Plants introduced into a new range are expected to harbour fewer specialized herbivores and to receive less damage than conspecifics in native ranges. Datura stramonium was introduced in Spain about five centuries ago. Here, we compare damage by herbivores, plant size, and leaf trichomes between plants from non-native and native ranges and perform selection analyses. Non-native plants experienced much less damage, were larger and less pubescent than plants of native populations. While plant size was related to fitness in both ranges, selection to increase resistance was only detected in the native region. We suggest this is a consequence of a release from enemies in this new environment.
Keywords: Datura stramonium, enemy release hypothesis (ERH), invasive species, natural selection, plant defence, resistance to herbivores, Spain, specialist and generalist herbivores
Abstract
When plants are introduced into new regions, the absence of their co-evolved natural enemies can result in lower levels of attack. As a consequence of this reduction in enemy pressure, plant performance may increase and selection for resistance to enemies may decrease. In the present study, we compared leaf damage, plant size and leaf trichome density, as well as the direction and magnitude of selection on resistance and plant size between non-native (Spain) and native (Mexico) populations of Datura stramonium. This species was introduced to Spain about five centuries ago and constitutes an ideal system to test four predictions of the enemy release hypothesis. Compared with native populations, we expected Spanish populations of D. stramonium to have (i) lower levels of foliar damage; (ii) larger plant size; (iii) lower leaf trichome density that is unrelated to foliar damage by herbivores; and (iv) weak or no selection on resistance to herbivores but strong selection on plant size. Our results showed that, on average, plants from non-native populations were significantly less damaged by herbivores, were less pubescent and were larger than those from native populations. We also detected different selection regimes on resistance and plant size between the non-native and native ranges. Positive selection on plant size was detected in both ranges (though it was higher in the non-native area), but consistent positive selection on relative resistance was detected only in the native range. Overall, we suggest that changes in selection pressure on resistance and plant size in D. stramonium in Spain are a consequence of ‘release from natural enemies’.
Introduction
The occurrence of biological invasions has increased dramatically over the past few decades (Vitousek et al. 1996; Sakai et al. 2001). This increase has been attributed to human activities (i.e. global trade and transport) extending the range of distribution of many species to novel areas (Bossdorf et al. 2005; Genton et al. 2005; Hierro et al. 2005). Besides their economic impact (Drake et al. 1989; Pimentel et al. 2000; Sakai et al. 2001), biological invasions are recognized as one of the greatest threats to biodiversity and the integrity of natural ecosystems (Sala et al. 2000; Sakai et al. 2001; Wolfe et al. 2004; Agrawal et al. 2005; Thelen et al. 2005). Nonetheless, not all alien introductions become successful invasions (Groves 1986, 1991; van Kleunen and Johnson 2007; Moles et al. 2008). When invasive species are transported to new areas, their abundance and performance will be affected by a different set of parameters than in their native range (Maron et al. 2004; Hierro et al. 2005). Thus, a relevant question is to determine the conditions that allow alien species become successfully established. Many studies have shown that most successful invasions occur in disturbed habitats (Sax and Brown 2000; Moles et al. 2008) and/or where environmental conditions such as the availability of resources (Moles et al. 2008) have been altered by human activities. However, only a small fraction of introduced plant species become invasive (Joshi and Vrieling 2005). To invade, alien plants must be able to establish and successfully compete with resident species or occupy empty niches, which may depend on, among other factors, release from their natural enemies (i.e. herbivores, pathogens and parasites) in the non-native habitat (Sax and Brown 2000; Colautti et al. 2004; Wolfe et al. 2004; Moles et al. 2008).
The enemy release hypothesis (ERH, Elton 1958; Crawley 1987; Keane and Crawley 2002) is one of the most commonly considered explanations for the success of invasive plant species (reviewed by Colautti et al. 2004; Liu and Stiling 2006). The ERH postulates that during the introduction into a novel region, plant populations experience a decrease in regulation by their co-evolved natural enemies (Keane and Crawley 2002). This liberation from natural enemies can result in reduced levels of herbivory and parasitism in introduced compared with native plant species (Keane and Crawley 2002; Agrawal et al. 2005; Mitchell et al. 2006), which should result in increased plant size, fecundity (Crawley 1987; Blossey and Notzold 1995; Keane and Crawley 2002; Jakobs et al. 2004; Joshi and Vrieling 2005) and population growth in an invasive species’ introduced range compared with its native range (Crawley 1987; Keane and Crawley 2002; Maron et al. 2004; Vilà et al. 2005; Dawson et al. 2014). However, this plastic response to the release from damage by herbivores may also have evolutionary consequences (Jakobs et al. 2004; Maron et al. 2004; Wolfe et al. 2004). If specialist herbivores of an alien plant species are absent in the introduced areas (Keane and Crawley 2002), it is expected that selection for resistance against them may also be absent (van Kleunen and Schmid 2003). Although the production of chemical and mechanical defences may be adaptive in the presence of natural herbivores, the expression of plant resistance to herbivores can be costly when they are scarce or absent (Purrington 2000; Koricheva 2002; Strauss et al. 2002; Wolfe et al. 2004). For this reason, natural selection in the new environment would reduce resource allocation to defence against herbivores and favour genotypes with improved competitive abilities (i.e. increasing vegetative growth or reproductive effort) (Blossey and Notzold 1995). This is the essence of the ‘Evolution of Increased Competitive Ability (EICA)’ hypothesis proposed by Blossey and Notzold (1995). Obviously, ERH and EICA are not mutually exclusive hypotheses and can be seen as a two-step process, where increased ecological performance follows rapid evolutionary change.
Datura stramonium (Solanaceae) is an annual weed that inhabits open, cultivated and disturbed sites where it grows to an average height of 1 m (Núñez-Farfán 1991). Datura stramonium is native to Mexico (Jiao et al. 2002) and it is widely distributed in warm regions around the world (van Kleunen et al. 2007). In its native range, this species is attacked by a wide variety of herbivores (Núñez-Farfán 1991) and leaf damage significantly reduces plant fitness (Núñez-Farfán and Dirzo 1994; Fornoni et al. 2003; Valverde et al. 2003). Previous field and experimental studies have found that leaf trichomes and tropane alkaloids are two defensive traits that prevent herbivory (Shonle and Bergelson 2000; Valverde et al. 2001; Castillo et al. 2013; Kariñho-Betancourt and Núñez-Farfán 2015). Moreover, significant selection on resistance (measured as 1—damage) and on defensive traits (trichomes and alkaloids) had been detected in natural and experimental populations (Núñez-Farfán and Dirzo 1994; Núñez-Farfán et al. 1996; Shonle and Bergelson 2000; Valverde et al. 2001, 2003; Fornoni et al. 2004; Castillo et al. 2014). In the present study, we investigated the case of D. stramonium in Spain, a country where it was introduced about five centuries ago and is presently considered an invasive species (Dana-Sánchez et al. 2004; Sanz-Elorza et al. 2004). Owing to early trade across the Atlantic, this introduction and invasion were probably the first intercontinental invasion achieved by the species, which actually faced a new biotic environment. If this is the case, Spanish populations have had the highest number of generations in their new range, and therefore the most opportunity for evolutionary change. Datura stramonium is therefore an ideal system in which to test some of the predictions of the ERH. In order to accomplish this, we conducted a field survey in southern Spain to examine leaf damage, plant size and trichome density and compare these characters with Mexican populations. We also compared phenotypic selection gradients on resistance and plant size in this species’ non-native and native ranges. In this study, we tested four predictions of the ERH. If specialist herbivore insects are absent in the new region (Keane and Crawley 2002), we predicted that populations in Spain would have (i) lower levels of foliar damage and (ii) larger plant size. We also expected that since resistance traits can be costly (Strauss et al. 2002), trichome density would be lower and unrelated to foliar damage by herbivores in Spain. Finally, we expected weak or no selection on resistance to herbivores in the non-native region but strong selection on plant size. Recently, several studies evaluating invasive plant species in their native and introduced ranges with natural populations have found evidence that supports some ecological expectations of the ERH (e.g. Memmott et al. 2000; Wolfe 2002; Jakobs et al. 2004; Blaisdell and Roy 2014; Huberty et al. 2014; Kambo and Kotanen 2014). However, to our knowledge, this is one of the first attempts to evaluate the joint pattern of selection on plant resistance to herbivores and plant size in the non-native and native range.
