Abstract
Purpose
Endoglin, an endothelial cell membrane receptor expressed on angiogenic tumor vessels, is essential for angiogenesis and upregulated in the setting of VEGF inhibition. TRC105 is an anti-endoglin IgG1 monoclonal antibody that potentiates VEGF inhibitors in preclinical models. This study assessed safety, pharmacokinetics, and anti-tumor activity of TRC105 in combination with bevacizumab.
Patients and Methods
Patients (n=38) with advanced solid tumors, Eastern Cooperative Group performance status 0–1, and normal organ function were treated with escalating doses of TRC105 plus bevacizumab until disease progression or unacceptable toxicity using a standard 3 + 3 phase 1 design.
Results
TRC105 and bevacizumab were well tolerated at their recommended single agent doses (10 mg/kg) when the initial dose of TRC105 was delayed by one week and divided over two days to limit the frequency of headache. The concurrent administration of bevacizumab and TRC105 did not otherwise potentiate known toxicities of TRC105 or bevacizumab. Hypertension and proteinuria were observed, though not at rates expected for single agent bevacizumab. Several patients who had previously progressed on bevacizumab or VEGF receptor tyrosine kinase inhibitor (VEGFR TKI) treatment experienced reductions in tumor volume, including two partial responses by RECIST, and six remained without progression for longer periods than during their prior VEGF inhibitor therapy.
Conclusion
TRC105 was well tolerated with bevacizumab and clinical activity was observed in a VEGF inhibitor refractory population. Ongoing clinical trials are testing TRC105 in combination with bevacizumab in glioblastoma, and with VEGFR TKIs in renal cell carcinoma, hepatocellular carcinoma, and soft tissue sarcoma.
Keywords: Endoglin, CD105, TRC105, Vascular endothelial growth factor, Antibody
INTRODUCTION
Angiogenesis is a complex process that is regulated by multiple pathways (1). Approved antiangiogenic drugs, including bevacizumab, sorafenib, sunitinib, and pazopanib, primarily target the vascular endothelial growth factor (VEGF) signaling pathway and are associated with modest survival advantages in select indications (2–7). Inhibition of complementary, non-VEGF angiogenic pathways is a strategy that may improve antitumor activity and address resistance to anti-VEGF therapies.
Endoglin (CD105) is a homodimeric TGF-β coreceptor expressed on proliferating vascular endothelium in solid tumors (8, 9). Endoglin is selectively expressed at high density on proliferating endothelial cells and is up-regulated by hypoxia through the induction of hypoxia-inducible factor-1-α (HIF-1-α) (8, 10). Endoglin expression is also up-regulated on tumor endothelial cells in response to inhibitors of the VEGF pathway and allows continued tumor growth (11, 12). Loss of endoglin expression reverses resistance to large and small molecule inhibitors of the VEGF pathway (13).
Endoglin is essential for normal vascular development, (14) and loss of endoglin expression is associated with the Osler-Webber-Rendu syndrome, a disease characterized by ectatic blood vessel formation that is associated with improved cancer survival, suggesting that targeting endoglin may have beneficial clinical effects (15, 16). In patients with solid tumors, high tumor microvessel density as assessed by endoglin immunohistochemistry has been correlated with poor prognosis (17–30).
TRC105 (TRACON Pharmaceuticals, Inc.) is a chimeric IgG1 antibody that binds human endoglin with high avidity, induces antibody-dependent cellular cytotoxicity (ADCC) and apoptosis of human vascular endothelial cells (HUVECs) and endoglin-positive tumor cells (8), and inhibits angiogenesis in response to VEGF and fibroblast growth factor (31). TRC105 potentiates bevacizumab in preclinical models of angiogenesis and was well tolerated at 10 mg/kg every week and 15 mg/kg every 2 weeks as a single agent in a Phase 1 trial, with a safety profile that was distinct from that of VEGF inhibitors, that included the distinct lack of hypertension and proteinuria (32).
Here we report the results of an open label phase 1 clinical study that assessed the safety, tolerability, pharmacokinetics (PK), and antitumor activity of TRC105 when given concurrently with bevacizumab to adult patients with advanced refractory solid tumors.
PATIENTS AND METHODS
Patient Eligibility
Patients with histologically proven advanced or metastatic solid cancer for which curative therapy was unavailable were eligible for this trial. Further inclusion criteria were an Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate organ function as demonstrated by an absolute neutrophil count ≥1,500 cells/μL, hemoglobin ≥ 9 g/dL, platelets ≥100,000/μL, prothrombin time or international normalized ratio within normal limits, creatinine ≤1.5 times the upper limit of normal (ULN), bilirubin ≤1.5 mg/dL, and aspartate and alanine transaminases ≤2.5 times the ULN (or ≤ 5 times the ULN in patients with liver metastases). Exclusion criteria included a known history of central nervous system metastases, lung cancer with a central chest lesion, thromboembolic disease, clinically significant ascites or pleural effusions, uncontrolled hypertension, required anticoagulation, and cancer therapy within 4 weeks prior to study entry. Additional exclusion criteria were a history of hemorrhage or unhealed surgical wounds within 30 days of study entry or were pregnant or lactating. All patients signed an institutional review board (IRB)-approved informed consent form prior to undertaking study-related procedures. The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice (ICH GCP) guidelines and all applicable local regulatory requirements and laws.
Study Design and Treatments
This was a multicenter, open-label, nonrandomized, phase 1b, dose-finding study of TRC105 in combination with bevacizumab in patients with advanced or metastatic solid cancer for whom curative therapy was unavailable (NCT01332721). TRC105 dose was escalated in serial cohorts of patients with a fixed dose of bevacizumab using a standard 3 + 3 design. Dose-limiting toxicity was defined as any grade ≥ 3 hematologic or nonhematologic adverse event related to TRC105. Intrapatient dose escalation was not permitted. Patients were allowed to dose reduce and continue treatment for adverse events that resolved to grade 1 or baseline, and were allowed to discontinue bevacizumab and interrupt TRC105 dosing for up to 6 weeks, following the dose-limiting toxicity evaluation period. Dose expansion was planned at the top dose level.
