Abstract
Inflammatory bowel disease (IBD) is a chronic and relapsing inflammatory disorder of the gastrointestinal (GI) tract, and currently no curative treatment available. Mangiferin, a natural glucosylxanthone mainly from the fruit, leaves and stem bark of the mango tree, has strong anti-inflammatory activity. We sought to investigate whether mangiferin attenuates inflammation in a mouse model of chemically induced IBD. Pre-administration of mangiferin significantly attenuated dextran sulfate sodium (DSS)-induced body weight loss, diarrhea, colon shortening and histological injury, which correlated with the decline in the activity of myeloperoxidase (MPO) and the level of tumor necrosis factor-α (TNF-α) in the colon. DSS-induced degradation of inhibitory κBα (IκBα) and the phosphorylation of nuclear factor-kappa B (NF-κB) p65 as well as the mRNA expression of pro-inflammatory mediators (inducible NO synthase (iNOS), intercellular adhesion molecule-1 (ICAM-1), TNF-α, interleukin-1β (IL-1β) and IL-6) in the colon were also downregulated by mangiferin treatment. Additionally, the phosphorylation/activation of DSS-induced mitogen-activated protein kinase (MAPK) proteins was also inhibited by mangiferin treatment. In accordance with the in vivo results, mangiferin exposure blocked TNF-α-stimulated nuclear translocation of NF-κB in RAW264.7 mouse macrophage cells. Transient transfection gene reporter assay performed in TNF-α-stimulated HT-29 human colorectal adenocarcinoma cells indicated that mangiferin inhibits NF-κB transcriptional activity in a dose-dependent manner. The current study clearly demonstrates a protective role for mangiferin in experimental IBD through NF-κB and MAPK signaling inhibition. Since mangiferin is a natural compound with little toxicity, the results may contribute to the effective utilization of mangiferin in the treatment of human IBD.
Keywords: Inflammatory bowel disease, dextran sulfate sodium, NF-κB, MAPK, Mangiferin
1. Introduction
Inflammatory bowel disease (IBD), which primarily manifests as ulcerative colitis (UC) and Crohn’s disease (CD), is a chronic and relapsing inflammatory condition of the gastrointestinal (GI) tract [1]. It is associated with a considerable reduction in the quality of life and an increased risk of colorectal cancer [2,3]. Although many advances have been made in the management of IBD, it is still incurable [4]. Glucocorticoid, sulfasalazine and immunosuppressive drugs have been mainly used for the treatment of IBD; however, their side effects and toxicity remain a major clinical concern [5,6]. As a result, there is an increasing interest in using herbal medicines as an alternative and adjunct treatment in addition to the conventional therapies [7,8].
Although the exact etiologies of IBD remain elusive, it has been generally accepted that the excessive production of pro-inflammatory cytokines and mediators, such as interleukin-1 (IL-1), IL-6, IL-12 and tumor necrosis factor-α (TNF-α), plays an important role in the pathogenesis of IBD [9,10]. Recently, anti-TNF agents have dramatically influenced the clinical management of IBD [11,12]. However, not all patients respond to treatment, and some become intolerant over time. Nuclear factor-kappa B (NF-κB) is a critical mediator of immune and inflammatory responses that controls the transcription of inflammatory cytokine and downstream mediator genes. Increased NF-κB activation has been detected in the mucosa of IBD patients and in murine IBD models [13–15]. Inhibition of NF-κB with a specific p65 antisense oligonucleotide was shown to be an effective approach for preventing IBD symptoms in IBD experimental mouse models and blocking the production of pro-inflammatory cytokines in CD patients [16–18]. We and others have demonstrated that specifically inhibiting NF-κB activity in IBD animal models repressed a subset of pro-inflammatory mediators, such as inducible NO synthase (iNOS), intercellular adhesion molecule-1 (ICAM-1), monocyte chemotactic protein-1 (MCP-1), cyclooxygenase 2 (COX2), interferon-gamma (IFN-γ ), TNF-α, IL-1β, IL-6 and IL-17 [19–21]. For this reason, inhibiting NF-κB activation is thought to be an effective strategy for UC therapy.
Mangiferin (2-C-β-D-glucopyranosyl-1,3,6,7-tetrahydroxyxanthone) is a natural glucosylxanthone present in many plant species, while it is primarily found in the fruit, leaves and stem bark of the mango tree (Mangifera indica) [22]. Mangiferin has multiple pharmacological activities, including the inhibition of inflammatory mediators, induction of antioxidation and apoptosis in cancer cells, protection against liver and lung injury, improvement of glucose intolerance, prevention of gene mutation and photoaging, and amelioration of memory deficit [22–25]. Recent studies have demonstrated that mangiferin is a potent inhibitor of NF-κB and MAPK pathways [24,26]. However, the direct effect of mangiferin on intestinal inflammation remains unknown. Thus, we hypothesized that mangiferin may block NF-κB and MAPK signaling pathways and thereby attenuate experimental IBD in mice.
2. Materials and Methods
2.1. Cell lines and reagents
The HT-29 human colorectal adenocarcinoma cell line and RAW264.7 mouse macrophage cell line were obtained from the American Type Culture Collection (ATCC) and cultured according to ATCC recommendations. The human HT-29 human colorectal adenocarcinoma cell line, as an in vitro model, is usually used to investigate the anti-colitis effects of drugs [27]. The murine RAW 264.7 macrophage cell line is a well-established model system for many inflammatory studies [28]. Mangiferin (Lot No. 05–1020, HPLC purity ≥ 98%) was kindly provided by the Shanghai R&D Center for Standardization of Chinese Medicine, Shanghai, China. DSS (MW 36–50 KDa) was acquired from MP Biochemical LLC, Solon, OH. Donkey serum, paraformaldehyde, methylcellulose, formalin, Tween-20, ethanol and DMSO were obtained from Sigma-Aldrich, St.Louis, MO. SYBR Premix ExTaq Mix was obtained from TAKARA Bio, Otsu, Japan. The SuperScript III first-strand synthesis system, Fluor 488-conjugated anti-rabbit IgG (A-21206), lipofectamine 2000 transfection reagent, Triton X-100, Trizol, and DAPI reagents were obtained from Invitrogen, Carlsbad, CA. Bovine serum albumin and protease inhibitor cocktail tablets were obtained from Roche Diagnostics, Mannheim, Germany. 1× passive lysis buffer and luciferase assay system were from Promega, Madison, WI. TNF-α reagent and mouse antibodies to the following proteins: NF-κB p65 (#8242), phospho-p65 (#3033), phospho-IκBα (#2859), IκBα (#4812), phospho-ERK1/2 (#4377), ERK1/2 (#4348), phospho-JNK (#9255), JNK (#9252), phospho-p38 (#9215), p38 (#9212) and β-actin (#4970) were obtained from Cell Signaling Technology Inc., Danvers, MA. TNF-α ELISA kit was from R&D Systems, Minneapolis, MN. Anti-phospho-NF-κB p65-NLS (RIPA 523170) was from Thermo Scientific Inc., Waltham, MA. Envision-HRP reagent and diaminobenzidine were purchased from DakoCytomation, Carpinteria, CA.
2.2. Mice
Healthy 8-week-old female C57BL/6 mice (20 ± 2 g) were obtained from the Shanghai Laboratory Animal Center, and the subsequent studies were performed in accordance with the guidelines approved by the Animal Ethics Committee of Shanghai University of TCM (SHUTCM). Standard mouse chow pellets and water were supplied ad libitum. All mice were housed under a specific pathogen-free facility at SHUTCM and kept under the same temperature (25 ± 2°C) and lighting (12-h light-dark cycle) conditions.
