Abstract
We report the primary structures of human and rabbit brush border membrane beta-glycosidase complexes (pre-pro-lactase-phlorizin hydrolase, or pre-pro-LPH, EC 3.2.1.23-62), as deduced from cDNA sequences. The human and rabbit primary translation products contain 1927 and 1926 amino acids respectively. Based on the data, as well as on peptide sequences and further biochemical data, we conclude that the proteins comprise five domains: (i) a cleaved signal sequence of 19 amino acids; (ii) a large 'pro' portion of 847 amino acids (rabbit), none of which appears in mature, membrane-bound LPH; (iii) the mature LPH, which contains both the lactase and phlorizin hydrolase activities in a single polypeptide chain; (iv) a membrane-spanning hydrophobic segment near the carboxy terminus, which serves as membrane anchor; and (v) a short hydrophilic segment at the carboxy terminus, which must be cytosolic (i.e. the protein has an Nout-Cin orientation). The genes have a 4-fold internal homology, suggesting that they evolved by two cycles of partial gene duplication. This repetition also implies that parts of the 'pro' portion are very similar to parts of mature LPH, and hence that the 'pro' portion may be a water-soluble beta-glycosidase with another cellular location than LPH. Our results have implications for the decline of LPH after weaning and for human adult-type alactasia, and for the evolutionary history of LPH.
Full text
PDF


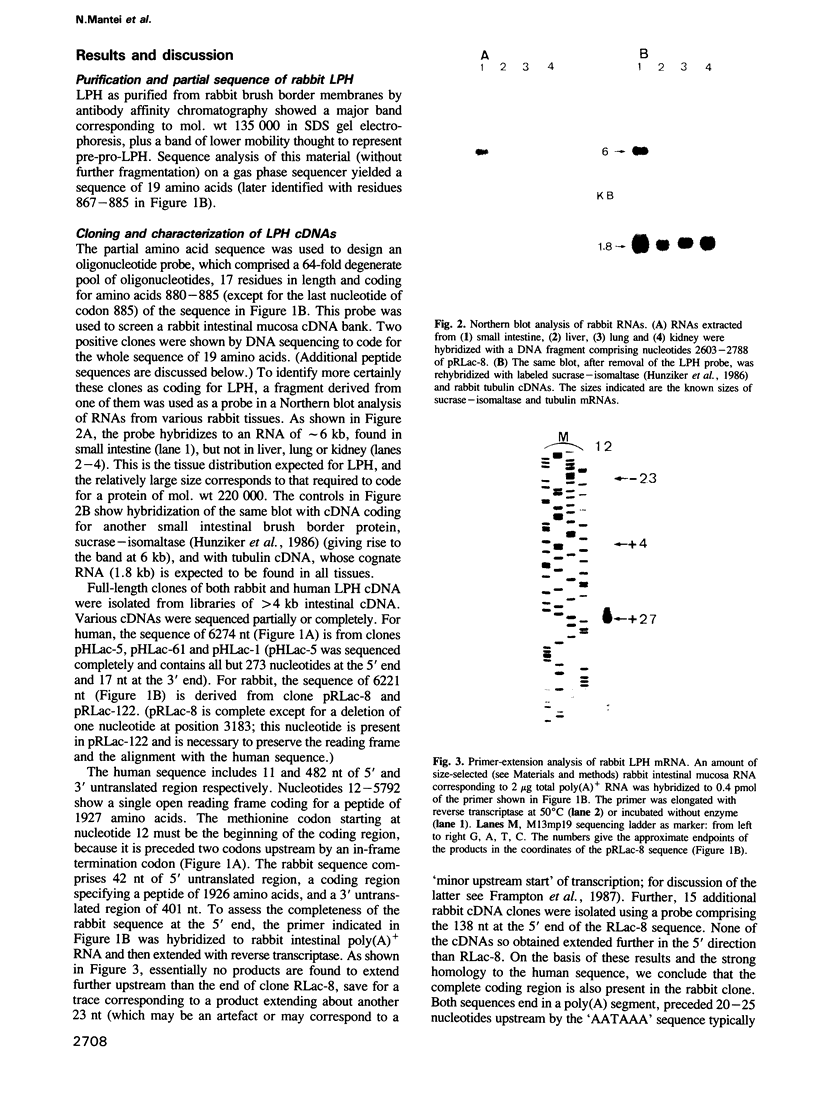
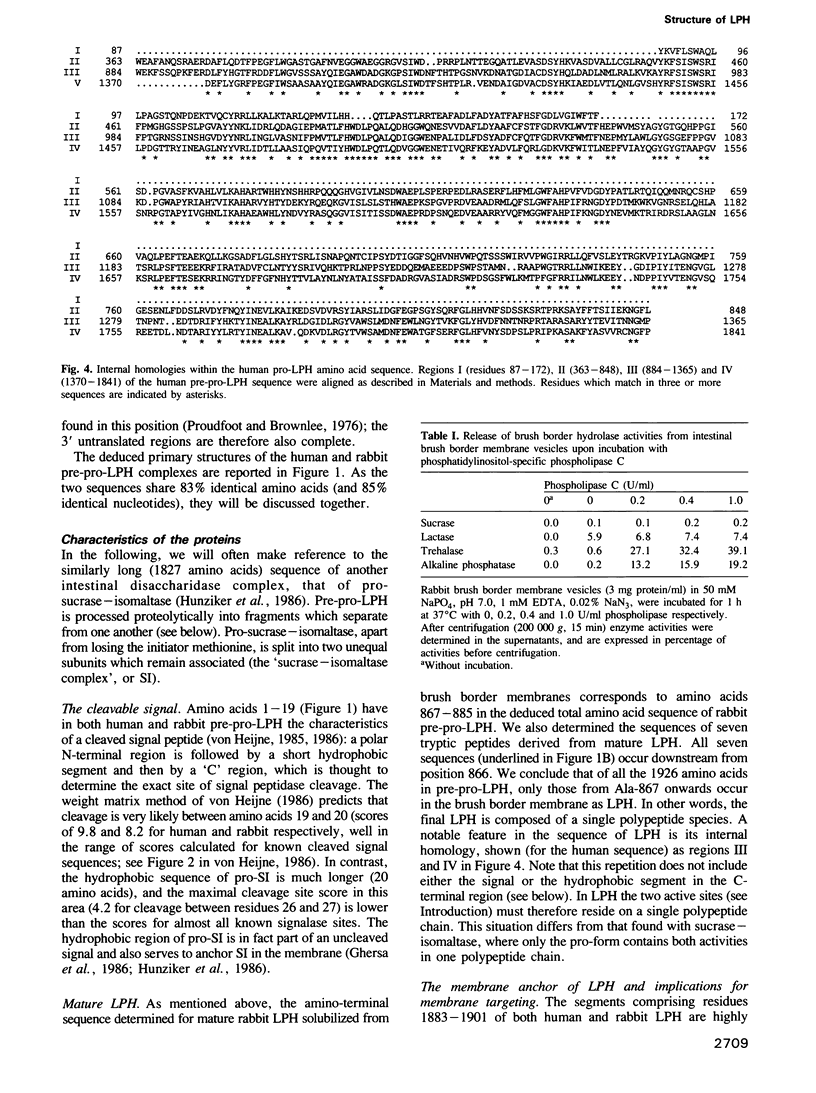




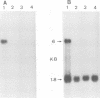
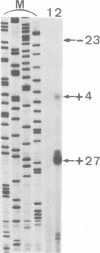
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AURICCHIO S., RUBINO A., LANDOLT M., SEMENZA G., PRADER A. ISOLATED INTESTINAL LACTASE DEFICIENCY IN THE ADULT. Lancet. 1963 Aug 17;2(7303):324–326. doi: 10.1016/s0140-6736(63)92991-x. [DOI] [PubMed] [Google Scholar]
- Aramayo L. A., De Silva D. G., Hughes C. A., Brown G. A., McNeish A. S. Disaccharidase activities in jejunal fluid. Arch Dis Child. 1983 Sep;58(9):686–691. doi: 10.1136/adc.58.9.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asp N. G., Dahlqvist A. Rat small-intestinal beta-galactosidases. Separation by ion-exchange chromatography and gel filtration. Biochem J. 1968 Feb;106(4):841–845. doi: 10.1042/bj1060841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Berger J., Garattini E., Hua J. C., Udenfriend S. Cloning and sequencing of human intestinal alkaline phosphatase cDNA. Proc Natl Acad Sci U S A. 1987 Feb;84(3):695–698. doi: 10.1073/pnas.84.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. Proteins of the kidney microvillar membrane. Asymmetric labelling of the membrane by lactoperoxidase-catalysed radioiodination and by photolysis of 3,5-di[125I]iodo-4-azidobenzenesulphonate. Biochem J. 1980 Apr 1;187(1):31–44. doi: 10.1042/bj1870031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner J., Semenza G. Selective labeling of the hydrophobic core of membranes with 3-(trifluoromethyl)-3-(m-[125I]iodophenyl)diazirine, a carbene-generating reagent. Biochemistry. 1981 Dec 8;20(25):7174–7182. doi: 10.1021/bi00528a019. [DOI] [PubMed] [Google Scholar]
