Abstract
Background
Although emerging evidence suggests that intra-abdominal hypertension (IAH) is a predictor of the development of acute kidney injury (AKI), it remains unclear whether the presence of IAH is a predictor of prognosis in patients with AKI. The purpose of this study was to assess whether the presence of IAH could predict prognosis in critically ill patients with AKI. The prognostic value of urinary biomarkers was also determined.
Methods
In this prospective observational study, we enrolled 57 patients with established AKI, who were admitted to the intensive care unit between February 2012 and June 2014. IAH was defined as a sustained elevation in intra-abdominal pressure of ≥12 mmHg, in three consecutive measurements performed daily on the first 3 days. Urinary neutrophil gelatinase-associated lipocalin (NGAL), liver-type fatty acid-binding protein, and simplified acute physiology score II score at the time of admission were also examined.
Results
IAH was observed in 78.9% of patients. The in-hospital mortality was 21.1%, and renal recovery during hospitalization was achieved in 40.4% of patients. Although high urinary NGAL [odds ratio (OR), 1.015] and liver-type fatty acid-binding protein (OR, 1.003) were found to be independent predictors of renal recovery, IAH was not. High urinary NGAL (OR, 1.003) and a high simplified acute physiology score II score (OR, 1.102) were independent predictors of in-hospital mortality, while IAH or urinary liver-type fatty acid-binding protein was not.
Conclusion
Although IAH is prevalent in critically ill patients with AKI, it did not predict AKI prognosis. However, urinary NGAL was found to be a useful predictor of both renal recovery and in-hospital mortality.
Keywords: Acute kidney injury, Intra-abdominal hypertension, Prognosis
Introduction
Elevated intra-abdominal pressure (IAP) has long been known to be associated with altered renal function. Bradley and Bradley [1], more than 60 years ago, found that intra-abdominal hypertension (IAH) induced by abdominal compression resulted in reduced renal plasma and urine flow in humans, and suggested that an increase in renal venous pressure accounted for this change in renal function.
IAH, defined as a pathological increase in IAP, is commonly found in critically ill patients admitted to intensive care units (ICUs), and recently has been implicated as a possible cause of acute kidney injury (AKI). Although several epidemiological studies indicated that IAH is a useful predictor for AKI development [2–5], the predictive value of IAH for renal recovery or mortality remains unclear.
The purpose of this study was to determine the prevalence of IAH and to examine whether the presence of IAH during hospitalization could predict short-term renal recovery or in-hospital mortality in critically ill patients with established AKI. The prognostic value of urinary biomarkers, including neutrophil gelatinase-associated lipocalin (NGAL) and liver-type fatty acid-binding protein (L-FABP), in renal recovery or in-hospital mortality was also investigated.
Methods
Patients
This was a single-center, prospective observational study conducted between February 2012 and June 2014. This study included 57 consecutive patients who were at least 18 years old and admitted with a diagnosis of AKI to the medical ICU of the Korea University Medical Center, Seoul, Korea. Exclusion criteria were patients with end-stage renal failure who were receiving maintenance dialysis or had contraindications to the insertion of a urinary catheter for intravesical pressure measurement, such as patients with pelvic fracture or urethral injury. The study protocol was approved by the institutional review board of this center, and written informed consent was obtained.
Data collection
Data on age, sex, predisposing condition for increased IAP, length of ICU and hospital stay, and in-hospital mortality were collected. Patients were followed up until death or discharge from hospital. The calculation of the Simplified Acute Physiology Score II (SAPS II) [6] was based on the worst values recorded on the day of ICU admission. The primary endpoint was renal recovery from AKI on Day 7, and the secondary endpoint was in-hospital mortality.
Measurement of IAP
IAP was measured intravesically once a day for the first 3 days via a Foley catheter, according to the U-tube manometer technique [7,8], and the mean value was calculated. The sterile saline instillation volume was no more than 25 mL, according to the World Society of the Abdominal Compartment Syndrome consensus [9]. IAP was measured in the supine position at the end of expiration after ensuring that abdominal muscle contractions were absent. The symphysis pubis was considered the reference line, and the pressure was expressed in mmHg (1 mmHg = 1.36 cmH2O) [7–9].
Measurement of urinary NGAL and L-FABP
Urine samples were collected on ICU admission and centrifuged at 2,500 rpm at 4°C for 5 minutes. The supernatants were frozen at −80°C until further biomarker analysis. Urinary NGAL and L-FABP were measured using the NGAL ELISA (BioPorto, Gentofte, Denmark), and human L-FABP Assay kits (CMIC Co. Ltd, Tokyo, Japan), respectively, according to the manufacturer’s instructions.