2.3. Experimental design
Colitis was induced in mice by administering DSS in the drinking water as described previously [29]. The experiment lasted for 10 days. A mangiferin stock solution was prepared in 0.5% methylcellulose and administered to mice at a dose of 50 mg/kg/day by oral gavage. Mangiferin dosing (50 mg/kg per body weight) was based on previous report and our preliminary studies [30]. Mice were randomly distributed into the following four groups (n = 10–15 mice per group): Group 1 comprised the vehicle controls, which were administered 100 µl of 0.5% methylcellulose by oral gavage once per day; Group 2 comprised mangiferin treated mice at a dose of 50 mg/kg of body weight via oral gavage once per day; Group 3 comprised mice administered 100 µl of 0.5% methylcellulose by oral gavage once per day and 4% DSS in the drinking water from d 3 to d 10; and Group 4 received mangiferin by oral gavage 3 days prior to DSS treatment and maintained until the end of DSS treatment. The total gavage volume was identical for each group.
2.4. Clinical and histological scores of colitis
Mice were monitored daily for body weight, diarrhea and bloody stool changes. The mice were sacrificed under anesthesia 4 h after receiving the last gavage. The entire colon was removed and the total length was measured. The distal colons were taken and fixed in 10% buffered formalin for 24 h at room temperature, embedded in paraffin and stained with hematoxylin-eosin (H&E) for histological evaluation. Histological damage was assessed as a combined score of inflammatory cell infiltration (score 0–3) and mucosal damage (score 0–3) using a previously described method [20].
2.5. Western blot
Colon tissues were disrupted by homogenization on ice and centrifuged at 4°C (12,000 g, 15 min), and the supernatants were collected. Equal amounts of protein (40 µg) were separated by 10% SDS-PAGE and transferred to a nitrocellulose membrane. Membranes were blocked in 5% (w/v) skim milk and incubated with antibodies against mouse phospho-p65 (1:1000), phospho-IκBα (1:1000), IκBα (1:1000), phospho-ERK1/2 (1:1000), ERK1/2 (1:1000), phospho-JNK (1:2000), JNK (1:1000), phospho-p38 (1:1000), p38 (1:1000) and β-actin (1:2000), respectively. Blots were incubated with horseradish peroxidase conjugated secondary antibodies (Santa Cruz Biotechnology, Dallas, TX) and developed by enhanced chemiluminescence (ECL) western blotting detection reagents (Thermo Scientific, Waltham, MA). Protein expression quantification was performed by densitometric analysis of the blots.
2.6. RNA analysis
RNA was extracted from colon samples using TRIzol reagent. Quantitative real-time polymerase chain reaction (qPCR) was performed using cDNA generated from 3 µg of total RNA with the SuperScript II Reverse Transcriptase kit. The following PCR primer sequences were used: 5’ -GGGAATCTTGGAGCGAGTTG-3’/5’ -GTGAGGGCTTGGCTGAGTGA-3’ for iNOS, 5’-CGCTGTGCTTTGAGAACTGT-3’/5’-AGGTCCTTGCCTACTTGCTG-3’ for ICAM-1, 5’ -GAAGTCTTTGGTCTGGTGCCT-3’/5’ -GCTCCTGCTTGAGTATGTCG-3’ for COX2, 5’ -GGCTGGACTGTTTCTAATGC-3’/5’ - ATGGTTTCTTGTGACCCTGA-3’ for IL-1 β, 5’ -ACCACGGCCTTCCCTACTTC-3’/5’ -CATTTCCACGATTTCCCAGA-3’ for IL-6, 5’ -CGTGGAACTGGCAGAAGAGG-3’/5’ -AGACAGAAGAGCGTGGTGGC-3’ for TNF-α, and 5’ -C AGCCTTCCTTCTTGGGTAT-3 ‘/5’ -TGGCATAGAGGTCTTTACGG-3’ for β-actin. PCR reactions were carried out using SYBR Premix ExTaq Mix and quantitatively measured with an ABI Prism 7900HT Sequence Detection System (Life Technologies, Carlsbad, CA). The following thermal cycler parameters were used: 1 cycle of 95°C for 30 s and 40 cycles of denaturation (95°C, 5 s) and combined annealing/extension (60°C, 30 s). Gene expression changes were calculated by the comparative Ct method, and the values were normalized to the β-actin endogenous reference.
2.7. NF-κB immunohistochemistry
The paraffin-embedded colon tissue slides were incubated with rabbit polyclonal antibody against mouse phospho-NF-κB p65-NLS (1:50) in a humid chamber at 4°C overnight. After further washing, the slides were incubated with Envision/HRP at 37°C for 30 min. Finally, immune complexes were visualized by incubating with diaminobenzidine for 10 min and counterstained with hematoxylin.
2.8. NF-κB immunofluorescence
RAW264.7 cells were seeded in 8-chamber slides (BD Biosciences, Bedford, MA) at a density of 5×104 cells per well. Cells were incubated with or without mangiferin (25 µM) for 2 hours and then treated with TNF-α (20 ng/ml) for an additional 12 hours. Cells were fixed with a 4% paraformaldehyde solution at 20°C for 10 min. After washing in PBS, cells were permeabilized with 0.3% Triton X-100 in PBS at room temperature for 20 min. After incubation in 0.1% Triton X-100 blocking buffer, 1% BSA, and 3% donkey serum, cells were incubated with a rabbit NF-κB p65 antibody (1:50) at 4°C overnight and then incubated with an Alexa Fluor 488-conjugated anti-rabbit IgG secondary antibody (1:500) at room temperature for 45 min. DAPI at a concentration of 1 µg/ml in PBS was added to stain the nuclei. Fluorescence photographs were obtained using a CKX41 inverted fluorescence microscope (Olympus, Shanghai, China).
2.9. Determination of TNF-α level
Colon segments were homogenized in ice-cold PBS. The homogenates were centrifuged at 3,000 g for 10 min, and the supernatants were assayed for TNF-α level using ELISA kit as described previously [20]. The results were expressed as pg/mg of protein in each sample.
2. 10. Myeloperoxidase (MPO) assay
Tissue MPO activity, which is linearly related to neutrophil infiltration in inflamed tissue, was assayed to monitor the degree of inflammation. MPO activity was measured in pieces of the colon according to the manufacturer’s instruction (CytoStore, Alberta, Canada) and our report [21]. The results were expressed as units/mg of protein.
2.11. NF-κB luciferase reporter assay
HT-29 cells were seeded in a 24-well plate at a density of 1.5 × 105 cells/well one day before transfection. The cells were transfected with 0.8 µg of pGL4.32 [luc2P/NF-κB-RE/Hygro] vector (Promega, Madison, WI) using the lipofectamine 2000 reagent as previously described [31]. The pGL4.32 plasmid is a NF-κB reporter vector that contains NF-κB response elements and the firefly luciferase gene. Twelve hours after the transient transfection, cells were incubated with or without mangiferin (1, 10, and 25 µmol/L) for 2 hours and then treated with TNF-α (20 ng/ml) for an additional 12 hours. Cells were washed once with PBS and lysed in 100 µl of 1× passive lysis buffer. Cell-free lysates were obtained by centrifugation at 10,000 g for 2 minutes at 4°C. Luciferase activity from cell lysates was quantified using a luciferase assay system and a Glomax 20/20 luminometer (Promega). Results are expressed as fold induction of control cells.