Methods
Study species
Datura stramonium (Solanaceae) is a cosmopolitan annual weed occurring in a wide variety of plant communities in North America (Avery et al. 1959; Weaver and Warwick 1984), and Mexico is likely to be its centre of origin (Symon and Haegi 1991; Jiao et al. 2002). In its native range, this herbaceous plant inhabits open, cultivated lands, roadsides and disturbed sites (Núñez-Farfán 1991). In central Mexico, leaves of this species are consumed by at least two specialist herbivorous beetles, Epitrix parvula and Lema daturaphila (Coleoptera: Chrysomelidae), and by a generalist grasshopper, Sphenarium purpurascens (Orthoptera: Pyrgomorphidae) (Núñez-Farfán and Dirzo 1994). Datura stramonium is also attacked by a specialist pre-dispersal seed predator, Trichobaris soror (Coleoptera: Curculionidae) (Cabrales-Vargas 1991; Bello-Bedoy et al. 2011). A complete description of the plant, herbivorous insects and damage types produced by them can be found elsewhere (Núñez-Farfán 1991; Núñez-Farfán and Dirzo 1994; Carmona and Fornoni 2013). Previous studies in natural and experimental populations of D. stramonium in their native range have reported that foliar damage caused by herbivorous insects imposes selection on resistance and/or tolerance (Núñez-Farfán and Dirzo 1994; Shonle and Bergelson 2000; Valverde et al. 2001, 2003; Fornoni et al. 2004). Moreover, leaf trichomes and tropane alkaloids, two putative components of defence, can evolve as a result of natural selection imposed by herbivorous insects (Shonle and Bergelson 2000; Castillo et al. 2014; Kariñho-Betancourt and Núñez-Farfán 2015).
Datura stramonium is an invasive species in almost all temperate and tropical regions of the world (van Kleunen et al. 2007). In Spain, D. stramonium was introduced, probably from Mexico, by Post-Columbian expeditionists between the years 1540 and 1577 (Sanz-Elorza et al. 2004). In this country D. stramonium is considered an invasive species since it mainly inhabits agricultural fields, waste lands and natural habitats like riparian areas and wetlands in warm regions with moderate to high human influence. Currently, the main problem is that this species reaches high densities in soils with high nitrogen content that prevent the development of native species (Dana-Sánchez et al. 2004; Sanz-Elorza et al. 2004).
Sampling populations and data collection in Spain
From September to November 2010 and 2011, we sampled 14 populations of D. stramonium occurring in different habitats and environmental conditions in southern Spain (Table 1). The populations were sampled in the regions of Andalousia, Extremadura and Murcia. While all are described as having a Mediterranean climate, yearly mean rainfall varied by 4-fold among sites, with wettest conditions in the west (more than 800 mm) and driest conditions in the east (∼200 mm, see Table 1). Thus, a wide range of conditions was represented within the widespread Mediterranean climate of the Iberian Peninsula. The linear distance between pairs of populations ranged from 5 to 468 km. In a sample of mature plants in each population (mean sample size: 26.74 ± 1.69 SE individual plants, Table 1), we measured the basal stem diameter as an estimate of plant size, collected a sample of 8–40 fully expanded leaves and collected all the fruits produced.
Table 1.
Geographic location and environmental characteristics of 14 populations of D. stramonium in the non-native range (southern Spain. n = sample size). 1Data taken from Ninyerola et al. (2005). a.s.l, above sea level
| Number and locality of each population (Province) (n) | Geographical coordinates | Habitat | Altitude (m a.s.l.) | Mean annual precipitation (mm)1 | Mean annual temperature (°C)1 |
|---|---|---|---|---|---|
| 1. Hinojos 1 (Huelva) (18) | 37°18′0.39″N 6°22′41.72″W | River bank | 67 | 503.3 | 18 |
| 2. Hinojos 2 (Huelva) (22) | 37°19′28.36″N 6°25′32.45″W | River bank | 88 | 515.8 | 18 |
| 3. Bolonia (Cádiz) (30) | 36°5′9.99″N 5°46′7.57″W | Stream in seashore | 3 | 693.3 | 18 |
| 4. Gerena (Sevilla) (30) | 37°31′28.86″N 6°11′24.76″W | River bank | 55 | 501.6 | 18 |
| 5. Zubia (Granada) (30) | 37°7′47.28″N 3°35′57.06″W | Cropland edge | 692 | 337.5 | 15 |
| 6. Castañuelos (Huelva) (9) | 37°56′19.83″N 6°35′2.97″W | Oak forest edge | 437 | 825.8 | 16 |
| 7. El Higueral (Almería) (30) | 37°23′12.61″N 2°29′56.48″W | Dry riverbed | 880 | 255.0 | 14 |
| 8. Pinilla (Murcia) (30) | 37°41′3.10″N 1°17′0.62″W | Wasteland | 240 | 236.6 | 17 |
| 9. Don Fadrique (Granada) (25) | 37°57′39.75″N 2°26′8.75″W | Abandoned orchard | 1161 | 422.5 | 13 |
| 10. Lora del Río (Sevilla) (30) | 37°39′33.28″N 5°32′5.93″W | River bank | 36 | 483.3 | 18 |
| 11. El Pedroso (Sevilla) (30) | 37°50′12.81″N 5°45′58.67″W | Roadside | 383 | 501.6 | 17 |
| 12. Cardeña (Córdoba) (30) | 38°14′56.63″N 4°12′58.45″W | Dry riverbed | 351 | 645.8 | 17 |
| 13. Valdeflores (Sevilla) (30) | 37°43′2.23″N 6°18′50.44″W | River bank | 287 | 598.2 | 17 |
| 14. Cabeza La Vaca (Badajoz) (30) | 38°6′48.00″N 6°24′23.36″W | River bank | 548 | 625.0 | 16 |
We measured the total and damaged areas for each collected leaf using free ImageJ v1.47 software (National Institutes of Health, Bethesda, MD, USA). For a given plant i, relative resistance to herbivores (Ri) was estimated as the converse of the average relative leaf damage (Di) as:
where AD and AT are the damaged and total leaf areas, respectively, and n is the number of leaves sampled (following Núñez-Farfán and Dirzo 1994; Bello-Bedoy and Núñez-Farfán 2010). This estimate of resistance to herbivores (Ri) is broadly interpreted as a measure of total resistance (see Leimu and Koricheva 2006) and has been used in previous studies with D. stramonium (Núñez-Farfán and Dirzo 1994; Fornoni et al. 2003, 2004).
We measured leaf trichome density as the total number of trichomes within a 2.5 mm2 area on the basal central area of the adaxial side of the leaf following Valverde et al. (2001), using a dissecting microscope. This sampled area of the leaf gives a good estimate of the whole-leaf average trichome density (Valverde et al. 2001). In each population, we estimated the average trichome density per plant from a sample of 8–10 fully expanded, mature leaves, obtained from the same sample of leaves used to estimate relative damage.