Case definition
IAH was defined as a sustained pathological elevation in IAP of ≥12 mmHg, in three consecutive measurements performed daily on the first 3 days, according to the World Society of the Abdominal Compartment Syndrome consensus [9]. We defined the following risk factors of IAH [10–13]:
-
(1)
Mechanical ventilation, defined as the use of invasive positive pressure ventilation through an endotracheal tube or a tracheostomy tube
-
(2)
Liver dysfunction, defined as decompensated or compensated cirrhosis or other liver failure with ascites
-
(3)
Positive fluid balance, defined as >1.5 L of total input–output in the initial 72 hours
-
(4)
Ileus, defined as abdominal distension or failure of enteral feeding evidenced by gastric dilatation or gastroparesis with gastric residual >1,000 mL in 24 hours
-
(5)
Acidosis, defined as an arterial pH of <7.2
-
(6)
Hypothermia, defined as a core temperature of <33°C
-
(7)
Polytransfusion, defined as the transfusion of >6 units of packed red cells in 24 hours
-
(8)
Coagulopathy, defined as a platelet count of <55,000/mm3 or an activated partial thromboplastin time more than two times normal or a prothrombin time of >50% or an international standardized ratio of >1.5
-
(9)
Sepsis, defined according to the American–European consensus conference definitions
-
(10)
Shock, defined as a cardiovascular Sequential Organ Failure Assessment (SOFA) subscore of >3, i.e., hypotension requiring dopamine >5 μg/kg/min, or norepinephrine and/or epinephrine <0.1 μg/kg/min.
According to the types of changes in IAP during admission, we categorized these IAP changes into downtrend or uptrend and fluctuation. Downtrend of IAP was defined as a continuous decrease in IAP during initial 3 days. AKI was diagnosed and staged on the day of admission according to the risk–injury–failure–loss–end-stage kidney disease (RIFLE) criteria. Renal recovery during hospitalization was defined as a serum creatinine level of <0.45 mg/dL or within 20% above the baseline value, and without a requirement for dialysis [14] on Day 7 postadmission.
Statistical analysis
All statistical analyses were performed using SPSS version 20.0 (IBM Corp, Armonk, NY, USA). Continuous variables, including IAP and SAPS II score, were expressed as mean ± standard deviation, calculated using the Student t test. Skewed data, including urinary NGAL and L-FABP levels, were expressed as median and interquartile ranges, and comparisons were assessed using the Mann–Whitney U test. Categorical variables were expressed as proportions, and the chi-square test was used for comparisons. Univariate and multivariate logistic regression analyses were conducted to examine predictors of renal recovery and in-hospital mortality. A P value of <0.05 was considered statistically significant.
Results
Baseline characteristics
Fifty-seven patients were enrolled during the study period. Their baseline clinical and demographic information is summarized in Table 1. Forty-five (78.9%) of study patients had IAH and mean IAP was 22.8 ± 9.6 mmHg. The mean age was 68.7 ± 13.7 years, and 52.6% were men. There were no statistically significant differences in baseline and clinical characteristics, including predisposing conditions for IAH, AKI severity, or short-term prognosis between patients with or without IAH. Urinary level of NGAL or L-FABP did not differ either. Exception was that baseline serum creatinine level was significantly lower in patients with IAH, compared to those with no IAH.
Table 1.