2.12. Statistics
All data are expressed as the mean ± SD. The differences between groups were analyzed by one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) for post-hoc test. Statistical analysis was performed by the SPSS 16.0 software package. A value of p <0.05 was considered statistically significant.
3. Results
3.1. Mangiferin treatment attenuated DSS-induced colitis
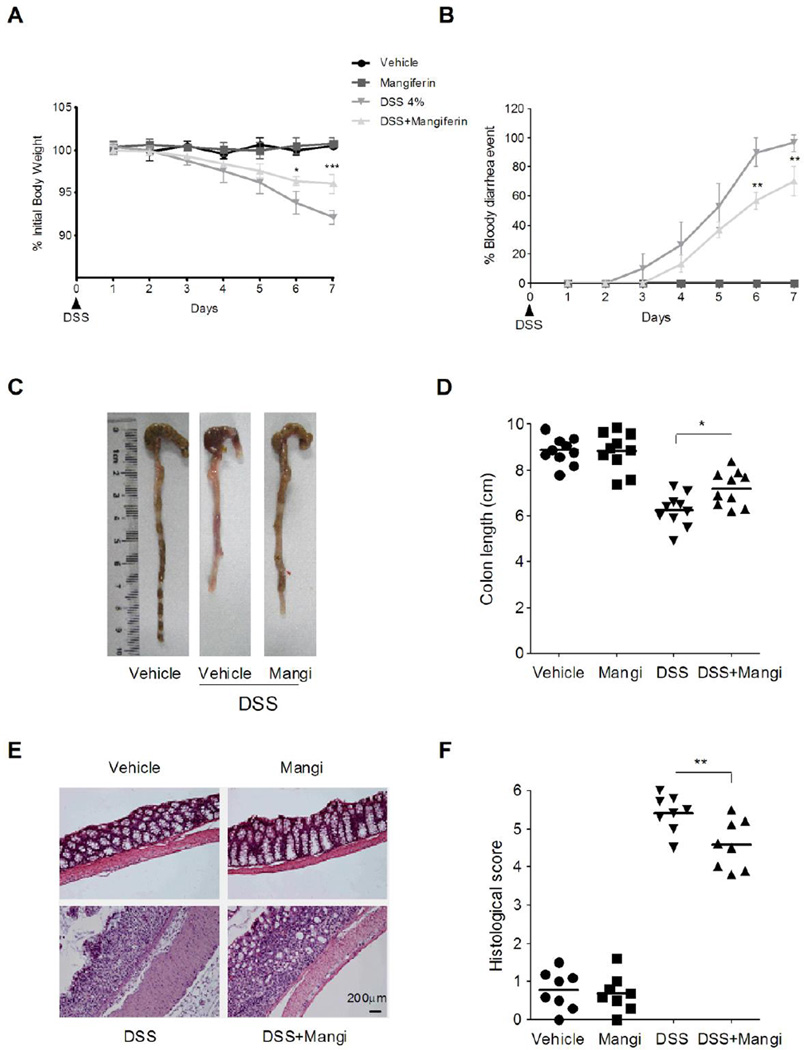
Oral administration of DSS in mice induces colitis that resembles human UC, and the inflammation is mainly localized to the colon [32]. In mice receiving DSS from day 3 onward, we observed a significant body weight loss and bloody diarrhea incidence. Administration of mangiferin significantly ameliorated the weight loss and bloody diarrhea symptoms (Fig. 1A and 1B). Colon shortening is an indication of colitis and is therefore measured as an inflammatory marker [33]. After 7 days of treatment with DSS in the drinking water, there was a significant shortening of the colon length compared with the healthy control mice, and this parameter was reversed by mangiferin treatment (Fig. 1C and 1D). The colon histologic sections from mice exposed to DSS exhibited a discontinuous disruption of the epithelial layer and marked inflammatory cell infiltration (predominantly neutrophils) within the mucosa and sub-mucosa. These parameters were attenuated in mice administered mangiferin (Fig. 1E and 1F). In addition, there was no weight loss, diarrhea, colon shortening or mucosal disruption observed in control mice receiving vehicle or mangiferin alone during the study.
Fig. 1.
Mangiferin attenuated DSS-induced colitis in mice. (A) Body weight changes following DSS induction of colitis. Data are plotted as the percentage of basal body weight. (B) The occurrence of bloody diarrhea. Data plotted as percentage of total mice that had bloody diarrhea at different time points of DSS treatment. (C) and (D) Macroscopic observation and assessment of colon shortening. (E) Representative H&E-stained colon sections and histology score. Scale bar corresponds to 200 µ m and applies throughout. (F). Values were expressed as the mean ± SD of n = 10 mice in each group. * p<0.05, ** P< 0.01, *** p<0.001 vs. DSS-treated group.
3.2. Mangiferin decreased the activity of MPO and the level of TNF-α in the colon
MPO is an enzyme produced mainly by neutrophils. It is a marker of inflammation, tissue injury and neutrophil infiltration in a given tissue [34]. Histological images and enhanced MPO activity in colon tissues showed an increase in neutrophil infiltration in tissues receiving DSS administration, which were improved by mangiferin treatment (Fig. 1E and Table 1). Moreover, TNF-α is indicated to play a vital role in the UC inflammatory process [9]. A significant increase in the content of TNF-α was observed in mice exposed to DSS and treatment with mangiferin reduced the level of TNF-α in the inflamed colon (Table 1). These data suggest that mangiferin might exert anti-inflammatory effect by reducing neutrophil infiltration and the production of pro-inflammatory cytokines in the colonic mucosa.
Table 1.
Effects of mangiferin on the activity of MPO and the level of TNF-α in DSS-induced colitis mice.
| Group | TNF-α (pg/mg pr.) | MPO (U/mg pr.) |
|---|---|---|
| Vehicle | 23.9 ± 1.8 | 5.7 ± 0.3 |
| DSS | 198.1 ± 8.3### | 20.3 ± 1.8#### |
| DSS+Mangiferin | 134.8 ±11.6* | 14.1 ± 0.5** |
Colon segments from mice (n = 6 per group) were excised and homogenized. The supernatants were assayed for the determination of the activity of MPO and the level of TNF-α as described in the Methods. Values are expressed as the mean ± SD (n = 6).
p<0.001 vs. vehicle-treated group;
p < 0.05, ** P < 0.01 vs. DSS-treated group.
