Abstract
Background
Although cross-sectional studies have suggested a relationship between proton pump inhibitor (PPI) use and hypomagnesemia, no large-scale cohort study has been conducted to date. Here, we examined the changes in serum magnesium levels in response to PPI use. We hypothesized that PPI use might change the serum magnesium concentration.
Methods
Of the 2,892 patients hospitalized for percutaneous coronary intervention between January 2007 and May 2012, 1,076 patients with normal baseline (1.6–2.5 mg/dL) and follow-up serum magnesium concentrations were enrolled. These patients were divided into two groups: the PPI group and the control group.
Results
The mean follow-up period was 9.51 ± 2.94 months. The incidence of hypomagnesemia (< 1.6 mg/dL) was 0.4% (3/834) in the PPI group and 0.4% (1/242) in the control group (P = 0.904). The change in magnesium levels did not differ between the two groups, and this result was maintained in the analysis of covariance after adjusting for confounding factors (P = 0.381). Moreover, magnesium levels did not significantly differ between the long-term (duration of use ≥ 12 months, n = 71) and short-term PPI groups (duration of use < 12 months, n = 763), and the control group (n = 242; P = 0.620). The effect of PPI use on change in serum magnesium concentration was affected by the use of multiple diuretics (−0.01 ± 0.25 mg/dL; P = 0.025), although a single diuretic use with PPI did not alter the change in magnesium level (0.12 ± 0.27 mg/dL).
Conclusion
Changes in magnesium levels might be subtle after PPI use in patients with normal baseline magnesium values.
Keywords: Diuretics, Magnesium, Proton pump inhibitor
Introduction
Magnesium is the second most common intracellular cation and is involved in a wide range of cellular functions, including protein synthesis, enzymatic reactions, and the regulation of ion channels. Significantly low serum magnesium levels have been associated with bradycardia, hypotension, seizures, tetany, and death [1,2].
Proton pump inhibitors (PPI) are widely used worldwide. They have been associated with a wide variety of side effects including renal failure, respiratory infections, Clostridium difficile colitis, and hip fractures [3–7]. There have been several case reports and case series of PPI-induced hypomagnesemia (PIH) with a wide array of symptoms, including cardiac arrhythmias and seizures [8–13]. A common characteristic of PIH might be the long-term use of PPIs (> 1 year duration) and the presence of severe hypomagnesemia. If the long-term use of PPIs could decrease the serum magnesium level, PPI use might be a risk factor in patients with coronary heart disease. Serum magnesium levels might be particularly important in patients with coronary heart disease who are taking PPIs.
Although some cross-sectional studies have suggested that PPI use could be related with hypomagnesemia [14–17], there was a lack of causal relation between PPI use and hypomagnesemia.
To address these questions, we examined the change in serum magnesium level after use of PPI in a retrospective cohort of 1,076 patients, who underwent percutaneous coronary intervention (PCI) in a tertiary medical center.
Methods
Study population
The study population consisted of all patients undergoing PCI from January 2007 to May 2012 at the Soonchunhyang University Cheonan Hospital, Korea.
Our exclusion criteria included the following: patients without follow-up magnesium data, baseline magnesium < 1.6 mg/dL, baseline magnesium > 2.5 mg/dL, hemodialysis patients, estimated glomerular filtration rate (GFR) of < 15 mL/min/1.73 m2, and patients who were prescribed laxatives including magnesium or histamine-2 receptor antagonist. Baseline magnesium level was measured at admission to undergo PCI, and follow-up magnesium level was measured at readmission for follow-up coronary angiography or cardiac event. During this time, the patients taking PPIs were assigned to the PPI group. Blood sampling was carried out before the intravenous fluid therapy.
