Abstract
Background
Pulmonary hypertension (PHT) is a recently recognized complication of chronic kidney disease. In this study, we investigated the association between PHT, peripheral vascular calcifications (VCs), and major cardiovascular events.
Methods
In this retrospective study, we included 172 end-stage renal disease (ESRD) patients undergoing dialysis [hemodialysis (HD)=84, peritoneal dialysis=88]. PHT was defined as an estimated pulmonary artery systolic pressure>37 mmHg using echocardiography. The Simple Vascular Calcification Score (SVCS) was measured using plain radiographic films of the hands and pelvis.
Results
The prevalence of PHT was significantly higher in HD patients (51.2% vs. 22.7%). Dialysis patients with PHT had a significantly higher prevalence of severe VCs (SVCS≥3). In multivariate analysis, the presence of severe VCs [odds ratio (OR), 2.68], mitral valve disease (OR, 7.79), HD (OR, 3.35), and larger left atrial diameter (OR, 11.39) were independent risk factors for PHT. In addition to the presence of anemia, severe VCs, or older age, the presence of PHT was an independent predictor of major cardiovascular events in ESRD patients.
Conclusion
The prevalence of PHT was higher in HD patients and was associated with higher rates of major cardiovascular events. Severe VCs are thought to be an independent risk factor for predicting PHT in ESRD patients. Therefore, in dialysis patients with PHT, careful attention should be paid to the presence of VCs and the occurrence of major cardiovascular events.
Keywords: Cardiovascular disease, Dialysis, Pulmonary hypertension
Introduction
Emerging evidence suggests that pulmonary hypertension (PHT), a recently recognized complication of end-stage renal disease (ESRD), is closely associated with cardiac, pulmonary, and systemic diseases and also with increased mortality [1]. The prevalence of PHT is known to range from 18.8% to 68.8% in hemodialysis (HD) patients and from 0% to 42% in peritoneal dialysis (PD) patients [2]. The possible causes of PHT in dialysis patients are classified into three categories: (1) increased cardiac output caused by an arteriovenous fistula, anemia, or hypervolemia; (2) increased pulmonary vascular resistance caused by uremic endothelial dysfunction, pulmonary embolism, pulmonary artery calcifications, or other comorbid diseases including chronic obstructive pulmonary disease or connective tissue disease; and (3) elevated pulmonary capillary wedge pressure caused by systolic and diastolic heart failure or mitral valve disease [3]. In a previous study on dogs, high parathyroid hormone levels provoked pulmonary artery calcifications, and this resulted in decreased pulmonary vascular compliance [4]. Although this result indicated that extraosseous calcifications might be a risk factor for PHT, results in human studies about the association between PHT and pulmonary artery calcification or parathyroid hormone levels have been conflicting [5–9]. Medial artery calcification is a common complication in patients with chronic kidney disease (CKD) contributing to increased cardiovascular mortality, and multiple factors including abnormal calcium phosphate metabolism, uremia, oxidative stress, and inflammation are known to be important in vascular calcification (VC) development. In this study, we examined the association between PHT, peripheral VCs, and major cardiovascular events in dialysis patients.
Methods
Study population
In this retrospective study, we included 172 ESRD patients undergoing dialysis (HD=84, PD=88) who had been enrolled in previous cross-sectional studies performed in March 2009 [10,11]. In the previous studies, 198 stable dialysis patients (HD=105, PD=93) who had been followed in the dialysis center of Korea University Anam Hospital with over 6-mo duration of dialysis were enrolled. Among 198 patients, 26 without echocardiographic data were excluded from this study. Baseline characteristics, including sex, age, body weight, height, dialysis duration, causes of ESRD, and history of hypertension, diabetes, ischemic heart disease, and chronic obstructive pulmonary disease were recorded. The mean values of serum albumin, low-density lipoprotein (LDL) cholesterol, alkaline phosphatase, calcium, phosphate, hemoglobin, and C-reactive protein (CRP) that had been measured every month for 18 months were used. The data collection on baseline characteristics, laboratory tests, and echocardiographic findings was based on the enrollment date of the previous studies. The electronic medical records of enrolled patients from March 2009 to December 2013 were reviewed to check for the development of major cardiovascular events including acute myocardial infarction and stroke. For acute myocardial infarction, we included non-ST elevation and ST elevation myocardial infarction. We defined stroke as an abrupt onset of focal neurological deficit with intracranial infarction or hemorrhage on brain imaging studies, during the follow-up period. This study was approved by the Institutional Review Board of Korea University, Anam Hospital.
Echocardiography
Standard two-dimensional, M-mode, Doppler echocardiography was performed for all patients. We used the modified Bernoulli equation to calculate pulmonary artery systolic pressure (PASP) as follows: PASP=4×(tricuspid systolic jet)2+10 mmHg (estimated arterial pressure). PHT was defined as an estimated PASP>37 mmHg. Left ventricular (LV) mass was calculated with the following formula: LV mass (g)=0.8×{1.04 [(LVIDd+PWTd+SWTd)3 – (LVIDd)3]}+0.6, where LVIDd is the left ventricle internal diameter, diastole; PWTd is the posterior wall thickness, diastole; and SWTd is the septum wall thickness, diastole. This was indexed for body surface area to yield the LV mass index [12]. LV systolic dysfunction was defined as LV fractional shortening≤25%. Mitral valve disease was defined as mitral valve stenosis or regurgitation with more than moderate degree. Aortic valve disease was also defined as stenosis or regurgitation of aortic valve with more than moderate degree.