3.3. Mangifern downregulated the mRNAs expression of pro-inflammatory mediators in the colon
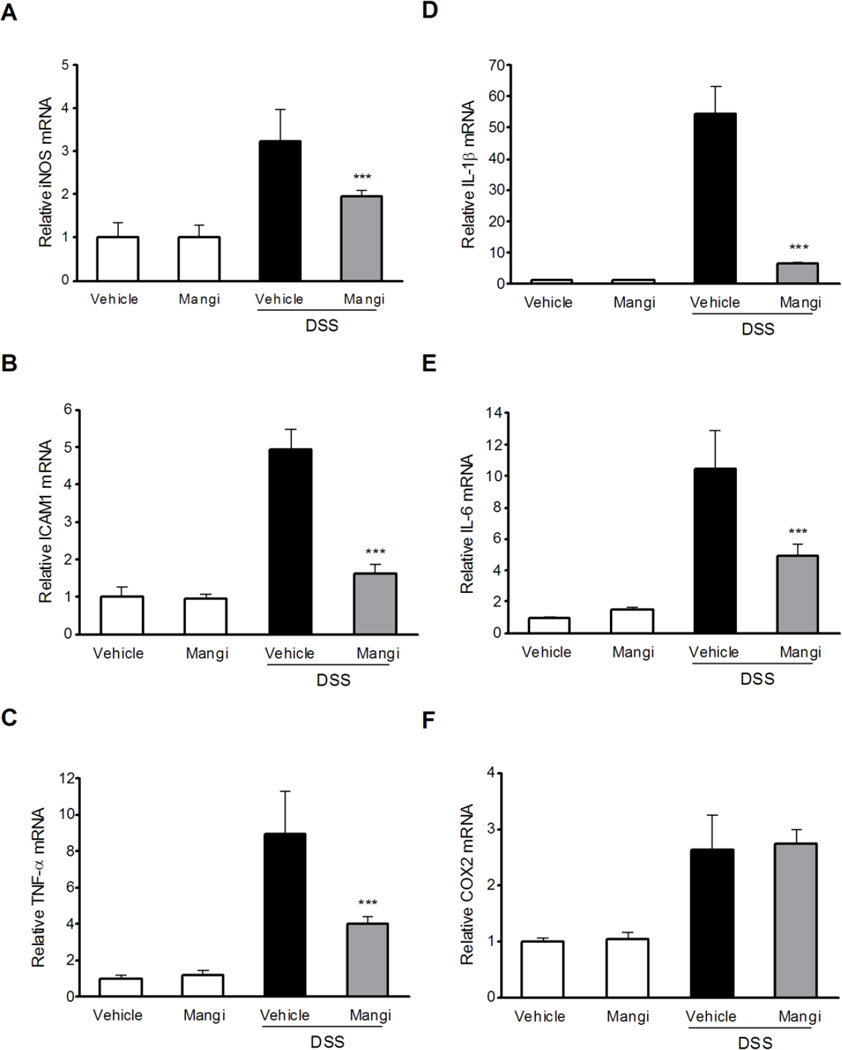
To determine the effect of mangiferin on pro-inflammatory mediator genes expression, real-time quantitative reverse transcription PCR (qRT-PCR) analysis of iNOS, ICAM-1, TNF-α, COX2, IL-1β and IL-6 genes in the colon was carried out. The relative increase in mRNA expression of iNOS, ICAM-1, TNF-α, IL-1β and IL-6 after DSS treatment was significantly downregulated in mice pretreated with mangiferin (Fig. 2A-2E), whereas no downregulation of COX2 mRNA expression was observed in DSS-induced colitic mice after mangiferin treatment (Fig. 2F). Thus, suppression of the pro-inflammatory genes may be related to the attenuated effect of mangiferin on experimental IBD.
Fig. 2.
The effects of mangiferin on NF-κB target genes expression in the colon. mRNA expression of iNOS (A), ICAM-1 (B), TNF-α (C), IL-1β (D), IL-6 (E) and COX2 (F) was determined by qRT-PCR in colon samples isolated from mice (n = 6 per group). Expression was normalized to β-actin, and each bar represents the mean ± SD of two independent experiments with samples in triplicate. * p < 0.05, ** p < 0.01, ***P < 0.001 vs. DSS-treated group.
3.4. Mangiferin blocked the activation of NF-κB in the colon
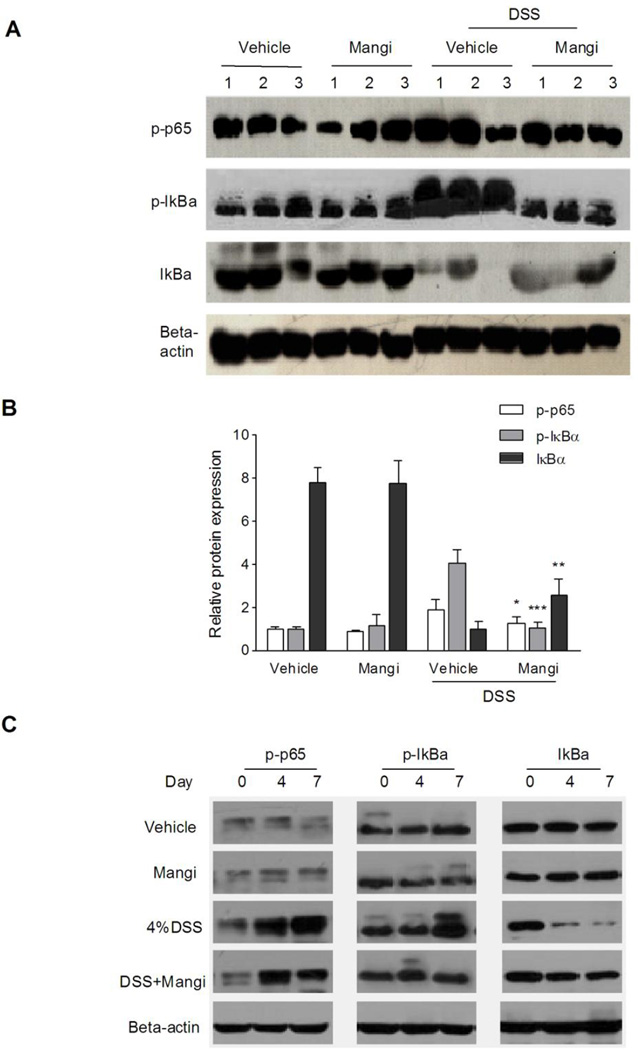
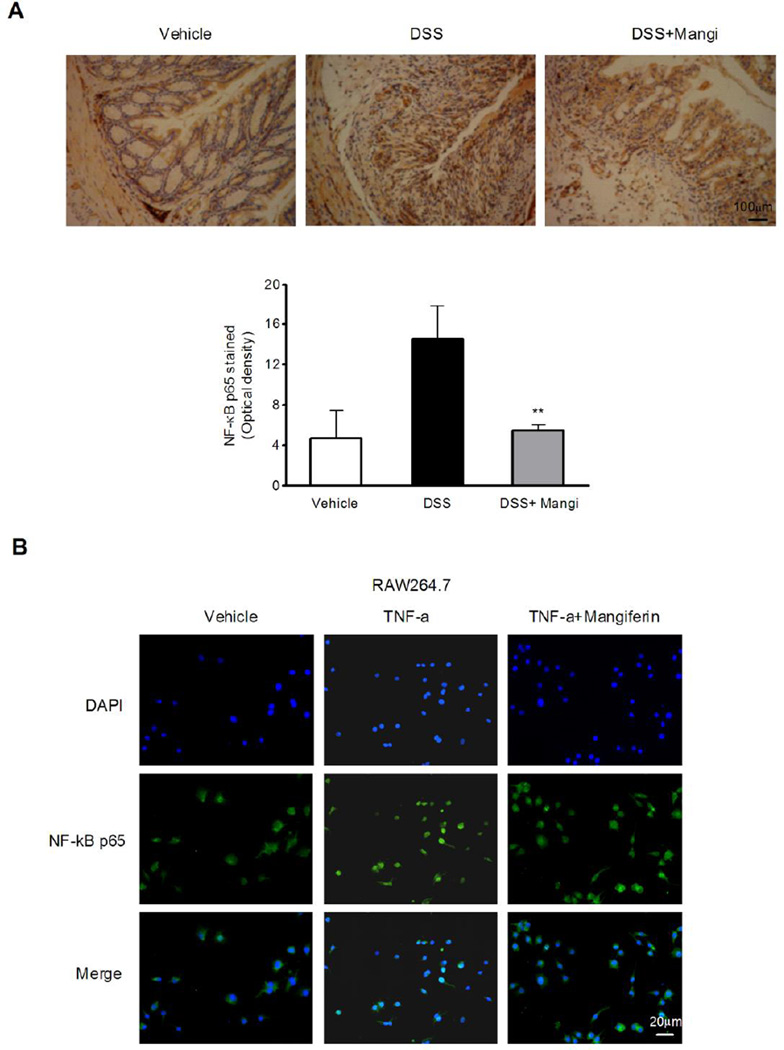
NF-κB is a key transcription factor that regulates the expression of genes encoding pro-inflammatory mediators. To determine whether the inhibitory effect of mangiferin on the expression of pro-inflammatory mediator genes was due to NF-κB activation inhibition, we examined phospho-p65, phospho-IκBα and IκBα protein levels by western blotting. The results showed that the DSS-induced phosphorylation/degradation of IκBα and the phosphorylation of NF-κB p65 were blocked by mangiferin treatment (Fig. 3A and 3B). Next, we examined the effects of mangiferin on colonic NF-κB activity in different point of time. As shown in Fig. 3C, the DSS-induced phosphorylation/degradation of IκBα and the phosphorylation of NF-κB p65 were up-regulated from d 4 to d 7. Mangiferin treatment had little effects on the phosphorylation of IκBα and NF-κB p65 at d 4 compared to DSS alone treatment group, while significantly inhibited the DSS-induced phosphorylation/degradation of IκBα and the phosphorylation of NF-κB p65 at d 7. To provide further insight into the effects of mangiferin on colonic NF-κB inhibition, we performed immunohistochemical analysis on paraffin-embedded colon tissue using anti-phospho-NF-κB p65 antibody. As expected, after 7 days of DSS exposure, a pronounced phosphorylation of NF-κB p65 in the colonic tissue was observed (Fig. 4A). Mangiferin treatment markedly reduced the NF-κB p65 activation in the colon.
