Abstract
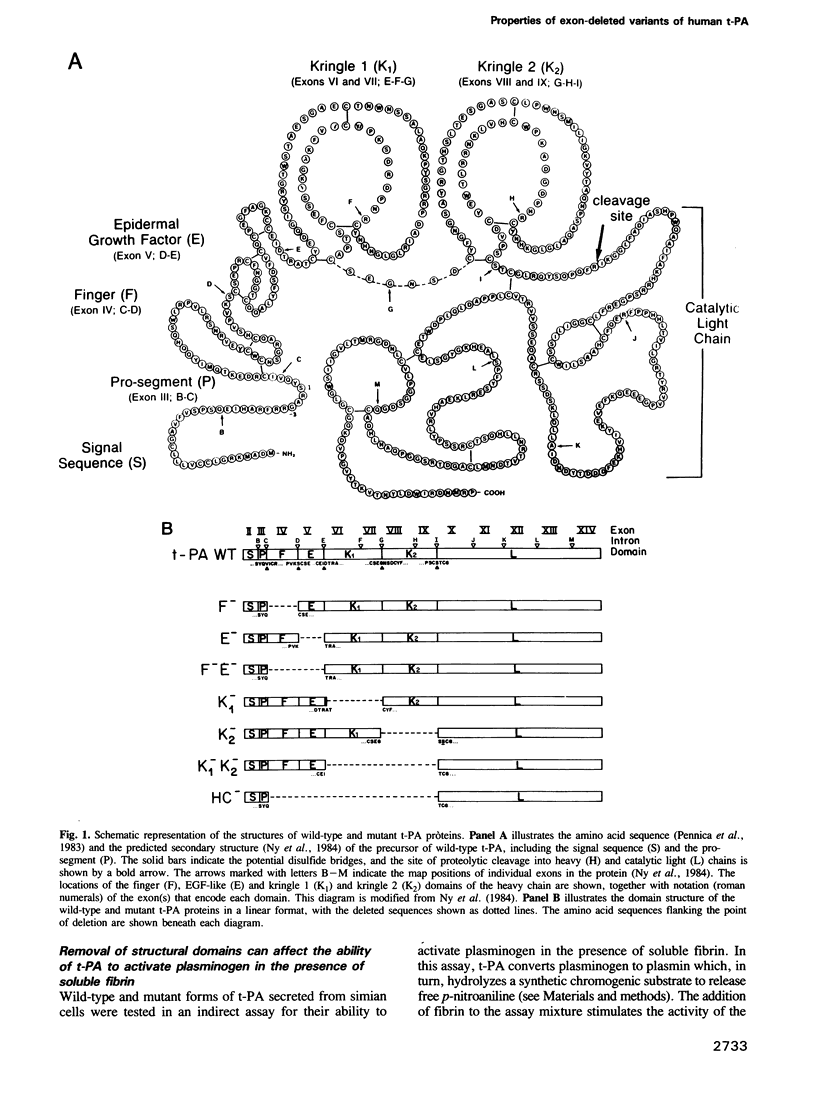
The heavy chain of tissue plasminogen activator (t-PA) consists of four domains [finger, epidermal-growth-factor (EGF)-like, kringle 1 and kringle 2] that are homologous to similar domains present in other proteins. To assess the contribution of each of the domains to the biological properties of the enzyme, site-directed mutagenesis was used to generate a set of mutants lacking sequences corresponding to the axons encoding the individual structural domains. The mutant proteins were assayed for their ability to hydrolyze artificial and natural substrates in the presence and absence of fibrin, to bind to lysine-Sepharose and to be inhibited by plasminogen activator inhibitor-1. All the deletion mutants exhibit levels of basal enzymatic activity very similar to that of wild-type t-PA assayed in the absence of fibrin. A mutant protein lacking the finger domain has a 2-fold higher affinity for plasminogen than wild-type t-PA, while the mutant that lacks both finger and EGF-like domains is less active at low concentrations of plasminogen. Mutants lacking both kringles neither bind to lysine-Sepharose nor are stimulated by fibrin. However, mutants containing only one kringle (either kringle 1 or kringle 2) behave indistinguishably from one another and from the wild-type protein. We conclude that kringle 1 and kringle 2 are equivalent in their ability to mediate stimulation of catalytic activity by fibrin.