Escalating doses of intravenous (IV) TRC105 were administered weekly beginning with Dose Level 1 in combination with bevacizumab (Table 1). Patients received bevacizumab 15 mg/kg every three weeks in combination with TRC105 weekly in cohorts 1 and 2. Patients received bevacizumab 10 mg/kg on days 1 and 15 of each 28 day cycle and received TRC105 weekly starting on day 8 in cohorts 3 and 4. Patients received bevacizumab 10 mg/kg on days 1 and 15 of each 28 day cycle and received TRC105 on days 8 (3 mg/kg), 11 (balance of the weekly dose), 15 (initial full weekly dose) and weekly thereafter in cohorts 5 and 6.
Table 1.
Dose Levels
| Cohort | TRC105 | Bevacizumab | Cycle Duration | DLT (G3 headache) |
|---|---|---|---|---|
| 1 | 3 mg/kg (weekly) | 15 mg/kg (every 3 weeks) | 3 weeks | 0/3 |
| 2 | 6 mg/kg (weekly) | 15 mg/kg (every 3 weeks) | 3 weeks | 2/5 |
| 3 | 6 mg/kg (weekly starting on day 8) | 10 mg/kg (every 2 weeks) | 4 weeks | 0/4 |
| 4 | 8 mg/kg (weekly starting on day 8) | 10 mg/kg (every 2 weeks) | 4 weeks | 1/4 |
| 5 | 8 mg/kg (weekly starting on day 8 with the initial TRC105 dose divided over two 2 days) | 10 mg/kg (every 2 weeks) | 4 weeks | 0/3 |
| 6 | 10 mg/kg (weekly starting on day 8 with the initial TRC105 dose divided over two 2 days) | 10 mg/kg (every 2 weeks) | 4 weeks | 1/19 |
TRC105 was manufactured in Chinese hamster ovary cells and supplied as a phosphate buffered saline solution in single-use glass vials for IV administration. Prior to infusion, the agent was diluted in normal saline and infused using an in-line 0.2 micron low protein binding filter. Premedication regimen included acetaminophen 650 mg, diphenhydramine 50 mg (or similar H1 receptor antagonist), famotidine 20 mg (or similar H2 receptor antagonist), and dexamethasone 20 mg. The dexamethasone dose was tapered and discontinued as tolerated.
Safety Assessments
Safety was evaluated at baseline, at regular intervals during treatment, and for 28 days after completing study therapy. Safety assessments included vital signs before, during and after every infusion, and, physical examination performed every 4 weeks. Hematologic parameters were assessed weekly during the initial two cycles of treatment and then every 4 weeks. Serum chemistry was analyzed weekly during the initial cycle and every 4 weeks thereafter. Urinalysis was performed prior to dosing and every 4 weeks thereafter. Coagulation parameters were assessed every 4 weeks during the initial two cycles of treatment. Adverse events were graded using National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Pharmacokinetics and Immunogenicity
Peak (sampled within 5 minutes prior to the completion of TRC105 infusion) and trough (sampled immediately prior to subsequent dosing with TRC105) serum samples for assessment of TRC105 serum concentrations were collected on the first day of dosing (including both dosing days when the initial dose of TRC105 was administered in divided doses on cycle 1 day 8 and cycle 1 day 11), on the third week (cycle 1 day 15), in cycle 2 (cycle 2 day 1), at end of study and approximately 4 weeks after the last dose of TRC105. TRC105 concentration was determined using a validated enzyme-linked immunosorbent assay (ELISA) with a limit of quantitation of 78 ng/mL (33).
Serum for assessment of human antibody formation to the murine portion of TRC105 (human anti-murine antibody, HAMA) and human portion of TRC105 (human anti-chimeric antibody, HACA) was collected prior to dosing, 4 weeks thereafter, at end of study and approximately 4 weeks following the last dose of TRC105. HAMA and HACA concentrations were determined by validated ELISAs.
Evaluation of Tumor Response
Tumor responses were evaluated using CT or MRI per Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1) (34) and Choi criteria (35). Evaluations were performed at 2 month intervals or earlier if disease progression was suspected. Serum tumor markers as appropriate for the tumor type were assessed at baseline and monthly thereafter.
Statistical Analysis
The safety population included all patients who received at least a portion of the initial TRC105 infusion. The evaluable population for determination of response included all patients with a baseline and a follow-up radiographic assessment for response at designated time points (e.g., 2 months and 4 months). Descriptive statistics (means, medians, standard deviations and ranges for continuous data and percentages for categorical data) were used to summarize patient characteristics, treatment administration, safety, efficacy, and pharmacokinetic parameters.
RESULTS
Maximum Tolerated Dose, Dose-Limiting Toxicity, and Disposition
Between May 5, 2011 and May 8, 2013, 38 patients with advanced or metastatic solid tumors were enrolled at four sites in the United States and treated with escalating doses of TRC105 at 3, 6, 8, or 10 mg/kg. TRC105 and bevacizumab were studied over 6 cohorts (Table 1). During dose escalation, the initial starting dose and schedule of bevacizumab of 15 mg/kg every three weeks was modified to 10mg/kg every two weeks and the schedule of TRC105 was modified to divide the initial TRC105 dose over two days beginning one week following initial bevacizumab dosing, to reduce the frequency of headache. Headache was the only DLT reported and was significantly mitigated by administering the first dose of TRC105 one week following the initial bevacizumab 10 mg/kg dose and dividing the initial TRC105 dose over two days. Headaches were not associated with hypertension, focal neurologic signs, or radiographic abnormalities in those patients in whom imaging was performed. Demographics of enrolled patients are presented in Table 2.