Summary of baseline and clinical characteristics of the patients
| Parameters | All patients | IAH group | Non-IAH group | P |
|---|---|---|---|---|
| (n = 57) | (n = 45) | (n = 12) | ||
| Demographic factor | ||||
| Male, n (%) | 30 (52.6) | 21 (46.7) | 9 (75.0) | 0.081 |
| Age (y)⁎ | 68.7±13.7 | 68.6±14.1 | 69.0±12.5 | 0.929 |
| Predisposing conditions for IAH, n (%) | ||||
| Mechanical ventilation | 10 (17.5) | 8 (17.8) | 2 (16.7) | 0.928 |
| Liver dysfunction | 3 (5.3) | 2 (4.4) | 1 (8.3) | 0.592 |
| Positive fluid balance | 10 (17.5) | 10 (22.2) | 0 (0.0) | 0.072 |
| Ileus | 15 (26.3) | 12 (26.7) | 3 (25.0) | 0.790 |
| Acidosis | 6 (10.5) | 5 (11.1) | 1 (8.3) | 0.781 |
| Hypothermia | 5 (8.8) | 5 (11.1) | 0 (0.0) | 0.227 |
| Polytransfusion | 1 (1.8) | 1 (2.2) | 0 (0.0) | 0.602 |
| Coagulopathy | 12 (21.1) | 11 (24.4) | 1 (8.3) | 0.224 |
| Sepsis | 24 (42.1) | 21 (46.7) | 3 (25.0) | 0.177 |
| Shock | 27 (47.4) | 23 (51.1) | 4 (33.3) | 0.273 |
| RIFLE (failure), n (%) | 40 (70.2) | 31 (68.9) | 9 (75.0) | 0.681 |
| SAPS II score⁎ | 44.9±14.7 | 44.8±14.9 | 45.2±14.6 | 0.940 |
| Mean CVP for initial 3 d (mmHg)† | 9.7 (6.7–11.8) | 10.6 (7.0–18.4) | 8.5 (5.0–11.0) | 0.138 |
| Hb (g/dL)⁎ | 10.6±2.5 | 10.7±2.7 | 10.3±2.0 | 0.635 |
| Baseline Cr (mg/dL)† | 1.0 (0.9–1.7) | 0.9 (0.6–1.2) | 1.8 (1.3–4.2) | 0.044 |
| Urinary NGAL (ng/mL)† | 173.3 (65.6–557.7) | 210.2 (63.0–451.7) | 347.5 (198.6–682.6) | 0.314 |
| Urinary L-FABP (ng/mL)† | 19.1 (6.8–79.6) | 26.5 (1.6–51.3) | 20.7 (14.3–30.2) | 0.826 |
| Mean IAP (mmHg)⁎ | 22.8±9.6 | 25.6±8.4 | 12.2±5.6 | <0.001 |
| IAP (mmHg), Day 1 (on admission)⁎ | 24.1±10.7 | 26.3±9.7 | 15.7±11.0 | 0.002 |
| IAP (mmHg), Day 2⁎ | 22.2±9.9 | 25.2±9.6 | 9.9±5.2 | <0.001 |
| IAP (mmHg), Day 3⁎ | 21.7±11.4 | 25.2±9.6 | 8.1±6.4 | <0.001 |
| IAP: downtrend, n (%) | 24 (42.1) | 17 (37.8) | 7 (58.3) | 0.200 |
| IAP: fluctuation + uptrend, n (%) | 33 (57.9) | 28 (62.2) | 5 (41.7) | |
| Clinical course | ||||
| RRT, n (%) | 26 (45.6) | 19 (42.2) | 7 (58.3) | 0.319 |
| Renal recovery on Day 7, n (%) | 23 (40.4) | 20 (44.4) | 3 (25.0) | 0.223 |
| ICU length of stay (d)† | 6.0 (3.0–11.0) | 5.5 (3.0–9.0) | 5.0 (2.5–8.0) | 0.479 |
| Length of hospital stay (d)† | 15.0 (9.0–27.0) | 13.0 (8.0–18.0) | 14.0 (13.5–16.5) | 0.638 |
| In-hospital mortality, n (%) | 12 (21.1) | 10 (22.2) | 2 (16.7) | 0.675 |
Cr, creatinine; CVP, central venous pressure; Hb, hemoglobin; IAH, intra-abdominal hypertension; IAP, intra-abdominal pressure; ICU, intensive care unit; L-FABP, liver-type fatty acid binding protein; NGAL, neutrophil gelatinase-associated lipocalin; RIFLE, risk–injury–failure–loss–end-stage kidney disease; RRT, renal replacement therapy; SAPS, simplified acute physiology score.
Mean ± SD.
Median (interquartile range).
Prediction of renal recovery
Renal recovery, as assessed on Day 7 postadmission, was observed in 23 (40.4%) patients. Univariate analysis showed that acidosis, sepsis, severe AKI (failure in RIFLE) (47.8% vs. 85.3%, P = 0.002), and a higher SAPS II score (39.9 ± 12.0 vs. 48.2 ± 15.5, P = 0.035) were predictors of nonrecovery of renal function, while the mean IAP, presence of IAH, or change in IAP during the first 3 days were not different between the recovery and nonrecovery groups. Urinary NGAL (median 110.8 ng/mL vs. 239.2 ng/mL, P = 0.050) and L-FABP at the time of ICU admission (median 12.3 ng/mL vs. 20.7 ng/mL, P = 0.028) were also significantly higher in the nonrecovery group. To identify risk factors that independently predict renal recovery from AKI, a multivariate analysis, which included variables giving P values of <0.1 in univariate analysis, was conducted. The presence of ileus, acidosis, sepsis, RIFLE criteria, SAPS II score, hemoglobin, NGAL, and L-FABP were included as variables in a binary logistic regression analysis (backward method). Severe AKI [odds ratio (OR), 14.8], high urinary NGAL level (OR, 1.015), and high urinary L-FABP level (OR, 1.003) were found to be independent predictors of nonrecovery (Table 2), but the presence of IAH was not (OR, 0.417; P = 0.231).