The number of fruits per plant was used as a measure of individual maternal plant fitness. Since D. stramonium has a mixed mating system (Motten and Antonovics 1992; Motten and Stone 2000) with a high level of selfing (Núñez-Farfán et al. 1996; van Kleunen et al. 2007), the number of fruits and seeds is a good estimator of reproductive success of the female function (see Mauricio and Rausher 1997), and male and female functions are probably highly correlated in selfing plants (Charlesworth and Charlesworth 1981; Bertin 1988).
Data from Mexican populations
Measurements of leaf damage, plant diameter, leaf trichome density and number of fruits per plant from seven populations of D. stramonium in its native range were obtained from a previous study (Castillo et al. 2014). These populations occur in different plant communities (Castillo et al. 2014). The chosen populations were Acolman, Patria Nueva, San Martín, Sanabria, Santo Domingo, Tzin Tzun Tzan and Valsequillo. The mean sample size was 29 (±1.88 SE) individual plants per population. Data collection procedures in Mexico were similar to those conducted in Spain. Further details on geographic location and environmental characteristics of the seven Mexican populations of D. stramonium are available elsewhere (Table S1 in Castillo et al. 2014).
Statistical analysis
We performed a nested analysis of variance to test differences in relative leaf damage, plant diameter and leaf trichome density due to range (non-native vs. native) and population (within range). Range was considered as a fixed factor and population (within range) as a random factor. We used regression analyses to assess the effect of leaf trichome density on relative leaf damage for each population. Prior to analyses, plant diameter and leaf trichome density were natural log-transformed, while relative leaf damage was arcsine-transformed (Sokal and Rohlf 2012).
Directional selection on relative resistance and plant size
In order to estimate the magnitude and direction of selection gradients (βi) on relative resistance and plant size, we performed multiple regression analysis (Lande and Arnold 1983) in each population. For these analyses, directional selection gradients (βi) were estimated as the standardized partial linear regression coefficients of relative fitness as a function of relative resistance and plant diameter. For each population, relative resistance and plant diameter were standardized ( and S = 1) and plant fitness was relativized (the number of fruits divided by the corresponding population mean fitness) (Lande and Arnold 1983).
Comparing directional selection gradients between ranges
In order to compare the consistency of the patterns of selection on relative resistance and plant size between ranges, we performed a meta-analysis to estimate the effect size of each selection gradient (Castillo et al. 2014). The effect size is a value that reflects the strength of a relationship between two variables (Borenstein et al. 2009). Mean effect sizes were used to compare estimates of phenotypic selection on relative resistance and/or plant size corresponding to populations of each range. We estimated effect sizes using the partial regression coefficients (i.e. selection gradients) weighted by their variances (following Castillo et al. 2014). Assuming that the true effect size varies from population to population, we estimated mean effect sizes and their 95 % confidence intervals by applying a random-effect model (Borenstein et al. 2009). If the confidence interval around the mean effect size did not include zero, we concluded that there is a significant effect on the pattern and intensity of selection on relative resistance and/or plant size in a particular range. The meta-analyses were performed using the metaphor package (Viechtbauer 2010) for R v3.0.2 software (R Development Core Team 2011).
Results
Between-range and among-population variation
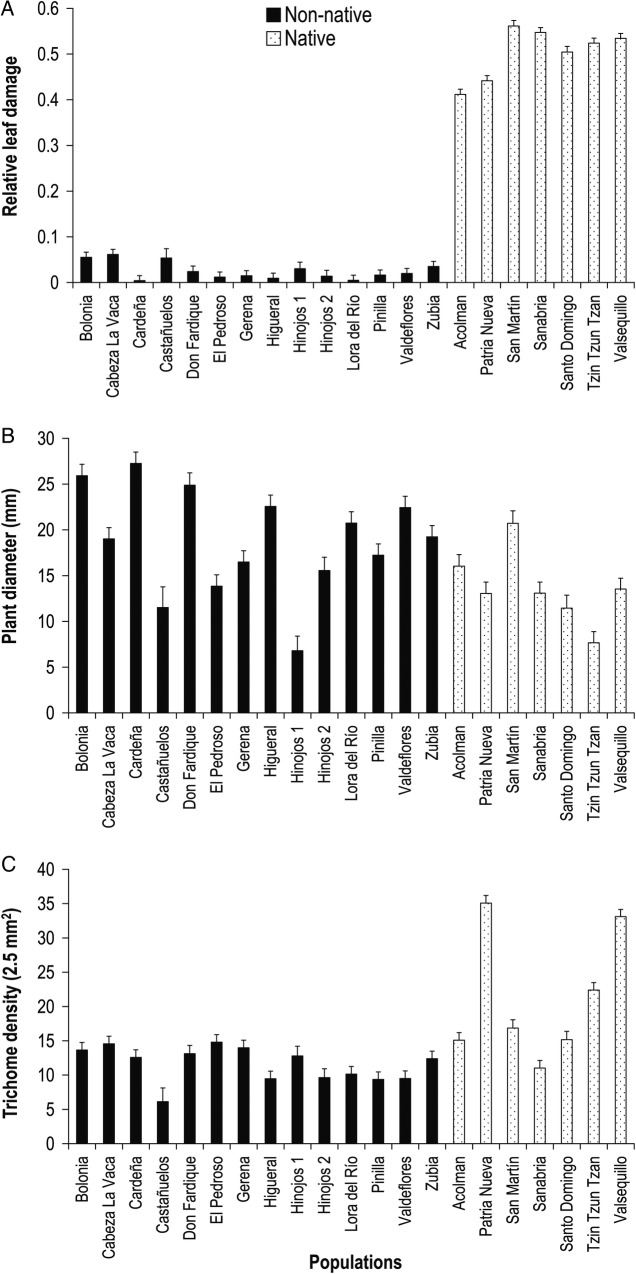
Relative leaf damage, plant diameter and leaf trichome density differed significantly between the non-native and native ranges and among populations within each range (Fig. 1). In the non-native range, populations experienced low average levels of relative leaf damage ( 0.025 ± 0.0102, Fig. 1A). On average, Spanish populations had 20 times less damage than Mexican populations (0.503 ± 0.013, F1, 19.04 = 623.07, P < 0.0001, Fig. 1A). Similarly, Spanish populations are 1.84 times less pubescent (11.57 ± 1.66 trichomes × 2.5 mm2) than Mexican populations (21.24 ± 2.14, F1, 19.03 = 13.47, P = 0.0016, Fig. 1C). Conversely, on average, Spanish plant populations had a significantly larger average diameter than Mexican plant populations (18.81 ± 1.37 and 13.64 ± 1.77 mm, respectively, F1, 19.04 = 4.54, P = 0.0463, Fig. 1B).
Figure 1.
Mean (±SE) relative leaf damage by herbivores (A), plant diameter (B) and leaf trichome density (C) of populations of D. stramonium in the non-native (southern Spain) and native (Mexico) ranges.
In the non-native range, field observations indicated a low richness of phytophagous invertebrates on individual plants of D. stramonium (n = 6 species, Table 2). The generalist moth Helicoverpa armigera (Noctuidae: Lepidoptera) was the main herbivore in all populations. Besides leaves, larvae of H. armigera also bored into fruits to consume immature seeds until pupation (P. L. Valverde, pers. obs.).
Table 2.