Table 2.
Baseline Patient Characteristics (N=38)
| Age | Median: 63 |
| Range: 42 – 82 | |
|
| |
| Gender | Female: 23 |
| Male: 15 | |
|
| |
| Baseline ECOG Performance Status | ECOG PS 0: 14 |
| ECOG PS 1: 24 | |
|
| |
| Number of Prior Regimens | Median: 5 |
| Range: 0 – 10 | |
|
| |
| Prior Anti-VEGF Therapy | Yes: 30 |
| No: 8 | |
|
| |
| Cancer Type | Colorectal: 17 |
| Ovarian: 11 | |
| Hepatocellular: 2 | |
| Renal: 2 | |
| Lung: 2 | |
| Cervical: 1 | |
| Endometrial: 1 | |
| Esthesioneuroblastoma: 1 | |
| Peritoneal: 1 | |
Nineteen patients received treatment at the recommended single agent doses of both drugs following dose level expansion, which was the recommended phase 2 dose of the combination of TRC105 and bevacizumab, and consisted of 10 mg/kg bevacizumab every 2 weeks beginning on cycle 1 day 1 and TRC105 10 mg/kg weekly beginning on cycle 1 day 8 with the first TRC105 dose divided such that 3 mg/kg was given on cycle 1 day 8 and 7 mg/kg was given on cycle 1 day 11. Twelve patients progressed based on radiographic or clinical criteria. One patient discontinued treatment for grade 3 pulmonary embolism detected on CT scan and considered related to bevacizumab, and one patient discontinued treatment for MRSA sepsis considered unrelated to study treatment. Two patients withdrew secondary to fatigue. Three patients demonstrated clinical benefit throughout the study (including two patients with partial responses by RECIST) and continued treatment on a separate continuation protocol.
Overall, all doses of both drugs were administered as planned to thirty-four patients (90%) for at least one cycle, 28 patients (74%) for at least two cycles, 18 patients (47%) for at least three cycles, 14 patients (37%) for at least 4 cycles, 10 patients (26%) for at least 5 cycles, 7 patients (18%) for at least 6 cycles, 3 patients (8%) for at least 10 cycles, and two patients for greater than 19 cycles. The median and mean number of doses of TRC105 received across all dose levels were 9 and 14, respectively. The median and mean number of doses of bevacizumab received at the 15 mg/kg and 10 mg/kg dose levels were 3 and 4.2 and 5.5 and 8.4, respectively.
Safety and Tolerability
A total of 38 patients received TRC105 and bevacizumab on study across six cohorts and four dose levels. The incidence of headache was mitigated by adjusting the dosing schedule of TRC105 and dose reduction of bevacizumab to 10 mg/kg. At the 10 mg/kg dosing of both agents, the combination of TRC105 and bevacizumab was well tolerated (Table 3: related AEs). Most adverse events were graded as 1 or 2 and grade 4 and 5 suspected adverse reactions were not observed. Grade 3 suspected adverse reactions included anemia (the dose limiting toxicity of TRC105 established as a single agent; 9 patients), headache (4 patients; three of which occurred prior to adjusting the schedule of TRC105), and fatigue (2 patients). Headache was the most common suspected adverse reaction and was treated with 5-hydroxytryptophan antagonists and non-steroidal anti-inflammatory drugs. Headaches generally started the evening following initial dosing of both agents and tended to reduce in frequency and intensity with continued treatment.
Table 3.
Most Common (n > 1) and all Grade 3 and Above TRC105 Drug-related Adverse Events.
| Adverse Event | TRC105 | ||||||
|---|---|---|---|---|---|---|---|
| Maximum Grade | Total N = 38 | ||||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | n | Percent | |
| Headache | 14 | 14 | 4 | 0 | 0 | 32 | (84.2%) |
| Epistaxis | 22 | 3 | 0 | 0 | 0 | 25 | (65.8%) |
| Telangiectasia | 17 | 2 | 0 | 0 | 0 | 19 | (50.0%) |
| Gingival bleeding | 11 | 1 | 0 | 0 | 0 | 12 | (31.6%) |
| Anemia | 0 | 3 | 8 | 0 | 0 | 11 | (28.9%) |
| Infusion reaction | 0 | 10 | 1 | 0 | 0 | 11 | (28.9%) |
| Fatigue | 1 | 7 | 2 | 0 | 0 | 10 | (26.3%) |
| Flushing | 7 | 1 | 0 | 0 | 0 | 8 | (21.1%) |
| Decreased appetite | 0 | 3 | 1 | 0 | 0 | 4 | (10.5%) |
| Facial edema | 0 | 4 | 0 | 0 | 0 | 4 | (10.5%) |
| Gingival pain | 4 | 0 | 0 | 0 | 0 | 4 | (10.5%) |
| Nasal congestion | 2 | 1 | 0 | 0 | 0 | 3 | (7.9%) |
| Nausea | 1 | 2 | 0 | 0 | 0 | 3 | (7.9%) |
| Oral pain | 3 | 0 | 0 | 0 | 0 | 3 | (7.9%) |
| Rash | 3 | 0 | 0 | 0 | 0 | 3 | (7.9%) |
| Dyspnea | 2 | 0 | 0 | 0 | 0 | 2 | (5.3%) |
| Hypothyroidism | 0 | 2 | 0 | 0 | 0 | 2 | (5.3%) |
| Periorbital edema | 1 | 1 | 0 | 0 | 0 | 2 | (5.3%) |
| Vomiting | 0 | 2 | 0 | 0 | 0 | 2 | (5.3%) |
| Brain abscess | 0 | 0 | 1 | 0 | 0 | 1 | (2.6%) |
Percents are computed by using the number of patients in the safety population as the denominator. Adverse Events are coded by using MedDRA dictionary version 14.1.