Simple Vascular Calcification Score and parathyroid hormone level
The Simple Vascular Calcification Score (SVCS) was measured using plain radiographic films of both hands and pelvis. The pelvic radiographic film was arbitrarily divided into four sections by two imaginary lines (a horizontal line over the upper limit of both femoral heads and a median vertical line over the vertebral column). For each hand, the film was divided by a horizontal line over the upper limit of the metacarpal bones. Only the linear calcifications in the iliac, femoral, radial, and digital arteries were counted. The presence of linear calcifications in each section was counted as a score of 1, and the absence of calcifications was counted as a score of 0; the SVCS was the sum of all sections, ranging from 0 to 8. Adragao et al [13] found that an SVCS≥3 was an independent predictor of cardiovascular mortality and cardiovascular events. According to this result, we defined severe VCs as an SVCS≥3. We also defined an intact parathyroid hormone level<150 pg/mL as the low parathyroid hormone group and an intact parathyroid hormone level>300 pg/mL as the high parathyroid hormone group based on the Kidney Disease Outcomes Quality Initiative guidelines [14].
Statistical analysis
Statistical analyses were performed using SPSS, version 20.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as the means±standard deviation or median (interquartile range), according to distribution. Continuous variables were analyzed using the Student t test for normally distributed data or the Mann–Whitney test for non-normally distributed data. The Pearson χ2 test was used to analyze nominal data. In multivariate analysis, a binary logistic regression model was used. The major cardiovascular events-free survivals were determined based on survival curves using the Kaplan–Meier method and log-rank test. The effect of prognostic factors on major cardiovascular events was examined using the Cox proportional regression model. All parameters used in the cross-sectional study were evaluated using a univariate Cox proportional hazard model. Those covariates with P<0.20 in the univariate model were considered for the multivariate Cox regression analysis. Patients were censored at kidney transplantation (n=28), last follow-up (n=45), and death (n=21). A P value<0.05 was considered statistically significant.
Results
Baseline characteristics according to dialysis modality
The mean age of the patients was 56.3±13.2 years. Seventy-four patients (43.0%) had a history of diabetes, and 152 patients (88.4%) had hypertension. The most common cause of ESRD was diabetes mellitus, followed by hypertension, glomerulonephritis, and polycystic kidney disease. There was no statistically significant difference in the causes of ESRD or comorbidities according to dialysis modality. Of the 172 dialysis patients, 63 (36.6%) had PHT, and the median dialysis duration before enrollment was 35.0 (18.5–64.0) months. The prevalence of PHT was significantly higher in HD patients (51.2% vs. 22.7%, P<0.001). ESRD patients undergoing PD had lower serum albumin and CRP levels. Serum LDL cholesterol, high-density lipoprotein (HDL) cholesterol, triglyceride, alkaline phosphatase, and intact parathyroid hormone levels were significantly higher in PD patients. However, there were no statistically significant differences in echocardiographic findings such as LV mass index, left atrial diameter, LV segmental abnormalities, LV systolic dysfunction, and the prevalence of mitral valve disease between HD and PD patients (Table 1).
Table 1.
Baseline characteristics according to the dialysis modality
| All (n=172) | HD (n=84) | PD (n=88) | P | |
|---|---|---|---|---|
| Age, y | 56.3±13.2 | 58.3±14.3 | 54.8±12.3 | 0.093 |