Full text
PDF

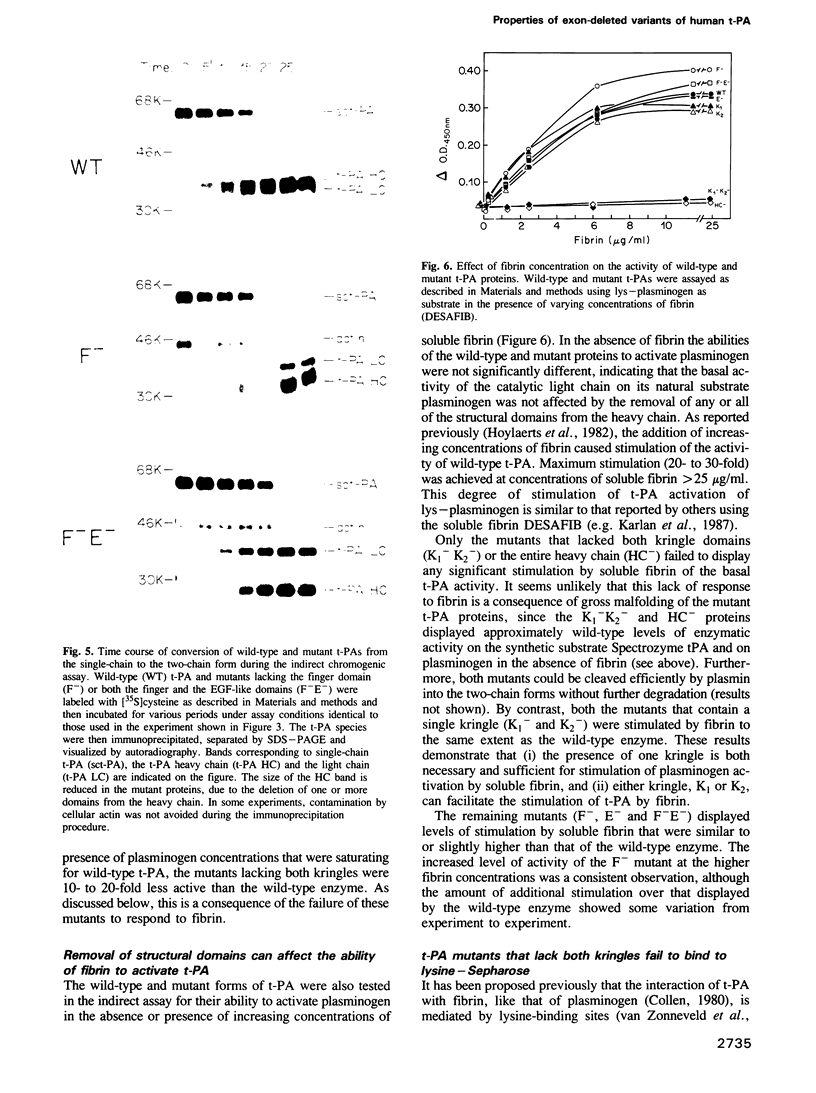
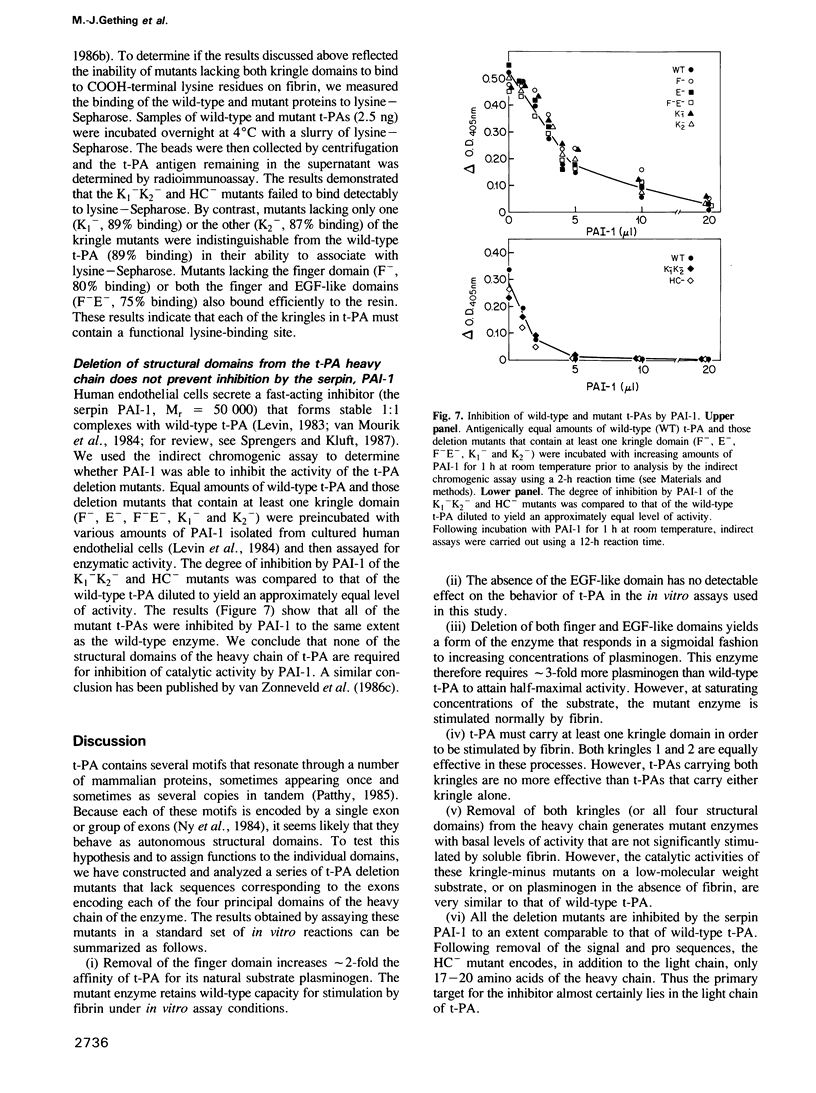




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anson D. S., Choo K. H., Rees D. J., Giannelli F., Gould K., Huddleston J. A., Brownlee G. G. The gene structure of human anti-haemophilic factor IX. EMBO J. 1984 May;3(5):1053–1060. doi: 10.1002/j.1460-2075.1984.tb01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird P., Gething M. J., Sambrook J. Translocation in yeast and mammalian cells: not all signal sequences are functionally equivalent. J Cell Biol. 1987 Dec;105(6 Pt 2):2905–2914. doi: 10.1083/jcb.105.6.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake C. C., Harlos K., Holland S. K. Exon and domain evolution in the proenzymes of blood coagulation and fibrinolysis. Cold Spring Harb Symp Quant Biol. 1987;52:925–931. doi: 10.1101/sqb.1987.052.01.101. [DOI] [PubMed] [Google Scholar]
- Browne M. J., Carey J. E., Chapman C. G., Tyrrell A. W., Entwisle C., Lawrence G. M., Reavy B., Dodd I., Esmail A., Robinson J. H. A tissue-type plasminogen activator mutant with prolonged clearance in vivo. Effect of removal of the growth factor domain. J Biol Chem. 1988 Feb 5;263(4):1599–1602. [PubMed] [Google Scholar]
- Bányai L., Váradi A., Patthy L. Common evolutionary origin of the fibrin-binding structures of fibronectin and tissue-type plasminogen activator. FEBS Lett. 1983 Oct 31;163(1):37–41. doi: 10.1016/0014-5793(83)81157-0. [DOI] [PubMed] [Google Scholar]
- Collen D. On the regulation and control of fibrinolysis. Edward Kowalski Memorial Lecture. Thromb Haemost. 1980 Jun 18;43(2):77–89. [PubMed] [Google Scholar]
- Degen S. J., Rajput B., Reich E. The human tissue plasminogen activator gene. J Biol Chem. 1986 May 25;261(15):6972–6985. [PubMed] [Google Scholar]
- Doolittle R. F., Feng D. F., Johnson M. S. Computer-based characterization of epidermal growth factor precursor. Nature. 1984 Feb 9;307(5951):558–560. doi: 10.1038/307558a0. [DOI] [PubMed] [Google Scholar]