If more than 1 event is recorded for a patient, the patient is only counted once at the highest grade.
Two patients experienced serious adverse events considered related to TRC105. One grade 3 headache (in a patient dosed at 8 mg/kg prior to dividing the initial TRC105 dose over two days) resulted in hospitalization for analgesia and patient discontinuation. One patient with colorectal cancer dosed at 10 mg/kg of TRC105 experienced a grade 3 brain abscess requiring open drainage. Serious adverse events, considered unrelated to TRC105 treatment, included: grade 3 pneumonia and subsequent grade 4 MRSA sepsis that was complicated by a non Q-wave myocardial infarction during a period of hemodynamic instability; grade 3 ileus at the time of symptomatic disease progression; death from disease progression; grade 3 left foot cellulitis; grade 3 recurrent pneumothorax; grade 3 small bowel obstruction and grade 4 urosepsis.
Other than headache, adverse events characteristic of each individual drug were not increased in frequency or severity when the two drugs were administered together. Of note, the concurrent administration of bevacizumab and TRC105 did not potentiate known bevacizumab toxicities including hypertension, hemorrhage (including tumor-associated hemorrhage, and pulmonary hemorrhage or hemoptysis), or proteinuria. Reversible posterior leukoencephalopathy syndrome, congestive heart failure, fistulae, gastrointestinal perforation impaired wound healing, and arterial thromboembolic events were not observed.
Notably, hypertension and proteinuria, known adverse events of bevacizumab, were rarely observed when bevacizumab was given with TRC105. Mild and transient hypertension was observed in five patients (13%; grade 3 in one case prior to TRC105 study drug treatment and grade 2 in four cases) and mild transient proteinuria was observed in two patients (5%; both grade 2).
At least one sign of the triad of epistaxis, gingival bleeding and telangiectasia, reflecting vascular ectasia characteristic of the Osler-Weber-Rendu syndrome, was observed frequently. At least one of these signs or symptoms (of grade 1 or 2 severity) was noted in one of three patients treated at 3 mg/kg, four of eight patients treated at 6 mg/kg, four of eight patients treated at 8 mg/kg and in all nineteen patients treated at 10 mg/kg of TRC105, generally with onset within the first month of dosing. These reversible signs and symptoms are expected pharmacologic effects of TRC105 binding to the endoglin receptor and resemble characteristics of the Osler-Weber-Rendu syndrome. They were also observed routinely within the first month of dosing of 10 mg/kg weekly in the single agent TRC105 dose escalation study (36).
Consistent with the dose escalation trial of TRC105 as a single agent (36), infusion reactions were more notable at lower doses, and were rare at the MTD of TRC105 of 10 mg/kg, when TRC105 serum concentrations were maintained continuously. Two of nineteen patients (10%) dosed with 10 mg/kg of TRC105 each experienced a single grade 2 infusion reaction, both with the initial dose of TRC105, that required a brief interruption of the infusion prior to completion of the scheduled dose. At all dose levels, including the maximum tolerated dose level of 10 mg/kg, patients were permitted to taper the infusion duration from 4 hours to 1 hour and then taper steroid premedication, such that steroids could be permanently discontinued by cycle 2. Dexamethasone was discontinued in all but one of 16 patients dosed with 10 mg/kg TRC105 who completed two cycles of treatment.
Clinically significant hypoproliferative anemia, the DLT of TRC105 given as a single agent, was reported in 3 of 7 patients (43%; all grade 3) dosed with 8 mg/kg of TRC105, and was observed in nine of 19 (47%; three of grade 2 and six of grade 3 severity) patients dosed with 10 mg/kg of TRC105. Anemia prompted transfusion of packed red blood cells in 10 patients and growth factors (i.e., erythropoietin) were used in five patients. TRC105 dose reductions due to anemia occurred in five patients.
Pharmacokinetics and Immunogenicity
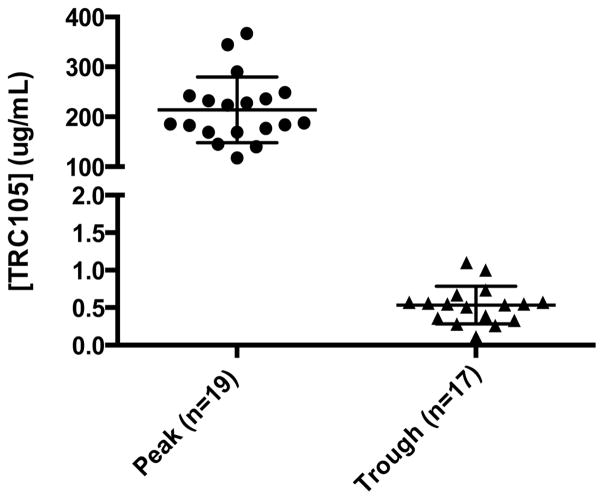
TRC105 was measurable above the target concentration that saturates endoglin receptors (0.200 ug/mL) in all patients immediately following dosing. The mean concentration immediately following dosing across all doses in a cohort (for which peak concentration data were available) was proportional to dose: 43 μg/ml (3 mg/kg), 59 μg/mL (6 mg/kg cohorts), 134 μg/mL (8 mg/kg cohorts, including only peak concentrations following dosing of undivided 8 mg/kg doses) and 214 μg/mL (10 mg/kg cohort, including only peak concentrations following dosing of undivided 10 mg/kg doses). Trough concentrations were not detected above the level of detection of 0.078μg/mL in the 3 mg/kg cohort. Trough concentrations above the target concentration of 0.200 μg/mL were detected in five of six patients dosed with 6 mg/kg of TRC105 for whom trough concentration data were available (and were undetectable in one patient who developed an infusion reaction when dosed in the absence of steroid premedication). Trough concentrations above the target concentration of 0.200 μg/mL were detected in four of six patients dosed with 8 mg/kg of TRC105 for whom trough concentration data were available (and were undetectable in two patients who were unable to discontinue steroid premedication). Trough concentrations were detected in all 18 patients dosed with 10 mg/kg for whom trough concentrations were available, and were above the target concentration of 0.200 μg/mL in 17 of 18 patients. Of the 18 patients with trough data, one patient’s trough concentration was 77 ug/mL and was excluded as a statistical outlier. The mean trough concentration following 10 mg/kg doses was 0.54 μg/mL, (Figure 1). HAMA and HACA were detected in two patients (dosed at 6 and at 8 mg/kg, respectively), within 4 weeks following dosing with TRC105, and were absent in the one patient for whom serum was available for testing at the end of study assessment.