Phytophagous invertebrate species sampled on leaves of D. stramonium in 14 populations in the non-native range (southern Spain). The number of populations as in Table 1.
| Class | Order | Family | Species | Population |
|---|---|---|---|---|
| Insecta | Lepidoptera | Noctuidae | Helicoverpa armigera | 1–14 |
| Hemiptera | Pentatomidae | Nezara viridula | 11 and 12 | |
| Pyrrhocoridae | Pyrrhocoris apterus | 11 and 13 | ||
| Coleoptera | Curculionidae | Coniatus repandus | 13 | |
| Orthoptera | Tettigoniidae | Phaneroptera sp. | 12 | |
| Gastropoda | Pulmonata | Helicidae | Theba pisana | 11 |
Effect of leaf trichome density on relative leaf damage
We did not detect a consistent effect of trichome density on relative leaf damage in either range. In the non-native range, one population showed a negative relationship (Castañuelos population, F1, 7 = 14.13, P = 0.0071). In the native range, two populations showed a positive relationship, Patria Nueva (F1, 28 = 15.19, P = 0.0006) and Tzin Tzun Tzan (F1, 29 = 11.19, P = 0.0023), and one showed a negative relationship (San Martín population, F1, 23 = 10.84, P = 0.0032).
Directional phenotypic selection on relative resistance and plant size
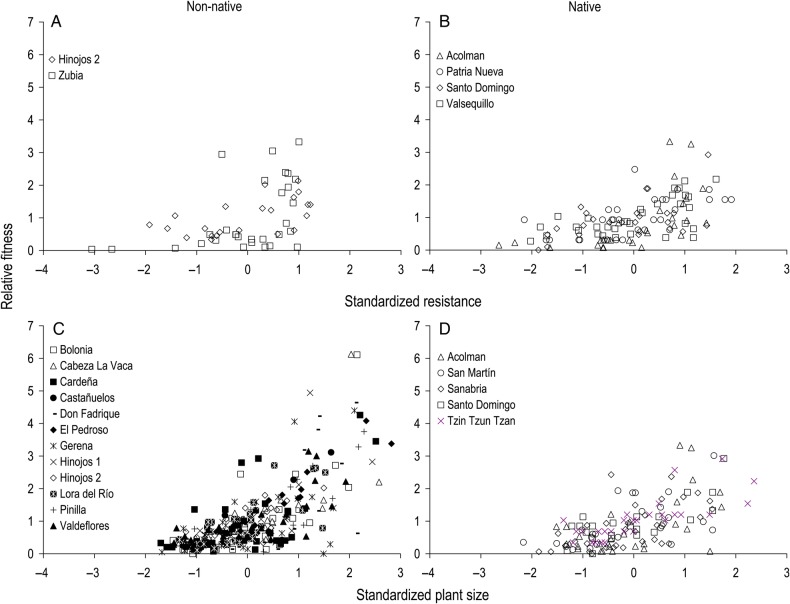
We found positive selection on relative resistance in two populations (Table 3, Fig. 2A) and on plant size in 12 populations (Table 3, Fig. 2C) of the 14 populations measured in the introduced range. In the seven native range populations measured, we found positive selection on relative resistance in four populations (Table 3, Fig. 2B) and on plant size in five populations (Table 3, Fig. 2D).
Table 3.
Standardized directional (βi) selection gradients (±SE) on relative resistance and plant diameter in populations of D. stramonium from the non-native (southern Spain) and native (México) ranges. Significant gradients appear in bold type face.
| Range | Population | Plant trait | β (±SE) | t (df) | P |
|---|---|---|---|---|---|
| Non-native | Hinojos 1 | Relative resistance | 0.074 (0.220) | 0.34 (15) | 0.7422 |
| Diameter | 0.827 (0.069) | 3.75 (15) | 0.0019 | ||
| Hinojos 2 | Relative resistance | 0.167 (0.075) | 2.20 (19) | 0.0403 | |
| Diameter | 0.365 (0.075) | 4.86 (19) | 0.0001 | ||
| Bolonia | Relative resistance | 0.271 (0.164) | 1.65 (27) | 0.1099 | |
| Diameter | 0.927 (0.164) | 5.65 (27) | <0.0001 | ||
| Gerena | Relative resistance | 0.026 (0.182) | 0.15 (27) | 0.8840 | |
| Diameter | 0.551 (0.182) | 3.02 (27) | 0.0050 | ||
| Zubia | Relative resistance | 0.480 (0.179) | 2.67 (27) | 0.0126 | |
| Diameter | −0.013 (0.179) | −0.07 (27) | 0.9414 | ||
| Castañuelos | Relative resistance | −0.307 (0.243) | −1.26 (6) | 0.2533 | |
| Diameter | 0.716 (0.243) | 2.95 (6) | 0.0257 | ||
| El Higueral | Relative resistance | 0.200 (0.207) | 0.97 (27) | 0.3428 | |
| Diameter | 0.191 (0.207) | 0.92 (27) | 0.3641 | ||
| Pinilla | Relative resistance | −0.004 (0.096) | −0.05 (27) | 0.9630 | |
| Diameter | 0.810 (0.096) | 8.37 (27) | <0.0001 | ||
| Don Fadrique | Relative resistance | 0.175 (0.216) | 0.81 (22) | 0.4261 | |
| Diameter | 0.978 (0.200) | 4.89 (22) | <0.0001 | ||
| Lora del Río | Relative resistance | 0.036 (0.107) | 0.34 (27) | 0.7383 | |
| Diameter | 0.545 (0.107) | 5.07 (27) | <0.0001 | ||
| El Pedroso | Relative resistance | −0.046 (0.058) | −0.8 (27) | 0.4279 | |
| Diameter | 0.954 (0.058) | 16.43 (27) | <0.0001 | ||
| Cardeña | Relative resistance | −0.157 (0.156) | −1.01 (27) | 0.3217 | |
| Diameter | 0.667 (0.156) | 4.27 (27) | 0.0002 | ||
| Valdeflores | Relative resistance | 0.123 (0.096) | 1.27 (27) | 0.2135 | |
| Diameter | 0.532 (0.096) | 5.52 (27) | <0.0001 | ||
| Cabeza La Vaca | Relative resistance | 0.231 (0.153) | 1.51 (27) | 0.1421 | |
| Diameter | 0.877 (0.153) | 5.73 (27) | <0.0001 | ||
| Native | Acolman | Relative resistance | 0.336 (0.142) | 2.58 (26) | 0.0311 |
| Diameter | 0.337 (0.147) | 2.58 (26) | 0.0311 | ||
| Patria Nueva | Relative resistance | 0.343 (0.099) | 3.45 (27) | 0.0018 | |
| Diameter | 0.042 (0.099) | 0.43 (27) | 0.6739 | ||
| San Martín | Relative resistance | 0.145 (0.131) | 1.10 (22) | 0.2822 | |
| Diameter | 0.376 (0.127) | 2.94 (22) | 0.0076 | ||
| Sanabria | Relative resistance | 0.025 (0.108) | 0.24 (29) | 0.8157 | |
| Diameter | 0.451 (0.120) | 3.75 (29) | 0.0008 | ||
| Santo Domingo | Relative resistance | 0.225 (0.091) | 2.47 (20) | 0.0228 | |
| Diameter | 0.418 (0.091) | 4.59 (20) | 0.0002 | ||
| Tzin Tzun Tzan | Relative resistance | 0.018 (0.087) | 0.21 (28) | 0.8325 | |
| Diameter | 0.488 (0.087) | 5.55 (28) | <0.0001 | ||
| Valsequillo | Relative resistance | 0.324 (0.081) | 3.98 (30) | 0.0004 | |
| Diameter | 0.063 (0.081) | 0.77 (30) | 0.4459 |
Figure 2.