- Doyle C., Roth M. G., Sambrook J., Gething M. J. Mutations in the cytoplasmic domain of the influenza virus hemagglutinin affect different stages of intracellular transport. J Cell Biol. 1985 Mar;100(3):704–714. doi: 10.1083/jcb.100.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle C., Sambrook J., Gething M. J. Analysis of progressive deletions of the transmembrane and cytoplasmic domains of influenza hemagglutinin. J Cell Biol. 1986 Oct;103(4):1193–1204. doi: 10.1083/jcb.103.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D., Davie E. W. Characterization of a cDNA coding for human protein C. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4766–4770. doi: 10.1073/pnas.81.15.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Greenwald I. lin-12, a nematode homeotic gene, is homologous to a set of mammalian proteins that includes epidermal growth factor. Cell. 1985 Dec;43(3 Pt 2):583–590. doi: 10.1016/0092-8674(85)90230-2. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Harris T. J., Patel T., Marston F. A., Little S., Emtage J. S., Opdenakker G., Volckaert G., Rombauts W., Billiau A., De Somer P. Cloning of cDNA coding for human tissue-type plasminogen activator and its expression in Escherichia coli. Mol Biol Med. 1986 Jun;3(3):279–292. [PubMed] [Google Scholar]
- Harris T. J. Second-generation plasminogen activators. Protein Eng. 1987 Dec;1(6):449–458. doi: 10.1093/protein/1.6.449. [DOI] [PubMed] [Google Scholar]
- Hekman C. M., Loskutoff D. J. Endothelial cells produce a latent inhibitor of plasminogen activators that can be activated by denaturants. J Biol Chem. 1985 Sep 25;260(21):11581–11587. [PubMed] [Google Scholar]
- Higgins D. L., Vehar G. A. Interaction of one-chain and two-chain tissue plasminogen activator with intact and plasmin-degraded fibrin. Biochemistry. 1987 Dec 1;26(24):7786–7791. doi: 10.1021/bi00398a038. [DOI] [PubMed] [Google Scholar]
- Holland S. K., Harlos K., Blake C. C. Deriving the generic structure of the fibronectin type II domain from the prothrombin Kringle 1 crystal structure. EMBO J. 1987 Jul;6(7):1875–1880. doi: 10.1002/j.1460-2075.1987.tb02446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
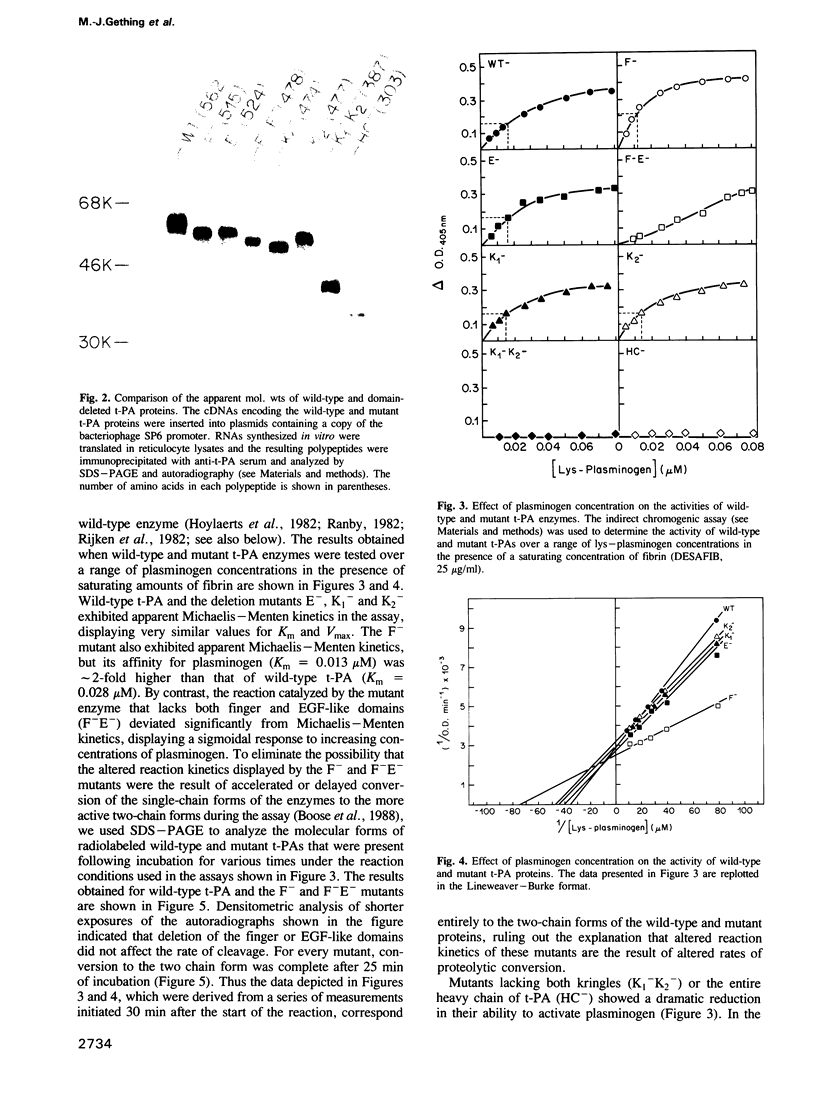
- Hoylaerts M., Rijken D. C., Lijnen H. R., Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982 Mar 25;257(6):2912–2919. [PubMed] [Google Scholar]