Figure 1.
Peak and trough serum values of TRC105 at 10 mg/kg in combination with bevacizumab at 10 mg/kg every 2 weeks, assessed 2 and 6 weeks following initiation of dosing
Antitumor Activity
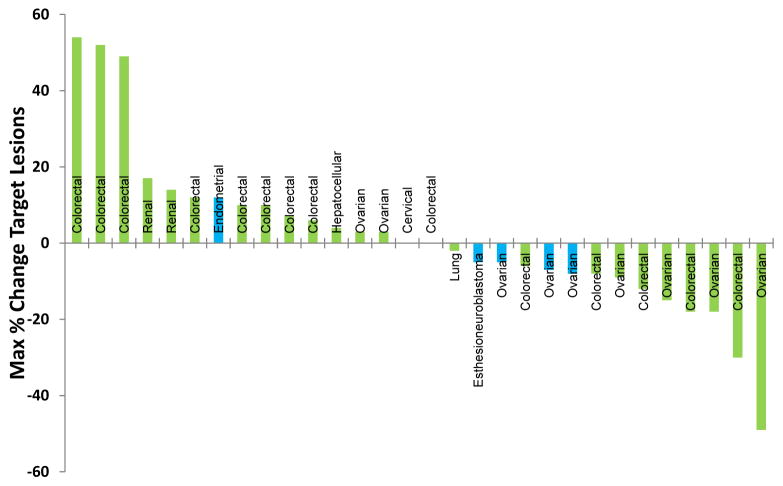
The combination of TRC105 and bevacizumab was active, including among patients with advanced refractory cancer who had progressed on prior bevacizumab or other VEGF inhibitor treatment. Of 38 patients enrolled, the majority had colorectal cancer (45%) or ovarian cancer (29%), as detailed in Figure 2. Thirty-three patients had either measurable (31 patients) or evaluable disease (2 patients) at baseline and received at least one follow up scan and were therefore evaluable for the primary outcome of overall response rate by RECIST 1.1. Eighteen patients with measurable disease (58%) had a best response of stable disease (n=16) or partial response (n=2), including 15 of 30 patients previously treated with inhibitors of the VEGF pathway. Time to progression ranged from 0 to 437+ days. Two patients (6%), both of whom had been treated with bevacizumab and chemotherapy prior to study entry, had RECIST 1.1-defined partial responses, including one patient with colorectal cancer who continues on treatment after 22 cycles and another patient with ovarian cancer who was progression free for 8 cycles. A total of 14 patients (45%) had decreases in overall tumor burden, of whom 10 had received and progressed on prior VEGF inhibitor treatment (usually bevacizumab with chemotherapy) (Figure 2). Reductions in tumor markers ranging from 5% to 85% were observed in 15 of 28 (54%) patients with relevant tumor markers.
Figure 2.
Waterfall plot of best response of measurable lesions by RECIST 1.1
Notably, the duration of treatment with TRC105 and bevacizumab in 6 patients with reductions in measurable disease (representing 19% of those patients with measurable disease) exceeded the duration of treatment of the most recent treatment regimen containing a VEGF inhibitor (i.e., VEGFR TKI or bevacizumab), received prior to study entry. These 6 patients had decreases in tumor burden and were responders by Choi criteria or RECIST 1.1 (37) (Table 4). One of these patients, with colorectal cancer metastatic to multiple locations in the liver with radiographic progression following 5-fluorouracil, irinotecan, leucovorin, and bevacizumab, remains on treatment with bevacizumab and TRC105 at cycle 23 with a partial response by RECIST 1.1 (33% reduction in tumor burden) and normalization of serum CEA. An additional patient, with a primary central nervous system tumor (esthesioneuroblastoma) who was treatment naïve, remains on treatment with bevacizumab and TRC105 at cycle 19 with stable disease by RECIST 1.1.
Table 4.
