments, Clear Brook, VA, USA) and had to be replaced after every 150–200 biological samples to avoid online sample oxidation. If samples contained excess uric acid, the mobile phase pH was increased to 5.7.
Dehydroascorbic acid was measured by reducing it to ascorbate with tris(2-carboxyethyl)phosphine and then using HPLC as above. To produce dehydroascorbic acid, 2 ml of 10 mM ascorbic acid in water was prepared, 4 μl bromine solution was added, and the mixture was vortexed for 30 s and bubbled with nitrogen gas until clear (2–4 min) [32]. The product, dehydroascorbic acid, was immediately diluted with cold phosphate-buffered saline (PBS) to 1, 2, 5, and 10 μM and reduced to ascorbic acid by addition of tris(2-carboxyethyl)phosphine (final concentration 0.5 μM). For HPLC analyses, the solution was diluted 1:1 with 90% methanol/1 mM EDTA. All procedures were conducted on ice. For sample determination, dehydroascorbic acid was measured by paired ascorbate measurement of nonreduced and reduced samples, using a predicted minimum 20-fold molar excess of tris(2-carboxyethyl)phosphine to ascorbate [33,34].
Animals
Wild-type mice (C57BL/6) were obtained from Charles River Laboratories (Wilmington, MA, USA). Gulo+/− mice (B6.129P2-GulotmlUmc/mmced, backcrossed to C57BL/6 for 10 generations) were obtained from the Mutant Mouse Regional Resource Center (University of California at Davis, USA), bred to obtain homozygous gulo−/− mice, and confirmed by RT-PCR and vitamin C deprivation to lack l-gulono-γ-lactone oxidase and the ability to synthesize vitamin C, respectively [35]. Gulo−/− mice used for experiments were 12–18 weeks of age. Gulo−/− mice do not make ascorbate, and ascorbate for these mice must come from either oral or parenteral sources [35]. Gulo−/− mice were supplemented with ascorbate in drinking water and achieved steady-state plasma concentrations corresponding to those of wild-type mice (50–65 μM; 330 mg/L for 1 week). To achieve lower ascorbate concentrations in gulo−/− mice, ascorbate supplements were withheld for 1 to 6 weeks. All animal experiments were conducted according to protocol K032 DDB 11 approved by the Animal Care and Use Committee of the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health (Bethesda, MD, USA). Mice were fed chow that had no detectable ascorbate as measured by HPLC (detection limit 10 nM).
Human subjects
Whole-blood samples were obtained from healthy subjects as approved by the National Institutes of Health per NIH Protocols 04-DK-0021 and 99-CC-0168. Whole-blood samples were placed immediately on ice and processed within 2 h unless otherwise described.
Preparation of plasma and RBC samples for HPLC analyses
Rodent samples
Mouse whole blood obtained by mandibular puncture was collected in heparinized plastic tubes (Microtainer; Becton–Dickinson) and drawn by capillary action into heparinized capillary tubes (Fisher Scientific) (maximal capillary tube volume 70 μl). Capillary tubes were sealed at the bottom using vinyl plastic putty (Critoseal; Leica) and centrifuged at 13,700g (12,000 rpm, Haemotkrit 210; Hettich, Tuttlingen, Germany) for 2 min at room temperature. All subsequent procedures were carried out on ice or at 4 °C. The centrifuged blood was separated into three parts by cutting the tube with a razor blade. The plasma (top section) was removed by pipette into an Eppendorf tube and processed as below. The buffy coat section (middle) was cut and discarded. The bottom sealed portion of the tube was cut and discarded. The remaining, cut, portion of the capillary tube contained RBCs. To elute them, 100–200 μl iced PBS (containing 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2.0 mM KH2PO4, pH adjusted to 7.4; Mediatech, Manassas, VA, USA) was gently pipetted into the top of the cut tube using a 200-μl pipette tip, which fits easily into the top of the cut tube. The bottom of the capillary tube section was immersed in 1 ml PBS in an Eppendorf tube, and RBCs were eluted by gentle pipetting. The capillary tube was washed with an additional 100–200 μl iced PBS. The eluted RBCs were washed three times using 1 ml PBS and centrifuged at 100g for 5 min for each wash. After the third wash, 1 volume of packed RBCs (minimal volume 10 μl or as otherwise indicated in text) was added to 4 volumes of ice-cold HPLC-grade water and the lysate incubated on ice for 1–2 min. The lysate was transferred to a centrifugal filter unit (Amicon Ultra 0.5 ml 10K Ultracel; Millipore) [28] followed by centrifugation at 14,000g for 10 min at 4 °C. An equal volume of ice-cold 90% methanol/1 mM EDTA was added to the ultrafiltrate. The mixture was vortexed for 10 s and then was either frozen at −80 °C or analyzed immediately by HPLC.
Mouse plasma was obtained from the top (cut) section of the capillary tube (see previous paragraph). One volume of plasma was added to 4 volumes of 90% methanol/1 mM EDTA in an Eppendorf tube, vortexed for 30–60 s, placed on ice for 10 min, and centrifuged at 41,600g for 10 min. The clear supernatant was transferred to another Eppendorf tube and either frozen at −80 °C or analyzed immediately by HPLC.
Human samples
Human whole blood obtained by venipuncture was drawn by vacuum into lithium heparin tubes (Becton–Dickinson), mixed gently by inverting several times, and placed on ice. Either immediately or after the indicated times, 100–200 μl whole blood was pipette into an Eppendorf tube and centrifuged at 1000g for 5 min to separate plasma, buffy coat, and RBCs. The plasma (top layer) was removed and processed as above for mouse plasma, and the buffy coat was discarded. From the bottom layer containing the RBCs, 50–100 μl was pipetted into 10-fold excess of PBS. RBC samples were washed and processed as above for mouse RBCs. For human blood samples of volume equal to or less than 70 μl, blood was processed as above for mouse blood using capillary tubes.
Minimal volume experiments
To determine the minimal amount of blood and packed red cell volume from mice and humans needed for analyses (see Fig. 3), the volumes listed in Table 1 were used.
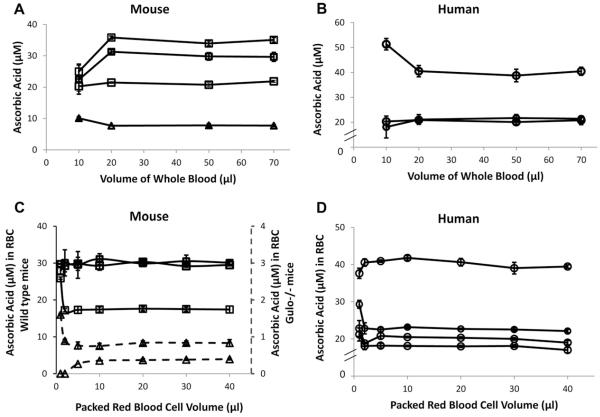
Fig.3.