- Jørgensen M., Philips M., Thorsen S., Selmer J., Zeuthen J. Plasminogen activator inhibitor-1 is the primary inhibitor of tissue-type plasminogen activator in pregnancy plasma. Thromb Haemost. 1987 Oct 28;58(3):872–878. [PubMed] [Google Scholar]
- Kagitani H., Tagawa M., Hatanaka K., Ikari T., Saito A., Bando H., Okada K., Matsuo O. Expression in E. coli of finger-domain lacking tissue-type plasminogen activator with high fibrin affinity. FEBS Lett. 1985 Sep 9;189(1):145–149. doi: 10.1016/0014-5793(85)80860-7. [DOI] [PubMed] [Google Scholar]
- Kalyan N. K., Lee S. G., Wilhelm J., Fu K. P., Hum W. T., Rappaport R., Hartzell R. W., Urbano C., Hung P. P. Structure-function analysis with tissue-type plasminogen activator. Effect of deletion of NH2-terminal domains on its biochemical and biological properties. J Biol Chem. 1988 Mar 15;263(8):3971–3978. [PubMed] [Google Scholar]
- Karlan B. Y., Clark A. S., Littlefield B. A. A highly sensitive chromogenic microtiter plate assay for plasminogen activators which quantitatively discriminates between the urokinase and tissue-type activators. Biochem Biophys Res Commun. 1987 Jan 15;142(1):147–154. doi: 10.1016/0006-291x(87)90463-3. [DOI] [PubMed] [Google Scholar]
- Larsen G. R., Henson K., Blue Y. Variants of human tissue-type plasminogen activator. Fibrin binding, fibrinolytic, and fibrinogenolytic characterization of genetic variants lacking the fibronectin finger-like and/or the epidermal growth factor domains. J Biol Chem. 1988 Jan 15;263(2):1023–1029. [PubMed] [Google Scholar]
- Levin E. G. Latent tissue plasminogen activator produced by human endothelial cells in culture: evidence for an enzyme-inhibitor complex. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6804–6808. doi: 10.1073/pnas.80.22.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E. G., Marzec U., Anderson J., Harker L. A. Thrombin stimulates tissue plasminogen activator release from cultured human endothelial cells. J Clin Invest. 1984 Dec;74(6):1988–1995. doi: 10.1172/JCI111620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leytus S. P., Chung D. W., Kisiel W., Kurachi K., Davie E. W. Characterization of a cDNA coding for human factor X. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3699–3702. doi: 10.1073/pnas.81.12.