Table 2.
Binary logistic regression analysis in predicting nonrecovery of renal function from AKI in critically ill patients (backward method)
| Parameters | OR | 95% CI | P |
|---|---|---|---|
| Urinary NGAL | 1.015 | 1.000–1.006 | 0.018 |
| Urinary L-FABP | 1.003 | 1.000–1.030 | 0.006 |
| RIFLE (failure) | 14.8 | 2.250–97.060 | 0.001 |
Excluded variables: acidosis, age, hemoglobin, intra-abdominal hypertension, intra-abdominal pressure on admission, ileus, polytransfusion, Simplified Acute Physiology Score II, sepsis, and shock.
AKI, acute kidney injury; CI, confidence interval; L-FABP, liver-type fatty acid binding protein; NGAL, neutrophil gelatinase-associated lipocalin; OR, odds ratio; RIFLE, risk–injury–failure–loss–end-stage kidney disease.
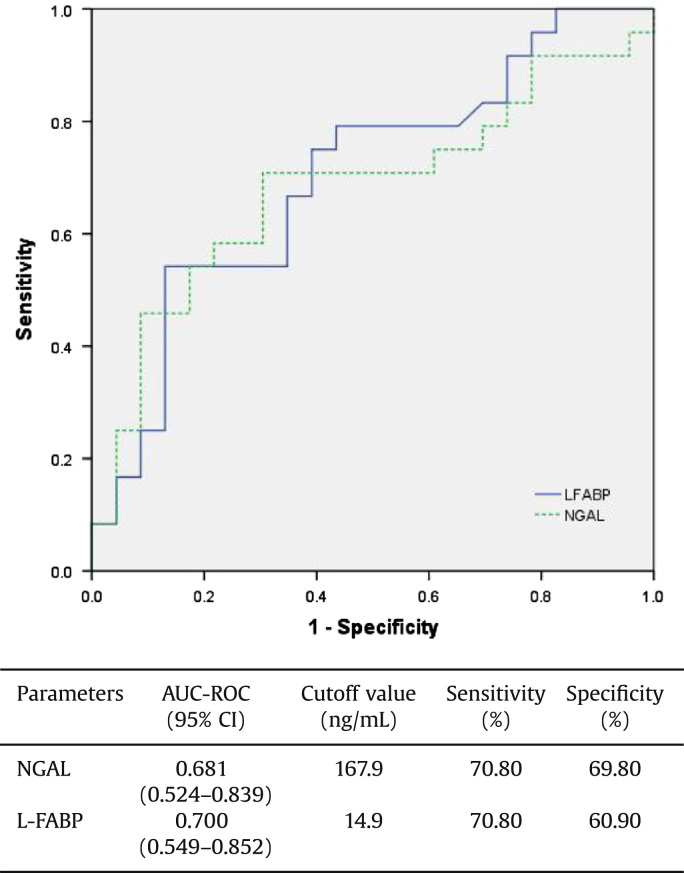
We also evaluated and compared the diagnostic performances of urinary biomarkers for predicting nonrecovery of renal function by calculating the area under the receiver operating characteristic (ROC) curves. Fig. 1 displays the ROC curves of both urinary NGAL and FABP. Performance of urinary L-FABP was better than that of NGAL (area under the curve, 0.700 vs. 0.681).
Figure 1.
Diagnostic performance of biomarker for predicting nonrecovery from AKI, based on the calculation of the area under the receiver operating characteristic (ROC) curves. The area under the ROC curves and the cutoff value of each urinary biomarker are presented in a separate table below the figure. AKI, acute kidney injury; AUC-ROC, area under the receiver operating characteristic curve; CI, confidence interval; L-FABP, liver-type fatty acid binding protein; NGAL, neutrophil gelatinase-associated lipocalin.
Prediction of in-hospital mortality
In-hospital mortality was 21.1% in this study. Patients who did not survive showed a significantly higher prevalence of shock (40% vs. 75%, P = 0.031), lower hemoglobin levels (11.0 ± 2.4 g/dL vs. 9.2 ± 2.8 g/dL, P = 0.028), and higher SAPS II scores (14.9 ± 12.3 vs. 55.8 ± 18.1, P = 0.003) in univariate analysis.