Vitamin C measurements in RBCs: effect of (A, B) various volumes of whole blood drawn initially and (C, D) volume of packed RBCs prepared for assay. Each line indicates that blood was obtained from the same mouse or human. On each line, every symbol represents the mean value ± SD for three individually processed blood samples, with variation of the whole-blood volume used or packed red cell volume used as indicated on the x axis. For all samples, final lysate volume after purified water addition was 100 μl. See Methods for details. (A, B) Volume indicates starting whole blood volume used for vitamin C RBC assay from (A) mice or (B) humans. (C, D) Packed RBC volumes prepared for assay from (C) mice and (D) humans. Packed RBC volumes varied from 1 to 40 μl as indicated. Right axis in (C) represents expanded scale for low ascorbic acid concentrations in RBCs from gulo−/− mice.
Table 1.
Volumes of blood, RBCs, and water used to determine minimal volumes needed for experimentation.
| Blood (μl) | Packed RBCs (μl) | H2O (μl) | RBC% |
|---|---|---|---|
| 10 | 2 | 48 | 4 |
| 20 | 5 | 45 | 10 |
| 50 | 15 | 60 | 20 |
| 70 | 20 | 80 | 20 |
Collection of human blood and storage of RBCs
Following standard transfusion medicine procedures [36], whole blood was collected from healthy human subjects and processed using a CP2D/AS-3 collection system with a leukocyte filter (RC2D 500-ml Triple CP2D/AS-3; Pall Medical, Covina, CA, USA). The final packed RBC product was stored as if for transfusion at 4 °C for 6 weeks.
Statistics
All error bars represent the standard deviation. Unless otherwise indicated, each point represents a minimum of three measurements; see figure legends for details. The correlation coefficient (R) was calculated as follows:
Results
Ascorbate was measured using HPLC with coulometric electrochemical detection. Dehydroascorbic acid was determined by paired measurement of ascorbate in unreduced samples and samples reduced with tris(2-carboxyethyl)phosphine. The method was validated, including demonstration of long-term reproducibility (Table 2). Precision was determined for ascorbate (Table 3) and dehydroascorbic acid (Table 4).
Table 2.
Summary of the method for measurement of ascorbic acid and dehydroascorbic acid.
| Characteristic | Activity |
|---|---|
| Range of ascorbic acid | 0.025–10 μM |
| Linearity |
y = 1361.4x; R = 0.9996 (n = 6, February 2012) y = 1341.2X; R = 0.9999 (n = 6, February 2011) |
| Precision | CV% < 10% |
| Accuracy | Recovery% > 90% |
| LOQ | 0.025 μM ascorbic acid; 1 μM dehydroascorbic acid |
| LOD | 42.2 (peak area) |
The linearity of ascorbic acid was tested by measuring ascorbic acid dissolved in 30% methanol/1 mM EDTA, using concentrations from 0.025 to 10 μM. Accuracy represents recovery of added ascorbic acid to red blood cell lysates (data displayed in Fig. 2 and Table 5). The LOQ (limit of quantitation) is the lowest ascorbic acid concentration that was determined with defined precision (CV% < 20%, see Tables 3 and 4). The LOD (limit of detection) was determined by six repeat analyses of the lowest value measured. The lowest ascorbic acid standard produced a peak with y area. The average response (area y) and the standard deviation (SD) were calculated. The LOD is y + (3 × SD).
Table 3.
Precision of ascorbic acid measurements.
| Ascorbic acid | Mean (μM) | SD | CV%a | Accuracy (%)b |
|---|---|---|---|---|
| Intraday (n = 6) | ||||
| 20 μM | 20.5 | 0.2 | 1.1 | 2.5 |
| 10 μM | 9.7 | 0.2 | 1.9 | −3.0 |
| 1 μM | 1.0 | 0.0 | 1.6 | 0.0 |
| 0.1 μM | 0.1 | 0.0 | 3.7 | 8.0 |
| Interday (n = 12) | ||||
| 20 μM | 20.3 | 0.3 | 1.7 | 1.5 |
| 10 μM | 9.8 | 0.2 | 2.2 | −2.0 |
| 1 μM | 1.0 | 0.0 | 2.8 | −1.0 |
| 0.1 μM | 0.1 | 0.0 | 8.3 | 8.0 |
Ascorbic acid was dissolved in PBS, diluted 1:1 with 90% methanol/1 mM EDTA, and analyzed by HPLC.
Precision is expressed as correlation value (CV)%. CV% = (standard deviation/mean) × 100%.
Accuracy (%) = [(mean – original value)/original value] × 100%.
Table 4.
Precision of dehydroascorbic acid measurements.
| Dehydroascorbic acid |
n | Average (μM) |
SD | Precision (CV%) |
Accuracy (%) |
|---|---|---|---|---|---|
| Intraday | |||||
| 10 μM | 6 | 8.8 | 0.3 | 2.9 | 11.8 |
| 5 μM | 6 | 4.5 | 0.2 | 4.9 | 11.0 |
| 2 μM | 3 | 1.9 | 0.0 | 0.8 | 6.5 |
| 1 μM | 5 | 0.9 | 0.0 | 2.5 | 13.0 |
| Interday | |||||
| 10 μM | 14 | 9.4 | 1.1 | 12.0 | 6.2 |
| 5 μM | 14 | 4.7 | 0.4 | 8.8 | 6.0 |
| 2 μM | 6 | 1.9 | 0.0 | 2.3 | 7.5 |
| 1 μM | 8 | 0.9 | 0.1 | 10.7 | 8.0 |
CV% and accuracy were calculated as in Table 3. Dehydroascorbic acid was produced by oxidizing ascorbic acid with bromine and measured by HPLC after reduction with tris(2-carboxyethyl)phosphine as described under Methods.