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean J. W., Tomlinson J. E., Kuang W. J., Eaton D. L., Chen E. Y., Fless G. M., Scanu A. M., Lawn R. M. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 1987 Nov 12;330(6144):132–137. doi: 10.1038/330132a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny T., Elgh F., Lund B. The structure of the human tissue-type plasminogen activator gene: correlation of intron and exon structures to functional and structural domains. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5355–5359. doi: 10.1073/pnas.81.17.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C. H., Tulinsky A. Three-dimensional structure of the kringle sequence: structure of prothrombin fragment 1. Biochemistry. 1986 Jul 15;25(14):3977–3982. doi: 10.1021/bi00362a001. [DOI] [PubMed] [Google Scholar]
- Patthy L. Evolution of the proteases of blood coagulation and fibrinolysis by assembly from modules. Cell. 1985 Jul;41(3):657–663. doi: 10.1016/s0092-8674(85)80046-5. [DOI] [PubMed] [Google Scholar]
- Patthy L., Trexler M., Váli Z., Bányai L., Váradi A. Kringles: modules specialized for protein binding. Homology of the gelatin-binding region of fibronectin with the kringle structures of proteases. FEBS Lett. 1984 Jun 4;171(1):131–136. doi: 10.1016/0014-5793(84)80473-1. [DOI] [PubMed] [Google Scholar]
- Pennica D., Holmes W. E., Kohr W. J., Harkins R. N., Vehar G. A., Ward C. A., Bennett W. F., Yelverton E., Seeburg P. H., Heyneker H. L. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983 Jan 20;301(5897):214–221. doi: 10.1038/301214a0. [DOI] [PubMed] [Google Scholar]
- Petersen L. C., Johannessen M., Foster D., Kumar A., Mulvihill E. The effect of polymerised fibrin on the catalytic activities of one-chain tissue-type plasminogen activator as revealed by an analogue resistant to plasmin cleavage. Biochim Biophys Acta. 1988 Feb 10;952(3):245–254. doi: 10.1016/0167-4838(88)90123-9. [DOI] [PubMed] [Google Scholar]