Fig. 3.
Mangiferin blocked the activation of NF-κB in the colon. (A) Mice (n = 6 per group) were sacrificed after 7 days of 4% DSS exposure, and total protein (40 µg) from colon samples was loaded. Western blot protein levels were detected with phospho-p65 (1:1000), phospho-IκBα (1:1000) and IκBα (1:1000) antibodies. One representative experiment is shown. (B) Quantification of the protein expression was performed by densitometric analysis of the blots. (C) Mice (n = 6 per group) were sacrificed on day 0, 4 and 7, and total protein (40 µg) from colon samples was loaded. Western blot protein levels were detected. One representative experiment is shown. Data were expressed as the mean ± SD of two independent experiments with samples in triplicate. * p < 0.05, ** p < 0.01, ***P < 0.001 vs. DSS-treated group.
Fig. 4.
Mangiferin attenuated NF-κB activation in the colon tissue and inhibited the nuclear translocation of NF-κB p65 in RAW264.7 cells. (A) Representative images of phospho-NF-κB p65 immunostaining in colon tissue (upper panel). Scale bar corresponds to 100 µm and applies throughout. Graphical representation of the expression for phospho-NF-κB p65 in colon tissue (lower panel). The mean intensity of phospho-NF-κB p65 staining was determined by image analysis and are represented as optical density. Each column represents the mean ± SD of two independent experiments (n=6 per group). ** p < 0.01 vs. DSS-treated group. (B) RAW264.7 cells were pretreated with or without mangiferin (25 µM) for 2 h followed by an additional treatment with TNF-α (20 ng/ml) for 12 h. NF-κB p65 localization was observed under a fluorescence microscope (magnification 200×) using an NF-κB p65 antibody (1:50) followed by an Alexa 488-conjugated secondary antibody (1:500). Scale bar corresponds to 20 µ m and applies throughout.
3.5. Mangiferin inhibited the nuclear translocation of NF-κB p65 in mouse macrophage cells
To investigate the cellular mechanism by which mangiferin attenuated chemically induced IBD, we evaluated the activation state of NF-κB after mangiferin treatment in RAW264.7 mouse macrophage cells. Consistent with the in vivo results, TNF-α-stimulated nuclear translocation of NF-κB p65 was blocked by mangiferin treatment (Fig. 4B).
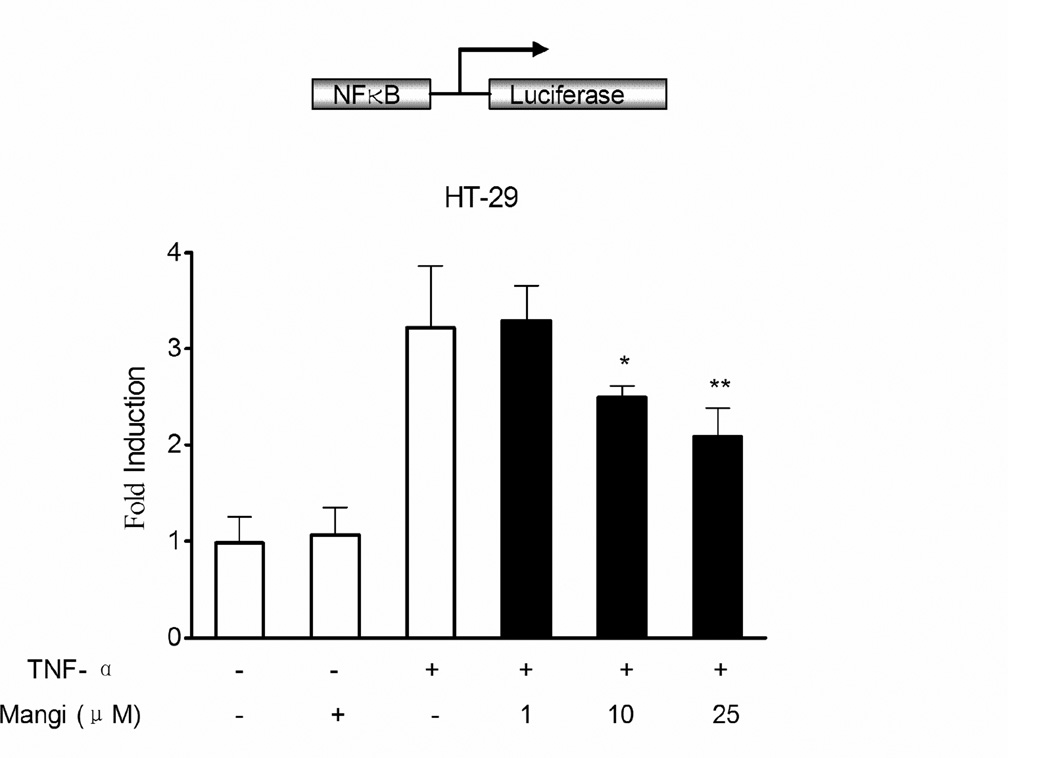
3.6. Mangiferin decreased NF-κB-luciferase activity
Using a luciferase reporter assay, we confirmed the effect of mangiferin on the transcriptional activity of NF-κB. HT-29 human colorectal adenocarcinoma cells were transiently transfected with NF-κB reporter construct pretreated with mangiferin and stimulated with TNF-α overnight. TNF-α caused a significant increase in NF-κB reporter activity, which was reduced in a dose dependent manner by pretreatment with mangiferin (Fig. 5).
Fig. 5.
Mangiferin reduced NF-κB-luciferase activity. HT-29 cells were transiently transfected with pGL4.32 [luc2P/NF-κB-RE/Hygro] construct. 12 h after transfection, cells were incubated with or without mangiferin (1, 10, and 25 µmol/l) for 2 hours and then treated with TNF-α (20 ng/ml) for an additional 12 hours. Cell lysates were analyzed for the NF-κB promoter-driven luciferase activity, which was expressed as the fold change in values compared with the control cells (designated as 1). Data are expressed as the mean ± SD of quadruplicates from two independent experiments. *P < 0.05, **P < 0.01 vs. TNF-α alone treatment group.