| Sex (male) | 86 (50.0%) | 44 (52.4%) | 42 (47.7%) | 0.647 |
| Body weight (kg) | 59.9±10.2 | 59.3±10.6 | 60.4±9.7 | 0.486 |
| Height (m) | 1.60±0.08 | 1.60±0.08 | 1.59±0.08 | 0.598 |
| BMI (kg/m2) | 22.8 (21.0–25.1) | 22.7 (21.0–24.2) | 23.1 (20.9–25.5) | 0.261 |
| Dialysis duration (mo) | 35.0 (18.5–64.0) | 35.0 (21.0–63.0) | 36.5 (15.0–67.0) | 0.980 |
| Comorbidities | ||||
| Diabetes mellitus | 74 (43.0%) | 34 (40.5%) | 40 (45.5%) | 0.540 |
| Hypertension | 152 (88.4%) | 75 (89.3%) | 77 (87.5%) | 0.814 |
| Ischemic heart disease | 15 (8.7%) | 9 (10.7%) | 6 (6.8%) | 0.425 |
| COPD | 7 (4.1%) | 5 (6.0%) | 2 (2.3%) | 0.269 |
| Causes of renal failure | ||||
| Glomerulonephritis | 25 (14.5%) | 14 (16.7%) | 11 (12.5%) | 0.667 |
| Hypertension | 52 (30.2%) | 28 (33.3%) | 24 (24.3%) | |
| Diabetes mellitus | 69 (40.1%) | 30 (35.7%) | 39 (44.3%) | |
| Polycystic kidney disease | 5 (2.9%) | 3 (3.6%) | 2 (2.3%) | |
| Others | 21 (12.2%) | 9 (10.7%) | 12 (13.6%) | |
| Intact parathyroid hormone (pg/mL) | 191 (109–391) | 176 (86–312) | 225 (134–440) | 0.019 |
| Intact parathyroid hormone group (150–300) | ||||
| Low | 49 (28.5%) | 24 (28.6%) | 25 (28.4%) | 0.031 |
| Intermediate | 65 (37.8%) | 39 (46.4%) | 26 (29.5%) | |
| High | 58 (33.7%) | 21 (25.0%) | 37 (42.0%) | |
| SVCS≥3 | 48 (27.9%) | 26 (31.0%) | 22 (25.0%) | 0.401 |
| Albumin (g/dL) | 3.87±0.42 | 4.02±0.37 | 3.73±0.43 | <0.001 |
| LDL cholesterol (mg/dL) | 90.3±20.2 | 85.3±20.3 | 95.1±19.0 | 0.001 |
| HDL cholesterol (mg/dL) | 40.6 (35.0–47.5) | 40.0 (32.1–45.5) | 41.1 (35.4–48.7) | 0.027 |
| Triglyceride (mg/dL) | 127.2 (91.9–160.3) | 110.3 (76–144) | 144.5 (111–175) | <0.001 |
| ALP (IU/L) | 67.8 (57.6–89.6) | 65.3 (52.5–84.0) | 73.4 (60.4–100.8) | 0.029 |
| Ca–P (mg2/dL2) | 44.7 (37.8–51.0) | 42.8 (37.2–49.4) | 45.8 (38.4–51.5) | 0.236 |
| Hemoglobin (g/dL) | 10.3 (9.9–10.7) | 10.2 (9.9–10.6) | 10.4 (9.8–10.8) | 0.519 |
| CRP (mg/L) | 2.25 (1.16–4.81) | 4.54 (1.51–8.29) | 1.79 (1.08–2.51) | <0.001 |
| Pulmonary hypertension | 63 (36.6%) | 43 (51.2%) | 20 (22.7%) | <0.001 |
| Echo findings | ||||
| LV mass index | 129.4±40.4 | 133.3±41.5 | 125.6±39.2 | 0.214 |
| Left atrial diameter (cm/m2) | 2.46±0.48 | 2.50±0.55 | 2.42±0.42 | 0.295 |
| LV segmental abnormality | 11 (6.4%) | 6 (7.1%) | 5 (5.7%) | 0.763 |
| LV systolic dysfunction (LVFS<25%) | 16 (9.3%) | 6 (7.1%) | 10 (11.4%) | 0.434 |
| MV disease | 18 (10.5%) | 12 (14.3%) | 6 (6.8%) | 0.137 |
| AV disease | 10 (5.8%) | 5 (6.0%) | 5 (5.7%) | 1.000 |
All values are expressed as mean±standard deviation, number of patients (%), or median (confidence interval 25–75%).
ALP, alkaline phosphatase; AV, aortic valve; BMI, body mass index; COPD, chronic obstructive lung disease; CRP, C-reactive protein; Hb, hemoglobin; HD, hemodialysis; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LV, left ventricle; LVFS, left ventricular fractional shortening; MV, mitral valve; PD, peritoneal dialysis; SVCS, simple vascular calcification score.
Risk factors that predict the presence of PHT
Univariate analysis of the various factors predicting the presence of PHT is presented in Table 2. Dialysis patients with PHT had a significantly higher prevalence of severe VCs (SVCS≥3), LV systolic dysfunction, and mitral valve disease. They also had higher LV mass indices and larger left atrial diameters. However, the difference in the causes of ESRD or comorbidities between the PHT and no PHT groups was not statistically significant. In multivariate analysis, we used a binary logistic regression model and included all variables with a P value<0.2 in the univariate analysis (Table 3). Severe VCs [odds ratio (OR), 2.68, P=0.013], mitral valve disease (OR, 7.79, P=0.005), HD (OR, 3.35, P=0.003), and larger left atrial diameter (OR, 11.39, P=0.005) were independent risk factors for PHT.
Table 2.