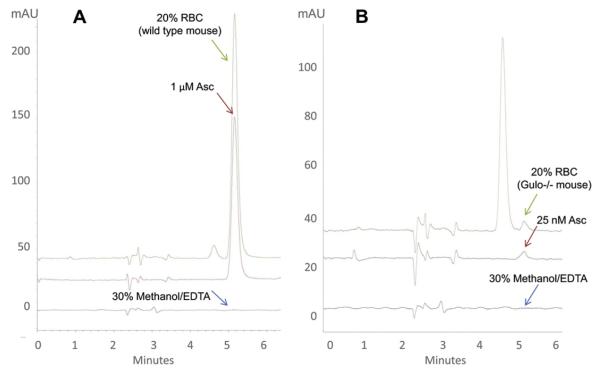
Representative chromatograms are shown in Fig. 1A for an ascorbate standard of 1 μM, for ascorbate found in 20 μl of diluted wild-type mouse RBCs, and for carrier (30% methanol/1 mM EDTA) without ascorbate. The ascorbate value shown in the chromatogram of RBCs was approximately 1.5 μM. With dilutions accounted for, the final RBC ascorbate concentration was 20.8 μM. The RBC chromatogram shows that minimal interfering peaks were present compared to the ascorbate standard. Because of the nonspecificity of the detection methods, interference was routinely reported with spectrophotometric and colorimetric assays for plasma and RBC ascorbate [3–5]. The source of interference could not always be identified, but was generally believed to be other reducing compounds and/or dietary components that were present in blood. Interference is minimized by the HPLC separation and electrochemical detection technique presented here. The HPLC method allows detection of as little as 26 nM ascorbate in RBCs and without interfering peaks (Fig. 1B). With dilutions accounted for, the final RBC ascorbate concentration was 370 nM. Samples were of 20 μl RBCs that were obtained from gulo−/− mice, which are unable to synthesize ascorbate and that received an ascorbate-free diet. A 25 nM standard and blank injection without ascorbate are also displayed.
Fig.1.
Representative chromatograms of standards (red), RBC samples in 30% methanol/1 mM EDTA (green), and 30% methanol/1 mM EDTA alone (gray). 20 μl of packed mouse red blood cells from (A) wild-type mouse or (B) gulo−/− mouse was prepared as described under Methods. Injection volume was 10 μl. (A) Red, 1 μM ascorbate standard in 30% methanol/1 mM EDTA. 30% methanol/1 mM EDTA alone is shown for comparison. RBC concentration in the chromatogram as shown was 1.5 μM. When dilutions are considered, the final ascorbate concentration in wild-type RBCs was 20.8 μM. (B) Red, 25 nM ascorbate standard in 30% methanol/1 mM EDTA. 30% methanol/1 mM EDTA alone is shown for comparison. RBC concentration in the chromatogram as shown was approximately 26 nM. When dilutions are considered, the final ascorbate concentration in RBCs from this gulo−/− mouse was 370 nM. The gulo−/− mouse was on an ascorbate-free diet for approximately 8 weeks. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
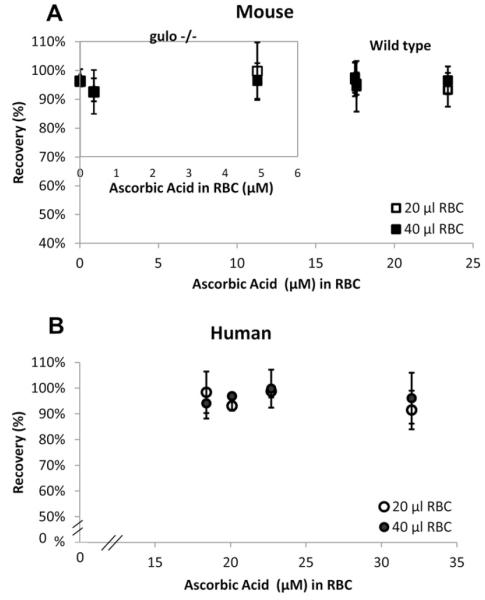
A key concern for ascorbate measurement in RBCs using the HPLC method was inadvertent oxidation by endogenous iron. Recovery of a known amount of ascorbate added was an essential step for assay fidelity. No ascorbate was recovered using standard ascorbate assay conditions of methanol/EDTA addition to RBCs, which is done to cause simultaneous lysis and protein precipitation [4,5]. Addition of other chelators did not improve ascorbate loss. These findings suggested that for ascorbate stability, protein should remain intact after RBC lysis [28,29]. Therefore, after three washes, RBCs were lysed in ice-cold HPLC-grade water and protein was removed by rapid centrifugal ultrafiltration. To assess recovery, samples were processed either with or without ascorbate addition immediately at lysis but before ultrafiltration. RBC samples were taken from healthy human subjects; from wild-type mice, which synthesize ascorbate; and from gulo−/− mice, which do not. Gulo−/− mice were given variable amounts of ascorbate in their diets so that ascorbate concentrations in RBCs would vary. Recovery was ~100% from all mouse and human samples and was independent of the initial RBC ascorbate concentration that was determined as part of recovery assessment (Fig. 2, Table 5). Recovery was 91–99% even when endogenous RBC concentration was as low as 380 nM or was below the detection limit of 25 nM for RBCs. Recovery was the same whether the processed hematocrit was 20%, from 60 μl of whole blood, or 40%, from 60–120 μl of whole blood. Because recovery of added ascorbate was ~100%, these data also indicate that ascorbate is not bound to hemoglobin or to RBC proteins >10,000 Da.
Fig.2.
Recovery of ascorbate added to RBCs. RBC ascorbate was measured before and after ascorbate addition to water-lysed RBCs: from (A) wild-type (C57BL/6) mice or mice unable to synthesize vitamin C (gulonolactone oxidase knockout mice, gulo−/−) (inset) and from (B) human subjects. For all samples, the amount of added ascorbate for recovery of 100% would have changed the final internal RBC concentration by 3.6 μM. Internal RBC concentration prior to addition is indicated on the x axis. Packed RBC volumes were 20 or 40 μl, as indicated. Three samples were obtained from each of three wild-type mice, three gulo−/− mice, and four human subjects.
Table 5.
Recovery of ascorbic acid spiked into 20% and 40% RBCs.