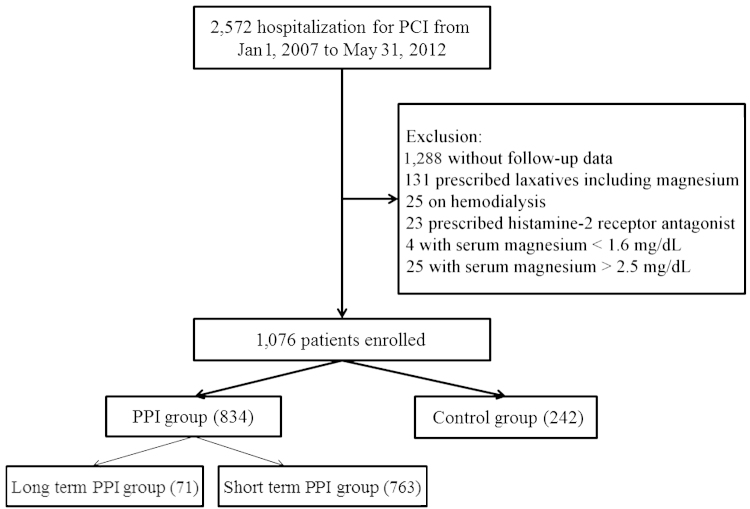
To investigate PPI use in relation to change in magnesium levels, patients were divided into groups (Fig. 1). First, patients were divided into the PPI group and the control group (those who did not use PPIs). Second, the PPI group was further divided into the short-term PPI group (duration of use < 12 months) and the long-term PPI group (duration of use ≥ 12 months). The Institutional Review Board of Soonchunhyang University Cheonan Hospital approved this study.
Figure 1.
The flowchart illustrates the patients’ inclusion and exclusion in this study. PCI, percutaneous coronary intervention; PPI, proton pump inhibitor.
Study variables
To investigate the prescription of PPIs, details on duration of use and type were obtained. Using a dose conversion factor, a daily omeprazole equivalent dose was also calculated when the daily PPI dose was documented. In brief, a 20-mg oral dose of omeprazole was considered to have an equivalent therapeutic efficacy to 20 mg esomeprazole, 30 mg lansoprazole, 40 mg pantoprazole, and 20 mg rabeprazole. PPI intensity was defined as equivalent dose multiplied by duration (months) of PPI use.
To improve clinical relevance, several covariates of interest were collected: age, sex, diabetes mellitus, history of diuretics use, and selected laboratory variables (serum creatinine, calcium, albumin, potassium) obtained at the time of hospital admission or, if they are missing, within the 1st day of hospitalization. The GFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation [18].
Statistical analysis
Data are presented as the mean ± standard deviation for continuous variables and frequency (in percent) for the categorical variables. The difference between groups was compared using the Student t test or analysis of variance for continuous variables and a Chi-square test for categorical variables. The effects of PPIs on changes in serum magnesium level were analyzed using analysis of covariance (ANCOVA), with the PPI group as the main factor, and adjusting for factors influencing the baseline magnesium value. A P value of < 0.05 was considered statistically significant. The statistical analysis was performed using SPSS for Windows version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient baseline characteristics
Of the 2,892 individuals hospitalized for PCI, 1,076 were enrolled in this study (Fig. 1). All patients were admitted for coronary heart disease and received PCI. These patients were followed up to monitor their condition. The mean follow-up duration was 9.51 ± 2.94 months. Among these patients, 834 received PPI agents according to the clinician’s opinion. Table 1 lists the characteristics of the study population. None of the patients received digoxin. After PCI, all patients were observed in the outpatient clinic. The baseline magnesium levels did not differ between the control group (2.02 ± 0.18 mg/dL) and the PPI group (2.03 ± 0.18 mg/dL; P = 0.948). There was no difference in baseline characteristics between the two groups except for use of potassium-sparing diuretics (Table 1).
Table 1.
Baseline characteristics of the study population
| Control group (n = 242) | PPI group (n = 834) | P | |
|---|---|---|---|
| Age (y) | 62.39±11.00 | 63.20±11.26 | 0.324 |
| Male sex | 166 (68.6%) | 528 (63.3%) | 0.130 |
| Diabetes mellitus | 81 (33.5%) | 268 (32.1%) | 0.696 |
| Underlying cardiac disease | 0.197 | ||
| Angina pectoris | 160 (66.1%) | 571 (68.5%) | |
| NSTEMI | 29 (12.0%) | 118 (14.1%) | |
| STEMI | 44 (18.2%) | 130 (15.6%) | |
| ICMP | 9 (3.7%) | 15 (1.8%) | |
| Serum magnesium (mg/dL) | 2.02±0.18 | 2.03±0.18 | 0.948 |
| Serum creatinine (mg/dL) | 0.86±0.34 | 0.84±0.30 | 0.415 |
| eGFR (mL/min/1.73 m2) | 88.62±20.02 | 88.26±19.66 | 0.801 |
| Serum albumin (g/dL) | 4.40±0.38 | 4.37±0.40 | 0.211 |
| Serum calcium (mg/dL) | 9.03±0.51 | 8.97±0.56 | 0.251 |
| Serum phosphate (mg/dL) | 3.35±0.80 | 3.37±0.76 | 0.841 |
| Serum potassium (mEq/L) | 4.17±0.48 | 4.16±0.44 | 0.925 |
| Use of diuretics during follow-up period | 76 (31.4%) | 218 (26.1%) | 0.106 |
Values are N, mean ± SD.
eGFR, estimated glomerular filtration rate; ICMP, ischemic cardiomyopathy; NSTEMI, non-ST elevation myocardial infarction; STEMI, ST elevation myocardial infarction.