Characteristics according to the pulmonary hypertension
| No PHT (n=109) | PHT (n=63) | P | |
|---|---|---|---|
| Age, y | 55.0±13.9 | 59.0±12.2 | 0.059 |
| Sex (male) | 54 (49.5%) | 32 (50.8%) | 1.000 |
| Body weight (kg) | 59.9±10.5 | 59.9±9.6 | 0.974 |
| Height (m) | 1.59±0.08 | 1.60±0.08 | 0.634 |
| BMI (kg/m2) | 22.9 (20.8–25.5) | 22.5 (21.3–24.3) | 0.833 |
| Hemodialysis | 41 (37.6%) | 43 (68.3%) | <0.001 |
| Dialysis duration (mo) | 34.0 (18.0–65.0) | 40.0 (19.5–63.0) | 0.927 |
| Comorbidities | |||
| Diabetes mellitus | 43 (39.4%) | 31 (49.2%) | 0.263 |
| Hypertension | 93 (85.3%) | 59 (93.7%) | 0.139 |
| Ischemic heart disease | 7 (6.4%) | 8 (12.7%) | 0.172 |
| COPD | 3 (2.8%) | 4 (6.3%) | 0.261 |
| Causes of renal failure | |||
| Glomerulonephritis | 16 (14.7%) | 9 (14.3%) | 0.709 |
| Hypertension | 34 (31.2%) | 18 (28.6%) | |
| Diabetes mellitus | 40 (36.7%) | 29 (46.0%) | |
| Polycystic kidney disease | 4 (3.7%) | 1 (1.6%) | |
| Others | 15 (13.8%) | 6 (9.5%) | |
| Intact parathyroid hormone (pg/mL) | 198 (124–398) | 174 (77–422) | 0.534 |
| Intact parathyroid hormone group (150–300) | |||
| Low | 38 (34.9%) | 27 (42.9%) | 0.352 |
| Intermediate | 35 (32.1%) | 14 (22.2%) | |
| High | 36 (33.0%) | 22 (34.9%) | |
| SVCS≥3 | 23 (21.1%) | 25 (39.7%) | 0.013 |
| Albumin (g/dL) | 3.98 (3.65–4.17) | 3.97 (3.64–4.12) | 0.648 |
| LDL cholesterol (mg/dL) | 90.7±18.4 | 89.7±23.1 | 0.752 |
| HDL cholesterol (mg/dL) | 43.0±12.0 | 40.6±10.8 | 0.192 |
| Triglyceride (mg/dL) | 145.8±76.5 | 132.9±81.1 | 0.298 |
| ALP (IU/L) | 67.8 (59.5–91.5) | 68.1 (53.7–85.9) | 0.468 |
| Ca–P (mg2/dL2) | 45.6 (37.8–50.5) | 43.7 (38.0–51.8) | 0.695 |
| Hb (g/dL) | 10.3 (9.9–10.7) | 10.3 (9.9–10.6) | 0.598 |
| CRP (mg/L) | 2.17 (1.08–4.16) | 2.49 (1.42–7.57) | 0.072 |
| Echo findings | |||
| LV mass index | 119.2±37.7 | 146.8±39.2 | <0.001 |
| Left atrial diameter (cm/m2) | 2.33±0.48 | 2.69±0.40 | <0.001 |
| LV segmental abnormality | 7 (6.4%) | 4 (6.3%) | 1.000 |
| LV systolic dysfunction (LVFS<25%) | 6 (5.5%) | 10 (15.9%) | 0.031 |
| MV disease | 3 (2.8%) | 15 (23.8%) | <0.001 |
| AV disease | 6 (5.5%) | 4 (6.3%) | 1.000 |
All values are expressed as mean±standard deviation, number of patients (%), or median (confidence interval 25–75%).
ALP, alkaline phosphatase; AV, aortic valve; BMI, body mass index; COPD, chronic obstructive lung disease; CRP, C-reactive protein; Hb, hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LV, left ventricle; LVFS, left ventricular fractional shortening; MV, mitral valve; PHT, pulmonary hypertension; SVCS, simple vascular calcification score.
Table 3.
Multivariate logistic regression analysis of risk factors associated with pulmonary hypertension (backward method)
| OR | 95% CI | P | |
|---|---|---|---|
| Hemodialysis | 3.35 | 1.59–7.03 | 0.003 |
| Hypertension | 3.42 | 0.77–15.22 | 0.106 |
| SVCS≥3 | 2.68 | 1.22–5.86 | 0.013 |
| Left atrial diameter (cm/m2) | 11.39 | 10.40–12.47 | 0.005 |
| MV disease | 7.79 | 1.88–32.20 | 0.005 |
Excluded variables: age, ischemic heart disease, high-density lipoprotein cholesterol, C-reactive protein, left ventricular mass index, and left ventricular systolic dysfunction.
CI, confidence interval; MV, mitral valve; OR, odds ratio; SVCS, simple vascular calcification score.
Risk factors that predict the severe VCs
Patients with severe VCs were older and had a significantly higher prevalence of diabetes and PHT. They also had significantly lower intact parathyroid hormone and HDL cholesterol levels (Table 4). To identify the risk factors that independently predict severe VCs, we applied a binary logistic regression model and included all variables with a P value<0.2 in the univariate analysis (Table 5). The presence of diabetes mellitus (OR, 14.23, P<0.001), PHT (OR, 2.66, P=0.030), low parathyroid hormone level (OR, 12.63, P<0.001), and also high parathyroid hormone level (OR, 5.25, P=0.017) were independent risk factors for severe VCs.
Table 4.