- Büller H. A., Montgomery R. K., Sasak W. V., Grand R. J. Biosynthesis, glycosylation, and intracellular transport of intestinal lactase-phlorizin hydrolase in rat. J Biol Chem. 1987 Dec 15;262(35):17206–17211. [PubMed] [Google Scholar]
- Cogoli A., Semenza G. A probable oxocarbonium ion in the reaction mechanism of small intestinal sucrase and isomaltase. J Biol Chem. 1975 Oct 10;250(19):7802–7809. [PubMed] [Google Scholar]
- Colombo V., Lorenz-Meyer H., Semenza G. Small intestinal phlorizin hydrolase: the "beta-glycosidase complex". Biochim Biophys Acta. 1973 Dec 19;327(2):412–424. doi: 10.1016/0005-2744(73)90425-7. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLQVIST A., HAMMOND J. B., CRANE R. K., DUNPHY J. V., LITTMAN A. INTESTINAL LACTASE DEFICIENCY AND LACTOSE INTOLERANCE IN ADULTS. PRELIMINARY REPORT. Gastroenterology. 1963 Oct;45:488–491. [PubMed] [Google Scholar]
- Danielsen E. M., Skovbjerg H., Norén O., Sjöström H. Biosynthesis of intestinal microvillar proteins. Intracellular processing of lactase-phlorizin hydrolase. Biochem Biophys Res Commun. 1984 Jul 18;122(1):82–90. doi: 10.1016/0006-291x(84)90442-x. [DOI] [PubMed] [Google Scholar]
- Devault A., Lazure C., Nault C., Le Moual H., Seidah N. G., Chrétien M., Kahn P., Powell J., Mallet J., Beaumont A. Amino acid sequence of rabbit kidney neutral endopeptidase 24.11 (enkephalinase) deduced from a complementary DNA. EMBO J. 1987 May;6(5):1317–1322. doi: 10.1002/j.1460-2075.1987.tb02370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Feracci H., Maroux S., Bonicel J., Desnuelle P. The amino acid sequence of the hydrophobic anchor of rabbit intestinal brush border aminopeptidase N. Biochim Biophys Acta. 1982 Jan 4;684(1):133–136. doi: 10.1016/0005-2736(82)90057-8. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Low M. G., Cross G. A. Glycosyl-sn-1,2-dimyristylphosphatidylinositol is covalently linked to Trypanosoma brucei variant surface glycoprotein. J Biol Chem. 1985 Nov 25;260(27):14547–14555. [PubMed] [Google Scholar]
- Frampton J., Conkie D., Chambers I., McBain W., Dexter M., Harrison P. Changes in minor transcripts from the alpha 1 and beta maj globin and glutathione peroxidase genes during erythropoiesis. Nucleic Acids Res. 1987 May 11;15(9):3671–3688. doi: 10.1093/nar/15.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frielle T., Curthoys N. P. Characterization of the membrane binding domain of gamma-glutamyltranspeptidase by specific labeling techniques. Biochemistry. 1983 Dec 6;22(25):5709–5714. doi: 10.1021/bi00294a005. [DOI] [PubMed] [Google Scholar]