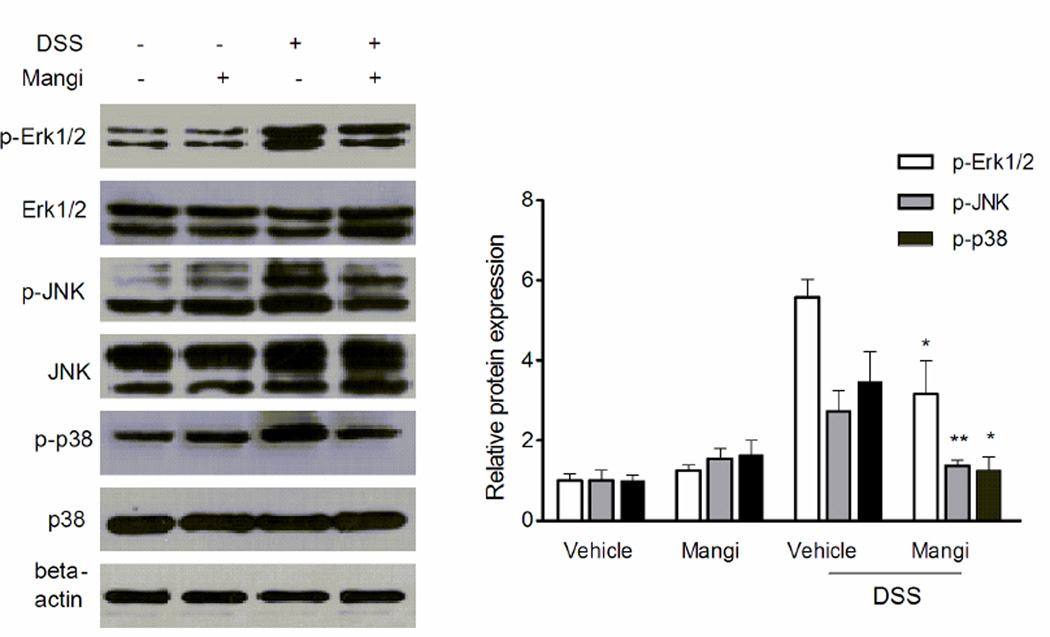
3.7. Mangiferin suppressed MAPK phosphorylation/activation in the colon
The activation of MAPK pathway has been implicated in the pathogenesis of IBD [35]. Finally, we assessed the effects of mangiferin on the activation of MAPK signaling molecules, including ERK1/2, JNK and p38. As shown in Fig. 6, DSS induced strong phosphorylation (activation) of ERK1/2, JNK and p38 in the inflamed colon. Interestingly, mangiferin treatment pronouncely inhibited the DSS-induced increase of phosphorylation of ERK1/2, JNK and p38. The results suggest that MAPK signaling suppression may also contribute to the anti-inflammatory effect of mangiferin.
Fig. 6.
Mangiferin inhibited MAPK phosphorylation/activation. Expression of the phospho-ERK1/2 (1:1000), ERK1/2 (1:1000), phospho-JNK (1:2000), JNK (1:1000), phospho-p38 (1:1000), p38 (1:1000) and β-actin (1:2000) in the colon tissue was analysed by western blot (n=4). Quantification of the protein expression was performed by densitometric analysis of the blots. *p < 0.05, **P< 0.01 vs. DSS-treated group.
4. Discussion
DSS-induced colitis is a well-established experimental model in which the inflammation is mainly localized to the colon with features resembling human ulcerative colitis (UC), such as ulceration, epithelial damage, mucosal inflammatory cellular infiltration, and lymphoid hyperplasia [32]. UC reportedly affects 24 per 10,000 people in the United States, and the prevalence in northern Europe ranges from 4 to 24 per 100,000 people [36]. A recent report on the incidence of IBD indicated that the prevalence of UC is rapidly growing in Asian countries [37]. Lifestyle changes may contribute to this increased incidence. Dietary habits in Asian countries have changed, resulting in a western-style diet with fewer plant-based and more processed food. A recent systematic review reported a negative correlation between UC risk and vegetable intake and a positive correlation between UC risk and the intake of total fat, omega-6 fatty acids and meat [38,39]. Notably, the increased incidence of UC may be closely related to an increase in the prevalence of colorectal cancer [39]. Therapeutic options currently available for the management of UC are numerous and generally include the administration of 5-aminosalicylates or sulfasalazine, antibiotics, glucocorticoids, immunosuppressive agents (e.g., 6-mercaptopurine, azathioprine, methotrexate, cyclosporine and tacrolimus) and biological therapies, such as anti-TNF agents (e.g., infliximab, adalimumab and certolizumab). However, despite their efficacy, some patients do not respond to treatment or suffer from significant side effects or complications. Therefore, there is an unmet need for new therapeutics with fewer potential adverse reactions [40].
Although the etiology of UC is currently unknown, increasing evidence suggests that UC results from aberrant innate immune responses to the enteric microbiota in a genetically susceptible host [41]. The intestinal lumen contains a vast array of different substances that may interact with the host, such as dietary factors, microbial components (e.g., bacteria and viruses), and environmental pollutants. Many of these substances interact with NF-κB, a key transcription factor involved in the pathogenesis of IBD [15]. Upon activation, NF-κB regulates the expression of pro-inflammatory cytokines, adhesion molecules, growth factors, and proliferation and survival genes, which impact both the extent and duration of intestinal inflammation. In the current study, we showed that mangiferin inhibited IκBα degradation and NF-κB p65 phosphorylation in mice colon mucosa, blocked NF-κB nuclear translocation in TNF-α-stimulated RAW264.7 mouse macrophage cells, and decreased NF-κB-luciferase expression in HT-29 human colon adenocarcinoma cells. Furthermore, mangiferin pretreatment alleviated the symptoms of DSS-induced colitis and improved the disease histopathology by reducing the activity of MPO, the level of TNF-α, and the mRNA expression of various inflammatory mediators, such as iNOS, ICAM-1, TNF-α, IL-1β and IL-6 in the colon. These data clearly demonstrates a protective role for mangiferin in DSS-induced colitis. Moreover, the data indicate that NF-kB inactivation is, at least in part, the possible mechanism by which mangiferin decreased the susceptibility of mice to DSS-induced colitis. Notably, none of the mice receiving mangiferin alone exhibited apparent body weight loss, diarrhea, colon shortening and mucosal disruption throughout the study, which indicates the relative safety of mangiferin management.
Several previous studies have demonstrated that mangiferin downregulates key inflammatory molecules. A recent study has indicated that mangiferin decreases the levels of IL-1β and TNF-α in rats with diabetes-associated cognitive impairment [42]. Das et al. reported that the hepatoprotective role of mangiferin is due to the induction of antioxidant defense systems and the reduction of inflammation via NF-κB inhibition [43]. Moreover, pre-administration of mangiferin in mice was indicated to prevent from stress-induced neuroinflammation and oxidative damage in the brain via the modulation of multiple molecules, including glucocorticoids (GCs), NF-κB, iNOS, COX-2, IL-1β and TNF-α [30]. Wei et al. showed that mangiferin inhibits lipopolysaccharide (LPS)-induced chronic inflammation in rats by regulating the MAPK signaling pathway [44]. MAPKs are the upstream enzymes and signaling molecules for NF-κB [45]. Recent studies demonstrated that ERK1/2, JNK and p38 MAPKs, as well as NF-κB are dramatically activated during the development of colitis [20, 46]. It has been reported that the ethanol extract of Antrodia camphorate inhibits activated MAPK and NF-κB in the colon tissue of DSS-induced colitis mice [46]. In this study, we found that mangiferin not only inhibited NF-κB signaling but also inhibited phosphorylation (activation) of MAPK signaling molecules, including ERK1/2, JNK and p38. The results suggest that mangiferin may exert anti-inflammatory activities through the regulation of the NF-κB signaling pathway and its upstream MAPK signaling proteins.