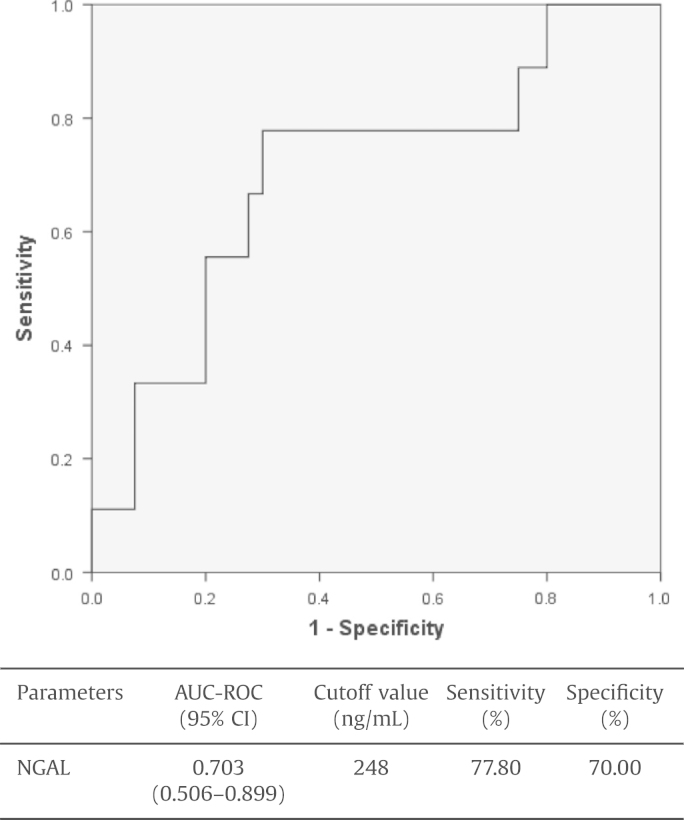
We performed a multivariate logistic regression analysis to identify factors that could predict in-hospital mortality, including variables giving P values of <0.1 in univariate analysis. Age, polytransfusion, shock, severe AKI (failure in RIFLE), SAPS II score, hemoglobin, urinary NGAL, and IAP on Day 1 were included as variables in a binary logistic regression analysis (backward method). In the final model, after backward variable selection, a high SAPS II score (OR, 1.102) and a high urinary NGAL level (OR, 1.003) were shown to be independent predictors of in-hospital mortality (Table 3). However, the presence of IAH did not predict in-hospital mortality in patients with established AKI (OR, 1.429; P = 0.676). The diagnostic performance of biomarkers for predicting in-hospital mortality was analyzed and the cutoff value of urine NGAL (248 ng/mL) was found to be able to predict mortality with 77.8% sensitivity and 70.0% specificity (Fig. 2).
Table 3.
Binary logistic regression analysis in predicting in-hospital mortality in critically ill patients with AKI (backward method)
| Parameters | OR | 95% CI | P |
|---|---|---|---|
| Urinary NGAL | 1.003 | 1.000–1.006 | 0.024 |
| SAPS II score | 1.102 | 1.027–1.182 | 0.007 |
Excluded variables: acidosis, age, hemoglobin, intra-abdominal hypertension, intra-abdominal pressure on admission, ileus, liver-type fatty acid binding protein, polytransfusion, risk–injury–failure–loss–end-stage kidney disease failure, sepsis, and shock.
CI, confidence interval; NGAL, neutrophil gelatinase-associated lipocalin; OR, odds ratio; SAPS, Simplified Acute Physiology Score.
Figure 2.
Diagnostic performance of urinary NGAL for predicting in-hospital mortality in critically ill patients with AKI, based on the calculation of the area under the receiver operating characteristic (ROC) curves. The area under the ROC curves and the cutoff value of each urinary biomarker are presented in a separate table below the figure. AKI, acute kidney injury; AUC-ROC, area under the receiver operating characteristic curve; CI, confidence interval; NGAL, neutrophil gelatinase-associated lipocalin.
Discussion
AKI is a significant medical problem associated with poor outcomes, including higher mortality, prolonged duration of hospitalization, and increased risk of progression to chronic kidney disease [15,16]. Therefore, predicting prognosis in patients with established AKI is important to avoid unnecessary delays in treatment and also exposure to nonbeneficial management such as excessive fluid management or diuretics. However, no specific predictive markers for renal recovery or mortality, or even a standard definition of renal recovery, currently exist [17].
With the purpose of determining whether various parameters could predict renal recovery or mortality, we performed a prospective observational study, enrolling patients with established AKI who were admitted to the medical ICU. We found that urinary NGAL, L-FABP, and failure grade according to the RIFLE criteria were independent predictors of nonrecovery of renal function. Urinary NGAL and SAPS II score were independent predictors of in-hospital mortality. However, the presence of IAH was not found to be useful in predicting either renal recovery or in-hospital mortality in patients with established AKI.