Relationship between standardized relative resistance and relative fitness of populations of D. stramonium in the non-native (southern Spain; A) and native (Mexico; B) ranges. Relationship between standardized plant size and relative fitness in the non-native (southern Spain; C) and native (Mexico; D) ranges. Only significant relationships are shown (see Table 3).
Comparing directional selection gradients among populations
In the native and non-native ranges, mean effect sizes of selection on plant size were 0.308 (95 % CI 0.135–0.46) and 0.516 (0.365–0.64), respectively (Fig. 3). Given that neither confidence interval overlaps zero, plant size was positively selected in both ranges, though this trend was stronger in the introduced range. On the other hand, mean effect sizes of relative resistance show a different pattern. We detected a consistent trend to positively select relative resistance in the native range (0.199, 95 % CI 0.096–0.297, Fig. 3), whereas no consistent trend was detected in the introduced range (0.075, 95 % CI −0.011–0.16, Fig. 3).
Figure 3.
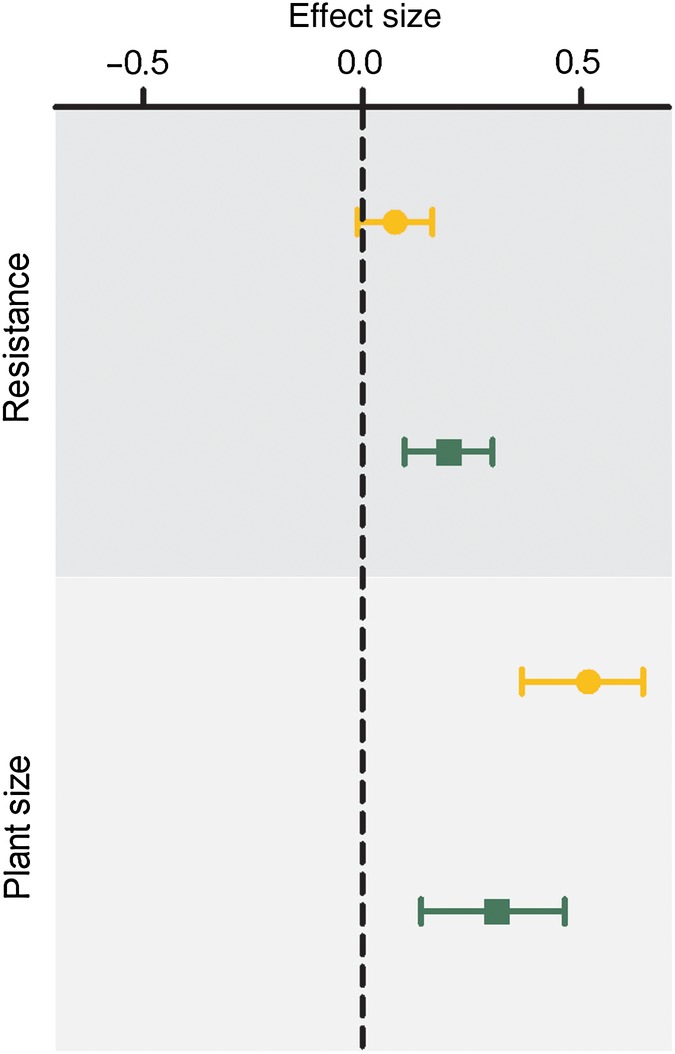
Forest plot of the mean effect sizes and 95 % confidence intervals for standardized selection gradients for relative resistance and plant size (plant diameter). Green squares correspond to the native range and yellow circles to the non-native range.
Discussion
Native and non-native populations of D. stramonium experience differential leaf damage by herbivores. In Spain, leaf damage was very low, averaging 2.5 % of total leaf area, thus supporting our first prediction. We also found that plants in the non-native range were larger than those of the native range, supporting our second prediction. In partial support of our third prediction, leaf trichome density was significantly lower in the non-native range. However, although Mexican populations had a higher mean leaf trichome density, its effect on leaf damage was not consistent. Our major finding was the detection of different selection regimes on resistance and plant size between the non-native and native ranges. While a trend of positive selection on plant size was detected in both ranges (though higher in the non-native area), positive selection on relative resistance was only detected in the native region, supporting our fourth prediction. Overall, our study suggests that changes in selection pressures on resistance and plant size in D. stramonium in Spain are the consequence of a ‘release from natural enemies’ in this new environment.
Introduced plant species commonly harbour fewer species of specialized natural enemies and are less attacked in their introduced vs. their native ranges (Memmott et al. 2000; Wolfe 2002; Torchin and Mitchell 2004; Wolfe et al. 2004; Agrawal et al. 2005; Genton et al. 2005; Blaisdell and Roy 2014). This appears to be the case in D. stramonium in southern Spain, where leaf damage was 20 times lower than in Mexico. This difference in leaf damage is in agreement with the ERH. There are at least two non-exclusive factors that could explain the lower leaf damage in the non-native area. First, the three specialist herbivores of D. stramonium commonly found in the native range are absent in populations of southern Spain. This is consistent with one of the basic predictions of ERH and is one of the main explanations cited for the success of introduced invasive plants species (Liu and Stiling 2006). Second, the generalist herbivores recorded during our field surveys in Spain (cf. Table 2; Torres-Vila et al. 2000; Torres-Vila et al. 2002; Ribes et al. 2004; Mata et al. 2013) consume a very small amount of foliar tissue of D. stramonium, and are therefore unlikely to exert strong selective pressure for plant resistance in the new environment.
Leaf trichome density is a plant resistance trait that prevents damage by herbivores (Levin 1973; Johnson 1975; Rodriguez et al. 1984; Handley et al. 2005; Holeski 2007), and previous studies on D. stramonium in its native range support this defensive function (Valverde et al. 2001; Kariñho-Betancourt and Núñez-Farfán 2015). Populations of D. stramonium surveyed in Spain were significantly less pubescent than populations in the native range. However, we did not detect a consistent effect of leaf trichomes on leaf damage by herbivores in the native or non-native areas. In this sense, the variation in leaf trichome density may be neutral in relation to resistance or related to the variation in other environmental factors (Valverde et al. 2001; Kariñho-Betancourt and Núñez-Farfán 2015). For instance, leaf trichomes might reduce water loss in dry environments (Turner and Kramer 1980; Fitter and Hay 1987; Larcher 2001). Further studies are therefore needed to explore whether the reduction in leaf trichomes or other components of defence in the non-native range is actually a consequence of relaxed selection pressure for resistance to generalist herbivores.
The ERH posits that plants introduced into a new range benefit from the absence of their natural enemies, resulting in larger and more vigorous plants than in their native ranges (Crawley 1987; Maron and Vilà 2001; Keane and Crawley 2002; Jakobs et al. 2004). Reduction of damage by herbivores would translate into higher allocation to plant growth in the non-native range (Keane and Crawley 2002; Vilà et al. 2005). Consistent with this idea, our study revealed that plants of D. stramonium in the non-native area are larger than in the native region. Furthermore, a reduction of resource allocation to defence in the new environment (where specialist herbivores are absent) would select for allocation to traits that enhance plants' competitive ability (Blossey and Notzold 1995). Our analysis of selection gradients on plant size showed that this trait was consistently favoured among populations of D. stramonium in both ranges. However, the mean effect size of selection gradients on plant size was higher in the non-native range, suggesting stronger selection. In this sense, it has been suggested that plant size promotes competitive ability (Kelly 1992; Cahill et al. 2008). In addition, our study also supports the prediction that plants from the introduced area experience weak or no selection on resistance to herbivores since the mean effect size of selection gradients on resistance revealed a no consistent trend in the non-native range. In contrast, mean effect size for selection to resistance showed a consistent positive trend in the native range.