| Type of RBC |
RBC ascorbate (μM) |
RBC% (0.1 ml) |
Endogenous RBC ascorbate (pmol/0.1 ml) |
Added ascorbate (pmol/ 0.1 ml) |
RBC ascorbate with addition (pmol/0.1 ml) |
Recovery (%)a |
|---|---|---|---|---|---|---|
| C57 (gulo−/−) | ||||||
| Gulo- 1 |
0.37 ± 0.2 | 20 | 4.9 ± 0.2 | 40 | 43.0 ± 2.3 | 94.9 ± 5.7 |
| Gulo- 1 |
0.37 ± 0.2 | 40 | 10.8 ± 0.3 | 40 | 49.7 ± 0.8 | 97.2 ± 2.7 |
| Gulo- 2 |
0 | 20 | 0 | 50 | 48.7 ± 1.5 | 97.4 ± 3.0 |
| Gulo- 2 |
0 | 40 | 0 | 50 | 48.1 ± 0.5 | 96.2 ± 1.1 |
| Gulo- 3 |
4.89 ± 0.4 | 20 | 70.9 ± 4.5 | 50 | 124.5 ± 8.5 | 99.7 ± 10. |
| Gulo- 3 |
4.89 ± 0.4 | 40 | 130.3 ± 6.8 | 50 | 178.4 ± 3.1 | 96.3 ± 6.2 |
| C57 (wild type) | ||||||
| Wt-1 | 17.5 ± 0.5 | 20 | 245.1 ± 4.6 | 50 | 292.9 ± 1.9 | 95.4 ± 3.9 |
| Wt-1 | 17.5 ± 0.5 | 40 | 486.0 ± 13.0 | 50 | 533.3 ± 4.4 | 94.5 ± 8.8 |
| Wt-2 | 17.5 ± 1.1 | 20 | 257.4 ± 13.0 | 50 | 305.9 ± 2.9 | 96.9 ± 5.9 |
| Wt-2 | 17.5 ± 1.1 | 40 | 467.7 ± 9.0 | 50 | 516.4 ± 2.8 | 97.6 ± 5.3 |
| Wt-3 | 23.4 ± 2.8 | 20 | 356.1 ± 8.9 | 50 | 402.7 ± 2.9 | 93.3 ± 5.9 |
| Wt-3 | 23.4 ± 2.8 | 40 | 571.6 ± 0.9 | 50 | 619.8 ± 2.4 | 96.6 ± 4.8 |
| Human | ||||||
| H-1 | 17.9 ± 0.5 | 20 | 252.1 ± 4.1 | 50 | 301.2 ± 4.0 | 98.4 ± 8.1 |
| H-1 | 17.9 ± 0.5 | 40 | 476.6 ± 20.7 | 50 | 523.6 ± 5.9 | 94.0 ± 5.9 |
| H-2 | 20.1 ± 0.7 | 20 | 283.5 ± 3.9 | 50 | 330.0 ± 0.9 | 93.1 ± 1.8 |
| H-2 | 20.1 ± 0.7 | 40 | 531.0 ± 15.8 | 50 | 579.5 ± 0.5 | 96.9 ± 1.0 |
| H-3 | 22.7 ± 0.4 | 20 | 317.6 ± 2.8 | 50 | 367.0 ± 1.2 | 98.8 ± 2.4 |
| H-3 | 22.7 ± 0.4 | 40 | 619.2 ± 12.8 | 50 | 669.1 ± 3.7 | 99.8 ± 7.4 |
| H-4 | 30.4 ± 3.5 | 20 | 447.6 ± 10.1 | 50 | 493.4 ± 3.8 | 91.5 ± 7.5 |
| H-4 | 30.4 ± 3.5 | 40 | 736.5 ± 39.8 | 50 | 782.4 ± 1.8 | 91.4 ± 3.7 |
We determined the limiting amount of whole blood that could be used for reproducible ascorbate measurement in RBCs from mice and humans (Fig. 3A and B). If measurement was reproducible, the ascorbate RBC concentration should remain unchanged despite differences in initial amount of whole blood used. RBC ascorbate concentrations were constant with as little as 20 μl initial starting volume of whole blood, from both mice and humans and across a range of internal ascorbate concentrations.
We also determined the minimum hematocrit that was necessary using RBCs from mice and humans (Fig. 3C and D). As above, if measurements were reproducible, ascorbate RBC concentrations should remain unchanged as hematocrit varied. Ascorbate RBC concentration was constant at a hematocrit as low as 10%, even with endogenous ascorbate concentrations as low as 400 nM. Taken together, the data in Fig. 3 indicate that a starting whole blood volume of 20 μl yielding a hematocrit as low as 10% was acceptable for reproducible ascorbate values in RBC, and 40 μl of whole blood was definitely sufficient. At a whole-blood volume of 40 μl, multiple samples are readily obtainable from the same mouse over time, making long-term experiments possible.
Dehydroascorbic acid within RBCs was determined by paired measurement of samples with and without the reducing agent tris(2-carboxyethyl)phosphine. As expected, based on multiple dehydroascorbic acid-reducing mechanisms within RBCs [37], no dehydroascorbic acid was detected in RBCs (Supplementary Fig. 1, Supplementary Table 1). As a control, dehydroascorbic acid was measured in and recovered from extracellular buffer in the presence of RBCs (Supplementary Table 2).
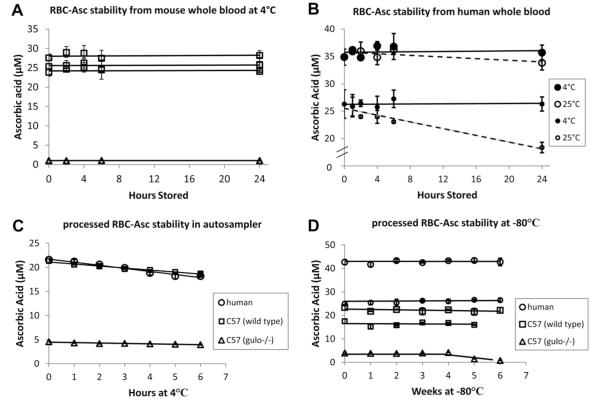
Ascorbate measurements in many sample types are prone to inadvertent oxidation [4,5]. Ascorbate oxidation in RBCs might occur not only during sample preparation, as with protein precipitation described above, but also as samples are processed and/or stored. We evaluated ascorbate stability in mouse and human RBC samples obtained from whole blood incubated at various temperatures before processing, for processed mouse and human samples in an HPLC autosampler at 4 °C, and in processed mouse and human samples stored at −80°C over weeks before analyses (Fig. 4A–D). Mimicking animal experimentation or clinical conditions, RBC ascorbate was stable if mouse or human whole blood was kept at 4 °C for as long as 24 h prior to processing (Fig. 4A and B). These data are also consistent with the stability of plasma ascorbate obtained from whole-blood samples that were stored at 4 °C for as long as 24 h prior to separation of plasma [38]. Processed samples kept in an HPLC autosampler at 4 °C were stable for several hours before analysis (Fig. 4C). Because each sample run required approximately 7 min, at least 30 samples could be loaded at one time. Processed samples stored at −80 °C were stable for at least 1 month, although for longer storage times RBC samples of <5 μM had less stability compared to those with higher concentrations (Fig. 4D).
Fig.4.