In addition to its anti-inflammatory effects, mangiferin has strong anti-cancer properties [22]. These properties have been associated with the inhibition of cellular proliferation, induction of apoptosis, and prevention of invasion [47]. Interestingly, mangiferin is suggested to enhance the inhibition of colon carcinogenesis induced by chemical carcinogens in rat [48]. Thus, the anti-cancer properties of mangiferin increase its promising medicinal value for patients with long-standing UC because the most important clinical issue for patients with long-standing UC is an increased risk for development of dysplasia and colon cancer [49].
In conclusion, The current study clearly demonstrates a protective role for mangiferin in experimental IBD possibly through NF-κB and MAPK signaling inhibition. Since mangiferin is a natural compound with little toxicity, these novel findings may contribute to the effective utilization of mangiferin or its derivatives in the treatment of human IBD.
Highlights.
► Mangiferin inhibited MPO activity, TNF-α level and the expressions of iNOS, ICAM-1, TNF-α, IL-1β and IL-6 in the colon. ► Mangiferin inhibited IκBα degradation and NF-κB/p65 phosphorylation in mice colon mucosa. ► Mangiferin blocked NF-κB nuclear translocation in RAW264.7 cells and decreased NF-κB-luciferase expression in HT-29 cells. ► Mangiferin inhibited the activation of MAPK signaling molecules, including ERK1/2, JNK and p38. ► Mangiferin MAPK significantly ameliorated DSS-induced colitis possibly via NF-κB and MAPK signaling inactivation.
Acknowlegements
This work was supported by National Natural Science Foundation of China (81273572, U1032604); Natural Science Foundation of Shanghai (12ZR1431400); Innovation Program of Shanghai Municipal Education Commission (13YZ043); National Institutes of Health (RO1CA127231); Damon Runyon Foundation Clinical Investigator Award (CI 1502).
Abbreviations
- CD
Crohn’s disease
- COX2
cyclooxygenase 2
- DSS
dextran sodium sulfate
- ELISA
enzyme linked immunosorbent assay
- ERK
extracellular signal-regulated kinase
- GC
glucocorticoid
- HPLC
high pressure liquid chromatography
- IBD
inflammatory bowel disease
- ICAM-1
intercellular adhesion molecule-1
- IFN-γ
interferon-gamma
- IL-1β/IL-2/IL-6/IL-12/IL-17
interleukin-1β/2/6/12/17
- IκBα
inhibitory κB-α
- iNOS
inducible NO synthase
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide; monocyte chemotactic protein-1
- Mangi
Mangiferin
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemotactic protein-1
- MPO
myeloperoxidase
- NF-κB
nuclear transcription factor-kappa B
- qRT-PCR
real-time quantitative reverse transcription-polymerase chain reaction
- TNF-α
tumor necrosis factor-α
- UC
ulcerative colitis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 2.Dyson JK, Rutter MD. Colorectal cancer in inflammatory bowel disease: what is the real magnitude of the risk? World J Gastroenterol. 2012;18:3839–3848. doi: 10.3748/wjg.v18.i29.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 4.van der Marel S, Majowicz A, van Deventer S, et al. Gene and cell therapy based treatment strategies for inflammatory bowel diseases. World J Gastrointest Pathophysiol. 2011;2:114–122. doi: 10.4291/wjgp.v2.i6.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nielsen OH, Bjerrum JT, Herfarth H, et al. Recent advances using immunomodulators for inflammatory bowel disease. J Clin Pharmacol. 2013;53:575–588. doi: 10.1002/jcph.2. [DOI] [PubMed] [Google Scholar]
- 6.Poitras P, Gougeon A, Binn M, et al. Extra digestive manifestations of irritable bowel syndrome: intolerance to drugs? Dig Dis Sci. 2008;53:2168–2176. doi: 10.1007/s10620-007-0123-8. [DOI] [PubMed] [Google Scholar]
- 7.Nunes C, Ferreira E, Freitas V, Laranjinha J, et al. Intestinal anti-inflammatory activity of red wine extract: unveiling the mechanisms in colonic epithelial cells. Food Funct. 2013;4:373–383. doi: 10.1039/c2fo30233k. [DOI] [PubMed] [Google Scholar]
- 8.Park MY, Ji GE, Sung MK. Dietary kaempferol suppresses inflammation of dextran sulfate sodium-induced colitis in mice. Dig Dis Sci. 2012;57:355–363. doi: 10.1007/s10620-011-1883-8. [DOI] [PubMed] [Google Scholar]
- 9.Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2000;51:289–298. doi: 10.1146/annurev.med.51.1.289. [DOI] [PubMed] [Google Scholar]
- 10.Rogler G, Andus T. Cytokines in inflammatory bowel disease. World J Surg. 1998;22:382–389. doi: 10.1007/s002689900401. [DOI] [PubMed] [Google Scholar]
- 11.Stokkers PC, Hommes DW. New cytokine therapeutics for inflammatory bowel disease. Cytokine. 2004;28:167–173. doi: 10.1016/j.cyto.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Coffin CS, Fraser HF, Panaccione R, et al. Liver diseases associated with anti-tumor necrosis factor-alpha (TNF-α) use for inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:479–484. doi: 10.1002/ibd.21336. [DOI] [PubMed] [Google Scholar]
- 13.Andresen L, Jørgensen VL, Perner A, et al. Activation of nuclear factor kappaB in colonic mucosa from patients with collagenous and ulcerative colitis. Gut. 2005;54:503–509. doi: 10.1136/gut.2003.034165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B infammatory bowel disease. Gut. 1998;42:477–484. doi: 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh K, Chaturvedi R, Barry DP, et al. The apolipoprotein E-mimetic Pettide COG112 inhibits NF-kappaB signaling, proinflammatory cytokine expression, and disease activity in murine models of colitis. J Biol Chem. 2011;286:3839–3850. doi: 10.1074/jbc.M110.176719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrance IC, Wu F, Leite AZ, et al. A murine model of chronic inflammation-induced intestinal fibrosis down-regulated by antisense NF-kappa B. Gastroenterology. 2003;125:1750–1761. doi: 10.1053/j.gastro.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Hibi T, Inoue N, Ogata H, et al. Introduction and overview: recent advances in the immunotherapy of inflammatory bowel disease. J Gastroenterol. 2003;38:36–42. [PubMed] [Google Scholar]
- 18.Li Z, Zhang deK, Yi WQ, et al. NF-kappaB p65 antisense oligonucleotides may serve as a novel molecular approach for the treatment of patients with ulcerative colitis. Arch Med Res. 2008;39:729–734. doi: 10.1016/j.arcmed.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Shah YM, Ma X, Morimura K, et al. Pregnane X receptor activation ameliorates DSS-induced inflammatory bowel disease via inhibition of NF-kappaB target gene expression. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1114–G1122. doi: 10.1152/ajpgi.00528.2006. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Dou W, Zhang E, et al. Paeoniflorin abrogates DSS-induced colitis via a TLR4-dependent pathway. Am J Physiol Gastrointest Liver Physiol. 2014;306:G27–G36. doi: 10.1152/ajpgi.00465.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dou W, Zhang J, Zhang E, et al. Chrysin ameliorates chemically induced colitis in the mouse through modulation of a PXR/NF-κB signaling pathway. J Pharmacol Exp Ther. 2013;345:473–482. doi: 10.1124/jpet.112.201863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matkowski A, Kuś P, Góralska E, et al. Mangiferin - a bioactive xanthonoid, not only from mango and not just antioxidant. Mini Rev Med Chem. 2013;13:439–455. [PubMed] [Google Scholar]