Conclusions
Since D. stramonium has experienced a much larger number of generations than minimum estimated for evolutionary change in a new range (Prentis et al. 2008), this constitutes an opportunity for selection to occur. Our study suggests that D. stramonium is not subject to demographic regulation by generalist herbivores in the new environment and that selection would not favour the maintenance of allocation to defence in Spain. Hence, a variation in resistance to herbivores among populations of D. stramonium in the new geographic range may not be adaptive. On the other hand, if genetic differentiation for traits that enhance competitive ability in the introduced range occur, and these are beneficial under the novel selective scenario (Bossdorf et al. 2005), further studies warrant genetic variation and selection. Such evidence will help us to better understand the adaptive evolutionary change in the introduced populations of D. stramonium in Spain.
Sources of Funding
This research was funded by the Universidad Autónoma Metropolitana-Iztapalapa, the Universidad de Sevilla and the PAPIIT UNAM grant IN212214.
Contributions by the Authors
P.L.V., J.A. and J.N-F. designed the study. P.L.V., J.A. and R.P-B. carried out the field work. P.L.V., J.N-F., A.C., G.C. and R.T-L. performed the statistical analyses. P.L.V., J.A. and J.N-F. drafted the manuscript. All authors read and approved the manuscript.
Conflict of Interest Statement
None declared.
Acknowledgments
We thank Alejandra de Castro and Jose Ruiz for field assistance and C. A. Antonietty for the identification of insects. The field survey was carried out during a sabbatical leave of P.L.V. at the Universidad de Sevilla, Spain. We thank the Universidad de Sevilla for logistic support.
Literature Cited
- Agrawal AA, Kotanen PM, Mitchell CE, Power AG, Godsoe W, Klironomos J. 2005. Enemy release? An experiment with congeneric plant pairs and diverse above- and belowground enemies. Ecology 86:2979–2989. 10.1890/05-0219 [DOI] [Google Scholar]
- Avery AG, Satina S, Rietsema J. 1959. Blakeslee: the genus Datura, Chronica Botánica. New York: Ronald Press Co. [Google Scholar]
- Bello-Bedoy R, Núñez-Farfán J. 2010. Cost of inbreeding in resistance to herbivores in Datura stramonium. Annals of Botany 105:747–753. 10.1093/aob/mcq038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello-Bedoy R, Cruz LL, Núñez-Farfán J. 2011. Inbreeding alters a plant-predispersal seed predator interaction. Evolutionary Ecology 25:815–829. 10.1007/s10682-010-9448-4 [DOI] [Google Scholar]
- Bertin RI. 1988. Paternity in plants. In: Lovett-Doust J, Lovett-Doust L, eds. Plant reproductive ecology: patterns and strategies. New York: Oxford University Press, 30–59. [Google Scholar]
- Blaisdell GK, Roy BA. 2014. Two tests of enemy release of commonly co-occurring bunchgrasses native in Europe and introduced in the United States. Biological Invasions 16:833–842. 10.1007/s10530-013-0541-9 [DOI] [Google Scholar]
- Blossey B, Notzold R. 1995. Evolution of increased competitive ability in invasive nonindigenous plants: a hypothesis. Journal of Ecology 83:887–889. 10.2307/2261425 [DOI] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. 2009. Introduction to meta-analysis. Chichester: Wiley. [Google Scholar]
- Bossdorf O, Auge H, Lafuma L, Rogers WE, Siemann E, Prati D. 2005. Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia 144:1–11. 10.1007/s00442-005-0070-z [DOI] [PubMed] [Google Scholar]
- Cabrales-Vargas RA. 1991. Demografía e historia natural de Datura stramonium L. en el Pedregal de San Ángel con algunas implicaciones evolutivas. BSc Thesis, Universidad Nacional Autónoma de México, Distrito Federal, p. 118. [Google Scholar]
- Cahill JF, Kembel SW, Lamb EG, Keddy PA. 2008. Does phylogenetic relatedness influence the strength of competition among vascular plants? Perspectives in Plant Ecology, Evolution and Systematics 10:41–50. 10.1016/j.ppees.2007.10.001 [DOI] [Google Scholar]
- Carmona D, Fornoni J. 2013. Herbivores can select for mixed defensive strategies in plants. New Phytologist 197:576–585. [DOI] [PubMed] [Google Scholar]
- Castillo G, Cruz LL, Hernández-Cumplido J, Oyama K, Flores-Ortiz CM, Fornoni J, Valverde PL, Núñez-Farfán J. 2013. Geographic association and temporal variation of chemical and physical defense and leaf damage in Datura stramonium. Ecological Research 28:663–672. 10.1007/s11284-013-1059-4 [DOI] [Google Scholar]
- Castillo G, Cruz LL, Tapia-López R, Olmedo-Vicente E, Carmona D, Anaya-Lang AL, Fornoni J, Andraca-Gómez G, Valverde PL, Núñez-Farfán J. 2014. Selection mosaic exerted by specialist and generalist herbivores on chemical and physical defense of Datura stramonium. PLoS ONE 9:e102478 10.1371/journal.pone.0102478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth D, Charlesworth B. 1981. Allocation of resources to male and female functions in hermaphrodites. Biological Journal of the Linnean Society 15:57–74. 10.1111/j.1095-8312.1981.tb00748.x [DOI] [Google Scholar]
- Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ. 2004. Is invasion success explained by the enemy release hypothesis? Ecology Letters 7:721–733. 10.1111/j.1461-0248.2004.00616.x [DOI] [Google Scholar]
- Crawley MJ. 1987. What makes a community invasible? In: Gray AJ, Crawley MJ, Edwards PJ, eds. Colonization, succession and stability. London: Blackwell, 429–453. [Google Scholar]
- Dana-Sánchez ED, Sobrino-Vespertino E, Sanz-Elorza M. 2004. Plantas invasoras en España: un nuevo problema en las estrategias de conservación. In: Bañares A, Blancas G, Güemes J, Moreno JC, Ortiz S, eds. Atlas y libro rojo de la flora vascular amenazada de España. Madrid: Dirección General de la Naturaleza, 1011–1029. [Google Scholar]
- Dawson W, Bottini A, Fischer M, van Kleunen M, Knop E. 2014. Little evidence for release from herbivores as a driver of plant invasiveness from a multi-species herbivore-removal experiment. Oikos 123:1509–1518. [Google Scholar]
- Drake JA, Mooney HA, di Castri F, Groves RH, Kruger FJ, Rejmánek M, Williamson M. 1989. Biological invasions: a global perspective. New York: Wiley and Sons. [Google Scholar]
- Elton CS. 1958. The ecology of invasions by animals and plants. London: Methuen. [Google Scholar]
- Fitter AH, Hay RKM. 1987. Environmental physiology of plants. 2nd edn London: Academic Press. [Google Scholar]
- Fornoni J, Valverde PL, Núñez-Farfán J. 2003. Quantitative genetics of plant tolerance and resistance against natural enemies of two natural populations of Datura stramonium. Evolutionary Ecology Research 5:1049–1065. [Google Scholar]