- Gee A. P., Borsos T., Boyle M. D. Interaction between components of the human classical complement pathway and immobilized Cibacron Blue F3GA. J Immunol Methods. 1979;30(2):119–126. doi: 10.1016/0022-1759(79)90086-3. [DOI] [PubMed] [Google Scholar]
- Geliebter J., Zeff R. A., Melvold R. W., Nathenson S. G. Mitotic recombination in germ cells generated two major histocompatibility complex mutant genes shown to be identical by RNA sequence analysis: Kbm9 and Kbm6. Proc Natl Acad Sci U S A. 1986 May;83(10):3371–3375. doi: 10.1073/pnas.83.10.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghersa P., Huber P., Semenza G., Wacker H. Cell-free synthesis, membrane integration, and glycosylation of pro-sucrase-isomaltase. J Biol Chem. 1986 Jun 15;261(17):7969–7974. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Quaroni A., Isselbacher K. J. Monoclonal antibodies to sucrase/isomaltase: probes for the study of postnatal development and biogenesis of the intestinal microvillus membrane. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6629–6633. doi: 10.1073/pnas.77.11.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser H., Howell K., Dawson R. M., Bowyer D. E. Rabbit small intestinal brush border membrane preparation and lipid composition. Biochim Biophys Acta. 1980 Nov 18;602(3):567–577. doi: 10.1016/0005-2736(80)90335-1. [DOI] [PubMed] [Google Scholar]
- Henning S. J. Ontogeny of enzymes in the small intestine. Annu Rev Physiol. 1985;47:231–245. doi: 10.1146/annurev.ph.47.030185.001311. [DOI] [PubMed] [Google Scholar]
- Hoefsloot L. H., Hoogeveen-Westerveld M., Kroos M. A., van Beeumen J., Reuser A. J., Oostra B. A. Primary structure and processing of lysosomal alpha-glucosidase; homology with the intestinal sucrase-isomaltase complex. EMBO J. 1988 Jun;7(6):1697–1704. doi: 10.1002/j.1460-2075.1988.tb02998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper N. M., Keen J., Pappin D. J., Turner A. J. Pig kidney angiotensin converting enzyme. Purification and characterization of amphipathic and hydrophilic forms of the enzyme establishes C-terminal anchorage to the plasma membrane. Biochem J. 1987 Oct 1;247(1):85–93. doi: 10.1042/bj2470085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper N. M., Low M. G., Turner A. J. Renal dipeptidase is one of the membrane proteins released by phosphatidylinositol-specific phospholipase C. Biochem J. 1987 Jun 1;244(2):465–469. doi: 10.1042/bj2440465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper N. M., Turner A. J. Ectoenzymes of the kidney microvillar membrane. Aminopeptidase P is anchored by a glycosyl-phosphatidylinositol moiety. FEBS Lett. 1988 Mar 14;229(2):340–344. doi: 10.1016/0014-5793(88)81152-9. [DOI] [PubMed] [Google Scholar]