- Petersen T. E., Thøgersen H. C., Skorstengaard K., Vibe-Pedersen K., Sahl P., Sottrup-Jensen L., Magnusson S. Partial primary structure of bovine plasma fibronectin: three types of internal homology. Proc Natl Acad Sci U S A. 1983 Jan;80(1):137–141. doi: 10.1073/pnas.80.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl G., Källström M., Bergsdorf N., Wallén P., Jörnvall H. Tissue plasminogen activator: peptide analyses confirm an indirectly derived amino acid sequence, identify the active site serine residue, establish glycosylation sites, and localize variant differences. Biochemistry. 1984 Jul 31;23(16):3701–3707. doi: 10.1021/bi00311a020. [DOI] [PubMed] [Google Scholar]
- Rijken D. C., Collen D. Purification and characterization of the plasminogen activator secreted by human melanoma cells in culture. J Biol Chem. 1981 Jul 10;256(13):7035–7041. [PubMed] [Google Scholar]
- Rijken D. C., Hoylaerts M., Collen D. Fibrinolytic properties of one-chain and two-chain human extrinsic (tissue-type) plasminogen activator. J Biol Chem. 1982 Mar 25;257(6):2920–2925. [PubMed] [Google Scholar]
- Russell D. W., Schneider W. J., Yamamoto T., Luskey K. L., Brown M. S., Goldstein J. L. Domain map of the LDL receptor: sequence homology with the epidermal growth factor precursor. Cell. 1984 Jun;37(2):577–585. doi: 10.1016/0092-8674(84)90388-x. [DOI] [PubMed] [Google Scholar]
- Rånby M. Studies on the kinetics of plasminogen activation by tissue plasminogen activator. Biochim Biophys Acta. 1982 Jun 24;704(3):461–469. doi: 10.1016/0167-4838(82)90068-1. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Hanahan D., Rodgers L., Gething M. J. Expression of human tissue-type plasminogen activator from lytic viral vectors and in established cell lines. Mol Biol Med. 1986 Dec;3(6):459–481. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi K., Fukuda M., Hakomori S. Domain structure of hamster plasma fibronectin. Isolation and characterization of four functionally distinct domains and their unequal distribution between two subunit polypeptides. J Biol Chem. 1981 Jun 25;256(12):6452–6462. [PubMed] [Google Scholar]
- Sprengers E. D., Kluft C. Plasminogen activator inhibitors. Blood. 1987 Feb;69(2):381–387. [PubMed] [Google Scholar]
- Tate K. M., Higgins D. L., Holmes W. E., Winkler M. E., Heyneker H. L., Vehar G. A. Functional role of proteolytic cleavage at arginine-275 of human tissue plasminogen activator as assessed by site-directed mutagenesis. Biochemistry. 1987 Jan 27;26(2):338–343. doi: 10.1021/bi00376a002. [DOI] [PubMed] [Google Scholar]