Duration of Treatment in Patients with Response by RECIST or Choi criteria
| Patient Demographic | Primary Site of Disease | Prior VEGF Inhibitor Containing Therapy | Duration of Most Recent VEGF Inhibitor Containing Treatment (days) | Duration of TRC105 + Bevacizumab Treatment (days) | Notes |
|---|---|---|---|---|---|
| 56 year old female | Ovarian | pegylated liposomal doxorubicin + Bevacizumab | 126 (RPD) | 162 | 9% tumor reduction |
| 71 year old female | Ovarian | investigational VEGFR TKI | 141 (RPD) | 218 | 18% tumor reduction and 51% reduction in CA-125 |
| 66 year old female | Colorectal | Cetuximab + Bevacizumab | 31 (RPD) | 162 | 18% tumor reduction and 35% reduction in CEA |
| 81 year old female | Ovarian | Topotecan + Bevacizumab | 71 | 224 | 49% tumor reduction and 80% reduction in CA-125 |
| 53 year old male | Colorectal | 5-fluorouracil+irinotecan + leucovorin + Bevacizumab | 33 (RPD) | 532+ | 30% tumor reduction and 82% reduction in CEA to normal |
| 55 year old male | Colorectal | FOLFIRI + Bevacizumab | 146 (CPD) | 164 | 12% tumor reduction |
RPD= radiographic progression documented; CPD= clinical progression documented
DISCUSSION
The trial of TRC105 with bevacizumab in solid tumors was remarkable for the fact that both drugs could be administered at their recommended single agent doses (10 mg/kg each) by modifying the schedule of TRC105 to administer the initial dose one week following the initial bevacizumab dose and dividing the initial TRC105 dose over two days, to nearly eliminate the frequency of significant headache. Other adverse events characteristic of the individual agents were not observed more frequently when the two drugs were given concurrently (32). The lack of hypertension and proteinuria was noteworthy, and will be assessed in additional studies of TRC105 with other VEGF inhibitors. Notably, patients who were refractory to bevacizumab or VEGF receptor tyrosine kinase inhibitor treatment experienced reductions in tumor volume including partial responses by RECIST 1.1 and Choi criteria, and remained without progression for longer than the duration of prior VEGF inhibitor therapy.
Since its approval in 2004, bevacizumab has been shown to be active when added to chemotherapy in many cancer types. However, bevacizumab has not been developed successfully as a single agent in colorectal cancer and multiple other tumors types. Overcoming resistance to bevacizumab and other inhibitors of the VEGF pathway represents an unmet medical need that has not been satisfied despite multiple attempts to combine it with targeted agents, including those addressing placental growth factor, angiopoietins, and the epidermal growth factor pathways (38). Unlike these targets, endoglin expression is required for angiogenesis, as deletion of the gene in utero is lethal as a result of absent vascular development (14).
Generally, it is hypothesized that resistance to antiangiogenic agents occurs through the emergence of escape pathways rather than by acquiring mutations to the VEGF receptor or its ligand (39). Activity of the combination of TRC105 and bevacizumab in a refractory clinical setting are consistent with preclinical observations that endoglin is an endothelial receptor that mediates VEGF resistance. Endoglin expressing vessels resist treatment with antibody targeting the VEGFR, allowing continued growth of human tumor xenografts (12). The endoglin ligand TGF-β is the most highly up-regulated angiogenic factor in spontaneous pancreatic tumors from RIP-Tag2 mice treated with antibody that binds VEGF (40). Tumors in these mice deficient in one copy of the endoglin gene become resensitized to large and small molecule inhibitors of the VEGF pathway. Likewise, endoglin conditional knock-out mice carrying subcutaneous lung tumors present with dramatically reduced lung metastases following treatment with a VEGFR TKI (41). Impressively, the tendency of agents targeting the VEGF or endoglin pathways individually to increase local invasion and distant metastasis (42) is reversed with concurrent therapies targeting of the VEGF and endoglin pathways.
Defining populations responsive to the combination of TRC105 and VEGF inhibitors is an area of active research. An assessment of TRC105 expression on sarcoma cells will be incorporated into a Phase 1b dose escalation study with pazopanib, to facilitate an enrichment strategy for sarcoma subtypes enrolled in a Phase 2 trial. Melanoma and leukemia also represent indications where stratification of patients based on endoglin expression on malignant tissue could be employed. Enrichment for epithelial tumors may be possible based on CT characteristics of metastatic deposits. An exploratory analysis, applying novel quantitative textural analysis measures of standard spiral CT scans, indicates markers of tumor heterogeneity and hypoxia at baseline correlate with individual lesion responses to TRC105 and bevacizumab, and are worthy of prospective evaluation as predictive imaging biomarkers (37). Soluble biomarker expression is also being assessed in ongoing TRC105 trials in an effort to identify a responsive profile. Marked elevations of TGF-β and VEGF-A levels at baseline were observed in a patient with castrate-resistant prostate cancer with an ongoing long term complete PSA key></foreignowing TRC105 monotherapy (32). These biomarkers are part of a panel of more than 30 soluble markers that will be evaluated in patients who participated in this trial of TRC105 and bevacizumab.
Although activity in refractory cancers is notable, it may be easier to prevent bevacizumab resistance than to treat patients following the development of bevacizumab resistance. The combination of TRC105 and bevacizumab is being studied in randomized trials of bevacizumab naïve patients with glioblastoma and renal cell cancer. Studies evaluating the combinations of TRC105 with small molecule inhibitors of the VEGF pathway (i.e., sorafenib, axitinib, and pazopanib) are also underway to plan randomized trials testing VEGF inhibitors with TRC105 in patients who have not been treated previously with VEGF inhibitors.
Translational Statement.
TRC105 is a therapeutic monoclonal antibody to endoglin (CD105), a receptor that is expressed at high levels on proliferating endothelial cells. Endoglin is an essential angiogenic target that is implicated as a mediator of resistance to inhibitors of the VEGF pathway. The present article describes the Phase1 evaluation of TRC105 given in combination with bevacizumab. Consistent with its unique mechanism of action and a safety profile that is distinct from that of inhibitors of the VEGF pathway, TRC105 combined safely with bevacizumab at the recommended single agent doses of each drug. The combination demonstrated durable activity in a VEGF inhibitor refractory population. Ongoing randomized Phase 2 trials are testing TRC105 in combination with bevacizumab in glioblastoma and renal cell carcinoma, and Phase 1 trials in renal cell carcinoma, hepatocellular carcinoma, and soft tissue sarcoma are assessing safety and activity in combination with small molecule inhibitors of the VEGF pathway.