- Hooper N. M., Turner A. J. Ectoenzymes of the kidney microvillar membrane. Differential solubilization by detergents can predict a glycosyl-phosphatidylinositol membrane anchor. Biochem J. 1988 Mar 15;250(3):865–869. doi: 10.1042/bj2500865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C. B., Spiess M., Semenza G. The mode of anchoring and precursor forms of sucrase-isomaltase and maltase-glucoamylase in chicken intestinal brush-border membrane. Phylogenetic implications. Biochim Biophys Acta. 1987 Jan 26;896(2):275–286. doi: 10.1016/0005-2736(87)90188-x. [DOI] [PubMed] [Google Scholar]
- Hunziker W., Spiess M., Semenza G., Lodish H. F. The sucrase-isomaltase complex: primary structure, membrane-orientation, and evolution of a stalked, intrinsic brush border protein. Cell. 1986 Jul 18;46(2):227–234. doi: 10.1016/0092-8674(86)90739-7. [DOI] [PubMed] [Google Scholar]
- Ikezawa H., Yamanegi M., Taguchi R., Miyashita T., Ohyabu T. Studies on phosphatidylinositol phosphodiesterase (phospholipase C type) of Bacillus cereus. I. purification, properties and phosphatase-releasing activity. Biochim Biophys Acta. 1976 Nov 19;450(2):154–164. [PubMed] [Google Scholar]
- Khandjian E. W. UV crosslinking of RNA to nylon membrane enhances hybridization signals. Mol Biol Rep. 1986;11(2):107–115. doi: 10.1007/BF00364822. [DOI] [PubMed] [Google Scholar]
- Kraml J., Kolínská J., Eddederová D., Hirsová D. -Glucosidase (phlorizin hydrolase) activity of the lactase fraction isolated from the small intestinal mucosa of infant rats, and the relationship between -glucosidases and -galactosidases. Biochim Biophys Acta. 1972 Feb 28;258(2):520–530. doi: 10.1016/0005-2744(72)90243-4. [DOI] [PubMed] [Google Scholar]
- Kretchmer N. Lactose and lactase--a historical perspective. Gastroenterology. 1971 Dec;61(6):805–813. [PubMed] [Google Scholar]
- Laperche Y., Bulle F., Aissani T., Chobert M. N., Aggerbeck M., Hanoune J., Guellaën G. Molecular cloning and nucleotide sequence of rat kidney gamma-glutamyl transpeptidase cDNA. Proc Natl Acad Sci U S A. 1986 Feb;83(4):937–941. doi: 10.1073/pnas.83.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leese H. J., Semenza G. On the identity between the small intestinal enzymes phlorizin hydrolase and glycosylceramidase. J Biol Chem. 1973 Dec 10;248(23):8170–8173. [PubMed] [Google Scholar]
- Low M. G. Biochemistry of the glycosyl-phosphatidylinositol membrane protein anchors. Biochem J. 1987 May 15;244(1):1–13. doi: 10.1042/bj2440001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnair D. C., Kenny A. J. Proteins of the kidney microvillar membrane. The amphipathic form of dipeptidyl peptidase IV. Biochem J. 1979 May 1;179(2):379–395. doi: 10.1042/bj1790379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfroy B., Schofield P. R., Kuang W. J., Seeburg P. H., Mason A. J., Henzel W. J. Molecular cloning and amino acid sequence of rat enkephalinase. Biochem Biophys Res Commun. 1987 Apr 14;144(1):59–66. doi: 10.1016/s0006-291x(87)80475-8. [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Tsuji A., Katunuma N. Studies on the structure of gamma-glutamyltranspeptidase. III. Evidence that the amino terminus of the heavy subunit is the membrane binding segment. J Biochem. 1983 May;93(5):1427–1433. doi: 10.1093/oxfordjournals.jbchem.a134278. [DOI] [PubMed] [Google Scholar]
