Abstract
Three different cDNA clones (pmcTyr1, pmcTyr2 and pmcTyr3) representing mRNAs originating by alternative splicing of the primary transcript of mouse tyrosinase gene, were identified and characterized by sequence analysis and by a functional assay. These cDNAs were subcloned into the newly constructed expression vector pHD. After electroporation of these hybrid clones into tyrosinase negative cells, protein extracts were prepared and tested for tyrosinase enzyme activity. Only the cDNA insert of pmcTyr1 was able to confer tyrosinase enzyme activity. This cDNA encodes a protein 533 amino acid residues in length containing a putative leader peptide of 18 amino acids and six putative glycosylation sites. Comparisons of the deduced amino acid sequence of the cDNA clone pmcTyr1 with the protein sequence of tyrosinases from man, Streptomyces, Neurospora and with haemocyanin subunits from a spider showed two regions of sequence conservation. One of these regions is known to be involved in copper binding. Since this gene with the coding capacity for tyrosinase is absent in all studied c-locus lethal deletion mutant mice, we have evidence that albinism in mice is caused by mutations of the tyrosinase gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Bernan V., Filpula D., Herber W., Bibb M., Katz E. The nucleotide sequence of the tyrosinase gene from Streptomyces antibioticus and characterization of the gene product. Gene. 1985;37(1-3):101–110. doi: 10.1016/0378-1119(85)90262-8. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnett J. B. The tyrosinases of mouse melanoma. Isolation and molecular properties. J Biol Chem. 1971 May 25;246(10):3079–3091. [PubMed] [Google Scholar]
- COLEMAN D. L. Effect of genic substitution on the incorporation of tyrosine into the melanin of mouse skin. Arch Biochem Biophys. 1962 Mar;96:562–568. doi: 10.1016/0003-9861(62)90337-5. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobner P. R., Kawasaki E. S., Yu L. Y., Bancroft F. C. Thyroid or glucocorticoid hormone induces pre-growth-hormone mRNA and its probable nuclear precursor in rat pituitary cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2230–2234. doi: 10.1073/pnas.78.4.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M., Taylor L. P., Walbot V. Expression of genes transferred into monocot and dicot plant cells by electroporation. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5824–5828. doi: 10.1073/pnas.82.17.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller B. B., Lunsford J. B., Iman D. S. Alpha-melanocyte-stimulating hormone regulation of tyrosinase in Cloudman S-91 mouse melanoma cell cultures. J Biol Chem. 1987 Mar 25;262(9):4024–4033. [PubMed] [Google Scholar]