Characteristics according to the severe vascular calcifications
| SVCS<2 (n=124) | SVCS≥3 (n=48) | P | |
|---|---|---|---|
| Age, y | 54.7±13.8 | 61.0±11.3 | 0.003 |
| Sex (male) | 59 (47.6%) | 27 (56.3%) | 0.395 |
| Body weight (kg) | 59.4±10.8 | 61.0±8.1 | 0.301 |
| Height (m) | 1.60±0.08 | 1.60±0.08 | 0.923 |
| BMI (kg/m2) | 22.5 (20.8–25.2) | 223.1 (22.0–25.0) | 0.125 |
| Hemodialysis | 58 (46.8%) | 26 (54.2%) | 0.401 |
| Dialysis duration (mo) | 34.5 (19.0–67.0) | 40.5 (16.0–62.5) | 0.782 |
| Comorbidities | |||
| Diabetes mellitus | 34 (27.4%) | 40 (83.3%) | 0.000 |
| Hypertension | 108 (87.1%) | 44 (91.7%) | 0.596 |
| Ischemic heart disease | 9 (7.3%) | 6 (12.5%) | 0.365 |
| COPD | 3 (2.4%) | 4 (8.3%) | 0.096 |
| Intact parathyroid hormone (pg/mL) | 237.2 (147.9–419.7) | 123.4 (71.1–293.0) | 0.001 |
| Intact parathyroid hormone group (150–300) | |||
| Low | 33 (26.6%) | 32 (66.7%) | 0.000 |
| Intermediate | 45 (36.3%) | 4 (8.3%) | |
| High | 46 (37.1%) | 12 (25.0%) | |
| Pulmonary hypertension | 38 (30.6%) | 25 (52.1%) | 0.013 |
| Albumin (g/dL) | 3.9 (3.6–4.1) | 3.8 (3.4–4.1) | 0.070 |
| LDL cholesterol (mg/dL) | 91.6±19.2 | 86.9±22.4 | 0.173 |
| HDL cholesterol (mg/dL) | 41.0 (35.3–48.8) | 37.0 (29.7–44.5) | 0.023 |
| Triglyceride (mg/dL) | 128.5 (98.5–159.2) | 117.3 (83.6–182.3) | 0.724 |
| ALP (IU/L) | 67.1 (58.1–88.5) | 71.6 (58.2–95.0) | 0.352 |
| Ca–P (mg2/dL2) | 44.4 (38.6–51.8) | 45.4 (36.5–50.1) | 0.424 |
| Hb (g/dL) | 10.3 (9.9–10.7) | 10.2 (9.8–10.7) | 0.586 |
| CRP (mg/L) | 2.15 (1.09–4.18) | 2.80 (1.25–5.75) | 0.087 |
All values are expressed as mean±standard deviation, number of patients (%), or median (confidence interval 25–75%).
ALP, alkaline phosphatase; BMI, body mass index; COPD, chronic obstructive lung disease; CRP, C-reactive protein; Hb, hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SVCS, simple vascular calcification score.
Table 5.
Multivariate logistic regression analysis of risk factors associated with severe vascular calcifications (backward method)
| OR | 95% CI | P | |
|---|---|---|---|
| Diabetes mellitus | 14.23 | 5.50–36.82 | <0.001 |
| Intact parathyroid hormone group (150–300) | |||
| Intermediate | – | – | – |
| Low | 12.63 | 3.53–45.14 | <0.001 |
| High | 5.25 | 1.34–20.49 | 0.017 |
| Pulmonary hypertension | 2.66 | 1.09–6.44 | 0.030 |
Excluded variables: age, chronic obstructive lung disease, albumin, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and C-reactive protein.
CI, confidence interval; OR, odds ratio.
Major cardiovascular event-free survival
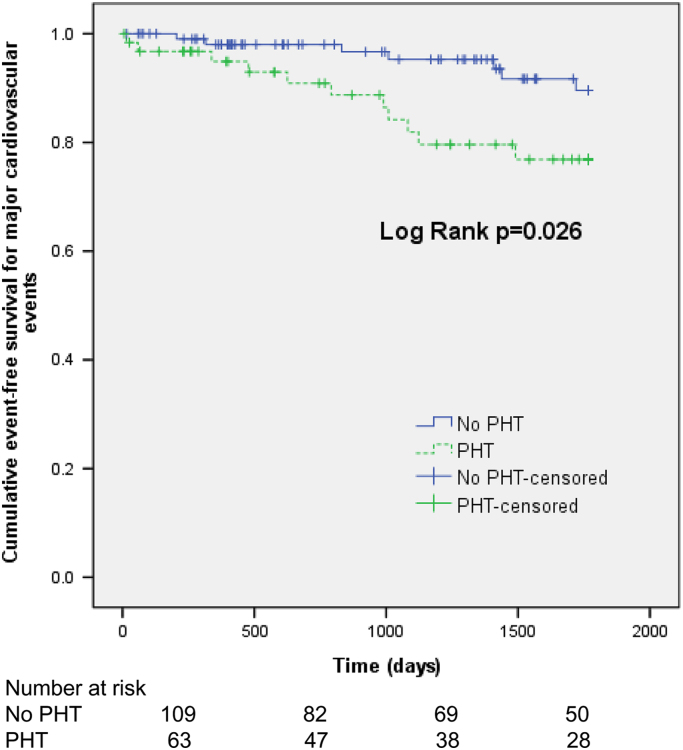
By December 2013, the median follow-up period was 46.9 months. The major cardiovascular events-free survival rates at 1, 2, and 4 years in dialysis patients with PHT were significantly lower than those in patients without PHT (94.9% vs. 98.0%, 92.7% vs. 90.9%, and 79.6% vs. 91.7%, log-rank test P=0.026; Fig. 1). All variables in the cross-sectional study were evaluated using the univariate Cox proportional regression model, and Table 6 shows all variables found to be associated with major cardiovascular events at the P<0.20 level. In a multivariate Cox proportional regression analysis, age [hazard ratio (HR) 1.05, P=0.044], the presence of severe VCs (HR, 4.09, P=0.010), PHT (HR, 3.11, P=0.035), and hemoglobin level (HR, 0.43, P=0.022) were significant predictors for major cardiovascular events.