Effect of PPI use on serum magnesium
The incidence rate of hypomagnesemia during the follow-up period was 0.4% (3/834) in the PPI group and 0.4% (1/242) in the control group (P = 0.904). To investigate PPI use in relation with change in magnesium level, the ANCOVA test was performed. After adjusting for baseline magnesium, the results showed that change in magnesium level was not influenced by PPI use (P = 0.179). After ANCOVA adjusting for age, sex, baseline GFR, baseline calcium, use of diuretics, and baseline potassium, it was noted that the change in magnesium level was not significantly different between the two groups (P = 0.103; Table 2).
Table 2.
Comparisons of serum magnesium concentration between PPI group and control group
| Control group | PPI group | P⁎ | P† | |
|---|---|---|---|---|
| Baseline (mg/dL) | 2.03±0.18 | 2.03±0.18 | 0.948 | |
| Follow-up (mg/dL) | 2.07±0.21 | 2.05±0.20 | 0.190 | |
| Change (mg/dL) | 0.04±0.23 | 0.02±0.23 | 0.269 | 0.103 |
Values are N, mean ± SD.
ANCOVA, analysis of covariance; Ca, calcium; GFR, glomerular filtration rate; K, potassium; PPI, proton pump inhibitor.
P value by Student t test.
P value by ANCOVA adjusting for age, sex, baseline GFR, baseline Ca, use of diuretics, and baseline K.
Effect of duration of PPI use on serum magnesium
To investigate the impact of prolonged use of PPIs on serum magnesium concentration, the PPI group was divided into the long-term group and the short-term group. The mean duration of PPI use was 6.39 ± 3.20 months in the short-term PPI group and 12.20 ± 0.62 months in the long-term PPI group (P < 0.001). Each PPI intensity was 4.47 ± 2.92 in the short-term PPI group and 7.39 ± 2.59 in the long-term PPI group (P < 0.001). The use of diuretics was not different between two groups (26.1% vs. 25.4%, P = 0.875). Based on the comparison of the short-term and the long-term PPI groups, the change in magnesium level was not significantly different using the Student t test and ANCOVA with or without adjustment for confounding factors. Also, when comparing the three groups (control, long term, short term), the change in magnesium levels was not significantly different (Table 3).
Table 3.
Comparisons of serum magnesium levels in controls, short-term PPI group, and long-term PPI group
| Control | Short-term PPI use (< 12 mo) | Long-term PPI use (≥ 12 mo) | P⁎ | P† | |
|---|---|---|---|---|---|
| Baseline (mg/dL) | 2.03±0.18 | 2.02±0.18 | 2.04±0.19 | 0.851 | |
| Follow-up (mg/dL) | 2.07±0.21 | 2.05±0.20 | 2.06±0.17 | 0.347 | |
| Change (mg/dL) | 0.04±0.23 | 0.02±0.23 | 0.03±0.22 | 0.540 | 0.213 |
Values indicate the mean ± SD.
ANCOVA, analysis of covariance; ANOVA, analysis of variance; GFR, glomerular filtration rate; K, potassium; Mg, magnesium; PPI, proton pump inhibitor.
P value by ANOVA
P value by ANCOVA adjusting for age, sex, baseline GFR, baseline Mg, use of diuretics, and baseline K
Effect of PPI use with diuretics on serum magnesium
Given the modifying effect of diuretics, we then assessed whether the type and combined use of multiple diuretics further influenced the change in serum magnesium level after PPI use. As shown in Table 4, the effects of PPI use on change in serum magnesium level were similar among individuals taking any type of diuretic agents. The effect of PPI use on change in serum magnesium level was altered by the use of multiple diuretics. However, the single diuretic use with PPI did not influence the change in magnesium level.