- Fornoni J, Valverde PL, Núñez-Farfán J. 2004. Population variation in the cost and benefit of tolerance and resistance against herbivory in Datura stramonium. Evolution 58:1696–1704. 10.1111/j.0014-3820.2004.tb00455.x [DOI] [PubMed] [Google Scholar]
- Genton BJ, Shykoff JA, Giraud T. 2005. High genetic diversity in French invasive populations of common ragweed, Ambrosia artemisiifolia, as a result of multiple sources of introduction. Molecular Ecology 14:4275–4285. 10.1111/j.1365-294X.2005.02750.x [DOI] [PubMed] [Google Scholar]
- Groves R. 1991. A short history of biological invasions of Australia. In: Groves R, di Castri F, eds. Biogeography of Mediterranean invasions. Cambridge: Cambridge University Press, 59–63. [Google Scholar]
- Groves RH. 1986. Invasions of Mediterranean ecosystems by weeds. In: Dell B, Hopkins AJ, Lamont BB, eds. Resilience in Mediterranean-type ecosystems. The Hague: Junk, 129–145. [Google Scholar]
- Handley R, Ekbom B, Ågren J. 2005. Variation in trichome density and resistance against a specialist insect herbivore in natural populations of Arabidopsis thaliana. Ecological Entomology 30:284–292. 10.1111/j.0307-6946.2005.00699.x [DOI] [Google Scholar]
- Hierro JL, Maron JL, Callaway RM. 2005. A biogeographical approach to plant invasions: the importance of studying exotics in their introduced and native range. Journal of Ecology 93:5–15. 10.1111/j.0022-0477.2004.00953.x [DOI] [Google Scholar]
- Holeski LM. 2007. Within and between generation phenotypic plasticity in trichome density of Mimulus guttatus. Journal of Evolutionary Biology 20:2092–2100. 10.1111/j.1420-9101.2007.01434.x [DOI] [PubMed] [Google Scholar]
- Huberty M, Tielbörger K, Harvey JA, Müller C, Macel M. 2014. Chemical defenses (Glucosinolates) of native and invasive populations of the range expanding invasive plant Rorippa austriaca. Journal of Chemical Ecology 40:363–370. 10.1007/s10886-014-0425-1 [DOI] [PubMed] [Google Scholar]
- Jakobs G, Weber E, Edwards PJ. 2004. Introduced plants of the invasive Solidago gigantea (Asteraceae) are larger and grow denser than conspecifics in the native range. Diversity and Distributions 10:11–19. 10.1111/j.1472-4642.2004.00052.x [DOI] [Google Scholar]
- Jiao M, Luna-Cavazos M, Bye R. 2002. Allozyme variation in Mexican species and classification of Datura (Solanaceae). Plant Systematics and Evolution 232:155–166. 10.1007/s006060200039 [DOI] [Google Scholar]
- Johnson B. 1975. Plant pubescence: an ecological perspective. Botanical Review 41:233–258. 10.1007/BF02860838 [DOI] [Google Scholar]
- Joshi J, Vrieling K. 2005. The enemy release and EICA hypothesis revisited: incorporating the fundamental difference between specialist and generalist herbivores. Ecology Letters 8:704–714. 10.1111/j.1461-0248.2005.00769.x [DOI] [Google Scholar]
- Kambo D, Kotanen PM. 2014. Latitudinal trends in herbivory and performance of an invasive species, common burdock (Arctium minus). Biological Invasions 16:101–112. 10.1007/s10530-013-0506-z [DOI] [Google Scholar]
- Kariñho-Betancourt E, Núñez-Farfán J. 2015. Evolution of resistance and tolerance to herbivores: testing the trade-off hypothesis. PeerJ 3:e789 10.7717/peerj.789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane RM, Crawley MJ. 2002. Exotic plant invasions and the enemy release hypothesis. Trends in Ecology and Evolution 17:164–170. 10.1016/S0169-5347(02)02499-0 [DOI] [Google Scholar]
- Kelly CA. 1992. Spatial and temporal variation in selection on correlated life-history traits and plant size in Chamaecrista fasciculata. Evolution 46:1658–1673. 10.2307/2410022 [DOI] [PubMed] [Google Scholar]
- Koricheva J. 2002. Meta-analysis of sources of variation in fitness costs of plant antiherbivore defenses. Ecology 83:176–190. 10.1890/0012-9658(2002)083[0176:MAOSOV]2.0.CO;2 [DOI] [Google Scholar]
- Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37:1210–1226. 10.2307/2408842 [DOI] [PubMed] [Google Scholar]
- Larcher W. 2001. Physiological plant ecology. Berlin: Springer. [Google Scholar]
- Leimu R, Koricheva J. 2006. A meta-analysis of tradeoffs between plant tolerance and resistance to herbivores: combining the evidence from ecological and agricultural studies. Oikos 112:1–9. 10.1111/j.0030-1299.2006.41023.x [DOI] [Google Scholar]
- Levin DA. 1973. The role of trichomes in plant defense. The Quarterly Review of Biology 48:3–15. 10.1086/407484 [DOI] [Google Scholar]
- Liu H, Stiling P. 2006. Testing the enemy release hypothesis: a review and meta-analysis. Biological Invasions 8:1535–1545. 10.1007/s10530-005-5845-y [DOI] [Google Scholar]
- Maron JL, Vilà M. 2001. When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos 95:361–373. 10.1034/j.1600-0706.2001.950301.x [DOI] [Google Scholar]
- Maron JL, Vilà M, Bommarco R, Elmendorf S, Beardsley P. 2004. Rapid evolution of an invasive plant. Ecological Monographs 74:261–280. 10.1890/03-4027 [DOI] [Google Scholar]
- Mata L, Grosso-Silva JM, Goula M. 2013. Pyrrhocoridae from the Iberian Peninsula (Hemiptera: Heteroptera). Heteropterus Revista de Entomología 13:175–189. [Google Scholar]
- Mauricio R, Rausher MD. 1997. Experimental manipulation of putative selective agents provides evidence for the role of natural enemies in the evolution of plant defense. Evolution 51:1435–1444. 10.2307/2411196 [DOI] [PubMed] [Google Scholar]
- Memmott J, Fowler SV, Paynter Q, Sheppard AW, Syrett P. 2000. The invertebrate fauna on broom, Cytisus scoparius, in two native and two exotic habitats. Acta Oecologica 21:213–222. 10.1016/S1146-609X(00)00124-7 [DOI] [Google Scholar]
- Mitchell CE, Agrawal AA, Bever JD, Gilbert GS, Hufbauer RA, Klironomos JN, Maron JL, Morris WF, Parker IM, Power AG, Seabloom EW, Torchin ME, Vázquez DP. 2006. Biotic interactions and plant invasions. Ecology Letters 9:726–740. 10.1111/j.1461-0248.2006.00908.x [DOI] [PubMed] [Google Scholar]
- Moles AT, Gruber MAM, Bonser SP. 2008. A new framework for predicting invasive plant species. Journal of Ecology 96:13–17. [Google Scholar]
- Motten AF, Antonovics J. 1992. Determinants of outcrossing rate in a predominantly self-fertilizing weed, Datura stramonium (Solanaceae). American Journal of Botany 79:419–427. 10.2307/2445154 [DOI] [PubMed] [Google Scholar]
- Motten AF, Stone JL. 2000. Heritability of stigma position and the effect of stigma-anther separation on outcrossing in a predominantly self-fertilizing weed, Datura stramonium (Solanaceae). American Journal of Botany 87:339–347. 10.2307/2656629 [DOI] [PubMed] [Google Scholar]
- Ninyerola M, Pons X, Roure JM. 2005. Atlas Climático Digital de la Península Ibérica. Metodología y aplicaciones en bioclimatología y geobotánica. Bellaterra: Universidad Autónoma de Barcelona. [Google Scholar]