Ascorbic acid stability in RBC samples from whole blood, prepared samples in an HPLC autosampler, and samples in frozen storage. For all experiments, 20 μl packed RBCs was prepared as described under Methods; final lysis volume was 100 μl. Each line indicates that blood was obtained from the same mouse or human. On each line, every symbol represents the mean value ± SD for three individually processed blood samples, with variation in time as indicated on the x axis. (A) Mouse whole-blood samples were stored at 4 °C for times indicated before processing. (B) Human whole-blood samples were stored at 4 or 25 °C for the times indicated before processing. (C) Processed mouse or human RBC samples were kept in an autosampler at 4 °C for the times indicated before analyses. (D) Processed mouse or human RBC samples were stored at −80 °C for the weeks indicated before analyses.
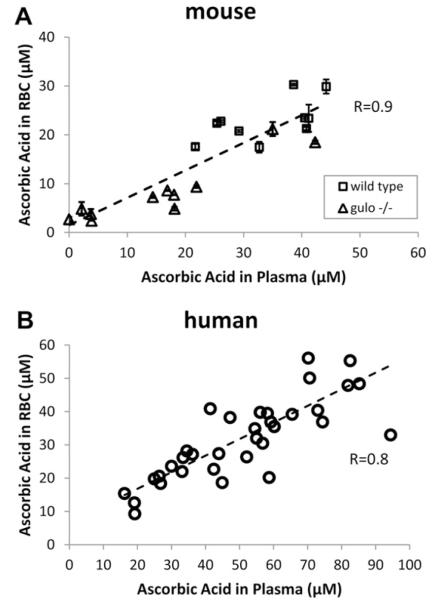
We utilized the assay to measure plasma and RBC ascorbate concentrations in samples obtained from mouse and human whole blood (Fig. 5A and B). Mouse samples were obtained from both wild-type and gulo−/− mice, to ensure a range of plasma ascorbate concentrations. Human samples were obtained from enough subjects to unmask the expected dietary variability of ascorbate ingestion [18,19,39]. The data show that RBC ascorbate was linearly related to plasma ascorbate for both mice and humans and that there was no ascorbate accumulation in RBC against a plasma concentration gradient. These findings are unique compared to other cell types, which accumulate ascorbate to millimolar concentrations against a concentration gradient and saturate at plasma concentrations of approximately 40–50 μM [14,18,19].
Fig.5.
RBC ascorbate concentrations as a function of plasma ascorbate concentrations in samples from (A) mouse and (B) human whole blood. (A) Mouse blood. Packed RBC volume was 20 μl, total water lysis volume 100 μl. Each symbol represents the mean ± SD of three separate samples from the same animal. The slope of the line is 0.6 and the y intercept at x = 0 is 1.5 μM. (B) Human blood. Packed RBC volume was 40 μl, total water lysis volume 200 μl. The slope of the line is 0.5 and the y intercept at x = 0 is 6.8 μM.
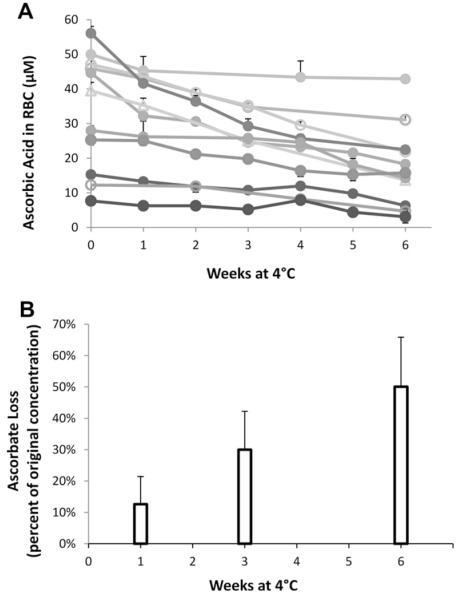
We measured RBC ascorbate in stored blood from human subjects as a function of storage time (Fig. 6A and B). Blood was stored under the same conditions as packed RBCs used for transfusion. The data show that initial RBC values varied approximately sevenfold and that all stored RBCs lost ascorbate over time. Although the rate of ascorbate loss showed individual variability, by 6 weeks the average ascorbate loss was approximately 50%.
Fig.6.
Ascorbate concentrations in stored human RBCs as a function of storage time. Packed RBCs from 11 healthy donors were prepared by standard transfusion medicine procedures and stored at 4 °C in a dark refrigerator for 6 weeks. Samples were taken at the weeks indicated. Packed RBC volume was 40 μl, total water lysis volume 200 μl. (A) RBC ascorbate concentration as a function of storage time. Each symbol represents the mean ± SD of three separate samples drawn from the same RBC storage bag. Each line and symbol type represents a different donor. (B) Loss of ascorbate in percentage compared to original RBC concentration.
Discussion
We describe here a new assay for ascorbate in RBCs that is sensitive, specific, free from interference, and accurate with small blood volumes and provides for ascorbate stability in samples processed immediately and stored for 1 month. This assay detected intracellular RBC ascorbate concentrations as low as 25 nM. Forty to fifty microliters of whole blood was routinely used for preparing RBC, and whole-blood volumes of 20 μl were sufficient. The assay was used to measure ascorbate in mouse and human RBCs and in packed RBCs stored under conditions for clinical use in transfusion. Although plasma ascorbate values were previously related to whole blood ascorbate concentrations [2,7], we describe for what we believe to be the first time a linear relationship between internal RBC and plasma ascorbate concentrations across a wide range for both mice and humans. We also believe that these measurements are the first to describe changes in ascorbate in stored blood over time.
Soon after its chemical identification as ascorbic acid, vitamin C was detected in whole blood and in RBCs. However, RBC measurements of ascorbate were considered unreliable because of assay interference. Ascorbate assays were based on either reduction of the dye 2,6-dichlorophenol indophenol or formation of a colored derivative of 2,4-dinitrophenylhydrazine. Neither of these reactions is specific, and assay interference from other reducing substances in biological samples was predicted and found [3–5,7,10,40]. Because of insensitivity, these assays required a sample volume of at least several hundred microliters, and animal experiments were difficult. With the advent of HPLC, there was an improvement in specificity and reduction of interference, but sensitivity and starting sample volume remained limiting factors. The lowest concentration of ascorbate that could be detected in RBC was approximately 10 μM, and measurement required several hundred microliters of blood [29,30]. The method we describe improves sensitivity ~100-fold and can detect ascorbate in RBC obtained from 20–50 μl of whole blood.
An inherent difficulty in measuring RBC ascorbate is the abundance of iron, which under some circumstances is an excellent catalyst for ascorbate oxidation, dependent on the coordination environment of the iron [41,42]. In RBCs lysed and prepared for analyses, it is a reasonable possibility that iron might oxidize ascorbate. Unless stability is accounted for, RBC ascorbate concentration values will be incorrectly low. After RBC isolation, we found that when RBC protein was precipitated, unintentional ascorbate oxidation was complete despite the addition of chelators. Given these findings, we surmised that hemoglobin would be an excellent iron chelator as long as hemoglobin was not denatured. We utilized the sensitivity of RBCs to hypotonicity with water lysis and removed hemoglobin from ascorbate within 10 min. These steps resulted in 100% recovery of added ascorbate, without oxidation artifact.