Table 4.
Effect of diuretics on association between PPI use and change in serum magnesium level
| Change in serum magnesium level (mg/dL) |
|||
|---|---|---|---|
| Control group | PPI group | P⁎ | |
| Loop diuretics (n = 69) | 0.02±0.23 | 0.09±0.27 | 0.282⁎ |
| Thiazide diuretics (n = 82) | 0.01±0.11 | 0.03±0.22 | 0.801⁎ |
| Potassium-sparing diuretics (n = 47) | 0.05±0.25 | −0.03±0.34 | 0.431⁎ |
| Multiple (n = 96) | 0.12±0.27 | −0.01±0.25 | 0.025⁎ |
| Loop + thiazide | 0.10±0.42 (n = 2) | 0.10±0.37 (n = 4) | 0.999† |
| Loop + potassium sparing | 0.12±0.20 (n = 15) | 0.03±0.24 (n = 43) | 0.188† |
| Thiazide + potassium sparing | 0.10±0.4 (n = 8) | −0.18±0.24 (n = 13) | 0.151† |
| More than 3 diuretic agents | 0.17±0.06 (n = 8) | −0.13±0.22 (n = 13) | 0.054† |
Values are N, mean ± SD.
ANCOVA, analysis of covariance; PPI, proton pump inhibitor.
P value by ANCOVA.
P value by t test.
Effect of PPI use according to GFR on serum magnesium
We investigated the effect of PPI use on serum magnesium in patients with low GFR (i.e., 15–60 mL/min/1.73 m2; n = 116, where PPI users = 90, controls = 26). The change in magnesium levels was not significantly different after PPI use (controls, 0.04 ± 0.27; PPI users, 0.03 ± 0.28; P = 0.818). Furthermore, in patients with GFR > 60 mL/min/1.73 m2, the change in magnesium levels was not significantly different after PPI use (controls, 0.04 ± 0.02; PPI users, 0.02 ± 0.22; P = 0.270).
Discussion
Several case reports suggested that PPI use could be related with hypomagnesemia [8,11,12,19,20]. Although these cases series showed that chronic use of PPIs could be a risk factor, there was lack of causal relationship to support the premise that long-standing use of PPIs could be linked with hypomagnesemia. In recent years, some cross-sectional studies supported these findings. Danziger et al [14] suggested that serum magnesium concentrations on admission were lower among patients who were taking both PPIs and diuretics than in patients without PPI use, as documented in a large sample of critically ill patients. No such relationship was found among PPI users who were not taking diuretics. Koulouridis et al [16] investigated whether there was an association in 402 adults between hypomagnesemia at the time of hospital admission and out-of-hospital use of PPIs. They found that out-of-hospital PPI use was not associated with hypomagnesemia at the time of hospital admission [16]. Kim retrospectively studied 112 consecutive patients aged 20 years or older who were treated with PPIs for ≥ 30 days and whose serum magnesium levels were available for the PPI treatment period [17]. This study included only 105 PPI users and 210 nonusers with matched controls. There was no difference in magnesium level between the two groups. In the PPI group, hypomagnesemia was observed in 32 patients. However, the mean magnesium value was 0.76mM (1.85 mg/dL) in the hypomagnesemia group. No incidence of hypomagnesemia was observed in the control group. Therefore the authors did not prove whether PPI itself is a risk factor of hypomagnesemia. Previous studies were mostly cross-sectional studies that did not reveal the causal relationship.
The purpose of our study is to investigate the change in magnesium level after PPI use. In Korea, regulations allow PPIs to be prescribed only in hospitals. We showed that PPI use was not related with change in serum magnesium. In particular, we showed that the long-term (> 1 year) use of PPI is not related with the change in magnesium level. This finding suggested that PIH might be not related with duration or dose. We thought that PIH may be attributed to individual sensitivity including gene variant. After adjusting for confounding factors, PPI use was not found to be related with change in serum magnesium. In our study, concomitant use of diuretics itself did not alter serum magnesium levels; however, use of multiple diuretics with concomitant use of PPIs decreased the serum magnesium. This finding is similar to that of a previous meta-analysis [21], which showed that PPI use may increase the risk of hypomagnesemia. However, the authors showed that, based only on hospitalized patients or 1.7 mg/dL of the cutoff value, PPI use was not associated with hypomagnesemia. Yet, when based on 1.8 mg/dL of the cutoff value, PPI use increased the risk of hypomagnesemia. Park et al [21] also demonstrated the significant heterogeneity among the included studies. In our study, we included only hospitalized patients and the cutoff value is less than 1.6 mg/dL (even though 1.7 or 1.8 mg/dL of the cutoff value did not influence the results; data not shown). We think that heterogeneity among studies prevented us from reaching a definite conclusion.