Acknowledgments
Research funding: This research was supported by TRACON Pharmaceuticals Inc, San Diego, CA and by the Center for Cancer Research of the National Cancer Institute.
The authors express their appreciation to the patients who participated in this investigational study and the study staff.
Footnotes
Presented in part at the following conferences: 2012 ASCO Annual Meeting, Chicago, IL; The 14th International Symposium on Anti-Angiogenic Therapy, San Diego, CA; 2013 ASCO Annual Meeting, Chicago, IL; 2013 AACR-NCI-EORTC Symposium on Molecular Targets and Cancer Therapeutics, Boston, MA
Trail registry number: NCT01332721
Conflicts of Interest: L. S. Rosen received funding from TRACON for a clinical trial. B.K. Seon is the inventor of TRC105. D. Alvarez, B.J. Adams and C.P. Theuer are employees of TRACON. No conflicts of interest were disclosed by the other authors.
Disclaimer: This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract Number HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States Government.
References
- 1.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–87. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 2.Escudier B, Bellmunt J, Negrier S, Bajetta E, Melichar B, Bracarda S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol. 2010;28(13):2144–50. doi: 10.1200/JCO.2009.26.7849. [DOI] [PubMed] [Google Scholar]
- 3.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23(4):792–9. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 6.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 7.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–40. doi: 10.1200/JCO.2008.19.8721. Epub 2009/09/02. [DOI] [PubMed] [Google Scholar]
- 8.Seon BK, Haba A, Matsuno F, Takahashi N, Tsujie M, She X, et al. Endoglin-targeted cancer therapy. Curr Drug Deliv. 2011;8(1):135–43. doi: 10.2174/156720111793663570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen L, Gordon M, Robert F, Matei D. Endoglin for Targeted Cancer Treatment. Current oncology reports. 2014;16(365) doi: 10.1007/s11912-013-0365-x. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Elsner T, Botella LM, Velasco B, Langa C, Bernabeu C. Endoglin expression is regulated by transcriptional cooperation between the hypoxia and transforming growth factor-beta pathways. J Biol Chem. 2002;277(46):43799–808. doi: 10.1074/jbc.M207160200. [DOI] [PubMed] [Google Scholar]
- 11.Bockhorn M, Tsuzuki Y, Xu L, Frilling A, Broelsch CE, Fukumura D. Differential vascular and transcriptional responses to anti-vascular endothelial growth factor antibody in orthotopic human pancreatic cancer xenografts. Clin Cancer Res. 2003;9(11):4221–6. [PubMed] [Google Scholar]
- 12.Davis DW, Inoue K, Dinney CP, Hicklin DJ, Abbruzzese JL, McConkey DJ. Regional effects of an antivascular endothelial growth factor receptor monoclonal antibody on receptor phosphorylation and apoptosis in human 253J B-V bladder cancer xenografts. Cancer Res. 2004;64(13):4601–10. doi: 10.1158/0008-5472.CAN-2879-2. [DOI] [PubMed] [Google Scholar]
- 13.Anderberg C, Cunha SI, Zhai Z, Cortez E, Pardali E, Johnson JR, et al. Deficiency for endoglin in tumor vasculature weakens the endothelial barrier to metastatic dissemination. The Journal of experimental medicine. 2013;210(3):563–79. doi: 10.1084/jem.20120662. Epub 2013/02/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, et al. Defective angiogenesis in mice lacking endoglin. Science. 1999;284(5419):1534–7. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- 15.Duarte C, Murray K, Lucas F, Fairfield K, Miller H, Brooks P, et al. Improved survival outcomes in cancer patients with hereditary hemorrhagic telangiectasia. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23(1):117–25. doi: 10.1158/1055-9965.EPI-13-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenato GM, Guanti G. Hereditary Haemorrhagic Telangiectasia (HHT): genetic and molecular aspects. Curr Pharm Des. 2006;12(10):1173–93. doi: 10.2174/138161206776361291. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S, Ghellal A, Li C, Byrne G, Haboubi N, Wang JM, et al. Breast carcinoma: vascular density determined using CD105 antibody correlates with tumor prognosis. Cancer Res. 1999;59(4):856–61. [PubMed] [Google Scholar]
- 18.Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: evidence and potential applications. Faseb J. 2003;17(9):984–92. doi: 10.1096/fj.02-0634rev. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Gardy R, Seon BK, Duff SE, Abdalla S, Renehan A, et al. Both high intratumoral microvessel density determined using CD105 antibody and elevated plasma levels of CD105 in colorectal cancer patients correlate with poor prognosis. Br J Cancer. 2003;88(9):1424–31. doi: 10.1038/sj.bjc.6600874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saad RS, El-Gohary Y, Memari E, Liu YL, Silverman JF. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in esophageal adenocarcinoma. Hum Pathol. 2005;36(9):955–61. doi: 10.1016/j.humpath.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Yao Y, Kubota T, Takeuchi H, Sato K. Prognostic significance of microvessel density determined by an anti-CD105/endoglin monoclonal antibody in astrocytic tumors: comparison with an anti-CD31 monoclonal antibody. Neuropathology. 2005;25(3):201–6. doi: 10.1111/j.1440-1789.2005.00632.x. [DOI] [PubMed] [Google Scholar]