Figure 1.
Majorcardiovascular event-free survival in end-stage renal disease patients with (dottedline) and without (solid line) pulmonary hypertension (PHT).
Table 6.
Univariate and multivariate Cox regression models for major cardiovascular events
| Univariate |
Multivariate (backward) |
|||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | 1.04 (1.00–1.09) | 0.021 | 1.05 (1.00–1.11) | 0.044 |
| BMI | 1.12 (0.99–1.27) | 0.056 | ||
| Diabetes mellitus | 1.95 (0.75–5.05) | 0.166 | ||
| Intact parathyroid hormone group (150–300) | ||||
| Intermediate | 1.00 | – | 1.00 | – |
| Low | 1.79 (0.32–9.82) | 0.498 | 0.41 (0.06–2.85) | 0.417 |
| High | 5.82 (1.30–26.04) | 0.021 | 4.55 (0.98–20.98) | 0.052 |
| SVCS≥3 | 2.35 (0.92–5.96) | 0.071 | 4.09 (1.40–11.92) | 0.010 |
| Albumin | 0.38 (0.13–1.08) | 0.070 | ||
| ALP (per 10 IU/L) | 1.05 (1.00–1.10) | 0.041 | ||
| Hb | 0.63 (0.34–1.14) | 0.131 | 0.43 (0.21–0.88) | 0.022 |
| LV mass index | 1.01 (1.00–1.02) | 0.044 | ||
| Pulmonary hypertension | 2.80 (1.08–7.23) | 0.033 | 3.11 (1.08–8.98) | 0.035 |
ALP, alkaline phosphatase; BMI, body mass index; CI, confidence interval; Hb, hemoglobin; HR, hazard ratio; LV, left ventricle; SVCS, simple vascular calcification score.
Discussion
In this study, we observed the following: (1) HD patients had a higher prevalence of PHT than PD patients; (2) severe peripheral VCs (SVCS≥3) were independent risk factors for PHT; and (3) in addition to the old age, presence of anemia, or severe VCs, the presence of PHT was an independent predictor of major cardiovascular events in dialysis patents.
PHT is defined as a mean pulmonary artery pressure >25 mmHg using right heart catheterization. However, clinical utility of right heart catheterization has been hampered by the invasive nature of this procedure. Therefore, we used echocardiography as a noninvasive method to diagnose PHT by the modified Bernoulli equation [5–9,15]. In previous studies with CKD patients, estimated PASP ranging from 25 to 45 mmHg was used as the cutoff value of PHT [2]. In the 2009 European Society of Cardiology (ESC) guideline, estimated PASP of 37–50 mmHg was defined as “PHT possible,” and estimated PASP>50 as “PHT likely.” In this study, we defined PHT as an estimated PASP≥37 mmHg based on the ESC guideline [16]. We first observed that a substantial number of dialysis patients had PHT (36.6%) and also that the prevalence of PHT in HD patients was significantly higher than that in PD patients (51.2% vs. 22.7%). This finding is in accordance with several previous studies demonstrating that the prevalence of PHT in HD patients (18.8–68.8%) was significantly higher than that in PD patients (0–42%) [2].
PHT can be classified into five categories according to the World Health Organization classification system: (1) pulmonary arterial hypertension (Group 1); (2) PHT caused by left heart disease (Group 2); (3) PHT caused by chronic lung disease and/or hypoxia (Group 3); (4) chronic thromboembolic PHT (Group 4); and (5) PHT caused by unclear multifactorial mechanisms (Group 5) [1]. PHT occurring in CKD patients can be classified into Groups 1–4 according to the underlying causes of PHT; however, a considerable number of patients (30–50%) are classified into Group 5, that is, PHT caused by unknown or multifactorial mechanisms. The possible causes of PHT in ESRD patients are thought to be increased cardiac output caused by the presence of an arteriovenous fistula, anemia, and/or hypervolemia. In addition, increased pulmonary vascular resistance caused by uremic endothelial dysfunction, pulmonary embolism, pulmonary artery calcification, and increased pulmonary capillary wedge pressure related to heart failure or mitral valve disease might also play an important role [3]. A significantly higher prevalence of PHT in HD patients compared with PD patients suggests that among these factors, increased LV filling pressure caused by the presence of an arteriovenous fistula, a unique feature of HD, is likely to be important because other factors such as anemia, prevalence of LV systolic dysfunction, left atrial diameter, or mitral valve disease did not differ according to dialysis modality in our study.
In the dialysis patients, VC can be measured by cardiac computed tomography (CT), plain X-ray, ultrasound, echocardiography, or pulse wave velocity [17]. Although CT-based imaging has an advantage in quantitative measurement or in sequential monitoring of the progression of calcification, difficulty in distinguishing intima from medial calcification, higher cost, or higher amount of radiation exposure are thought to be its limitations. On the contrary, plain X-ray has merits of lower cost, less radiation exposure, and ability to distinguish medial calcification, but quantitative measurement is impossible. Although ultrasound or pulse wave velocity can also be used for assessing VC, operator dependency or impossibility of direct measurement of calcification is a major limitation, respectively.