The mechanism of PIH remains unclear. PPI-induced hypochlorhydria has been postulated [8]. However, there was no evidence of hypomagnesemia after use of the other antacid. Some data also suggested that genetic factors might result in increased susceptibility to PIH. It is unknown if the prolonged use of PPIs could cause an altered regulation of the transient receptor potential melastin 6/7 (TRPM6/7), which is an active transcellular channel present in the gastrointestinal tract and kidneys involved in driving cations such as magnesium and calcium into the cells [22,23]. TRPM6/7 mutations are postulated to impair kidney function and, possibly, also the urinary excretion of magnesium. TRPM6/7 mutations have been shown in human disease only in association with magnesium urinary wasting or hypomagnesemia with secondary hypocalcemia, a rare autosomal recessive disorder characterized by diminished intestinal absorption and increased renal excretion of magnesium that is uniformly manifested in early infancy with generalized convulsions, muscle spasms, or tetany [24]. Further studies should therefore focus on the individual susceptibility of PIH.
The U.S. Food and Drug administration has issued a warning that the prolonged use of PPIs may cause low serum magnesium levels and recommended obtaining serum magnesium levels prior to prescribing PPI treatment in patients who are expected to be using these drugs for a long period. According to our results, we recommend that serum magnesium levels be obtained before prescribing PPI treatment in patients who are expected to use multiple diuretics.
Our study has several limitations. The incidence of hypomagnesemia is very small in our study. Hypomagnesemia is a common entity, occurring in up to 12% of hospitalized patients [25]. The incidence rises to as high as 60–65% of patients in the intensive care setting [26]. Previous cross-sectional studies included only patients admitted to intensive care units [14]. Patients in our study had a less severe condition compared with patients in previous cross-sectional studies. In conclusion, the change in magnesium level might be subtle after PPI use in patients with normal baseline magnesium values.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
This study was supported by the Soonchunhyang University Research Fund.
References
- 1.Flink EB. Magnesium deficiency. Etiology and clinical spectrum. Acta Med Scand Suppl. 1981;647:125–137. doi: 10.1111/j.0954-6820.1981.tb02648.x. [DOI] [PubMed] [Google Scholar]
- 2.Moore MJ, Flink EB. Magnesium deficiency as a cause of serious arrhythmias. Arch Intern Med. 1978;138:825–826. [PubMed] [Google Scholar]
- 3.Ray S, Delaney M, Muller AF. Proton pump inhibitors and acute interstitial nephritis. BMJ. 2010;341:c4412. doi: 10.1136/bmj.c4412. [DOI] [PubMed] [Google Scholar]
- 4.Sierra F, Suarez M, Rey M, Vela MF. Systematic review: proton pump inhibitor-associated acute interstitial nephritis. Aliment Pharmacol Ther. 2007;26:545–553. doi: 10.1111/j.1365-2036.2007.03407.x. [DOI] [PubMed] [Google Scholar]
- 5.Herzig SJ, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA. 2009;301:2120–2128. doi: 10.1001/jama.2009.722. [DOI] [PubMed] [Google Scholar]
- 6.Howell MD, Novack V, Grgurich P, Soulliard D, Novack L, Pencina M, Talmor D. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170:784–790. doi: 10.1001/archinternmed.2010.89. [DOI] [PubMed] [Google Scholar]
- 7.Faulhaber GA, Furlanetto TW. Could magnesium depletion play a role on fracture risk in PPI users? Arch Intern Med. 2010;170:1776. doi: 10.1001/archinternmed.2010.374. [DOI] [PubMed] [Google Scholar]
- 8.Cundy T, Dissanayake A. Severe hypomagnesaemia in long-term users of proton-pump inhibitors. Clin Endocrinol (Oxf) 2008;69:338–341. doi: 10.1111/j.1365-2265.2008.03194.x. [DOI] [PubMed] [Google Scholar]