- Naim H. Y., Sterchi E. E., Lentze M. J. Biosynthesis and maturation of lactase-phlorizin hydrolase in the human small intestinal epithelial cells. Biochem J. 1987 Jan 15;241(2):427–434. doi: 10.1042/bj2410427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Norén O., Sjöström H., Cowell G. M., Tranum-Jensen J., Hansen O. C., Welinder K. G. Pig intestinal microvillar maltase-glucoamylase. Structure and membrane insertion. J Biol Chem. 1986 Sep 15;261(26):12306–12309. [PubMed] [Google Scholar]
- Norén O., Sjöström H. The insertion of pig microvillus aminopeptidase into the membrane as probed by [125I]iodonaphthylazide. Eur J Biochem. 1980 Feb;104(1):25–31. doi: 10.1111/j.1432-1033.1980.tb04395.x. [DOI] [PubMed] [Google Scholar]
- Nsi-Emvo E., Launay J. F., Raul F. Is adult-type hypolactasia in the intestine of mammals related to changes in the intracellular processing of lactase? Cell Mol Biol. 1987;33(3):335–344. [PubMed] [Google Scholar]
- Potter J., Ho M. W., Bolton H., Furth A. J., Swallow D. M., Griffiths B. Human lactase and the molecular basis of lactase persistence. Biochem Genet. 1985 Jun;23(5-6):423–439. doi: 10.1007/BF00499084. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Quaroni A., Semenza G. Partial amino acid sequences around the essential carboxylate in the active sites of the intestinal sucrase-isomaltase complex. J Biol Chem. 1976 Jun 10;251(11):3250–3253. [PubMed] [Google Scholar]
- Ramaswamy S., Radhakrishnan A. N. Lactase-phlorizin hydrolase complex from monkey small intestine. Purification, properties and evidence for two catalytic sites. Biochim Biophys Acta. 1975 Oct 22;403(2):446–455. doi: 10.1016/0005-2744(75)90072-8. [DOI] [PubMed] [Google Scholar]
- Roth J., Taatjes D. J., Weinstein J., Paulson J. C., Greenwell P., Watkins W. M. Differential subcompartmentation of terminal glycosylation in the Golgi apparatus of intestinal absorptive and goblet cells. J Biol Chem. 1986 Oct 25;261(30):14307–14312. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel-Haueter S., Hore P., Kerry K. R., Semenza G. The preparation of lactase and glucoamylase of rat small intestine. Biochim Biophys Acta. 1972 Feb 28;258(2):506–519. doi: 10.1016/0005-2744(72)90242-2. [DOI] [PubMed] [Google Scholar]
- Semenza G. Anchoring and biosynthesis of stalked brush border membrane proteins: glycosidases and peptidases of enterocytes and renal tubuli. Annu Rev Cell Biol. 1986;2:255–313. doi: 10.1146/annurev.cb.02.110186.001351. [DOI] [PubMed] [Google Scholar]