Direct HPLC methods with dehydroascorbic acid detection have been available for many years, but detectors are insensitive and not suitable for many biological samples [43–46]. Other methods utilize HPLC separation of dehydroascorbic and ascorbic acids, followed by online reduction of dehydroascorbic acid to ascorbic acid and quantitation as ascorbic acid [47,48]. These methods are also relatively insensitive, have not been used with a variety of biological samples, and do not account for inadvertent online sample oxidation. Mass spectrometry has been used to detect dehydroascorbic acid directly, but prior methods have been unsuitable for biological samples [5,49] A recent direct technique was described for food samples using tandem mass spectrometry with triple quadrupole in selective reaction monitoring mode [50]. Although this method has promise, its limitations are described use only with food samples; high initial sample volume requirement, so that measurements in biological samples would be difficult; no information concerning whether inadvertent ascorbate oxidation occurs during analyses of biological samples; and the need for sample dilution because of matrix effects, so that detection limits are too high for use with biological samples. If these problems can be solved, this method may improve dehydroascorbic acid measurement in biological samples.
For ascorbate in RBCs, published data support an unusual transport mechanism and several different functions [16,51]. Unlike most cells, ascorbate is not transported into RBCs directly by sodium-dependent vitamin C transporters [52]. Instead, the oxidized species dehydroascorbic acid appears to be transported on facilitated glucose transporters, followed by internal reduction to ascorbate [17,34,51–53]. Potential functions of ascorbate in RBCs include RBC maintenance of plasma ascorbate concentrations by ascorbate or dehydroascorbic acid efflux from RBCs, transmembrane electron transfer from RBC ascorbate, and antioxidant functions to protect RBCs from oxidative damage or to recycle membrane tocopherol [16,17,51,54].
Despite available information, ascorbate transport into and function within RBCs is not definitive, in part because of prior difficulties in accurate ascorbate measurements and inability to use small blood volumes permissive for rodent experiments. Most data for transport and function are not based on measurements of ascorbate itself. Rather, [14C] dehydroascorbic acid is used to assess transport and accumulation and is prepared by oxidation of [14C] ascorbate. Despite known dehydroascorbic acid instability and ready hydrolysis, the purity of [14C]dehydroascorbic acid in experiments is usually not described. Using [14C]dehydroascorbic acid without verification of its purity may lead to errors in data interpretation. For example, others have described that [14C]dehydroascorbic acid is not transported into mouse RBCs at all [17]. Because RBCs from mice also lack ascorbate transporters [52], the conclusion is that mouse RBCs lack ascorbate. Our findings here, using direct mass measurement, indicate that RBCs from both mice and humans have internal ascorbate, and at similar concentrations. The new assay described here quantitates mass, so that the problem of assay artifact with [14C] dehydroascorbic acid is avoided. Also, as a consequence of prior assay insensitivity, dehydroascorbic acid experimental concentrations are often used that are 2 to 3 orders of magnitude above what appear to be physiologic concentrations [38]. The use of nonphysiologic dehydroascorbic acid concentrations may obfuscate ascorbate/dehydroascorbic acid transport into and function within RBCs. Direct measurement of ascorbate, using the assay described here, will allow the science of ascorbate in RBCs to advance.
For mice and humans, RBC ascorbate values were approximately 60% of those of plasma (Fig. 5). The x = 0 intercepts for both species had a y value >0. While it is not clear that this intercept is biologically significant considering the distribution of the data, one interpretation is that RBCs effectively conserve ascorbate, especially at low plasma values. Even in the presence of very low plasma ascorbate values (i.e., <1 μM), what little ascorbate is present in plasma might oxidize to dehydroascorbic acid, which is transported into RBCs by facilitated transport on glucose transporters and trapped by reduction to ascorbate [16,17,32,52]. Such a mechanism could exist if ascorbate function in RBCs was essential, although the identity of this function is unknown.
To conduct both transport and concentration-dependent function studies, it is ideal to have conditions under which ascorbate concentrations can be varied easily and over a wide range [14]. In humans, plasma ascorbate concentrations vary ~10- to 12-fold because of varying dietary ascorbate ingestion [18,19,39]. However, identification of human subjects with low ascorbate concentrations is time-consuming and experimentally limiting. A practical solution is to vary ascorbate intake in a small animal, but most synthesize ascorbate. An exception is the gulo−/− mouse, which cannot synthesize ascorbate and must ingest it to survive [35]. Using this mouse and the assay described here, we were able to vary ascorbate concentrations in RBCs over more than 2 orders of magnitude. Because required sample volumes are low as 20 μl, multiple blood samples can be taken from the same mouse acutely or over days or weeks. The ascorbate assay, used in the gulo−/− mouse model, has the potential to reveal new concentration-dependent functions of ascorbate in RBCs, findings that may translate to humans.
Similarly, the ascorbate assay described can be used to determine whether stored human RBCs are affected by various ascorbate concentrations, as a function of either initial concentration or loss over time. Thus, the ascorbate assay opens new possibilities for learning whether low ascorbate is related to the RBC storage lesion and, perhaps, for improving the quality of blood stored for transfusion.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program, National Institutes of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. We thank Cynthia Matthews and her staff for expert technical assistance, Drs. Harvey Klein and Susan Leitman for their keen insights, and all of the Department of Transfusion Medicine, Clinical Center, National Institutes of Health.
Footnotes
Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ab.2012.04.014.
Abbreviations used: gulo−/−, gulonolactone knockout mice; PBS, phosphate-buffered saline; RBC, red blood cell.