As the abdominal cavity is considered a semiclosed compartment, any changes in its content may increase IAP [12,13]. According to the World Society of the Abdominal Compartment Syndrome guidelines, IAH is defined as a sustained or repeated pathological elevation in IAP of ≥12 mmHg, whereas a sustained elevation of IAP of >20 mmHg, known as abdominal compartment syndrome, is associated with organ dysfunction [9,12]. Elevation of IAP above physiological limits has adverse effects on end-organ function, and the kidney is especially vulnerable to IAH-induced organ dysfunction [18]. Recent data have indicated that IAH is emerging as an important factor influencing AKI development in critically ill patients [2–5,19]. Numerous conditions such as abdominal surgery, severe pancreatitis, mechanical ventilation, sepsis, ileus, and massive fluid resuscitation are known risk factors for IAH [12,13,20]. Increased renal venous pressure resulting from IAH is thought to be directly responsible for renal function impairment [1]. Sepsis syndrome, the most common cause of admission to a medical ICU, requires massive fluid resuscitation to maintain hemodynamic stability, or mechanical ventilation to treat combined pneumonia or adult respiratory distress syndrome. All these measures are likely to increase IAH and can lead to the development of AKI in critically ill patients [12,13,20].
Although incidence of IAH is known to be approximately 37–43% in critically ill ICU patients, or even higher in patients who have two or more categorized risk factors for IAH (67.8%) [12,13], the prevalence rate in our study, which included patients with established AKI, was much higher (78.9%). However, there was no association between the presence of IAH and the severity of AKI. Because IAH was diagnosed after AKI onset, a direct causal relationship between IAH and AKI could not be determined. Instead, we tried to assess whether the presence of IAH in patients with established AKI would further compromise their outcome. We observed that the presence of IAH in patients with AKI did not predict renal recovery or in-hospital mortality. While data about the effect of IAH on renal recovery are scarce, several studies have demonstrated a relationship between IAH and mortality. In a prospective cohort of 83 heterogeneous ICU patients, those with IAH had significantly higher mortality than those with no IAH (53% vs. 27%, P = 0.02) [19]. However, in a larger study comprising a mixed population of 265 critically ill patients from 14 ICUs in six countries, Malbrain et al [13] found that IAH on Day 1 did not predict mortality, a result consistent with our findings. However, in another prospective cohort study of 151 medical ICUs, it was shown that patients with more than two categorized risk factors for IAH (41.4% vs. 14.3%, P<0.001) or nonresolution of IAH in the IAH group during their ICU stay (64.7% vs. 35.7%, P = 0.001) was associated with higher mortality [12]. Further larger studies on measurement of IAP for longer periods are needed to verify whether IAH can be used to predict mortality.
Simultaneously, we also determined the prognostic value of novel biomarkers, including NGAL and L-FABP, and that of the RIFLE criteria or SAPS II in predicting the outcome of patients with established AKI.
Since NGAL was first introduced as a sensitive and specific early marker of AKI, the utility of urinary NGAL as a predictive or prognostic marker has been investigated extensively in numerous patient groups with different characteristics [21]. Srisawat et al [22,23] have shown that plasma NGAL can predict renal recovery from AKI following community-acquired pneumonia, and that urinary NGAL and hepatocyte growth factor can predict renal recovery in critically ill patients with renal support. Our group also demonstrated that an initial NGAL was useful in predicting mortality or renal recovery in patients with AKI from mixed ICUs or general wards [24]. As expected, in this study, we found that NGAL on ICU admission was useful to predict both renal recovery and in-hospital mortality. A recent study has shown that a gradual increment in urinary NGAL appears superior to a single NGAL value in differentiating between transient and sustained AKI on ICU admission [25].
Urine NGAL with a cutoff value of 248 ng/mL was found to predict mortality with 77.8% sensitivity and 70.0% specificity.
However, urine L-FABP, another novel biomarker, was useful in predicting only renal recovery, but not in-hospital mortality. Yokoyama et al [26] reported that the amount of renal expression and urinary excretion of L-FABP significantly reflected the severity of tubulointerstitial damage. However, the results of the current study do not concur with those of previous studies, including ours, demonstrating the usefulness of L-FABP in predicting 30-day or 90-day mortality in critically ill patients [27,28]. Although it is not clear, these discrepancies between the studies might be derived from the differences in baseline characteristics of enrolled patients. In addition to initial urinary NGAL and L-FABP, severe AKI (failure grade according to RIFLE criteria) was also a good predictor of nonrecovery of renal function. In defining renal recovery, we used the following criteria: serum creatinine level <0.45 mg/dL or within 20% above the baseline value during hospitalization, without the requirement for dialysis on Day 7. Twenty-three patients (40.3%) showed renal recovery from AKI, but because the definition of renal recovery and patient baseline characteristics vary between studies, direct comparisons cannot be made.