In this study, we used plain X-ray to measure SVCS and observed that severe VC (SVCS≥3) is an independent predictor of PHT. VC, a major complication of CKD, theoretically could involve pulmonary vascular beds and subsequently increase PHT risk. Although no studies have demonstrated the association between peripheral and pulmonary VCs, CKD patients with a milieu of accelerated extraosseous calcification may have increased VC not only in the peripheral artery but also in the coronary or pulmonary arteries [10,18]. We previously showed that peripheral VC can predict coronary calcification in PD patients [10]. Although we did not assess pulmonary artery calcification, we observed the significantly higher prevalence of severe peripheral VC in patients with PHT and also that the presence of severe VCs is an independent risk factor for PHT, suggesting that peripheral VC is closely associated with PHT development.
A previous study on dogs reported that high parathyroid hormone levels provoked pulmonary VCs and subsequently reduced pulmonary vascular compliance [4]. Accordingly, several clinical trials also reported that high parathyroid hormone levels can be a risk factor for PHT. However, other clinical trials reported a lack of association between parathyroid hormone levels and PHT [6–9]. Although there were strong relationships between intact parathyroid hormone levels and severe VCs in our study, we observed no association between intact parathyroid hormone levels and PHT. However, further studies are needed to verify the exact relationship between parathyroid hormone and PHT development.
Cardiac dysfunction is a common cause of PHT, and as expected, we observed that the prevalence of mitral valve disease was 10.5% in dialysis patients, being an independent risk factor for PHT in our study. In addition, left atrial diameter but not LV systolic dysfunction or LV mass index was strongly associated with PHT, suggesting that chronic volume overload is a risk factor for PHT regardless of myocardial dysfunction in dialysis patients.
Although it is well-known that PHT is closely associated with volume overload or cardiac diseases, data showing the impact of PHT on cardiovascular mortality or events have been insufficient [7,19–21]. Only one study has reported that PHT can predict cardiovascular mortality and events in HD patients [22]. In this study, we defined major cardiovascular events as acute myocardial infarction or stroke and showed that dialysis patients with PHT had decreased major cardiovascular events-free survivals. In addition, the presence of severe VCs, older age, and decreased hemoglobin levels were also independent predictors for the development of major cardiovascular events in multivariate analysis. Although the precise mechanisms leading to increased cardiovascular events in dialysis patients with PHT are unclear, there is a possibility that increased VC, a well-known risk factor for cardiovascular disease, might be important in PHT development. Therefore, in addition to routine screening of PHT in CKD patients, efforts to maintain proper fluid volume status or to reduce left atrial size by surgical reduction of oversized arteriovenous fistula could be considered when clinically indicated [21,23]. In terms of VC, early initiation of hyperphosphatemia management or use of noncalcium containing phosphate binder is needed in CKD patients [24].
Despite several meaningful findings, there are some limitations to our study. First, this was a retrospective cohort study that enrolled only a limited number of patients with relatively short follow-up period in a single center. Second, the diagnosis of PHT might be inaccurate, because PHT was defined by the indirect method of echocardiography rather than by the direct measure of pulmonary arterial systolic pressure with right heart catheterization. Third, there is a possibility that inconsistency in timing of echocardiography in terms of HD session might affect the results. However, in our analysis, there was no statistically significant difference in the prevalence of PHT according to timing of echocardiography (before or after HD, data not shown). In conclusion, the prevalence of PHT in dialysis patients is substantially high, and the presence of PHT is an independent risk factor for major cardiovascular events. PHT is also closely associated with peripheral VC, suggesting that the mechanisms underlying the development of CKD–mineral bone disorder might be partially responsible for PHT. Careful attention should be paid to the relationship between PHT, peripheral VCs, and cardiovascular disease in the care of ESRD patients, and further studies that could clarify the underlying mechanism are needed.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgments
This study was supported by a grant from Korea University.