- Simoons F. J. Progress report. New light on ethnic differences in adult lactose intolerance. Am J Dig Dis. 1973 Jul;18(7):595–611. doi: 10.1007/BF01072224. [DOI] [PubMed] [Google Scholar]
- Skovbjerg H., Danielsen E. M., Noren O., Sjöström H. Evidence for biosynthesis of lactase-phlorizin hydrolase as a single-chain high-molecular weight precursor. Biochim Biophys Acta. 1984 Apr 10;798(2):247–251. doi: 10.1016/0304-4165(84)90312-x. [DOI] [PubMed] [Google Scholar]
- Skovbjerg H., Norén O., Sjöström H., Danielsen E. M., Enevoldsen B. S. Further characterization of intestinal lactase/phlorizin hydrolase. Biochim Biophys Acta. 1982 Sep 22;707(1):89–97. doi: 10.1016/0167-4838(82)90400-9. [DOI] [PubMed] [Google Scholar]
- Skovbjerg H., Sjöström H., Norén O. Purification and characterisation of amphiphilic lactase/phlorizin hydrolase from human small intestine. Eur J Biochem. 1981 Mar;114(3):653–661. doi: 10.1111/j.1432-1033.1981.tb05193.x. [DOI] [PubMed] [Google Scholar]
- Sorge J., West C., Westwood B., Beutler E. Molecular cloning and nucleotide sequence of human glucocerebrosidase cDNA. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7289–7293. doi: 10.1073/pnas.82.21.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess M., Brunner J., Semenza G. Hydrophobic labeling, isolation, and partial characterization of the NH2-terminal membranous segment of sucrase-isomaltase complex. J Biol Chem. 1982 Mar 10;257(5):2370–2377. [PubMed] [Google Scholar]
- Taatjes D. J., Roth J., Weinstein J., Paulson J. C. Post-Golgi apparatus localization and regional expression of rat intestinal sialyltransferase detected by immunoelectron microscopy with polypeptide epitope-purified antibody. J Biol Chem. 1988 May 5;263(13):6302–6309. [PubMed] [Google Scholar]
- Takesue Y., Yokota K., Nishi Y., Taguchi R., Ikezawa H. Solubilization of trehalase from rabbit renal and intestinal brush-border membranes by a phosphatidylinositol-specific phospholipase C. FEBS Lett. 1986 May 26;201(1):5–8. doi: 10.1016/0014-5793(86)80560-9. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Vasseur M., Tellier C., Alvarado F. Sodium-dependent activation of intestinal brush-border sucrase: correlation with activation by deprotonation from pH 5 to 7. Arch Biochem Biophys. 1982 Oct 1;218(1):263–274. doi: 10.1016/0003-9861(82)90345-9. [DOI] [PubMed] [Google Scholar]
- WALLENFELS K., FISCHER J. [Research on lactose-splitting enzymes. X. Lactase of calf intestines]. Hoppe Seylers Z Physiol Chem. 1960 Dec 2;321:223–245. doi: 10.1515/bchm2.1960.321.1.223. [DOI] [PubMed] [Google Scholar]
- Wacker H., Jaussi R., Sonderegger P., Dokow M., Ghersa P., Hauri H. P., Christen P., Semenza G. Cell-free synthesis of the one-chain precursor of a major intrinsic protein complex of the small-intestinal brush border membrane (pro-sucrase-isomaltase). FEBS Lett. 1981 Dec 28;136(2):329–332. doi: 10.1016/0014-5793(81)80647-3. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]