References
- [1].Mirsky A, Swadesh S, Soskin S. Total ascorbic acid content of human blood. Biochem. J. 1935;32:1130–1131. [Google Scholar]
- [2].Butler AM, Cushman M. Distribution of ascorbic acid in the blood and its nutritional significance. J. Clin. Invest. 1940;19:459–467. doi: 10.1172/JCI101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Iggo B, Owen JA, Stewart CP. The determination of vitamin C in normal human plasma and erythrocytes. Clin. Chim. Acta. 1956;1:167–177. doi: 10.1016/0009-8981(56)90032-8. [DOI] [PubMed] [Google Scholar]
- [4].Washko PW, Welch RW, Dhariwal KR, Wang Y, Levine M. Ascorbic acid and dehydroascorbic acid analyses in biological samples. Anal. Biochem. 1992;204:1–14. doi: 10.1016/0003-2697(92)90131-p. [DOI] [PubMed] [Google Scholar]
- [5].Rumsey SC, Wang Y, Levine M. Ascorbic acid and dehydroascorbic acid analyses in biological samples. In: Song WO, Beecher GR, Eitenmiller RE, editors. Modern Analytical Methodologies on Fat and Water Soluble Vitamins. Wiley; New York: 2000. pp. 411–445. [Google Scholar]
- [6].Stephens DJ, Hawley EE. The partition of reduced ascorbic acid in blood. J. Biol. Chem. 1936;115:653–658. [Google Scholar]
- [7].Lubschez R. Studies in ascorbic acid with especial reference to the white layer. I. Description of method and comparison of ascorbic acid levels in whole blood, plasma, red cells, and white layer. J. Clin. Invest. 1945;24:573–578. doi: 10.1172/JCI101637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sargent F. A study of the normal distribution of ascorbic acid between the red cells and plasma of human blood. J. Biol. Chem. 1947;171:471–476. [PubMed] [Google Scholar]
- [9].Barkhan P, Howard AN. Distribution of ascorbic acid in normal and leukaemic human blood. Biochem. J. 1958;70:163–168. doi: 10.1042/bj0700163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Westerman MP, Zhang Y, McConnell JP, Chezick PA, Neelam R, Freels S, Feldman LS, Allen S, Baridi R, Feldman LE, Fung LW. Ascorbate levels in red blood cells and urine in patients with sickle cell anemia. Am. J. Hematol. 2000;65:174–175. doi: 10.1002/1096-8652(200010)65:2<174::aid-ajh15>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- [11].Beutler E. Biochemistry and function of the erythrocyte: composition of the erythrocyte. In: Williams WJ, Beutler E, Erslev AJ, Lichtman M, editors. Hematology. McGraw–Hill; New York: 1983. pp. 280–287. [Google Scholar]
- [12].Hoffman R. Hematology: Basic Principles and Practice. Churchill Livingstone Elsevier; Philadelphia: 2009. [Google Scholar]
- [13].Lichtman MA. Williams Hematology. Mc Graw–Hill; New York: 2010. [Google Scholar]
- [14].Levine M, Espey MG, Padayatty SJ. Vitamin C: a concentration–function approach yields pharmacology and therapeutic discoveries. Adv. Nutr. 2011;2:78–88. doi: 10.3945/an.110.000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].May JM. Ascorbate function and metabolism in the human erythrocyte. Front. Biosci. 1998;3:d1–d10. doi: 10.2741/a262. [DOI] [PubMed] [Google Scholar]
- [16].Goldenberg H, Landertshamer H, Laggner H. Functions of vitamin C as a mediator of transmembrane electron transport in blood cells and related cell culture models. Antioxid. Redox Signal. 2000;2:189–196. doi: 10.1089/ars.2000.2.2-189. [DOI] [PubMed] [Google Scholar]
- [17].Montel-Hagen A, Kinet S, Manel N, Mongellaz C, Prohaska R, Battini JL, Delaunay J, Sitbon M, Taylor N. Erythrocyte Glut1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C. Cell. 2008;132:1039–1048. doi: 10.1016/j.cell.2008.01.042. [DOI] [PubMed] [Google Scholar]
- [18].Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich J, King J, Cantilena LR. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA. 1996;93:3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc. Natl. Acad. Sci. USA. 2001;98:9842–9846. doi: 10.1073/pnas.171318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Inai Y, Ohta Y, Nishikimi M. The whole structure of the human nonfunctional l-gulono-γ-lactone oxidase gene—the gene responsible for scurvy—and the evolution of repetitive sequences thereon. J. Nutr. Sci. Vitaminol. (Tokyo) 2003;49:315–319. doi: 10.3177/jnsv.49.315. [DOI] [PubMed] [Google Scholar]
- [21].Maeda N, Hagihara H, Nakata Y, Hiller S, Wilder J, Reddick R. Aortic wall damage in mice unable to synthesize ascorbic acid. Proc. Natl. Acad. Sci. USA. 2000;97:841–846. doi: 10.1073/pnas.97.2.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tinmouth A, Fergusson D, Yee IC, Hebert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014–2027. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- [23].Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N. Engl. J. Med. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- [24].Hajjar LA, Vincent JL, Galas FR, Nakamura RE, Silva CM, Santos MH, Fukushima J, Kalil FR, Sierra DB, Lopes NH, Mauad T, Roquim AC, Sundin MR, Leao WC, Almeida JP, Pomerantzeff PM, Dallan LO, Jatene FB, Stolf NA, Auler JO., Jr. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304:1559–1567. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- [25].Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, McMahon TJ. Evolution of adverse changes in stored RBCs. Proc. Natl. Acad. Sci. USA. 2007;104:17063–17068. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, Stamler JS. S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc. Natl. Acad. Sci. USA. 2007;104:17058–17062. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hess JR. Red cell changes during storage. Transfus. Apher. Sci. 2010;43:51–59. doi: 10.1016/j.transci.2010.05.009. [DOI] [PubMed] [Google Scholar]
- [28].Iheanacho EN, Stocker R, Hunt NH. Redox metabolism of vitamin C in blood of normal and malaria-infected mice. Biochim. Biophys. Acta. 1993;1182:15–21. doi: 10.1016/0925-4439(93)90147-s. [DOI] [PubMed] [Google Scholar]
- [29].Iheanacho EN, Hunt NH, Stocker R. Vitamin C redox reactions in blood of normal and malaria-infected mice studied with isoascorbate as a nonisotopic marker. Free Radic. Biol. Med. 1995;18:543–552. doi: 10.1016/0891-5849(94)00182-j. [DOI] [PubMed] [Google Scholar]