- Gluecksohn-Waelsch S. Genetic control of morphogenetic and biochemical differentiation: lethal albino deletions in the mouse. Cell. 1979 Feb;16(2):225–237. doi: 10.1016/0092-8674(79)90001-1. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing V. J., Ekel T. M., Montague P. M. Mammalian tyrosinase: isozymic forms of the enzyme. Int J Biochem. 1981;13(1):99–103. doi: 10.1016/0020-711x(81)90141-5. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Jiménez M. Mammalian tyrosinase--the critical regulatory control point in melanocyte pigmentation. Int J Biochem. 1987;19(12):1141–1147. doi: 10.1016/0020-711x(87)90095-4. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Jr Mammalian monophenol monooxygenase (tyrosinase): purification, properties, and reactions catalyzed. Methods Enzymol. 1987;142:154–165. doi: 10.1016/s0076-6879(87)42024-7. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Korner A. M., Pawelek J. M. New regulators of melanogenesis are associated with purified tyrosinase isozymes. J Invest Dermatol. 1982 Jul;79(1):16–18. doi: 10.1111/1523-1747.ep12510422. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Nicholson J. M., Montague P. M., Ekel T. M., Tomecki K. J. Mammalian tyrosinase. Structural and functional interraltionship of isozymes. Biochim Biophys Acta. 1978 Feb 10;522(2):327–339. doi: 10.1016/0005-2744(78)90067-0. [DOI] [PubMed] [Google Scholar]
- Hearing V. J. Tyrosinase activity in subcellular fractions of black and albino mice. Nat New Biol. 1973 Sep 19;245(142):81–83. doi: 10.1038/newbio245081a0. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., Zava D. T., Thilagar A. K., Jensen E. M., McGuire W. L. Steroid receptor analyses of nine human breast cancer cell lines. Cancer Res. 1978 Aug;38(8):2434–2437. [PubMed] [Google Scholar]
- Houghton A. N., Eisinger M., Albino A. P., Cairncross J. G., Old L. J. Surface antigens of melanocytes and melanomas. Markers of melanocyte differentiation and melanoma subsets. J Exp Med. 1982 Dec 1;156(6):1755–1766. doi: 10.1084/jem.156.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M., Hintermann G., Lerch K. Primary structure of tyrosinase from Streptomyces glaucescens. Biochemistry. 1985 Oct 22;24(22):6038–6044. doi: 10.1021/bi00343a003. [DOI] [PubMed] [Google Scholar]
- Jolley R. L., Jr, Evans L. H., Makino N., Mason H. S. Oxytyrosinase. J Biol Chem. 1974 Jan 25;249(2):335–345. [PubMed] [Google Scholar]
- Kwon B. S., Haq A. K., Pomerantz S. H., Halaban R. Isolation and sequence of a cDNA clone for human tyrosinase that maps at the mouse c-albino locus. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7473–7477. doi: 10.1073/pnas.84.21.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner A., Pawelek J. Activation of melanoma tyrosinase by a cyclic AMP-dependent protein kinase in a cell-free system. Nature. 1977 Jun 2;267(5610):444–447. doi: 10.1038/267444a0. [DOI] [PubMed] [Google Scholar]
- Körner A., Pawelek J. Mammalian tyrosinase catalyzes three reactions in the biosynthesis of melanin. Science. 1982 Sep 17;217(4565):1163–1165. doi: 10.1126/science.6810464. [DOI] [PubMed] [Google Scholar]
- Lerch K. Neurospora tyrosinase: structural, spectroscopic and catalytic properties. Mol Cell Biochem. 1983;52(2):125–138. doi: 10.1007/BF00224921. [DOI] [PubMed] [Google Scholar]
- Lerch K. Primary structure of tyrosinase from Neurospora crassa. II. Complete amino acid sequence and chemical structure of a tripeptide containing an unusual thioether. J Biol Chem. 1982 Jun 10;257(11):6414–6419. [PubMed] [Google Scholar]
- Makino N., Mason H. S. Reactivity of oxytyrosinase toward substrates. J Biol Chem. 1973 Aug 25;248(16):5731–5735. [PubMed] [Google Scholar]