- Núñez-Farfán J. 1991. Biología evolutiva de Datura stramonium L. en el Centro de México: selección natural de la resistencia a los herbívoros, sistema de cruzamiento y variación genética intra e inter-poblacional. PhD Thesis Mexico D. F: Universidad Nacional Autonóma de México. [Google Scholar]
- Núñez-Farfán J, Dirzo R. 1994. Evolutionary ecology of Datura stramonium L. in central Mexico: natural selection for resistance to herbivorous insects. Evolution 48:423–436. [DOI] [PubMed] [Google Scholar]
- Núñez-Farfán J, Cabrales-Vargas RA, Dirzo R. 1996. Mating system consequences on resistance to herbivory and life history traits in Datura stramonium. American Journal of Botany 83:1041–1049. 10.2307/2445993 [DOI] [Google Scholar]
- Pimentel D, Lach L, Zuniga R, Morrison D. 2000. Environmental and economic costs of nonindigenous species in the United States. BioScience 50:53–65. 10.1641/0006-3568(2000)050[0053:EAECON]2.3.CO;2 [DOI] [Google Scholar]
- Prentis PJ, Wilson JRU, Dormontt EE, Richardson DM, Lowe AJ. 2008. Adaptive evolution in invasive species. Trends in Plant Science 13:288–294. 10.1016/j.tplants.2008.03.004 [DOI] [PubMed] [Google Scholar]
- Purrington CB. 2000. Costs of resistance. Current Opinion in Plant Biology 3:305–308. 10.1016/S1369-5266(00)00085-6 [DOI] [PubMed] [Google Scholar]
- R Development Core Team. 2011. R: a language and environment for statistical computing. 2.15.2 edn Vienna, Austria: R Foundation for Statistical Software. [Google Scholar]
- Ribes J, Espadaler X, Piñol J. 2004. Heterópteros de un cultivo ecológico de cítricos de Tarragona (Cataluña, NE España) (Hemiptera: Heteroptera). Orsis 19:21–35. [Google Scholar]
- Rodriguez E, Healy PL, Mehta I, eds. 1984. Biology and chemistry of plant trichomes. New York: Plenum. [Google Scholar]
- Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O'Neil P, Parker IM, Thompson JN, Weller SG. 2001. The population biology of invasive species. Annual Review of Ecology and Systematics 32:305–332. 10.1146/annurev.ecolsys.32.081501.114037 [DOI] [Google Scholar]
- Sala OE, Chapin FS III, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, LeRoy Poff N, Sykes MT, Walker BH, Walker M, Wall DH. 2000. Global biodiversity scenarios for the year 2100. Science 287:1770–1774. 10.1126/science.287.5459.1770 [DOI] [PubMed] [Google Scholar]
- Sanz-Elorza M, Dana-Sánchez ED, Sobrino-Vespertinas E, eds. 2004. Atlas de las plantas alóctonas invasoras en España. Madrid: Dirección General para la Biodiversidad. [Google Scholar]
- Sax DF, Brown JH. 2000. The paradox of invasion. Global Ecology and Biogeography 9:363–371. 10.1046/j.1365-2699.2000.00217.x [DOI] [Google Scholar]
- Shonle I, Bergelson J. 2000. Evolutionary ecology of the tropane alkaloids of Datura stramonium L. (Solanaceae). Evolution 54:778–788. 10.1111/j.0014-3820.2000.tb00079.x [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. 2012. Biometry. 4th edn New York: Freeman and Company. [Google Scholar]
- Strauss SY, Rudgers JA, Lau JA, Irwin RE. 2002. Direct and ecological costs of resistance to herbivory. Trends in Ecology and Evolution 17:278–285. 10.1016/S0169-5347(02)02483-7 [DOI] [Google Scholar]
- Symon D, Haegi L. 1991. Datura (Solanaceae) is a new world genus. In: Hawkes JG, Lester RN, Nee M, Estrada N, eds. Solanaceae. III. Taxonomy, chemistry, and evolution. Kew: Royal Botanic Gardens Press, 197–210. [Google Scholar]
- Thelen GC, Vivanco JM, Newingham B, Good W, Bais HP, Landres P, Caesar A, Callaway RM. 2005. Insect herbivory stimulates allelopathic exudation by an invasive plant and the suppression of natives. Ecology Letters 8:209–217. 10.1111/j.1461-0248.2004.00713.x [DOI] [Google Scholar]
- Torchin ME, Mitchell CE. 2004. Parasites, pathogens, and invasions by plants and animals. Frontiers in Ecology and the Environment 2:183–190. 10.1890/1540-9295(2004)002[0183:PPAIBP]2.0.CO;2 [DOI] [Google Scholar]
- Torres-Vila LM, Rodríguez-Molina MC, Palo E, Bielza P, Lacasa A. 2000. La resistencia a insecticidas de Helicoverpa armigera Hübner en España. Boletín de Sanidad Vegetal Plagas 26:493–501. [Google Scholar]
- Torres-Vila LM, Rodrıguez-Molina MC, Lacasa-Plasencia A, Bielza-Lino P, Rodríguez-del-Rincón Á. 2002. Pyrethroid resistance of Helicoverpa armigera in Spain: current status and agroecological perspective. Agriculture, Ecosystems and Environment 93:55–66. 10.1016/S0167-8809(02)00003-8 [DOI] [Google Scholar]
- Turner NC, Kramer PJ. 1980. Adaptations of plants to water and high temperature stress. New York: Wiley-Interscience. [Google Scholar]
- Valverde PL, Fornoni J, Núñez-Farfán J. 2001. Defensive role of leaf trichomes in resistance to herbivorous insects in Datura stramonium. Journal of Evolutionary Biology 14:424–432. 10.1046/j.1420-9101.2001.00295.x [DOI] [Google Scholar]
- Valverde PL, Fornoni J, Núñez-Farfán J. 2003. Evolutionary ecology of Datura stramonium: equal plant fitness benefits of growth and resistance against herbivory. Journal of Evolutionary Biology 16:127–137. 10.1046/j.1420-9101.2003.00482.x [DOI] [PubMed] [Google Scholar]
- van Kleunen M, Johnson SD. 2007. Effects of self-compatibility on the distribution range of invasive European plants in North America. Conservation Biology 21:1537–1544. [DOI] [PubMed] [Google Scholar]
- van Kleunen M, Schmid B. 2003. No evidence for an evolutionary increased competitive ability in an invasive plant. Ecology 84:2816–2823. 10.1890/02-0494 [DOI] [Google Scholar]
- van Kleunen M, Fischer M, Johnson SD. 2007. Reproductive assurance through self-fertilization does not vary with population size in the alien invasive plant Datura stramonium. Oikos 116:1400–1412. 10.1111/j.0030-1299.2007.16004.x [DOI] [Google Scholar]
- Viechtbauer W. 2010. Conducting meta-analyses in R with the metaphor package. Journal of Statistical Software 36:1–48. [Google Scholar]
- Vilà M, Maron JL, Marco L. 2005. Evidence for the enemy release hypothesis in Hypericum perforatum. Oecologia 142:474–479. 10.1007/s00442-004-1731-z [DOI] [PubMed] [Google Scholar]
- Vitousek PM, D'Antonio CM, Loope LL, Westbrooks R. 1996. Biological invasions as global environmental change. American Scientist 84:218–28. [Google Scholar]
- Weaver SE, Warwick SI. 1984. The biology of Canadian weeds: 64. Datura stramonium L. Canadian Journal of Plant Science 64:979–991. 10.4141/cjps84-132 [DOI] [Google Scholar]
- Wolfe LM. 2002. Why alien invaders succeed: support for the escape-from-enemy hypothesis. The American Naturalist 160:705–711. 10.1086/343872 [DOI] [PubMed] [Google Scholar]
- Wolfe LM, Elzinga JA, Biere A. 2004. Increased susceptibility to enemies following introduction in the invasive plant Silene latifolia. Ecology Letters 7:813–820. 10.1111/j.1461-0248.2004.00649.x [DOI] [Google Scholar]