- 23.Telang M, Dhulap S, Mandhare A, et al. Therapeutic and cosmetic applications of mangiferin: a patent review. Expert Opin Ther Pat. 2013;23:1561–1580. doi: 10.1517/13543776.2013.836182. [DOI] [PubMed] [Google Scholar]
- 24.Pal PB, Sinha K, Sil PC. Mangiferin, a natural xanthone, protects murine liver in Pb(II) induced hepatic damage and cell death via MAP kinase, NF-κB and mitochondria dependent pathways. PLoS One. 2013;8:e56894. doi: 10.1371/journal.pone.0056894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong X, Zhang L, Jiang R, et al. Anti-inflammatory effects of mangiferin on sepsis-induced lung injury in mice via up-regulation of heme oxygenase-1. J Nutr Biochem. 2013;24:1173–1181. doi: 10.1016/j.jnutbio.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Zheng D, Hou J, Xiao Y, et al. Cardioprotective effect of mangiferin on left ventricular remodeling in rats. Pharmacology. 2012;90:78–87. doi: 10.1159/000339450. [DOI] [PubMed] [Google Scholar]
- 27.Thapa D, Lee JS, Park SY, et al. Clotrimazole ameliorates intestinal inflammation and abnormal angiogenesis by inhibiting interleukin-8 expression through a nuclear factor-kappaB-dependent manner. J Pharmacol Exp Ther. 2008;327:353–364. doi: 10.1124/jpet.108.141887. [DOI] [PubMed] [Google Scholar]
- 28.Wu TY, Khor TO, Saw CL, et al. Anti-inflammatory/Anti-oxidative stress activities and differential regulation of Nrf2-mediated genes by non-polar fractions of tea Chrysanthemum zawadskii and licorice Glycyrrhiza uralensis. AAPS J. 2011;13:1–13. doi: 10.1208/s12248-010-9239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dou W, Mukherjee S, Li H, et al. Alleviation of gut inflammation by Cdx2/Pxr pathway in a mouse model of chemical colitis. PLoS One. 2012;7:e36075. doi: 10.1371/journal.pone.0036075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Márquez L, García-Bueno B, Madrigal JL, et al. Mangiferin decreases inflammation and oxidative damage in rat brain after stress. Eur J Nutr. 2012;51:729–739. doi: 10.1007/s00394-011-0252-x. [DOI] [PubMed] [Google Scholar]
- 31.Dou W, Zhang J, Sun A, Zhang E, et al. Protective effect of naringenin against experimental colitis via suppression of Toll-like receptor 4/NF-κB signalling. Br J Nutr. 2013;110:599–608. doi: 10.1017/S0007114512005594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalmasso G, Nguyen HT, Ingersoll SA, et al. The PepT1-NOD2 signaling pathway aggravates induced colitis in mice. Gastroenterology. 2011;141:1334–1345. doi: 10.1053/j.gastro.2011.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siegmund B, Lehr HA, Fantuzzi G, et al. IL-1b eta-converting enzyme (caspase-1) in intestinal inflammation. Proc Natl Acad Sci USA. 2001;98:13249–13254. doi: 10.1073/pnas.231473998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- 35.Sánchez-Fidalgo S, Sánchez de Ibargüen L, Cárdeno A, et al. Influence of extra virgin olive oil diet enriched with hydroxytyrosol in a chronic DSS colitis model. Eur J Nutr. 2012;51:497–506. doi: 10.1007/s00394-011-0235-y. [DOI] [PubMed] [Google Scholar]
- 36.Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 37.Prideaux L, Kamm MA, De Cruz PP, et al. Inflammatory bowel disease in Asia: a systematic review. J Gastroenterol Hepatol. 2012;27:1266–1280. doi: 10.1111/j.1440-1746.2012.07150.x. [DOI] [PubMed] [Google Scholar]
- 38.Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106:563–573. doi: 10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- 39.Sung MK, Park MY. Nutritional modulators of ulcerative colitis: clinical efficacies and mechanistic view. World J Gastroenterol. 2013;19:994–1004. doi: 10.3748/wjg.v19.i7.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Speight RA, Mansfield JC. Drug advances in inflammatory bowel disease. Clin Med. 2013;13:378–382. doi: 10.7861/clinmedicine.13-4-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reiff C, Kelly D. Inflammatory bowel disease, gut bacteria and probiotic therapy. Int J Med Microbiol. 2010;300:25–33. doi: 10.1016/j.ijmm.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Liu YW, Zhu X, Yang QQ, et al. Suppression of methylglyoxal hyperactivity by mangiferin can prevent diabetes-associated cognitive decline in rats. Psychopharmacology (Berl) 2013;228:585–594. doi: 10.1007/s00213-013-3061-5. [DOI] [PubMed] [Google Scholar]
- 43.Das J, Ghosh J, Roy A, et al. Mangiferin exerts hepatoprotective activity against D-galactosamine induced acute toxicity and oxidative/nitrosative stress via Nrf2-NFκB pathways. Toxicol Appl Pharmacol. 2012;260:35–47. doi: 10.1016/j.taap.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 44.Wei Z, Yan L, Deng J, et al. Effects of mangiferin on MAPK signaling pathway in chronic inflammation. Zhongguo Zhong Yao Za Zhi. 2011;36:1798–1802. [Article in Chinese] [PubMed] [Google Scholar]
- 45.Park DK, Park HJ. Ethanol Extract of Antrodia camphorata Grown on Germinated Brown Rice Suppresses Inflammatory Responses in Mice with Acute DSS-Induced Colitis. Evid Based Complement Alternat Med. 2013;2013:914524. doi: 10.1155/2013/914524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sánchez-Fidalgo S, Cárdeno A, Sánchez-Hidalgo M, et al. Dietary extra virgin olive oil polyphenols supplementation modulates DSS-induced chronic colitis in mice. J Nutr Biochem. 2013;24:1401–1413. doi: 10.1016/j.jnutbio.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 47.Jung JS, Jung K, Kim DH, et al. Selective inhibition of MMP-9 gene expression by mangiferin in PMA-stimulated human astroglioma cells: involvement of PI3K/Akt and MAPK signaling pathways. Pharmacol Res. 2012;66:95–103. doi: 10.1016/j.phrs.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 48.Yoshimi N, Matsunaga K, Katayama M, et al. The inhibitory effects of mangiferin, a naturally occurring glucosylxanthone, in bowel carcinogenesis of male F344 rats. Cancer Lett. 2001;163:163–170. doi: 10.1016/s0304-3835(00)00678-9. [DOI] [PubMed] [Google Scholar]
- 49.Andersen NN, Jess T. Has the risk of colorectal cancer in inflammatory bowel disease decreased? World J Gastroenterol. 2013;19:7561–7568. doi: 10.3748/wjg.v19.i43.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]