- Verde P., Stoppelli M. P., Galeffi P., Di Nocera P., Blasi F. Identification and primary sequence of an unspliced human urokinase poly(A)+ RNA. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4727–4731. doi: 10.1073/pnas.81.15.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheijen J. H., Caspers M. P., Chang G. T., de Munk G. A., Pouwels P. H., Enger-Valk B. E. Involvement of finger domain and kringle 2 domain of tissue-type plasminogen activator in fibrin binding and stimulation of activity by fibrin. EMBO J. 1986 Dec 20;5(13):3525–3530. doi: 10.1002/j.1460-2075.1986.tb04678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallén P., Bergsdorf N., Rånby M. Purification and identification of two structural variants of porcine tissue plasminogen activator by affinity adsorption on fibrin. Biochim Biophys Acta. 1982 Nov 24;719(2):318–328. doi: 10.1016/0304-4165(82)90105-2. [DOI] [PubMed] [Google Scholar]
- Wharton K. A., Johansen K. M., Xu T., Artavanis-Tsakonas S. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell. 1985 Dec;43(3 Pt 2):567–581. doi: 10.1016/0092-8674(85)90229-6. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]
- van Mourik J. A., Lawrence D. A., Loskutoff D. J. Purification of an inhibitor of plasminogen activator (antiactivator) synthesized by endothelial cells. J Biol Chem. 1984 Dec 10;259(23):14914–14921. [PubMed] [Google Scholar]
- van Zonneveld A. J., Veerman H., MacDonald M. E., van Mourik J. A., Pannekoek H. Structure and function of human tissue-type plasminogen activator (t-PA). J Cell Biochem. 1986;32(3):169–178. doi: 10.1002/jcb.240320302. [DOI] [PubMed] [Google Scholar]
- van Zonneveld A. J., Veerman H., Pannekoek H. Autonomous functions of structural domains on human tissue-type plasminogen activator. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4670–4674. doi: 10.1073/pnas.83.13.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zonneveld A. J., Veerman H., Pannekoek H. On the interaction of the finger and the kringle-2 domain of tissue-type plasminogen activator with fibrin. Inhibition of kringle-2 binding to fibrin by epsilon-amino caproic acid. J Biol Chem. 1986 Oct 25;261(30):14214–14218. [PubMed] [Google Scholar]