References
- 1.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Bolignano D, Rastelli S, Agarwal R, Fliser D, Massy Z, Ortiz A, Wiecek A, Martinez-Castelao A, Covic A, Goldsmith D, Suleymanlar G, Lindholm B, Parati G, Sicari R, Gargani L, Mallamaci F, London G, Zoccali C. Pulmonary hypertension in CKD. Am J Kidney Dis. 2013;61:612–622. doi: 10.1053/j.ajkd.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 3.Sise ME, Courtwright AM, Channick RN. Pulmonary hypertension in patients with chronic and end-stage kidney disease. Kidney Int. 2013;84:682–692. doi: 10.1038/ki.2013.186. [DOI] [PubMed] [Google Scholar]
- 4.Akmal M, Barndt RR, Ansari AN, Mohler JG, Massry SG. Excess PTH in CRF induces pulmonary calcification, pulmonary hypertension and right ventricular hypertrophy. Kidney Int. 1995;47:158–163. doi: 10.1038/ki.1995.18. [DOI] [PubMed] [Google Scholar]
- 5.Amin M, Fawzy A, Hamid MA, Elhendy A. Pulmonary hypertension in patients with chronic renal failure: role of parathyroid hormone and pulmonary artery calcifications. Chest. 2003;124:2093–2097. doi: 10.1378/chest.124.6.2093. [DOI] [PubMed] [Google Scholar]
- 6.Havlucu Y, Kursat S, Ekmekci C, Celik P, Serter S, Bayturan O, Dinc G. Pulmonary hypertension in patients with chronic renal failure. Respiration. 2007;74:503–510. doi: 10.1159/000102953. [DOI] [PubMed] [Google Scholar]
- 7.Kumbar L, Fein PA, Rafiq MA, Borawski C, Chattopadhyay J, Avram MM. Pulmonary hypertension in peritoneal dialysis patients. Adv Perit Dial. 2007;23:127–131. [PubMed] [Google Scholar]
- 8.Nakhoul F, Yigla M, Gilman R, Reisner SA, Abassi Z. The pathogenesis of pulmonary hypertension in haemodialysis patients via arterio-venous access. Nephrol Dial Transplant. 2005;20:1686–1692. doi: 10.1093/ndt/gfh840. [DOI] [PubMed] [Google Scholar]
- 9.Abdelwhab S, Elshinnawy S. Pulmonary hypertension in chronic renal failure patients. Am J Nephrol. 2008;28:990–997. doi: 10.1159/000146076. [DOI] [PubMed] [Google Scholar]
- 10.Kim SC, Kim HW, Oh SW, Yang HN, Kim MG, Jo SK, Cho WY, Kim HK. Low iPTH can predict vascular and coronary calcifications in patients undergoing peritoneal dialysis. Nephron Clin Pract. 2011;117:c113–c119. doi: 10.1159/000319658. [DOI] [PubMed] [Google Scholar]
- 11.Oh SW, Cha JJ, Yang HN, Jo SK, Kim HK. A low intact PTH is associated with simple vascular calcifications in hemodialysis patients. Kidney Res Clin Pract. 2011;30:260–268. [Google Scholar]
- 12.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, John Sutton M, Stewart W. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Adragao T, Pires A, Lucas C, Birne R, Magalhaes L, Goncalves M, Negrao AP. A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1480–1488. doi: 10.1093/ndt/gfh217. [DOI] [PubMed] [Google Scholar]
- 14.National Kidney Foundation K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–S201. [PubMed] [Google Scholar]
- 15.Yigla M, Fruchter O, Aharonson D, Yanay N, Reisner SA, Lewin M, Nakhoul F. Pulmonary hypertension is an independent predictor of mortality in hemodialysis patients. Kidney Int. 2009;75:969–975. doi: 10.1038/ki.2009.10. [DOI] [PubMed] [Google Scholar]
- 16.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 17.Karohl C, D’Marco Gascon L, Raggi P. Noninvasive imaging for assessment of calcification in chronic kidney disease. Nat Rev Nephrol. 2011;7:567–577. doi: 10.1038/nrneph.2011.110. [DOI] [PubMed] [Google Scholar]
- 18.Shroff R, Long DA, Shanahan C. Mechanistic insights into vascular calcification in CKD. J Am Soc Nephrol. 2013;24:179–189. doi: 10.1681/ASN.2011121191. [DOI] [PubMed] [Google Scholar]
- 19.Cirit M, Ozkahya M, Cinar CS, Ok E, Aydin S, Akcicek F, Dorhout Mees EJ. Disappearance of mitral and tricuspid regurgitation in haemodialysis patients after ultrafiltration. Nephrol Dial Transplant. 1998;13:389–392. doi: 10.1093/oxfordjournals.ndt.a027835. [DOI] [PubMed] [Google Scholar]
- 20.Unal A, Sipahioglu M, Oguz F, Kaya M, Kucuk H, Tokgoz B, Buyukoglan H, Oymak O, Utas C. Pulmonary hypertension in peritoneal dialysis patients: prevalence and risk factors. Perit Dial Int. 2009;29:191–198. [PubMed] [Google Scholar]
- 21.Agarwal R. Prevalence, determinants and prognosis of pulmonary hypertension among hemodialysis patients. Nephrol Dial Transplant. 2012;27:3908–3914. doi: 10.1093/ndt/gfr661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z, Liu S, Liang X, Wang W, Fei H, Hu P, Chen Y, Xu L, Li R, Shi W. Pulmonary hypertension as an independent predictor of cardiovascular mortality and events in hemodialysis patients. Int Urol Nephrol. 2014;46:141–149. doi: 10.1007/s11255-013-0486-z. [DOI] [PubMed] [Google Scholar]
- 23.Clarkson MR, Giblin L, Brown A, Little D, Donohoe J. Reversal of pulmonary hypertension after ligation of a brachiocephalic arteriovenous fistula. Am J Kidney Dis. 2002;40:E8. doi: 10.1053/ajkd.2002.34932. [DOI] [PubMed] [Google Scholar]
- 24.Lu KC, Wu CC, Yen JF, Liu WC. Vascular calcification and renal bone disorders. Scientific World Journal. 2014;2014:637065. doi: 10.1155/2014/637065. [DOI] [PMC free article] [PubMed] [Google Scholar]