- 22.Ding S, Li C, Lin S, Yang Y, Liu D, Han Y, et al. Comparative evaluation of microvessel density determined by CD34 or CD105 in benign and malignant gastric lesions. Hum Pathol. 2006;37(7):861–6. doi: 10.1016/j.humpath.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Erdem O, Taskiran C, Onan MA, Erdem M, Guner H, Ataoglu O. CD105 expression is an independent predictor of survival in patients with endometrial cancer. Gynecol Oncol. 2006;103(3):1007–11. doi: 10.1016/j.ygyno.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Kyzas PA, Agnantis NJ, Stefanou D. Endoglin (CD105) as a prognostic factor in head and neck squamous cell carcinoma. Virchows Arch. 2006;448(6):768–75. doi: 10.1007/s00428-006-0195-4. [DOI] [PubMed] [Google Scholar]
- 25.Marioni G, Marino F, Giacomelli L, Staffieri C, Mariuzzi ML, Violino E, et al. Endoglin expression is associated with poor oncologic outcome in oral and oropharyngeal carcinoma. Acta Otolaryngol. 2006;126(6):633–9. doi: 10.1080/00016480500452558. [DOI] [PubMed] [Google Scholar]
- 26.Taskiran C, Erdem O, Onan A, Arisoy O, Acar A, Vural C, et al. The prognostic value of endoglin (CD105) expression in ovarian carcinoma. Int J Gynecol Cancer. 2006;16(5):1789–93. doi: 10.1111/j.1525-1438.2006.00658.x. [DOI] [PubMed] [Google Scholar]
- 27.Yang LY, Lu WQ, Huang GW, Wang W. Correlation between CD105 expression and postoperative recurrence and metastasis of hepatocellular carcinoma. BMC Cancer. 2006;6:110. doi: 10.1186/1471-2407-6-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Gohary YM, Silverman JF, Olson PR, Liu YL, Cohen JK, Miller R, et al. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in prostatic adenocarcinoma. Am J Clin Pathol. 2007;127(4):572–9. doi: 10.1309/X6NXYE57DLUE2NQ8. [DOI] [PubMed] [Google Scholar]
- 29.Rubatt JM, Darcy KM, Hutson A, Bean SM, Havrilesky LJ, Grace LA, et al. Independent prognostic relevance of microvessel density in advanced epithelial ovarian cancer and associations between CD31, CD105, p53 status, and angiogenic marker expression: A Gynecologic Oncology Group study. Gynecol Oncol. 2009;112(3):469–74. doi: 10.1016/j.ygyno.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 30.Dallas NA, Samuel S, Xia L, Fan F, Gray MJ, Lim SJ, et al. Endoglin (CD105): a marker of tumor vasculature and potential target for therapy. Clin Cancer Res. 2008;14(7):1931–7. doi: 10.1158/1078-0432.CCR-07-4478. [DOI] [PubMed] [Google Scholar]
- 31.Nolan-Stevaux O, Zhong W, Culp S, Shaffer K, Hoover J, Wickramasinghe D, et al. Endoglin Requirement for BMP9 Signaling in Endothelial Cells Reveals New Mechanism of Action for Selective Anti-Endoglin Antibodies. PloS one. 2012;7(12):e50920. doi: 10.1371/journal.pone.0050920. Epub December 27, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosen L, Hurwitz H, Wong M, Goldman J, Mendelson D, Figg W, et al. A phase I first-in-human study of TRC105 (Anti-Endoglin Antibody) in patients with advanced cancer. Clin Cancer Res. 2012;18(17):4820–9. doi: 10.1158/1078-0432.CCR-12-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiozaki K, Harada N, Greco WR, Haba A, Uneda S, Tsai H, et al. Antiangiogenic chimeric anti-endoglin (CD105) antibody: pharmacokinetics and immunogenicity in nonhuman primates and effects of doxorubicin. Cancer Immunol Immunother. 2006;55(2):140–50. doi: 10.1007/s00262-005-0691-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duffaud F, Therasse P. New guidelines to evaluate the response to treatment in solid tumors. Bull Cancer. 2000;87(12):881–6. Nouvelles recommandations pour l’evaluation de la reponse tumorale dans les tumeurs solides. [PubMed] [Google Scholar]
- 35.Chuslip Choi HC, Faria Silvana C, Macapinlac Homer A, Burgess Michael A, Patel SRC, Lei L, Podoloff Donald A, Benjamin Robert S. Correlation of Computed Tomography and Positron Emission Tomography in Patients With Metastatic Gastrointestinal Stromal Tumor Treated at a Single Institution With Imatinib Mesylate: Proposal of New Computed Tomography Response Criteria. Journal of Clinical Oncology. 2007;25(13) doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 36.Rosen LS, Gordon MS, Hurwitz HI, Wong MK, Adams BJ, Alvarez D, et al. Phase 1 study of TRC105 [anti-CD105 (endoglin) antibody] therapy in patients with advanced refractory cancer. Proceedings of ASCO. 2009;27(15S):3518. [Google Scholar]
- 37.Korn R, Gordon M, Rosen L, Robert F, Matei DJWG, et al. Exploratory evaluation of the combination of TRC105 (anti-endoglin antibody) and bevacizumab indicates partial response by Choi criteria in bevacizumab refractory advanced cancer patients and identifies condidate textural analysis imaging markers of response. Annual meeting of the EORTC-NCI-AACR; Boston MA. 2013.. [Google Scholar]
- 38.Bukowski R, Kabbinavar F, Figlin R, Flaherty K, Srinivas S, Vaishampayan U, et al. Randomized phase II study of erlotinib combined with bevacizumab compared with bevacizumab alone in metastatic renal cell cancer. J Clin Oncol. 2007;25(29):4536–41. doi: 10.1200/JCO.2007.11.5154. [DOI] [PubMed] [Google Scholar]
- 39.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sennino B, Ishiguro-Oonuma T, Wei Y, Naylor R, Williamson C, Bhagwandin V, et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discovery AACR. 2012;2(3):270–87. doi: 10.1158/2159-8290.CD-11-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderberg CCS, Zhai Z, et al. Deficiency for endoglin in tumor vasculature weakens the endothelial barrier to metastatic dissemination. J Exp Med. 2013;210:563–79. doi: 10.1084/jem.20120662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer cell. 2009;15(3):220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]