Despite several meaningful findings, our study had some limitations. First, the sample size was not large enough to generalize these findings. Second, as IAP was measured only for the first 3 days, the effect of a changing pattern of IAP during hospitalization could not be determined. However, IAP should be measured daily during ICU admission for evaluating it as a prognostic marker for AKI recovery, because IAP can change according to therapy or patient status during admission. Therefore, further multicenter studies with longer follow-up periods are needed to assess whether changes in IAP or its resolution may affect the outcomes of patients with AKI.
In conclusion, we found that IAH was prevalent in critically ill patients with established AKI. However, IAH on initial admission did not predict short-term prognosis including renal recovery or in-hospital mortality. Urinary NGAL was found to be a useful predictor of both renal recovery and in-hospital mortality. Large, multicenter prospective studies are necessary to confirm our results and validate the predictive value of these factors for renal function recovery.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgments
This study was supported by a grant from Korea University.
References
- 1.Bradley SE, Bradley GP. The effect of increased intra-abdominal pressure on renal function in man. J Clin Invest. 1947;26:1010–1022. doi: 10.1172/JCI101867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugrue M, Jones F, Deane SA, Bishop G, Bauman A, Hillman K. Intra-abdominal hypertension is an independent cause of postoperative renal impairment. Arch Surg. 1999;134:1082–1085. doi: 10.1001/archsurg.134.10.1082. [DOI] [PubMed] [Google Scholar]
- 3.Shu M, Peng C, Chen H, Shen B, Zhou G, Shen C, Li H. Intra-abdominal hypertension is an independent cause of acute renal failure after orthotopic liver transplantation. Front Med China. 2007;1:167–172. doi: 10.1007/s11684-007-0031-5. [DOI] [PubMed] [Google Scholar]
- 4.Mohmand H, Goldfarb S. Renal dysfunction associated with intra-abdominal hypertension and the abdominal compartment syndrome. J Am Soc Nephrol. 2011;22:615–621. doi: 10.1681/ASN.2010121222. [DOI] [PubMed] [Google Scholar]
- 5.Dalfino L, Tullo L, Donadio I, Malcangi V, Brienza N. Intra-abdominal hypertension and acute renal failure in critically ill patients. Intensive Care Med. 2008;34:707–713. doi: 10.1007/s00134-007-0969-4. [DOI] [PubMed] [Google Scholar]
- 6.Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 7.Lee RK. Intra-abdominal hypertension and abdominal compartment syndrome: a comprehensive overview. Crit Care Nurse. 2012;32:19–31. doi: 10.4037/ccn2012662. [DOI] [PubMed] [Google Scholar]
- 8.Malbrain ML. Different techniques to measure intra-abdominal pressure (IAP): time for a critical re-appraisal. Intensive Care Med. 2004;30:357–371. doi: 10.1007/s00134-003-2107-2. [DOI] [PubMed] [Google Scholar]
- 9.Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, Duchesne J, Bjorck M, Leppaniemi A, Ejike JC, Sugrue M, Cheatham M, Ivatury R, Ball CG, Reintam Blaser A, Regli A, Balogh ZJ, D׳Amours S, Debergh D, Kaplan M, Kimball E, Olvera C, Pediatric Guidelines Sub-Committee for the World Society of the Abdominal Compartment S Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190–1206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malbrain ML, Chiumello D, Pelosi P, Wilmer A, Brienza N, Malcangi V, Bihari D, Innes R, Cohen J, Singer P, Japiassu A, Kurtop E, De Keulenaer BL, Daelemans R, Del Turco M, Cosimini P, Ranieri M, Jacquet L, Laterre PF, Gattinoni L. Prevalence of intra-abdominal hypertension in critically ill patients: a multicentre epidemiological study. Intensive Care Med. 2004;30:822–829. doi: 10.1007/s00134-004-2169-9. [DOI] [PubMed] [Google Scholar]
- 11.Kim IB, Prowle J, Baldwin I, Bellomo R. Incidence, risk factors and outcome associations of intra-abdominal hypertension in critically ill patients. Anaesth Intensive Care. 2012;40:79–89. doi: 10.1177/0310057X1204000107. [DOI] [PubMed] [Google Scholar]