- Marshall R. D. The nature and metabolism of the carbohydrate-peptide linkages of glycoproteins. Biochem Soc Symp. 1974;(40):17–26. [PubMed] [Google Scholar]
- McCabe R. P., Quaranta V., Frugis L., Ferrone S., Reisfeld R. A. A radioimmunometric antibody-binding assay for evaluation of xenoantisera to melanoma-associated antigens. J Natl Cancer Inst. 1979 Mar;62(3):455–463. doi: 10.1093/jnci/62.3.455. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miksicek R., Heber A., Schmid W., Danesch U., Posseckert G., Beato M., Schütz G. Glucocorticoid responsiveness of the transcriptional enhancer of Moloney murine sarcoma virus. Cell. 1986 Jul 18;46(2):283–290. doi: 10.1016/0092-8674(86)90745-2. [DOI] [PubMed] [Google Scholar]
- Pomerantz S. H. L-tyrosine-3,5-3H assay for tyrosinase development in skin of newborn hamsters. Science. 1969 May 16;164(3881):838–839. doi: 10.1126/science.164.3881.838. [DOI] [PubMed] [Google Scholar]
- Pomerantz S. H., Li J. P. Tyrosinase in the skin of albino hamsters and mice. Nature. 1974 Nov 15;252(5480):241–243. doi: 10.1038/252241a0. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
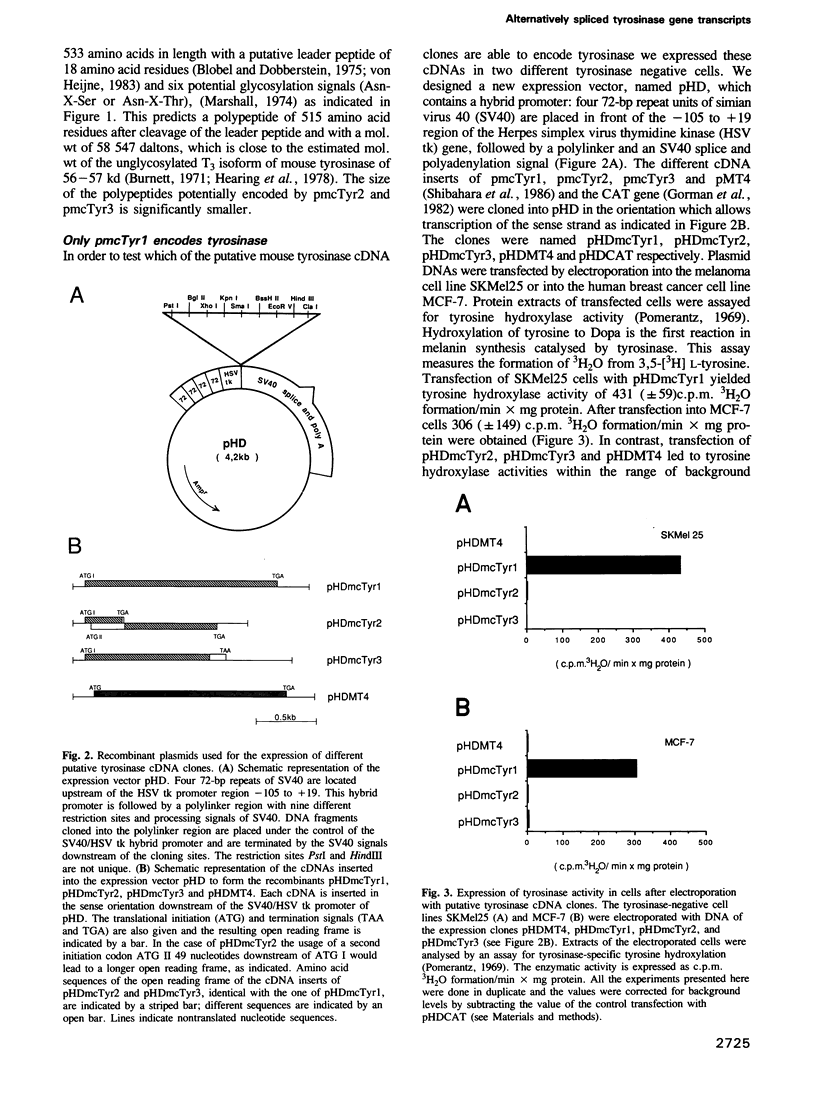
- Ruppert S., Müller G., Kwon B., Schütz G. Multiple transcripts of the mouse tyrosinase gene are generated by alternative splicing. EMBO J. 1988 Sep;7(9):2715–2722. doi: 10.1002/j.1460-2075.1988.tb03125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. B., Montgomery C. S., Raymer G. D. Analysis of the albino-locus region of the mouse: IV. Characterization of 34 deficiencies. Genetics. 1982 Mar;100(3):427–453. doi: 10.1093/genetics/100.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. B., Russell W. L. A Study of the Physiological Genetics of Coat Color in the Mouse by Means of the Dopa Reaction in Frozen Sections of Skin. Genetics. 1948 May;33(3):237–262. doi: 10.1093/genetics/33.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schartau W., Eyerle F., Reisinger P., Geisert H., Storz H., Linzen B. Hemocyanins in spiders, XIX. Complete amino-acid sequence of subunit d from Eurypelma californicum hemocyanin, and comparison to chain e. Hoppe Seylers Z Physiol Chem. 1983 Oct;364(10):1383–1409. doi: 10.1515/bchm2.1983.364.2.1383. [DOI] [PubMed] [Google Scholar]
- Schneider H. J., Drexel R., Feldmaier G., Linzen B., Lottspeich F., Henschen A. Hemocyanins in Spiders, XVIII. Complete amino-acid sequence of subunit e from Eurypelma californicum hemocyanin. Hoppe Seylers Z Physiol Chem. 1983 Oct;364(10):1357–1381. doi: 10.1515/bchm2.1983.364.2.1357. [DOI] [PubMed] [Google Scholar]
- Shibahara S., Tomita Y., Sakakura T., Nager C., Chaudhuri B., Müller R. Cloning and expression of cDNA encoding mouse tyrosinase. Nucleic Acids Res. 1986 Mar 25;14(6):2413–2427. doi: 10.1093/nar/14.6.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilgen W., Hellström I., Engstner M., Garrigues H. J., Riehl R., Hellström K. E. Localization of melanoma-associated antigen p97 in cultured human melanoma, as visualized by light and electron microscopy. J Invest Dermatol. 1983 May;80(5):459–463. doi: 10.1111/1523-1747.ep12558390. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]