- 9.Cundy T, Mackay J. Proton pump inhibitors and severe hypomagnesaemia. Curr Opin Gastroenterol. 2011;27:180–185. doi: 10.1097/MOG.0b013e32833ff5d6. [DOI] [PubMed] [Google Scholar]
- 10.Hess MW, Hoenderop JG, Bindels RJ, Drenth JP. Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther. 2012;36:405–413. doi: 10.1111/j.1365-2036.2012.05201.x. [DOI] [PubMed] [Google Scholar]
- 11.Hoorn EJ, van der Hoek J, de Man RA, Kuipers EJ, Bolwerk C, Zietse R. A case series of proton pump inhibitor-induced hypomagnesemia. Am J Kidney Dis. 2010;56:112–116. doi: 10.1053/j.ajkd.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Krupa LZ, Fellows IW: Lansoprazole-induced hypomagnesaemia. BMJ Case Rep 2014. Epub 2014 Jan 10. 10.1136/bcr-2012-006342 [DOI] [PMC free article] [PubMed]
- 13.Mackay JD, Bladon PT. Hypomagnesaemia due to proton-pump inhibitor therapy: a clinical case series. QJM. 2010;103:387–395. doi: 10.1093/qjmed/hcq021. [DOI] [PubMed] [Google Scholar]
- 14.Danziger J, William JH, Scott DJ, Lee J, Lehman LW, Mark RG, Howell MD, Celi LA, Mukamal KJ. Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int. 2013;83:692–699. doi: 10.1038/ki.2012.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faulhaber GA, Ascoli BM, Lubini A, Mossmann M, Rossi G, Geib G, Furlanetto TW. Serum magnesium and proton-pump inhibitors use: a cross-sectional study. Rev Assoc Med Bras. 2013;59:276–279. doi: 10.1016/j.ramb.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Koulouridis I, Alfayez M, Tighiouart H, Madias NE, Kent DM, Paulus JK, Jaber BL. Out-of-hospital use of proton pump inhibitors and hypomagnesemia at hospital admission: a nested case-control study. Am J Kidney Dis. 2013;62:730–737. doi: 10.1053/j.ajkd.2013.02.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S., Lee H., Park C.H., Shim C.N., Lee H.J., Park J.C., Shin S.K., Lee S.K., Lee Y.C., Kim H.Y., Kang D.R. Clinical predictors associated with proton pump inhibitor-induced hypomagnesemia. Am J Ther. 2015;22:14–21. doi: 10.1097/MJT.0b013e31829c4c71. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Famularo G, Gasbarrone L, Minisola G. Hypomagnesemia and proton-pump inhibitors. Expert Opin Drug Saf. 2013;12:709–716. doi: 10.1517/14740338.2013.809062. [DOI] [PubMed] [Google Scholar]
- 20.Delgado MG, Calleja S, Suarez L, Pascual J. Recurrent confusional episodes associated with hypomagnesaemia due to esomeprazol. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2013-200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park CH, Kim EH, Roh YH, Kim HY, Lee SK. The association between the use of proton pump inhibitors and the risk of hypomagnesemia: a systematic review and meta-analysis. PLoS One. 2014;9:e112558. doi: 10.1371/journal.pone.0112558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279:19–25. doi: 10.1074/jbc.M311201200. [DOI] [PubMed] [Google Scholar]
- 23.Quamme GA. Recent developments in intestinal magnesium absorption. Curr Opin Gastroenterol. 2008;24:230–235. doi: 10.1097/MOG.0b013e3282f37b59. [DOI] [PubMed] [Google Scholar]
- 24.Schlingmann KP, Sassen MC, Weber S, Pechmann U, Kusch K, Pelken L, Lotan D, Syrrou M, Prebble JJ, Cole DE, Metzger DL, Rahman S, Tajima T, Shu SG, Waldegger S, Seyberth HW, Konrad M. Novel TRPM6 mutations in 21 families with primary hypomagnesemia and secondary hypocalcemia. J Am Soc Nephrol. 2005;16:3061–3069. doi: 10.1681/ASN.2004110989. [DOI] [PubMed] [Google Scholar]
- 25.Wong ET, Rude RK, Singer FR. A high prevalence of hypomagnesemia in hospitalized patients. Am J Clin Pathol. 1983;79:348–352. doi: 10.1093/ajcp/79.3.348. [DOI] [PubMed] [Google Scholar]
- 26.Ryzen E. Magnesium homeostasis in critically ill patients. Magnesium. 1989;8:201–212. [PubMed] [Google Scholar]