- [30].Mendiratta S, Qu ZC, May JM. Erythrocyte ascorbate recycling: antioxidant effects in blood. Free Radic. Biol. Med. 1998;24:789–797. doi: 10.1016/s0891-5849(97)00351-1. [DOI] [PubMed] [Google Scholar]
- [31].Levine M, Wang Y, Rumsey SC. Analysis of ascorbic acid and dehydroascorbic acid in biological samples. Methods Enzymol. 1999;299:65–76. doi: 10.1016/s0076-6879(99)99009-2. [DOI] [PubMed] [Google Scholar]
- [32].Rumsey SC, Kwon O, Xu GW, Burant CF, Simpson I, Levine M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J. Biol. Chem. 1997;272:18982–18989. doi: 10.1074/jbc.272.30.18982. [DOI] [PubMed] [Google Scholar]
- [33].Rumsey SC, Welch RW, Garraffo HM, Ge P, Lu SF, Crossman AT, Kirk KL, Levine M. Specificity of ascorbate analogs for ascorbate transport: synthesis and detection of [125I]6-deoxy-6-iodo-l-ascorbic acid and characterization of its ascorbate-specific transport properties. J. Biol. Chem. 1999;274:23215–23222. doi: 10.1074/jbc.274.33.23215. [DOI] [PubMed] [Google Scholar]
- [34].Corpe CP, Lee JH, Kwon O, Eck P, Narayanan J, Kirk KL, Levine M. 6-Bromo-6-deoxy-l-ascorbic acid: an ascorbate analog specific for Na+-dependent vitamin C transporter but not glucose transporter pathways. J. Biol. Chem. 2005;280:5211–5220. doi: 10.1074/jbc.M412925200. [DOI] [PubMed] [Google Scholar]
- [35].Maeda H. Preparation of chemically modified carbon electrodes by anodization in 1-alkanols and their application to electrochemical analysis. Yakugaku Zasshi. 2000;120:170–182. doi: 10.1248/yakushi1947.120.2_170. [DOI] [PubMed] [Google Scholar]
- [36].Greene C, Hillyer CD. Component preparation and manufacturing. In: Hillyer CD, Shaz B, Zimring J, Shire T, editors. Transfusion Medicine and Hemostasis: Clinical and Laboratory Aspects. Elsevier; Burlington MA: 2009. pp. 45–50. [Google Scholar]
- [37].Xu DP, Washburn MP, Sun GP, Wells WW. Purification and characterization of a glutathione dependent dehydroascorbate reductase from human erythrocytes. Biochem. Biophys. Res. Commun. 1996;221:117–121. doi: 10.1006/bbrc.1996.0555. [DOI] [PubMed] [Google Scholar]
- [38].Dhariwal KR, Hartzell WO, Levine M. Ascorbic acid and dehydroascorbic acid measurements in human plasma and serum. Am. J. Clin. Nutr. 1991;54:712–716. doi: 10.1093/ajcn/54.4.712. [DOI] [PubMed] [Google Scholar]
- [39].Food and Nutrition Board, Panel on Dietary Antioxidants and Related Compounds, Vitamin C, Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Natl. Acad. Press; Washington, DC: 2000. pp. 95–185.pp. 1–20.pp. 434–435.pp. 442–443. [Google Scholar]
- [40].Okamura M. An improved method for determination of l-ascorbic acid and l-dehydroascorbic acid in blood plasma. Clin. Chim. Acta. 1980;103:259–268. doi: 10.1016/0009-8981(80)90144-8. [DOI] [PubMed] [Google Scholar]
- [41].Buettner GR. In the absence of catalytic metals ascorbate does not autoxidize at pH 7: ascorbate as a test for catalytic metals. J. Biochem. Biophys. Methods. 1988;16:27–40. doi: 10.1016/0165-022x(88)90100-5. [DOI] [PubMed] [Google Scholar]
- [42].Buettner GR, Jurkiewicz BA. Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiat. Res. 1996;145:532–541. [PubMed] [Google Scholar]
- [43].Rose RC, Nahrwold DL. Quantitative analysis of ascorbic acid and dehydroascorbic acid by high-performance liquid chromatography. Anal. Biochem. 1981;114:140–145. doi: 10.1016/0003-2697(81)90464-4. [DOI] [PubMed] [Google Scholar]
- [44].Farber CM, Kanengiser S, Stahl R, Liebes L, Silber R. A specific high-performance liquid chromatography assay for dehydroascorbic acid shows an increased content in CLL lymphocytes. Anal. Biochem. 1983;134:355–360. doi: 10.1016/0003-2697(83)90309-3. [DOI] [PubMed] [Google Scholar]
- [45].Bianchi J, Rose RC. Chromatographic separation and radiochemical quantification of ascorbic acid, dehydroascorbic acid, and ketogulonic acid. J. Micronutrient Anal. 1985;1:3–11. [Google Scholar]
- [46].Ibric LL, Benedict WF, Peterson AR. Simultaneous determination of ascorbic acid and dehydroascorbic acid in cultures of C3H/10T1/2 cells. In Vitro Cell Dev. Biol. 1988;24:669–676. doi: 10.1007/BF02623604. [DOI] [PubMed] [Google Scholar]
- [47].Ziegler SJ, Meier B, Sticher O. Rapid and sensitive determination of dehydroascorbic acid in addition to ascorbic acid by reversed-phase high-performance liquid chromatography using a post-column reduction system. J. Chromatogr. 1987;391:419–426. doi: 10.1016/s0021-9673(01)94343-2. [DOI] [PubMed] [Google Scholar]
- [48].Takayanagi T, Nishiuchi M, Ousaka M, Oshima M, Motomizu S. Monitoring of vitamin C species in aqueous solution by flow injection analysis coupled with an on-line separation with reversed-phase column. Talanta. 2009;79:1055–1060. doi: 10.1016/j.talanta.2009.02.036. [DOI] [PubMed] [Google Scholar]
- [49].Deutsch JC. Dehydroascorbic acid. J. Chromatogr. A. 2000;881:299–307. doi: 10.1016/s0021-9673(00)00166-7. [DOI] [PubMed] [Google Scholar]
- [50].Fenoll J, Martinez A, Hellin P, Flores P. Simultaneous determination of ascorbic and dehydroascorbic acids in vegetables and fruits by liquid chromatography with tandem-mass spectrometry. Food Chem. 2011;127:340–344. [Google Scholar]
- [51].May JM, Qu ZC, Mendiratta S. Protection and recycling of α-tocopherol in human erythrocytes by intracellular ascorbic acid. Arch. Biochem. Biophys. 1998;349:281–289. doi: 10.1006/abbi.1997.0473. [DOI] [PubMed] [Google Scholar]
- [52].May JM, Qu ZC, Qiao H, Koury MJ. Maturational loss of the vitamin C transporter in erythrocytes. Biochem. Biophys. Res. Commun. 2007;360:295–298. doi: 10.1016/j.bbrc.2007.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mendiratta S, Qu ZC, May JM. Enzyme-dependent ascorbate recycling in human erythrocytes: role of thioredoxin reductase. Free Radic. Biol. Med. 1998;25:221–228. doi: 10.1016/s0891-5849(98)00060-4. [DOI] [PubMed] [Google Scholar]
- [54].Su D, May JM, Koury MJ, Asard H. Human erythrocyte membranes contain a cytochrome b561 that may be involved in extracellular ascorbate recycling. J. Biol. Chem. 2006;281:39852–39859. doi: 10.1074/jbc.M606543200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.