- 12.Santa-Teresa P, Munoz J, Montero I, Zurita M, Tomey M, Alvarez-Sala L, Garcia P. Incidence and prognosis of intra-abdominal hypertension in critically ill medical patients: a prospective epidemiological study. Ann Intensive Care. 2012;2(Suppl 1):S3. doi: 10.1186/2110-5820-2-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malbrain ML, Chiumello D, Pelosi P, Bihari D, Innes R, Ranieri VM, Del Turco M, Wilmer A, Brienza N, Malcangi V, Cohen J, Japiassu A, De Keulenaer BL, Daelemans R, Jacquet L, Laterre PF, Frank G, de Souza P, Cesana B, Gattinoni L. Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: a multiple-center epidemiological study. Crit Care Med. 2005;33:315–322. doi: 10.1097/01.ccm.0000153408.09806.1b. [DOI] [PubMed] [Google Scholar]
- 14.Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL, Program to Improve Care in Acute Renal Disease Study G Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76:422–427. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 15.Thakar CV, Christianson A, Himmelfarb J, Leonard AC. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol. 2011;6:2567–2572. doi: 10.2215/CJN.01120211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bagshaw SM, Laupland KB, Doig CJ, Mortis G, Fick GH, Mucenski M, Godinez-Luna T, Svenson LW, Rosenal T. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care. 2005;9:R700–R709. doi: 10.1186/cc3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstein SL, Chawla L, Ronco C, Kellum JA. Renal recovery. Crit Care. 2014;18:301–307. doi: 10.1186/cc13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De laet I, Malbrain ML, Jadoul JL, Rogiers P, Sugrue M. Renal implications of increased intra-abdominal pressure: are the kidneys the canary for abdominal hypertension? Acta Clin Belg Suppl. 2007;62:119–130. doi: 10.1179/acb.2007.62.s1.015. [DOI] [PubMed] [Google Scholar]
- 19.Vidal MG, Ruiz Weisser J, Gonzalez F, Toro MA, Loudet C, Balasini C, Canales H, Reina R, Estenssoro E. Incidence and clinical effects of intra-abdominal hypertension in critically ill patients. Crit Care Med. 2008;36:1823–1831. doi: 10.1097/CCM.0b013e31817c7a4d. [DOI] [PubMed] [Google Scholar]
- 20.de Laet IE, Malbrain M. Current insights in intra-abdominal hypertension and abdominal compartment syndrome. Med Intensiva. 2007;31:88–99. doi: 10.1016/s0210-5691(07)74781-2. [DOI] [PubMed] [Google Scholar]
- 21.Ostermann M, Philips BJ, Forni LG. Clinical review: biomarkers of acute kidney injury: where are we now? Crit Care. 2012;16:233–245. doi: 10.1186/cc11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srisawat N, Murugan R, Lee M, Kong L, Carter M, Angus DC, Kellum JA, Genetic, Inflammatory Markers of Sepsis Study I Plasma neutrophil gelatinase-associated lipocalin predicts recovery from acute kidney injury following community-acquired pneumonia. Kidney Int. 2011;80:545–552. doi: 10.1038/ki.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srisawat N, Wen X, Lee M, Kong L, Elder M, Carter M, Unruh M, Finkel K, Vijayan A, Ramkumar M, Paganini E, Singbartl K, Palevsky PM, Kellum JA. Urinary biomarkers and renal recovery in critically ill patients with renal support. Clin J Am Soc Nephrol. 2011;6:1815–1823. doi: 10.2215/CJN.11261210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang HN, Boo CS, Kim MG, Jo SK, Cho WY, Kim HK. Urine neutrophil gelatinase-associated lipocalin: an independent predictor of adverse outcomes in acute kidney injury. Am J Nephrol. 2010;31:501–509. doi: 10.1159/000309841. [DOI] [PubMed] [Google Scholar]
- 25.de Geus HR, Woo JG, Wang Y, Devarajan P, Betjes MG, le Noble JL, Bakker J. Urinary neutrophil gelatinase-associated lipocalin measured on admission to the intensive care unit accurately discriminates between sustained and transient acute kidney injury in adult critically ill patients. Nephron Extra. 2011;1:9–23. doi: 10.1159/000330428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokoyama T, Kamijo-Ikemori A, Sugaya T, Hoshino S, Yasuda T, Kimura K. Urinary excretion of liver type fatty acid binding protein accurately reflects the degree of tubulointerstitial damage. Am J Pathol. 2009;174:2096–2106. doi: 10.2353/ajpath.2009.080780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho E, Yang HN, Jo SK, Cho WY, Kim HK. The role of urinary liver-type fatty acid-binding protein in critically ill patients. J Korean Med Sci. 2013;28:100–105. doi: 10.3346/jkms.2013.28.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doi K, Negishi K, Ishizu T, Katagiri D, Fujita T, Matsubara T, Yahagi N, Sugaya T, Noiri E. Evaluation of new acute kidney injury biomarkers in a mixed intensive care unit. Crit Care Med. 2011;39:2464–2469. doi: 10.1097/CCM.0b013e318225761a. [DOI] [PubMed] [Google Scholar]